Abstract
This population-based study examines the association of of lymph node dissection count with survival in patients with oral cavity squamous cell carcinoma using the National Cancer Database.
The standard treatment for early-stage oral cavity squamous cell carcinoma (OCSCC) is definitive surgery. A randomized clinical trial of ipsilateral elective vs therapeutic node dissection in cT1-2 patients with OCSCC demonstrated superior survival in the elective dissection group. Another recent analysis linked higher lymph node count to improved survival in all patients with head and neck cancer with heterogeneous clinical nodal presentations. However, the need for extensive neck dissection in cN0 patients with OCSCC remains unclear. We therefore evaluated the survival impact of lymph node count in these patients using the National Cancer Database (NCDB).
Methods
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It captures approximately 70% of newly diagnosed malignant diseases in the United States. Institutional review board approval was waived by Memorial Sloan Kettering Cancer Center because all data used were public and deidentified. We queried the NCDB to identify cN0, M0, OCSCC patients diagnosed from 2004 to 2012 using the International Classification of Diseases for Oncology (third edition) codes (C020-023, C030-031, C039, C040-041, C048-050, C060-063, and C068-069). We excluded C028-029 to eliminate possible base of tongue cancers. We selected patients who received definitive surgery, and excluded those who received neoadjuvant therapy, died or were lost to follow-up within 3 months of diagnosis or surgery (immortal time bias), had no information on neck nodes, or no neck dissection. The primary end point of the analysis was overall survival (OS), measured from 3 months after surgery to death or lost to follow-up.
We investigated the distribution of node counts and its association with OS using restricted cubic spline and maximally selected rank statistics. A cut-off of 24 was selected based on maximally selected rank statistics. We used Kaplan-Meier methods to estimate OS and Cox regressions to examine the association between OS and clinical and/or surgical factors. We examined model discriminative ability using bootstrapping bias-adjusted concordance probability (c-index) and calibration against the Kaplan-Meier estimated survival. The c-index ranges between 0 and 1 (1 means the model predictions are perfectly concordant with the observed outcomes). Internal validation was performed using bootstrapping with 200 replications. All analyses were performed using SAS statistical software (version 9.4, SAS Institute Inc) and R statistical software (version 3.1, the R Foundation for Statistical Computing).
Results
Final analysis included 7811 patients, with a median follow-up of 48.3 months (95% CI, 47.5-49.3) and OS of 92.2 months (95% CI, 87.0-96.2). The median node count was 23 (range, 1-90); 5998 (77%) patients were pN0. Compared with patients who had 24 or more nodes, those with less than 24 nodes were more likely to have a younger age, advanced clinical and/or pathologic categories, negative margins, adjuvant therapy, surgery at an academic facility, and private insurance.
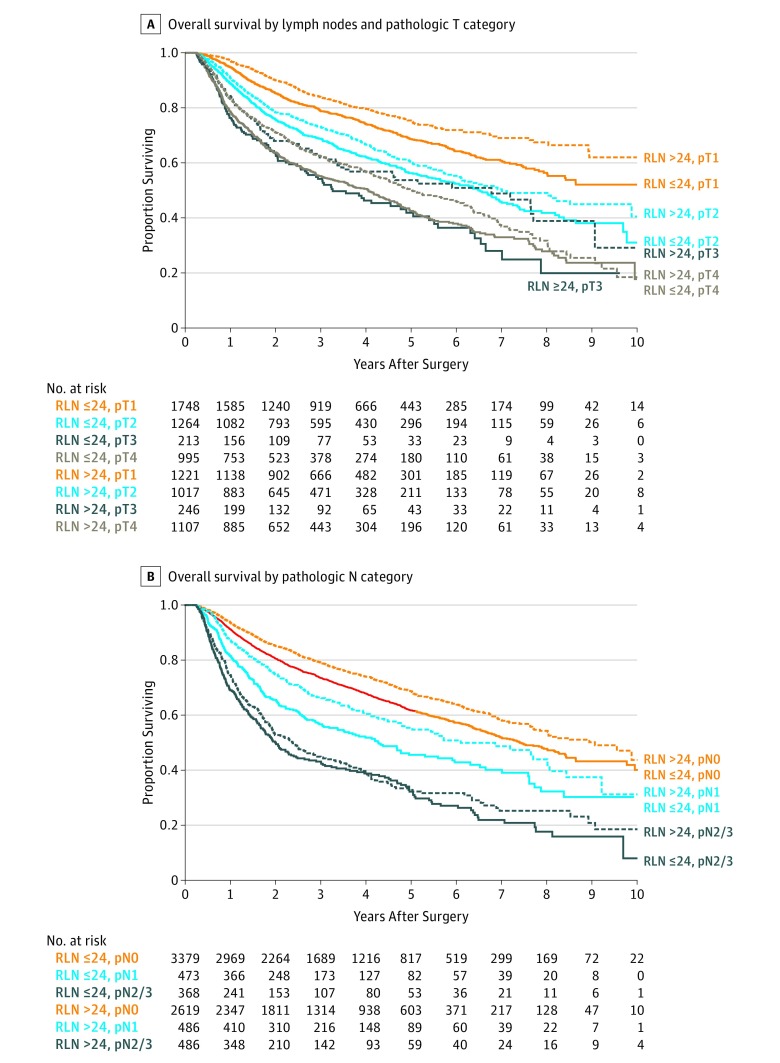
Patients with more than 24 nodes had longer OS compared with those who had 24 or fewer nodes in multivariable analyses (HR, 0.82; 95% CI, 0.75-0.88) (Figure) (Table). Increased age, comorbidity index, stage, and Medicare or Medicaid insurers were associated with increased mortality. Adjuvant radiation was associated with better OS. Similar results were obtained by using nodal cutoffs of 16 and 18 described elsewhere, and by analyzing the nodes by quartiles. The multivariable adjusted c-index was 0.66. There was no interaction of lymph node count and other covariates.
Figure. Overall Survival by Number of Resected Lymph Nodes (RLN).
Kaplan-Meier estimates of overall survival by number of dissected lymph nodes. A, Overall survival by lymph nodes and pathologic T category. B, Overall survival by pathologic N category.
Table. Overall Survival Analysis by Lymph Node Status and Patient Characteristics.
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P Value | HR (95%CI) | P Value | |
| No. of resected nodes, quartilesa | ||||
| 1-14 | 1 [Reference] | <.001 | NA | NA |
| 15-24 | 0.94 (0.86-1.04) | |||
| 25-35 | 0.80 (0.72-0.89) | |||
| 36-90 | 0.88 (0.79-0.98) | |||
| No. of resected nodes | ||||
| ≤24 | 1 [Reference] | NA | 1 [Reference] | <.001 |
| >24 | 0.86 (0.8-0.93) | 0.82 (0.75-0.88) | ||
| Age, y | ||||
| <60 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| ≥60 | 1.68 (1.55-1.82) | 1.3 (1.19-1.43) | ||
| Sex | ||||
| Male | 1 [Reference] | .07 | 1 [Reference] | .02 |
| Female | 0.93 (0.86-1.01) | 0.91 (0.85-0.99) | ||
| Race | ||||
| White | 1 [Reference] | .05 | 1 [Reference] | .61 |
| Black | 1.17 (1-1.37) | 1.07 (0.91-1.25) | ||
| Other | 0.88 (0.73-1.06) | 0.95 (0.78-1.15) | ||
| Comorbidity | ||||
| 0-1 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| 2 | 1.76 (1.53-2.01) | 1.57 (1.37-1.81) | ||
| Disease site | ||||
| Oral tongue | 1 [Reference] | <.001 | 1 [Reference] | .002 |
| Floor of mouth | 1.31 (1.19-1.44) | 1.01 (0.91-1.12) | ||
| Gum | 1.31 (1.17-1.46) | 0.81 (0.71-0.91) | ||
| Palate | 1.49 (1.08-2.05) | 0.99 (0.88-1.11) | ||
| Other | 1.44 (1.29-1.59) | 0.87 (0.62-1.2) | ||
| Payer | ||||
| Private | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Medicaid or Medicare | 1.9 (1.75-2.06) | 1.52 (1.38-1.66) | ||
| Not insured | 1.14 (0.93-1.4) | 1.02 (0.83-1.26) | ||
| Other | 1.54 (1.09-2.19) | 1.31 (0.92-1.87) | ||
| Pathologic T category | ||||
| 1 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| 2 | 1.68 (1.52-1.86) | 1.54 (1.39-1.7) | ||
| 3 | 2.5 (2.15-2.91) | 2.12 (1.81-2.48) | ||
| 4 | 2.52 (2.29-2.77) | 2.25 (2.01-2.52) | ||
| Pathologic N category | ||||
| 0 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| 1 | 1.62 (1.45-1.8) | 1.62 (1.45-1.8) | ||
| 2 | 2.82 (2.56-3.11) | 2.68 (2.41-2.99) | ||
| 3 | 8.75 (3.28-23.34) | 9.57 (3.51-26.05) | ||
| Depth of invasion, mm | ||||
| <40 | 1 [Reference] | 0.21 | 1 [Reference] | .64 |
| ≥40 | 1.02 (0.86-1.22) | 0.94 (0.79-1.12) | ||
| Unknown | 1.1 (0.98-1.24) | 0.95 (0.84-1.06) | ||
| Academic center | ||||
| No | 1 [Reference] | <.001 | 1 [Reference] | .12 |
| Yes | 0.92 (0.85-1.01) | 0.76 (0.58-0.99) | ||
| Unknown | 0.44 (0.34-0.57) | 0.97 (0.89-1.06) | ||
| Adjuvant chemotherapy | ||||
| None | 1 [Reference] | <.001 | 1 [Reference] | .11 |
| Yes | 1.62 (1.47-1.8) | 0.77 (0.6-0.99) | ||
| Unknown | 0.9 (0.7-1.16) | 1.03 (0.91-1.16) | ||
| Adjuvant radiation | ||||
| No or <5000 cGy | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| ≥5000 cGy | 1.31 (1.21-1.41) | 0.84 (0.77-0.92) | ||
| Margins | ||||
| Negative | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Positive | 1.9 (1.71-2.11) | 1.5 (1.35-1.68) | ||
| Unknown | 1.88 (1.4-2.51) | 1.5 (1.12-2.02) | ||
| ECE in pN+a | ||||
| No | 1 [Reference] | <.001 | 1 [Reference] | NA |
| Yes | 1.59 (1.26-1.99) | |||
| Unknown | 1.19 (1.01-1.4) | |||
Abbreviations: ECE, extracapsular extension; NA, not applicable.
Number of resected nodes in quartiles and ECE (only in pN+) are not included in the multivariable model.
Discussion
In this analysis, higher nodal yield (>24) was associated with lower mortality in cN0 patients with OCSCC receiving definitive surgery. Because OCSCC is treated primarily by curative surgery, it is imperative to analyze this disease as a separate entity and account for tumor depth of invasion, a crucial prognostic factor. Furthermore, since locoregional recurrent OCSCC patients have poor salvage outcomes, it is appropriate to use a more stringent nodal yield than previously described.
Limitations
Potential biases of this study include unmeasured confounders, missing data, selection bias, and reporting errors. Regardless, we were able to analyze a large homogeneous cohort to answer a clinically important question.
Conclusion
Based on this analysis, thorough surgical neck evaluation should be advocated for cN0 patients with OCSCC.
References
- 1.D’Cruz AK, Vaish R, Kapre N, et al. ; Head and Neck Disease Management Group . Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373(6):521-529. [DOI] [PubMed] [Google Scholar]
- 2.Divi V, Chen MM, Nussenbaum B, et al. Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol. 2016;(Aug):1. [DOI] [PubMed] [Google Scholar]
- 3.Lausen B, Hothorn T, Bretz F, Schumacher M. Assessment of Optimally Selected Prognostic Factors. Biom J. 2004;46(3):364-374. [Google Scholar]
- 4.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965-970. [Google Scholar]
- 5.Kuo P, Mehra S, Sosa JA, et al. Proposing prognostic thresholds for lymph node yield in clinically lymph node-negative and lymph node-positive cancers of the oral cavity. Cancer. 2016;122(23):3624-3631. [DOI] [PubMed] [Google Scholar]
- 6.Lok BH, Chin C, Riaz N, et al. Irradiation for locoregionally recurrent, never-irradiated oral cavity cancers. Head Neck. 2015;37(11):1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]