Abstract
This study of insurance claims data investigates whether the 2012 issuance of a black box warning against codeine use following tonsillectomy was followed by changes in prescribing patterns.
Tonsillectomy, a common ambulatory pediatric surgery, can result in substantial pain. It remains unclear what constitutes optimal analgesia following tonsillectomy.
Historically, codeine was commonly prescribed for acute pediatric pain, despite known variability in its metabolism. Case reports of respiratory depression and death following codeine administration for posttonsillectomy pain were published in 2009 and 2012, leading to a US Food and Drug Administration (FDA) “black box” warning against codeine use following tonsillectomy on August 15, 2012. This study investigates whether this regulatory change was followed by changes in prescribing patterns for analgesics following pediatric tonsillectomy.
Methods
This study analyzed the Truven Marketscan Commercial Claims and Encounters database, which contains deidentified insurance claims for approximately 50 million privately insured Americans annually. Research using Marketscan is considered non–human subjects research and is exempt from institutional review board review at the University of Washington.
Ambulatory tonsillectomies for patients younger than 18 years during the period 2010 through 2015 were identified. To capture all perioperative prescriptions, analgesics dispensed from 2 weeks prior until 2 days following tonsillectomy were collected and classified as follows: acetaminophen-codeine, acetaminophen-hydrocodone, oxycodone, or “any.” In addition, 14-day postoperative rates of emergency department (ED) visits, both for pain and for all causes, were examined. Cases were classified by period: prior to the black box warning for codeine on August 15, 2012 (“pre–black box”), and afterward (“post–black box”).
The monthly probability of receiving each analgesic was estimated, as well as the probability of a postoperative ED visit. Relative risks were calculated using the score test to calculate 95% confidence intervals. Statistics, graphics, and tables were prepared using R, version 3.2.3 (R Foundation for Statistical Computing).
Results
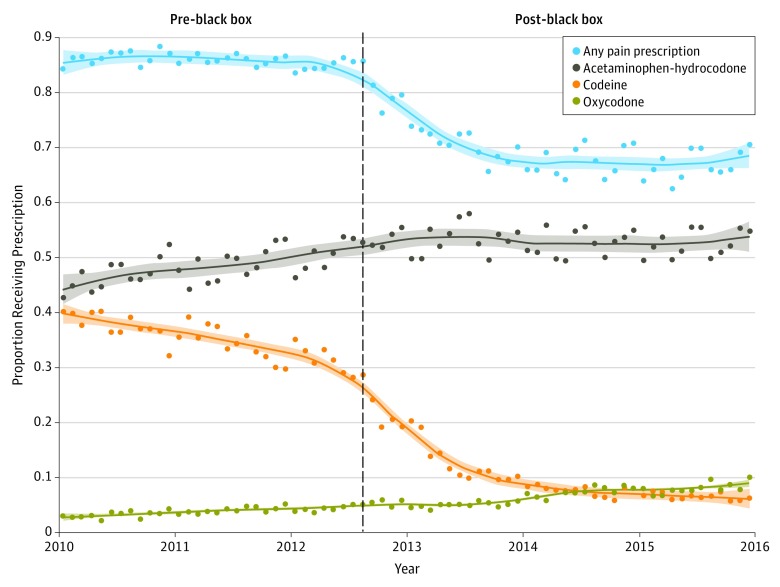
Data on 230 477 tonsillectomies were analyzed. The monthly probability of analgesic use is depicted in the Figure, and the pre–black box probability of analgesic use and ED visits and post–black box relative risks for each are presented in the Table. The relative risk of receiving codeine in the post–black box period was 0.31 (95% CI, 0.31-0.32). The decrease in codeine use appears to have temporally preceded the black box warning, likely as awareness of the safety concerns increased among prescribers prior to the publication of the black box warning.
Figure. Dispensed Postoperative Prescription Analgesics Following Ambulatory Pediatric Tonsillectomy, 2010 Through 2015.
Data include all prescribed analgesics dispensed from 2 weeks prior to surgery until 2 days following ambulatory pediatric tonsillectomy. Vertical dashed line indicates the date when the US Food and Drug Administration issued a black box warning on codeine. Loess curves depicting trends are plotted for each drug. Shading indicates 95% confidence interval.
Table. Relative Risks of Postoperative Prescription Analgesic Use and Relevant Outcomes Following Ambulatory Pediatric Tonsillectomy Before and After the US Food and Drug Administration Issued a Black Box Warning on Codeine.
| Parameter | % | Relative Risk Post Compared With Pre (95% CI)a | |
|---|---|---|---|
| Pre–Black Box (n = 125 354) |
Post–Black Box (n = 10 123) |
||
| Prescriptions | |||
| Any prescription analgesic | 86.2 | 70.5 | 0.82 (0.81-0.82) |
| Acetaminophen/hydrocodone | 49.1 | 53.6 | 1.09 (1.08-1.10) |
| Codeine | 34.7 | 10.9 | 0.31 (0.31-0.32) |
| Oxycodone | 4.0 | 6.7 | 1.66 (1.60-1.72) |
| 14-Day postoperative emergency department visits | |||
| For pain | 1.6 | 1.8 | 1.12 (1.05-1.19) |
| For any reason | 6.7 | 6.8 | 1.01 (0.98-1.04) |
Statistical significance for changes in relative risk is defined by confidence intervals that exclude a relative risk of 1.
Compared with the period before the FDA warning, the relative risk of receiving postoperative hydrocodone after the warning was 1.09 (95% CI, 1.08-1.10) and the relative risk for receiving oxycodone was 1.66 (95% CI, 1.60-1.72). The post–black box relative risk of receiving any prescription analgesic was 0.82 (95% CI, 0.81-0.82).
In the pre–black box period, 1.6% of patients returned to the ED for pain within 14 days following tonsillectomy, and 6.7% returned to the ED for any reason. In the post–black box period, the relative risk of an ED visit for pain was 1.12 (95% CI, 1.05-1.19), whereas no statistically significant change in all-cause ED visits was observed.
Discussion
The FDA black box warning against use of codeine following pediatric tonsillectomy appears to have been temporally associated with a substantially decreased risk of receiving codeine, as well as increases in use of alternative prescription analgesic agents. A small increase in the risk of visiting the ED for pain was observed in the same period, but no change in overall ED use was observed.
This study has 3 major limitations. First, it contains no data on publicly insured children. Second, physician-recommended use of over-the-counter drugs is not recorded. Finally, without granular outcome data, it is impossible to report on patient-centered outcomes (eg, quality of pain control). The effect of the observed decrease in codeine prescribing on outcomes such as hospital revisits and rare but devastating respiratory complications following tonsillectomy remains open for further research.
References
- 1.Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002;89(6):839-845. [DOI] [PubMed] [Google Scholar]
- 2.Ciszkowski C, Madadi P, Phillips MS, Lauwers AE, Koren G. Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med. 2009;361(8):827-828. [DOI] [PubMed] [Google Scholar]
- 3.Kelly LE, Rieder M, van den Anker J, et al. . More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129(5):e1343-e1347. [DOI] [PubMed] [Google Scholar]
- 4.Center for Drug Evaluation and Research Drug safety and availability - FDA drug safety communication: codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death. https://www.fda.gov/Drugs/DrugSafety/ucm313631.htm. Accessed February 24, 2016.
- 5.Koopman PAR. Confidence intervals for the ratio of two binomial proportions. Biometrics. 1984;40(2):513-517. [Google Scholar]
- 6.Fagerland MW, Lydersen S, Laake P. Recommended confidence intervals for two independent binomial proportions. Stat Methods Med Res. 2015;24(2):224-254. [DOI] [PubMed] [Google Scholar]