Key Points
Question
Is systemic inflammation (manifest as elevated neutrophil to lymphocyte ratio) associated with sarcopenia (reduced skeletal muscle mass), and are these 2 risk factors combined associated with survival after colorectal cancer (CRC) diagnosis?
Findings
In a cohort of 2470 patients with nonmetastatic CRC, elevated neutrophil to lymphocyte ratio before diagnosis was associated with at-diagnosis sarcopenia in a dose-response manner; patients with both sarcopenia and neutrophil to lymphocyte ratio of 3 or greater (vs neither) had double the risk of death overall and from CRC.
Meaning
Sarcopenia and inflammation predicted worse CRC prognosis regardless of stage. Because these 2 biomarkers are commonly collected and potentially modifiable, they have high potential for clinical use in prognostication and possibly in guiding intervention.
This cohort study of patients with early-stage colorectal cancer examines whether prediagnostic systemic inflammation is associated with at-diagnosis sarcopenia and explores whether these factors interact to predict cancer survival.
Abstract
Importance
Systemic inflammation and sarcopenia are easily evaluated, predict mortality in many cancers, and are potentially modifiable. The combination of inflammation and sarcopenia may be able to identify patients with early-stage colorectal cancer (CRC) with poor prognosis.
Objective
To examine associations of prediagnostic systemic inflammation with at-diagnosis sarcopenia, and determine whether these factors interact to predict CRC survival, adjusting for age, ethnicity, sex, body mass index, stage, and cancer site.
Design, Setting, and Participants
A prospective cohort of 2470 Kaiser Permanente patients with stage I to III CRC diagnosed from 2006 through 2011.
Exposures
Our primary measure of inflammation was the neutrophil to lymphocyte ratio (NLR). We averaged NLR in the 24 months before diagnosis (mean count = 3 measures; mean time before diagnosis = 7 mo). The reference group was NLR of less than 3, indicating low or no inflammation.
Main Outcomes and Measures
Using computed tomography scans, we calculated skeletal muscle index (muscle area at the third lumbar vertebra divided by squared height). Sarcopenia was defined as less than 52 cm2/m2 and less than 38 cm2/m2 for normal or overweight men and women, respectively, and less than 54 cm2/m2 and less than 47 cm2/m2 for obese men and women, respectively. The main outcome was death (overall or CRC related).
Results
Among 2470 patients, 1219 (49%) were female; mean (SD) age was 63 (12) years. An NLR of 3 or greater and sarcopenia were common (1133 [46%] and 1078 [44%], respectively). Over a median of 6 years of follow-up, we observed 656 deaths, 357 from CRC. Increasing NLR was associated with sarcopenia in a dose-response manner (compared with NLR < 3, odds ratio, 1.35; 95% CI, 1.10-1.67 for NLR 3 to <5; 1.47; 95% CI, 1.16-1.85 for NLR ≥ 5; P for trend < .001). An NLR of 3 or greater and sarcopenia independently predicted overall (hazard ratio [HR], 1.64; 95% CI, 1.40-1.91 and HR, 1.28; 95% CI, 1.10-1.53, respectively) and CRC-related death (HR, 1.71; 95% CI, 1.39-2.12 and HR, 1.42; 95% CI, 1.13-1.78, respectively). Patients with both sarcopenia and NLR of 3 or greater (vs neither) had double the risk of death, overall (HR, 2.12; 95% CI, 1.70-2.65) and CRC related (HR, 2.43; 95% CI, 1.79-3.29).
Conclusions and Relevance
Prediagnosis inflammation was associated with at-diagnosis sarcopenia. Sarcopenia combined with inflammation nearly doubled risk of death, suggesting that these commonly collected biomarkers could enhance prognostication. A better understanding of how the host inflammatory/immune response influences changes in skeletal muscle may open new therapeutic avenues to improve cancer outcomes.
Introduction
Identifying which patients with early-stage cancer are at high risk of adverse treatment outcomes and premature mortality is a clinical priority. Two novel prognostic indicators receiving increasing attention across cancer types are sarcopenia (low skeletal muscle mass) and an elevated neutrophil-to-lymphocyte ratio (NLR, a measure of systemic inflammation). Sarcopenia predicts poor surgical outcomes, treatment toxic effects, and reduced survival. Routine diagnostic imaging using computed tomography (CT) can be used to accurately quantify muscle mass, revealing sarcopenia that might otherwise go undetected. Similarly, pretreatment values of NLR and related blood biomarkers (eg, platelet-to-lymphocyte ratio [PLR] and lymphocyte-to-monocyte ratio [LMR]) are commonly measured and predict treatment response and survival. Whereas both sarcopenia and inflammation can be evaluated with existing clinical data and may be modifiable, the relationship between these 2 factors and their independent associations with survival are not well studied.
Recent trials treating cachexia with ω-3 fatty acid supplementation and nonsteroidal anti-inflammatory drugs underscore the importance of systemic inflammation as a driver of muscle degradation in patients with late-stage disease. Proinflammatory cytokines and growth factors released as part of the systemic inflammatory response to the tumor have profound catabolic effects on host metabolism, which lead to muscle breakdown. Low muscularity could contribute to local inflammation in the muscle, leading to further breakdown and driving systemic inflammation. In turn, this inflammatory cycle could enhance tumor aggressiveness or reduce treatment response, impairing the transition into survivorship. Whereas lower muscle mass also has deleterious consequences for morbidity and/or mortality in patients with early-stage disease, little research examines whether systemic inflammation predicts muscle mass in early-stage cancer.
To our knowledge, no prior study examines combined associations of the host systemic inflammatory response and sarcopenia with colorectal cancer (CRC) survival. Not only are these factors important as prognostic indicators, they are also potentially modifiable. Colorectal cancer, a leading cause of cancer death, is an ideal setting in which to evaluate this relationship because of the availability of CT images and laboratory blood biomarkers. Among 2470 patients with a diagnosis of American Joint Committee on Cancer stage I to III CRC, we examined the association of prediagnostic NLR with at-diagnosis sarcopenia. Subsequently, we assessed the independent and combined associations of these risk factors with survival. We also conducted sensitivity analyses to examine whether associations were consistent across other inflammatory biomarkers and subgroups defined by body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), stage, age, and sex.
Methods
Study Population
The “C SCANS” (Colorectal Cancer: Sarcopenia, Cancer, and Near-term Survival) cohort, described in detail elsewhere, included 3276 Kaiser Permanente Northern California (KPNC) health plan members who received a diagnosis of stage I to III CRC between 2006 and 2011 who underwent surgical resection and had an abdominal CT scan at diagnosis of sufficient image quality to analyze. We further restricted the sample to those with prediagnostic NLR values available from clinically acquired laboratory data (n = 2470). Patients included and excluded due to insufficient data were similar with respect to BMI, race/ethnicity, sex, age, stage, and survival duration. The study was approved by the KPNC Institutional Review Board. Informed consent was waived due to the retrospective nature of the study.
Body Composition and BMI
We selected the height and weight obtained closest to the CT scan measured by KPNC medical assistants and computed BMI, categorized as less than 20, 20 to less than 25, 25 to less than 30, 30 to less than 35, or at least 35. “At-diagnosis” muscle mass was assessed from a CT scan taken before chemotherapy or radiation therapy (if received). On average, scans were 2 days before diagnosis (range, −2 to 4 months; 1951 [79%] presurgical). A single, trained researcher at the University of Alberta (J.X.) selected the third lumbar vertebra and analyzed the cross-sectional area of muscle in centimeters squared according to predefined tissue-specific Hounsfield units ranges using Slice-O-Matic Software, version 5.0 (Tomovision). Single-slice muscle area at the third lumbar vertebra is strongly correlated with whole-body volume of muscle tissue and has been extensively used in oncology settings. As in prior publications, we used optimal stratification to select BMI and sex-specific cutoffs for skeletal muscle index (muscle area in centimeters squared divided by squared height in meters squared) to define sarcopenia: for normal or overweight patients (BMI < 30), these were less than 52 cm2/m2 for men and less than 38 cm2/m2 for women, while for obese patients (BMI ≥ 30), these were less than 54 cm2/m2 for men and less than 47 cm2/m2 for women.
Markers of Systemic Inflammation
Our primary measure of systemic inflammation was NLR from laboratory values obtained as part of routine blood tests (all measurements were prior to the diagnostic scan, surgery, or other treatment). We averaged all available NLR measures in the 24 months prior to diagnosis (mean number of NLR measures was 3; mean interval before diagnosis was 7 months) and categorized this average using standard cutoffs to define “normal” (<3), “moderate” (3 to <5), and “high” (≥5) inflammation. Secondary biomarkers of inflammation (eTable 1 in the Supplement) were available at the same time point; we averaged and categorized available measures using clinically relevant cutoffs (PLR of <150, 150 to <300, or ≥300 and LMR of <2, 2 to <3, 3 to <4, or ≥4 wherein higher PLR and lower LMR indicate more severe inflammation). Serum albumin level, of particular interest as a marker not only of nutritional status but also of systemic inflammation, was available for a subset of 716 participants, and dichotomized at less than 3.5 g/dL (the cutoff for hypoalbuminemia; to convert to grams per liter, multiply by 10).
Other Covariates and End Points
We reviewed the KPNC electronic medical record and Cancer Registry for information on disease stage, tumor characteristics, surgery, treatment (chemotherapy or radiation therapy), and demographic (eg, age, race/ethnicity, and sex) and health characteristics (Charlson comorbidity index). We obtained data on overall and CRC-specific mortality from the KPNC mortality file, composed of data from the California Department of Vital Statistics, US Social Security Administration, and KPNC health care utilization data.
Statistical Analysis
In exploratory analyses, we tabulated descriptive statistics (mean [SD] and percentages). Next, we conducted logistic regression for categorical NLR (<3, 3-5, ≥5) as a predictor of sarcopenia (yes or no), with the P value for a linear trend evaluated by treating the median of each NLR category in multivariable models as a continuous predictor. Using multivariable-adjusted linear regression, we evaluated differences in muscle in centimeters squared by category of NLR and secondary biomarkers. Models were adjusted for race/ethnicity, sex, cancer site, and at-diagnosis values of age, BMI category, and cancer stage.
In analyses in which survival was the outcome, we cross-classified NLR of 3 or greater and sarcopenia in 4 categories (inflammation only, sarcopenia only, both, or neither [reference]) and calculated Kaplan-Meier curves. Next, we examined NLR of 3 or greater and sarcopenia as independent (mutually adjusted) predictors of survival in multivariable-adjusted Cox proportional hazards models. Models were adjusted for race/ethnicity, sex, cancer site, and at-diagnosis values of age, BMI category, and cancer stage. We further adjusted for treatment (chemotherapy and/or radiation therapy) and Charlson comorbidity index. Participants were observed from diagnosis until death from any cause, death from CRC, or the end of follow-up (December 31, 2015). For overall mortality, individuals alive at the end of follow-up were censored at that time. For CRC mortality, individuals who died of other causes during follow-up were censored at that time. Evaluations for the presence of multiplicative interaction between NLR of 3 or greater and sarcopenia used the likelihood ratio test (LRT).
Finally, given the existing literature suggesting associations of BMI category, sex, age, and stage at diagnosis with CRC survival, we evaluated the main associations of each variable with survival, as well as possible interactions of these variables with NLR and/or sarcopenia using stratified analyses and LRTs.
Two-sided P < .05 was considered significant. We used Statistical Analysis Software, version 9.3 (SAS, Inc) for all statistical analyses.
Results
Table 1 presents the characteristics of 2470 patients with nonmetastatic CRC by category of prediagnostic NLR. Patients with a higher NLR in the 24 months prior to diagnosis had less favorable values for all other markers of systemic inflammation: higher PLR, lower LMR, and lower serum albumin level. Patients with greater systemic inflammation prior to diagnosis were older, more likely to be female, to be non-Hispanic white, to have colon (vs rectal) cancer, and to have stage II or III (vs I) cancer. A majority were overweight (864 [35%]) or obese (786 [32%]). The prevalence of sarcopenia and prediagnosis NLR of 3 or greater were 46% (n = 1133) and 44% (n = 1078), respectively.
Table 1. Characteristics by Level of Systemic Inflammation Prior to Diagnosis in 2470 Patients With Nonmetastatic Colorectal Cancer (CRC).
| Characteristica | NLR Prior to CRC Diagnosis | ||
|---|---|---|---|
| <3 (n = 1337) |
3 to <5 (n = 646) |
≥5 (n = 487) |
|
| NLR, median (IQR) | 2.0 (1.6-2.4) | 3.8 (3.4-4.3) | 6.9 (5.8-9.9) |
| Other inflammatory markers, median (IQR) | |||
| Platelet to lymphocyte ratio | 129 (103-166) | 185 (150-236) | 273 (205-370) |
| Lymphocyte to monocyte ratio | 3.6 (3.0-4.6) | 2.5 (2.0-3.1) | 1.8 (1.3-2.4) |
| Albumin level, g/dLb | 4.2 (3.8-4.4) | 3.9 (3.5-4.2) | 3.7 (3.1-4.2) |
| Time from NLR to scan, mean (SD), mo | −6 (6) | −5 (6) | −5 (6) |
| Age at diagnosis, mean (SD), y | 62 (11) | 63 (12) | 65 (12) |
| Charlson comorbidity score, mean (SD) | 0.8 (1.3) | 1.0 (1.6) | 1.4 (1.8) |
| Body mass index, mean (SD) | 28 (6) | 28 (6) | 28 (7) |
| Race/ethnicity, No. (%) | |||
| Non-Hispanic white | 842 (63) | 433 (67) | 346 (71) |
| Black | 120 (9) | 45 (7) | 24 (5) |
| Hispanic | 160 (12) | 84 (13) | 34 (7) |
| Asian/Pacific islander | 214 (16) | 84 (13) | 78 (16) |
| Other | 13 (1) | 6 (1) | 5 (1) |
| Sex, No. (%) | |||
| Female | 615 (46) | 336 (52) | 268 (55) |
| Male | 722 (54) | 310 (48) | 219 (45) |
| Cancer stage, No. (%) | |||
| I | 428 (32) | 155 (24) | 107 (22) |
| II | 388 (29) | 233 (36) | 185 (38) |
| III | 508 (38) | 258 (40) | 200 (41) |
| Cancer site, No. (%) | |||
| Colon | 936 (70) | 491 (76) | 394 (81) |
| Rectum | 401 (30) | 155 (24) | 93 (19) |
Abbreviations: IQR, interquartile range; NLR, neutrophil to lymphocyte ratio.
SI conversion factor: To convert albumin to grams per liter, multiply by 10.
Percentages may not total 100 because of rounding.
Albumin level was only available on a subset of 716 patients.
A higher prediagnostic NLR was associated in a dose-response manner with the odds of sarcopenia independent of race/ethnicity, cancer site, age, stage, and BMI: compared with patients with NLR of less than 3, the odds ratios (ORs) for sarcopenia were 1.35 (95% CI, 1.10-1.67) for NLR of 3 to less than 5 and 1.47 (95% CI, 1.16-1.85) for NLR of 5 or greater (P for trend across categories, <.001). Results were consistent across other markers of systemic inflammation, with higher PLR, lower LMR, and hypoalbuminemia all associated with higher odds of sarcopenia (eTable 1 in the Supplement), and, in continuous analyses, with lower skeletal muscle index (eTable 2 in the Supplement). Furthermore, the association of elevated NLR with increased odds of sarcopenia was consistent across stage, age, and sex (no evidence of interaction) (eTable 3 in the Supplement).
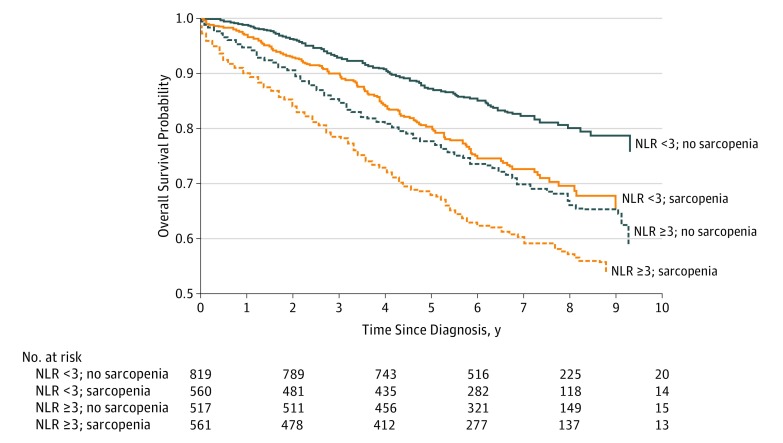
As observed in the Kaplan-Meier curves (Figure), patients with NLR of 3 or greater and sarcopenia had the worst survival, whereas patients with NLR of less than 3 and no sarcopenia survived the longest (log-rank P < .001) (Figure). eFigure 1 in the Supplement shows the multivariable-adjusted results for overall survival in each category defined by the presence (or absence) of NLR of 3 or greater and/or sarcopenia. These risks are overlaid on the distribution of the categories according to age at diagnosis, illustrating that although sarcopenia and inflammation were more common in older patients, both conditions occurred across age groups and were associated with survival: for example, 200 (16%) of patients younger than 65 years at diagnosis had both sarcopenia and inflammation. Survival probabilities for NLR of 3 or greater and sarcopenia are shown separately in eFigures 2 and 3 in the Supplement, respectively.
Figure. Kaplan-Meier Survivorship Function According to Neutrophil to Lymphocyte Ratio and Sarcopenia Status in 2470 Patients With Nonmetastatic Colorectal Cancer .
NLR indicates neutrophil to lymphocyte ratio.
In multivariable analyses, the interaction of sarcopenia with NLR was nonsignificant. Thus, we report the independent (mutually adjusted) associations of each risk factor with overall and CRC-specific survival (Table 2) in the form of hazard ratios (HRs). Neutrophil-to-lymphocyte ratio of 3 or greater and sarcopenia independently predicted overall survival (HR, 1.64; 95% CI, 1.40-1.91 and HR, 1.28; 95% CI, 1.10-1.53, respectively), as well as CRC-specific survival (HR, 1.71; 95% CI, 1.39-2.12 and HR, 1.42; 95% CI, 1.13-1.78, respectively). Under the model of independent effects (Table 2), a patient with both sarcopenia and NLR of 3 or greater was estimated to have much worse overall (HR, 2.12; 95% CI, 1.70-2.65) and CRC-specific survival (HR, 2.43; 95% CI, 1.79-3.29) than a patient with neither risk factor.
Table 2. Neutrophil to Lymphocyte Ratio (NLR), Sarcopenia, and Colorectal Cancer (CRC) Survival in 2470 Patients With Nonmetastatic CRCa.
| Independent, Mutually Adjusted Associations | HR (95% CI)b | |
|---|---|---|
| Overall Survival (674 Events) |
CRC Survival (357 Events) | |
| Sarcopenia | ||
| Yes | 1.28 (1.10-1.53) | 1.42 (1.13-1.78) |
| No | 1 [Reference] | 1 [Reference] |
| NLR | ||
| ≥3 | 1.64 (1.40-1.91) | 1.71 (1.39-2.12) |
| <3 | 1 [Reference] | 1 [Reference] |
| NLR ≥ 3 and sarcopenia | ||
| Both | 2.12 (1.70-2.65) | 2.43 (1.79-3.29) |
| Neither | 1 [Reference] | 1 [Reference] |
Abbreviations: HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio.
Cox proportional hazards models adjust for race/ethnicity (black, Hispanic, Asian/Pacific islander, other, or non-Hispanic white), cancer site (colon or rectum), and at-diagnosis values of age (years), body mass index category (<20, 20 to <25, 25 to <30, 30 to <35, or ≥35; calculated as weight in kilograms divided by height in meters squared), sex (male or female), and stage (I, II, or III).
All HR estimates are from the same model in which sarcopenia and NLR of 3 or greater are mutually adjusted, assuming independent effects.
As in previous studies, older age, later stage, and BMI of greater than 35 were associated with lower overall survival (data not shown). However, there was no statistical evidence that BMI, stage, age, or sex modified the associations of NLR of 3 or greater or sarcopenia with overall or CRC survival. Indeed, even stage I (HR, 1.81; 95% CI, 1.07-3.09) and younger patients (<65 years, HR, 1.65; 95% CI, 1.15-2.37) with NLR of 3 or greater and sarcopenia had nearly 2-fold increased risk of death from any cause compared with patients of similar age and stage but with neither condition (Table 3).
Table 3. Neutrophil to Lymphocyte Ratio (NLR), Sarcopenia, and Colorectal Cancer (CRC) Survival Stratified by Body Mass Index, Stage, Age, and Sex (n = 2470)a.
| Stratification Variable | HR (95% CI)b | |
|---|---|---|
| Overall Survival | CRC Survival | |
| BMI | ||
| <20 | 1.63 (0.48-5.59) | 4.02 (0.60-27.06) |
| 20 to <25 | 2.53 (1.66-3.87) | 3.24 (1.81-5.81) |
| 25 to <30 | 1.81 (1.22-2.70) | 2.01 (1.14-3.53) |
| 30 to <35 | 2.30 (1.38-3.82) | 2.07 (1.08-4.04) |
| ≥35 | 2.95 (1.58-5.54) | 2.78 (1.13-6.85) |
| Cancer stage | ||
| I | 1.81 (1.07-3.09) | 4.21 (1.45-12.23) |
| II | 1.54 (1.02-2.31) | 1.79 (0.96-3.33) |
| III | 2.79 (2.05-3.81) | 2.56 (1.77-3.73) |
| Age at diagnosis, y | ||
| <65 | 1.65 (1.15-2.37) | 1.70 (1.09-2.68) |
| ≥65 | 2.32 (1.75-3.08) | 3.15 (2.05 4.84) |
| Sex | ||
| Male | 2.05 (1.49-2.83) | 2.16 (1.39-3.35) |
| Female | 2.30 (1.67-3.17) | 2.71 (1.74-4.23) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio.
Cox proportional hazards models adjust for race/ethnicity (black, Hispanic, Asian/Pacific islander, other, or non-Hispanic white), cancer site (colon or rectum), and at-diagnosis values of age (years), BMI category (<20, 20 to <25, 25 to <30, 30 to <35, or ≥35), sex (male or female), and stage (I, II, or III), unless stratified by those variables.
All HRs compare patients with NLR of 3 or greater and sarcopenia vs patients with NLR less than 3 and no sarcopenia. All HR estimates are from the same model in which sarcopenia and NLR of 3 or greater are mutually adjusted, assuming independent effects.
In sensitivity analyses, further adjustment for cancer treatment and Charlson comorbidity index did not alter the HRs; these variables were excluded from models.
Discussion
To our knowledge, this is the largest study to examine the relationship of markers of systemic inflammation to muscle mass in CRC, and the only one to examine independent and combined associations with survival. In our cohort of 2470 patients with stage I to III CRC, we found that greater NLR in the months prior to diagnosis was associated with sarcopenia at diagnosis. In addition, we found that the co-occurrence of high NLR and sarcopenia was associated with double the mortality risk among patients with nonmetastatic CRC. Whereas both NLR and sarcopenia increase with age and stage, they identify high-risk subgroups of patients across the spectrum of age and stage. Measures of inflammation and sarcopenia are easily obtainable in the clinical setting and are each independently, and in combination, powerful prognostic indicators in patients with nonmetastatic cancer.
Although sarcopenia and systemic inflammation have previously been associated with prognosis in CRC and other cancers, most studies addressed them individually and in patients with metastatic disease. Our study newly suggests that similar processes occur in nonmetastatic CRC: elevated NLR in the months prior to diagnosis and other aberrations in inflammatory markers were present in nearly half of patients, were associated with sarcopenia, and were predictive of survival. Furthermore, we observed similar results regardless of stage at diagnosis, suggesting that muscle breakdown is not solely an artifact of increasing stage of disease; some of the same mechanisms implicated in cancer cachexia may be operating earlier on in the cancer trajectory and leading to relatively lower muscle mass long before the extreme muscle loss that is the hallmark of cachexia. Our findings that measures of inflammation were associated with sarcopenia and that the co-occurrence of inflammation and sarcopenia were associated with a high mortality risk are consistent with the limited prior literature on this topic. For example, a cross-sectional study of 763 patients with stage I to IV CRC found that preoperative NLR and serum albumin levels were associated with compromised muscle mass. With respect to survival, 1 prior study conducted in 117 men with small-cell lung cancer compared patients classified according to the co-occurrence of NLR and sarcopenia; consistent with our results, overall and progression-free survival were worse in the group with both sarcopenia and elevated NLR.
We hypothesize that inflammation both underlies and is enhanced by muscle breakdown, part of a mutually reinforcing cycle conducive to cancer progression. Markers of systemic inflammation such as NLR are correlated with elevated circulating concentrations of various cytokines in CRC, and these are implicated in the activation of several catabolic pathways. For example, cytokines such as tumor necrosis factor and interleukin 6 are produced by the tumor or surrounding cells and promote protein degradation and decreased synthesis. Tumor necrosis factor inhibits skeletal myocyte differentiation, promotes muscle atrophy, and contributes to insulin resistance by impairing the insulin signaling pathway. Interleukin 6 can further reduce muscle protein synthesis. Increases in inflammatory cytokines can also lead to insulin resistance and muscle wasting by activation of the ubiquitin-proteasome proteolytic pathway, while muscle loss itself further exacerbates insulin resistance. Low-grade, systemic inflammation caused by the tumor (and exacerbated possibly by obesity or insulin resistance) could drive local inflammation in the muscle. This, in turn, contributes further to systemic inflammation and muscle degradation (through both increased proteolysis, manifesting in decreased muscle mass [sarcopenia], and increased lipid deposition in skeletal muscle [measured via CT muscle radiodensity]). Although outside the scope of this analysis, excess fat may also play a role in this vicious cycle: most of our patients were overweight or obese. Intramuscular lipid levels, increased in obesity, can promote lipotoxicity and insulin resistance, as well as enhancing secretion of some proinflammatory myokines capable of both inducing muscle dysfunction and exacerbating chronic, low-grade systemic inflammation.
Importantly, low BMI is not sufficient to identify low muscle mass in an inflammatory and catabolic disease such as CRC, particularly because obesity is common. Among the patients with nonmetastatic disease in this study, fewer than 5% had a BMI of less than 20, yet 44% had sarcopenia and 46% had a prediagnosis NLR of 3 or greater. These factors may help identify patients with an elevated mortality risk where BMI and/or weight change cannot. Using clinically acquired CT images to aid in the early identification of sarcopenia could facilitate therapeutic intervention to ensure a successful transition into survivorship. Treatments might include anti-inflammatory drugs and/or resistance training, which is proven to be safe and effective in maintaining and/or increasing muscle mass and function in patients with cancer, improves quality of life, and is associated with longer survival.
This study cannot disentangle the web of bidirectional relationships among inflammation, body composition, and cancer progression. Although there was a temporal order to our data—patients who presented with sarcopenia at diagnosis had elevated levels of NLR over the 24 months prior to diagnosis—we cannot determine whether this consistently inflamed state precipitated sarcopenia or whether these are concurrent conditions. Cancer begins years before diagnosis, yet the appearance of sarcopenia was evaluated once, for the first time, at diagnosis; thus, we cannot determine how muscle mass changes in relation to cancer initiation or the onset of inflammation. Furthermore, whereas sarcopenia and elevated NLR were independently associated with survival, we do not know to what extent inflammation or sarcopenia are consequences of a more aggressive tumor or a shared pathologic mechanism vs conditions that enhance tumor growth.
We called the condition of low muscle “sarcopenia” in our study because patients had nonmetastatic disease and did not exhibit the degree of weight loss characteristic of cachexia. However, we cannot truly distinguish whether patients (1) have low muscle because of normal aging (sarcopenia) and sarcopenia exacerbates their mortality risk once they have cancer, (2) are precachexic, although early in the trajectory, or (3) both. Inflammation underlies both sarcopenia and cachexia, but recent reviews suggest that the pathogenesis of muscle loss in normal aging differs from cancer cachexia, in which activation of the ubiquitin-proteasome system and nuclear factor–κB signaling result in rapid atrophy. Further research is needed to elucidate the biological sequence of inflammation, changes in body composition, and cancer progression.
Limitations
To our knowledge, this is the largest study to examine the relationship between biomarkers of systemic inflammation and sarcopenia in CRC, and the only study to examine whether NLR and sarcopenia are independently associated with CRC survival. The use of clinically acquired CT scans makes our approach replicable at low cost in clinical practice. This link to clinical practice also presents a limitation: markers of systemic inflammation were obtained opportunistically through routine laboratory test results available in the electronic medical record. C-reactive protein level was available for few patients (<10%) and was therefore not used. However, results were largely consistent regardless of the metric of systemic inflammation examined, as well as across subgroups defined by age, BMI, sex, and stage. As in any observational study, residual confounding is possible; for example, physical activity is not well measured in the electronic medical record and patients with higher NLR may experience fatigue leading to inactivity and therefore to compromised muscle mass. Furthermore, socioeconomic status, diet, and alcohol consumption were not captured and could plausibly influence NLR, sarcopenia, and cancer death.
Conclusions
Both sarcopenia and high NLR were independent prognostic indicators in nonmetastatic CRC. If our findings are confirmed by additional studies, these 2 biomarkers are already collected in routine care and thus have high potential for use in clinical prognostication. We also found that the co-occurrence of sarcopenia and inflammation at diagnosis identified patients with a more than 2-fold risk of mortality compared with patients with neither condition; before this information can be used to influence treatment decisions or tailor interventions, future research must clarify whether reducing systemic inflammation or increasing muscle mass can enhance progression-free survival and through what mechanisms.
eTable 1. Association of Additional Markers of Systemic Inflammation with Sarcopenia at Colorectal Cancer Diagnosis
eTable 2. Association of Systemic Inflammation with Skeletal Muscle Index at Colorectal Cancer Diagnosis
eTable 3. Association of Neutrophil-to-Lymphocyte Ratio with Sarcopenia by Stage, Age and Sex
eFigure 1. Age-Distribution according to Neutrophil: Lymphocyte Ratio and Sarcopenia Status in Non-Metastatic Colorectal Cancer Patients and Risk of Death
eFigure 2. Kaplan-Meier Survivorship Function according to Neutrophil: Lymphocyte Ratio in Non-Metastatic Colorectal Cancer Patients
eFigure 3. Kaplan-Meier Survivorship Function according to Sarcopenia Status in Non-Metastatic Colorectal Cancer Patients
References
- 1.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67. [DOI] [PubMed] [Google Scholar]
- 2.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 3.Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol. 2015;112(5):503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali R, Baracos VE, Sawyer MB, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5(4):607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66(4):583-589. [DOI] [PubMed] [Google Scholar]
- 6.Jung HW, Kim JW, Kim JY, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23(3):687-694. [DOI] [PubMed] [Google Scholar]
- 7.Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study) [published online May 15, 2017]. Cancer Epidemiol Biomarkers Prev. doi: 10.1158/1055-9965.EPI-17-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204-1212. [DOI] [PubMed] [Google Scholar]
- 9.Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7(22):31926-31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab. 2014;39(6):654-662. [DOI] [PubMed] [Google Scholar]
- 11.Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC). 2016. https://clinicaltrials.gov/ct2/show/NCT02330926. Accessed November 10, 2016.
- 12.Baracos VE. Cancer-associated cachexia and underlying biological mechanisms. Annu Rev Nutr. 2006;26:435-461. [DOI] [PubMed] [Google Scholar]
- 13.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153-166. [DOI] [PubMed] [Google Scholar]
- 14.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200-221. [DOI] [PubMed] [Google Scholar]
- 16.Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2009;12(1):72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller MJ, Baracos V, Bosy-Westphal A, et al. Functional body composition and related aspects in research on obesity and cachexia: report on the 12th Stock Conference held on 6 and 7 September 2013 in Hamburg, Germany. Obes Rev. 2014;15(8):640-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke CH, Neugebauer R, Meyerhardt J, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2(9):1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Kroenke CH, Prado CM, et al. Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente Northern California Population. Cancer Epidemiol Biomarkers Prev. 2017;26(1):30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. J Clin Oncol. 2016;34(30):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188-198. [DOI] [PubMed] [Google Scholar]
- 23.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333-2338. [DOI] [PubMed] [Google Scholar]
- 24.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997-1006. [DOI] [PubMed] [Google Scholar]
- 25.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269-275. [DOI] [PubMed] [Google Scholar]
- 26.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663-2668. [DOI] [PubMed] [Google Scholar]
- 28.Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115(4):470-479. [DOI] [PubMed] [Google Scholar]
- 29.Malietzis G, Johns N, Al-Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263(2):320-325. [DOI] [PubMed] [Google Scholar]
- 30.Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7(8):e41883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24(5):2075-2084. [DOI] [PubMed] [Google Scholar]
- 32.Kantola T, Klintrup K, Väyrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. 2000;16(10):1015-1018. [DOI] [PubMed] [Google Scholar]
- 34.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363-2366. [DOI] [PubMed] [Google Scholar]
- 35.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245(6):621-625. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160-4168. [DOI] [PubMed] [Google Scholar]
- 37.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5(5):e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. [DOI] [PubMed] [Google Scholar]
- 39.Focht BC, Clinton SK, Devor ST, et al. Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol. 2013;11(2):45-60. [PubMed] [Google Scholar]
- 40.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45(11):2080-2090. [DOI] [PubMed] [Google Scholar]
- 41.Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11(3):339-349. [DOI] [PubMed] [Google Scholar]
- 42.Hardee JP, Porter RR, Sui X, et al. The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc. 2014;89(8):1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32(1):30-39. [DOI] [PubMed] [Google Scholar]
- 44.Sakuma K, Aoi W, Yamaguchi A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. 2017;469(5-6):573-591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association of Additional Markers of Systemic Inflammation with Sarcopenia at Colorectal Cancer Diagnosis
eTable 2. Association of Systemic Inflammation with Skeletal Muscle Index at Colorectal Cancer Diagnosis
eTable 3. Association of Neutrophil-to-Lymphocyte Ratio with Sarcopenia by Stage, Age and Sex
eFigure 1. Age-Distribution according to Neutrophil: Lymphocyte Ratio and Sarcopenia Status in Non-Metastatic Colorectal Cancer Patients and Risk of Death
eFigure 2. Kaplan-Meier Survivorship Function according to Neutrophil: Lymphocyte Ratio in Non-Metastatic Colorectal Cancer Patients
eFigure 3. Kaplan-Meier Survivorship Function according to Sarcopenia Status in Non-Metastatic Colorectal Cancer Patients