This randomized clinical trial compares the effects of metronomic chemotherapy on progression-free survival vs placebo in pediatric patients with primary extracranial, nonhematopoietic solid malignant tumors that progress after at least 2 lines of chemotherapy.
Key Points
Question
Does metronomic chemotherapy improve progression-free survival among pediatric patients with solid extracranial cancer that progresses after at least 2 lines of chemotherapy compared with placebo?
Findings
In this randomized clinical trial, the proportion of patients with progressive cancer at 6 months was 100% for those who received placebo and 94.6% for those who received metronomic chemotherapy, indicating no statistically significant difference. Those without bone sarcoma and those able to tolerate therapy for more than 3 cycles (9 weeks) appeared to benefit from metronomic chemotherapy.
Meaning
In solid extracranial pediatric cancers progressive after at least 2 lines of chemotherapy, metronomic chemotherapy does not prolong progression-free survival.
Abstract
Importance
Although oral metronomic chemotherapy is often used in progressive pediatric solid malignant tumors, a literature review reveals that only small single-arm retrospective or phase 1 and 2 studies have been performed. Skepticism abounds because of the lack of level 1 evidence.
Objectives
To compare the effect of metronomic chemotherapy on progression-free survival (PFS) with that of placebo in pediatric patients with primary extracranial, nonhematopoietic solid malignant tumors that progress after at least 2 lines of chemotherapy.
Design, Setting, and Participants
A double-blinded, placebo-controlled randomized clinical trial was conducted from October 1, 2013, through December 31, 2015, at the cancer center at All India Institute of Medical Sciences in children aged 5 to 18 years with primary extracranial, nonhematopoietic solid malignant tumors that progressed after at least 2 lines of chemotherapy and had no further curative options.
Interventions
One arm received a 4-drug oral metronomic regimen of daily celecoxib and thalidomide with alternating periods of etoposide and cyclophosphamide, whereas the other arm received placebo. Disease status was assessed at baseline, 9 weeks, 18 weeks, and 27 weeks or at clinical progression.
Main Outcomes and Measures
The primary end point was PFS as defined by the proportion of patients without disease progression at 6 months, and PFS duration and overall survival (OS) were secondary end points.
Results
A total of 108 of the 123 patients screened were enrolled, with 52 randomized to the placebo group (median age, 15 years; 40 male [76.9%]) and 56 to the metronomic chemotherapy group (median age, 13 years; 42 male [75.0%]). At a median follow-up of 2.9 months, 100% of the patients had disease progression by 6 months in the placebo group vs 96.4% in the metronomic chemotherapy group (P = .24). Median PFS and OS in the 2 groups was similar (hazard ratio [HR], 0.69; 95% CI, 0.47-1.03 [P = .07] for PFS; and HR, 0.74; 95% CI, 0.50-1.09 [P = .13] for OS). In post hoc subgroup analysis, cohorts receiving more than 3 cycles (HR for PFS, 0.46; 95% CI, 0.23-0.93; P = .03) and those without a bone sarcoma (ie, neither primitive neuroectodermal tumor nor osteosarcoma) (HR for PFS, 0.39; 95% CI, 0.18-0.81; P = .01) appeared to benefit from metronomic chemotherapy.
Conclusions and Relevance
Metronomic chemotherapy does not improve 6-month PFS, compared with placebo, among pediatric patients with extracranial progressive solid malignant tumors . However, patients without bone sarcoma and those able to tolerate therapy for more than 3 cycles (9 weeks) benefit.
Trial Registration
clinicaltrials.gov Identifier: NCT01858571.
Introduction
Despite remarkable treatment advances in childhood cancer in the past few decades that have resulted in a survival rate of more than 80% for childhood cancers in high-income countries, the survival rate among children with cancer in low- and middle-income countries can be as low as 10% in some settings, with a wide variability in survival by cancer type. Limited options usually remain when the malignant tumor progresses after 1 or 2 lines of a standard chemotherapy protocol. Repetitive administration of conventional chemotherapy at the maximum tolerated dose imposes many adverse effects that further limit dosing and quality of life. The goal of the oncologist at this point remains mainly palliative, with an effort to halt the progression of cancer and improve quality of life. Often the only therapy offered is best supportive care, which includes treatment of pain and other associated problems without any definite therapy for cancer control.
Metronomics have been used in this context with mixed results, some encouraging and some not encouraging. Metronomics as a new paradigm in the chemotherapeutics principle entered clinical practice in the early 21st century. Although metronomic chemotherapy is defined as long-term administration of chemotherapeutic agents at relatively low, minimally toxic doses and with no prolonged drug-free breaks, the term metronomics also includes repositioning and repurposing of nonchemotherapeutic agents. This type of therapy inhibits tumor growth primarily through antiangiogenic mechanisms (ie, shifting the target from tumor cells to tumor vasculature) while significantly reducing undesirable toxic effects.
Most studies on metronomic chemotherapy are retrospective or small single-arm studies, case series, and anecdotal case reports. To truly appreciate the effect of metronomic chemotherapy on progression-free survival (PFS) among pediatric patients with cancer after multiple relapses, we designed a double-blind randomized clinical trial to compare metronomic chemotherapy with placebo in cases of pediatric extracranial solid tumors that had progressed after at least 2 lines of chemotherapy.
Methods
Study Design and Patient Population
In this double-blinded, placebo-controlled randomized clinical trial with a superiority design, we recruited patients from October 1, 2013, through December 31, 2015, from among those registered at the cancer center at All India Institute of Medical Sciences, which is a tertiary care referral cancer center in North India. Inclusion criteria for study were as follows: age of 5 to 15 years, diagnosis of nonhematopoietic primarily extracranial solid tumor that was progressive after treatment with at least 2 lines of chemotherapy and had no other curative treatment options, performance status of 3 or less (at least ambulatory with the help of crutches or a wheelchair), recovered from all acute toxic effects of earlier therapy, absolute neutrophil count greater than 1000/µL (to convert to ×109/L, multiply by 0.001), absolute platelet count greater than 75 × 103/µL (to convert to ×109/L, multiply by 1), normal kidney function, serum bilirubin level less than 1.5 times the upper limit of normal, and serum aspartate aminotransferase and alanine aminotransferase levels less than 5 times the upper limits of normal. Exclusion criteria included uncontrolled concurrent illness or active infection, positive serologic test result for human immunodeficiency virus, and inability to swallow oral medication. All patients were included in the study after providing written informed consent. All data were deidentified. The study protocol was submitted to the All India Institute of Medical Sciences Ethics Committee, and approval was obtained. The trial protocol can be found in Supplement 1.
Randomization and Masking
Eligible patients underwent centralized simple 1:1 randomization based on a computer-generated table of random numbers by a single independent person (D.D.). This person was not involved in treatment, follow-up, or response assessment of patients.
Intervention
Group 1 received placebo and best supportive care, and group 2 received metronomic chemotherapy and best supportive care (eFigure 1 in Supplement 2). Treatment was continued until progression was documented. The metronomic chemotherapy schedule consisted of alternating cycles of cycle A and B (each cycle included 3 weeks of drug administration), with each drug rounded off to the nearest tablet or capsule size (eTable 1 in Supplement 2). The patients in the placebo group had similar alternating cycles of cycle A and B. Capsules of the same size and color as used in metronomic chemotherapy were given. The rationale for this combination is detailed in the eAppendix and eTable 2 in Supplement 2. Best supportive care included management of pain as per the World Health Organization standard for pain management, blood product transfusion, management of infection, and malodorous necrotic masses.
Patients were assessed at baseline with basic blood investigations (complete blood cell counts, liver function tests, and kidney function tests) and radiologic investigations (contrast-enhanced imaging of the chest and involved site and, in relevant cases, positron emission tomography–computed tomography, bone imaging, metaiodobenzylguanidine imaging, and magnetic resonance imaging). Before each cycle, complete blood cell counts, liver function tests, and kidney function tests were performed again. All patients were closely followed up for toxic effects at weekly visits and telephone contacts for an initial 6 weeks and then at longer intervals. Interim assessment was performed after 3 cycles (at 9 weeks), after 6 cycles (at 18 weeks), and after 9 cycles (at 27 weeks) or earlier if there were obvious symptoms and signs of progression. At interim assessments, response evaluation was performed clinically and radiologically according to the Response Evaluation Criteria in Solid Tumors, version 1.1.
In cases of hematologic toxic effects without progression, drug treatment was stopped for a few days, antibiotics and granulocyte colony-stimulating factor were used as necessary, and then drug treatment was started again after count recovery at 80% of the initial dose, rounded again to the nearest capsule or tablet size. In patients found to have progressive disease at any point, therapy was stopped but follow-up was continued with best supportive care until death. If patients preferred returning to their villages, then telephone contacts and WhatsApp messaging were continued at frequent intervals to provide emotional support and medical advice in coordination with local physicians in villages. Dates of death were obtained during these contacts.
Outcome Measures
The primary objective of the study was to compare PFS at 6 months in the intention-to-treat population between the 2 groups, which was defined as the proportion of patients free of disease progression at the end of 6 months. The secondary objectives of the study included analysis of (1) duration of PFS (defined as time from randomization to disease progression, relapse, or death) and (2) overall survival (OS) (defined as time from randomization to death from any cause). All toxic and adverse effects of the drugs were graded according to the National Cancer Center Common Terminology Criteria for Adverse Events, version 4.03.
Statistical Analysis
Descriptive statistics, such as mean, median, SD, and range, were used to describe baseline demographic and clinical profiles of all patients. To determine the association between 2 categorical variables, the χ2 test was used. Continuous variables were analyzed between the 2 groups using the unpaired t test or Wilcoxon rank sum test. Survivals were depicted using Kaplan-Meier plots. Difference between groups was analyzed using the log-rank test. Proportional survivals at specific times were determined using the Kaplan-Meier survival analysis. The Cox proportional hazards regression model was used to calculate the hazard ratios (HRs). Analysis was also performed on subgroups (eg, patients who completed a minimum of 3 cycles of therapy and tumors other than bone sarcomas). The proportional hazards assumption was checked using the test based on Schoenfeld residuals. P < .05 (1-sided) was considered to be statistically significant. Data analyses were performed using STATA statistical software, version 11.2 (StataCorp).
Sample Size Calculation
From an exhaustive review of the literature, data on PFS in solid extracranial pediatric tumors after 2 lines of chemotherapy failed, without any further therapy, were not available. From our experience, we knew that most patients with advanced cancer progress within a few weeks to a month’s time. Therefore, we made the modest assumption that 95% of such patients will progress by 6 months without therapy and that a 20% improvement in efficacy is standard in oncology patients. With a 2-sided α of 5% and a power of 80%, a sample size of 49 in each group would detect a 20% difference between the proportion of progression at 6 months between the placebo group (group 1) and metronomic chemotherapy group (group 2) (95% vs 75%). Assuming loss to follow-up of 15%, 54 individuals per group were required. Therefore, 108 patients were proposed to be randomized.
Results
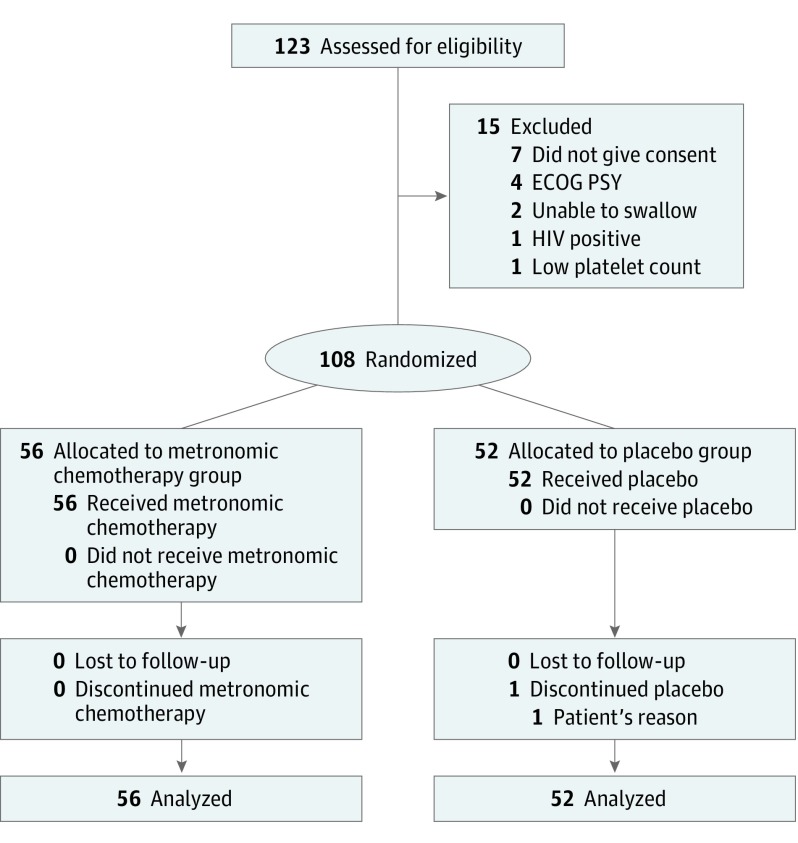
During the study period from October 1, 2013, through December 31, 2015, we screened 123 patients for eligibility and recruited 108 of them, with 52 randomized to the placebo group (median age, 15 years; 40 male [76.9%]) and 56 to the metronomic chemotherapy group (median age, 13 years; 42 male [75.0%]). Major reasons for ineligibility were unwillingness to give consent (7 patients), bedridden and therefore Eastern Cooperative Oncology Group performance status 4 (4 patients), thrombocytopenia (1 patient), active human immunodeficiency virus infection (1 patient), and inability to swallow capsules (2 patients). Fifty-two patients were randomly assigned to the placebo group (group 1) and 56 to the metronomic chemotherapy group (group 2) (Figure 1). One patient in the placebo group discontinued treatment on his own; the rest continued with treatment until progression. The cutoff date for data collection for this analysis was July 1, 2016. In the intention-to-treat analysis, there were 52 patients in the placebo group and 56 patients in the metronomic chemotherapy group. A total of 107 patients had progressed (placebo, 52; metronomic chemotherapy, 55) at this time, and 107 patients had died (placebo, 52; metronomic chemotherapy, 55). Median follow-up for all the patients was 2.9 months. Treatment groups were evenly balanced for baseline characteristics (eTable 3 in Supplement 2).
Figure 1. The CONSORT Diagram.
ECOG PS indicates Eastern Cooperative Oncology Group performance status; HIV, human immunodeficiency virus.
End Points
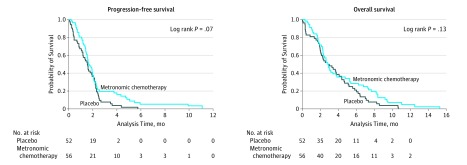
At a median follow-up of 2.9 months, the proportion of patients who progressed was 100% in the placebo group and 94.6% in the metronomic chemotherapy group (eTable 4 in Supplement 2). The median PFS in the entire cohort was 48 days (range, 1-333 days), and the median OS was 79.5 days (range, 1-458 days).
The median PFS was 46 days (95% CI, 33-58 days) in the placebo group and 49 days (95% CI, 43-59 days) in the metronomic chemotherapy group (P = .07). The HR of progression in the metronomic chemotherapy group compared with the placebo group was 0.69 (95% CI, 0.47-1.03; P = .07) (Figure 2). The OS was 85 days (95% CI, 61-123 days) in the placebo group and 85 days (95% CI, 69-113 days) in the metronomic chemotherapy group (P = .13); the HR of progression in the metronomic chemotherapy group compared with the placebo group was 0.74 (95% CI, 0.50-1.09; P = .13) (Figure 2).
Figure 2. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival.
Progression-free survival and overall survival in the intention-to-treat population.
Response Rates
There were no complete responses in either group. There were 8 stable disease cases and 2 partial responses in the metronomic chemotherapy group; 8 of 10 patients remained stable for more than 3 months, but none crossed the 6-month mark. The overall response rate for the metronomic chemotherapy group was therefore 3.5% (2 of 56 patients), and the disease control rate was 17.8% (10 of 56) (eTable 4 in Supplement 2).
Subgroup Analysis
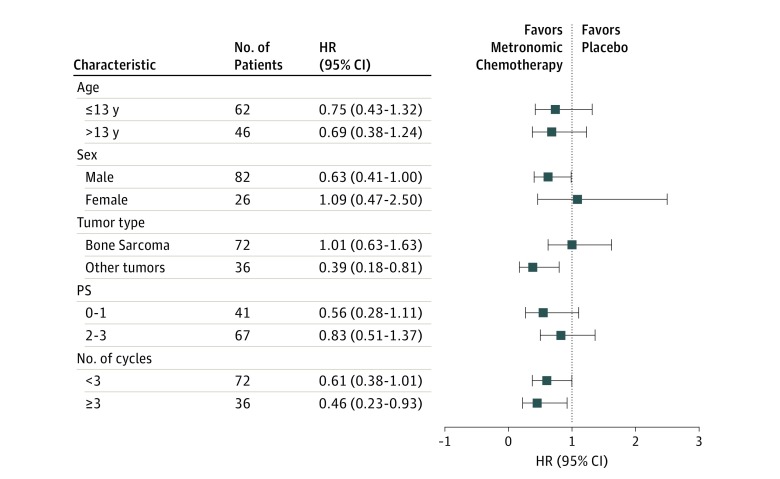
We analyzed the effect of several baseline factors (age, sex, Eastern Cooperative Oncology Group performance status, diagnosis [bone sarcoma vs other], number of cycles ≥3 vs <3) on PFS by the Cox proportional hazards regression model in an unplanned post hoc analysis to gain insights into some predictive factors (Figure 3). This was a stratified analysis within each category of these factors, and the values represent the HRs for PFS.
Figure 3. Forest Plot Representing Hazard Ratios With 95% CIs of Progression-Free Survival in Selected Subgroups.
HR indicates hazards ratio; PS, Eastern Cooperative Oncology Group performance status.
In the subgroup of patients who had received more than 3 cycles of chemotherapy (n = 40), metronomic chemotherapy significantly increased PFS (HR, 0.46; 95% CI, 0.23-0.93; P = .03) (eFigure 2A in Supplement 2). However, benefit was not statistically significant for OS in this cohort (HR, 0.56; 95% CI, 0.28-1.17; P = 0.08) (eFigure 2B in Supplement 2). In addition, in patients who did not have a bone tumor (osteosarcoma or Ewing sarcoma) (ie, other than bone sarcoma tumors [n = 36]), metronomic chemotherapy was associated with a statistically significant benefit for PFS (HR, 0.39; 95% CI, 0.18-0.81; P = .01) and OS (HR, 0.44; 95% CI, 0.21-0.90; P = .02) (eFigure 2C and D in Supplement 2).
Toxicity Data and Dose Adjustments
The complete list of adverse effects and supportive care administered is given in eTable 5 and eTable 6 in Supplement 2. The most common grade 3 to 4 adverse effects were hematologic. Anemia (7.1% vs 11.7%), neutropenia (0% vs 10.7%), thrombocytopenia (0% vs 10.7%), and febrile neutropenia (0% vs 8.8%) were the significant grade 3 to 4 hematologic toxic effects encountered in the placebo vs metronomic chemotherapy groups. Among the nonhematologic adverse effects, mucositis was the most common (grade 1-2, 8.8%; grade 3-4, 5.3%) in the metronomic chemotherapy group.
In the metronomic chemotherapy group, the dose had to be decreased in 8 patients (14.2%) and was delayed in 9 patients (16%). In the placebo group, although none needed a dose decrease, 2 had interruptions because of adherence issues (forgot or misplaced medicines), causing dose delay (eTable 6 in Supplement 2).
Discussion
Although metronomic chemotherapy is regarded as an innovative multitargeted therapy and is being promoted as an attractive option for low- and middle-income countries, limited phase 1 and 2 data exist. In most of the published literature on pediatric metronomic chemotherapy, studies have found benefit in terms of disease stabilization and have recorded it as clinical benefit. We do not actually have randomized comparative data to indicate that indeed this stabilization of disease is attributable to action of metronomic chemotherapy and not merely because of the natural history of the disease. The disease may have remained in control on its own for some time, which is what many of the clinicians might have observed during practice. In other words, many metastatic solid tumors, after becoming progressive and refractory, may remain stable for some period on their own before eventually progressing. No data exist on this phenomenon. The only answer to this question could come from a randomized clinical study in which the comparator receives no therapy.
We observed during our analysis that 5 patients in the placebo group remained stable (without receiving any therapy) for 9 weeks and 1 patient remained stable for 18 weeks. Although there were objective responses in the metronomic chemotherapy group (ie, 2 partial responses and 8 stable disease cases for 9 weeks and 3 maintaining that stable disease until the 18th week), these responses did not ultimately culminate in improved PFS or OS. Therefore, for this heterogeneous group of pediatric malignant tumors, this particular combination of metronomic chemotherapy did not improve PFS. However, there were some encouraging insights after the subgroup analysis, although we admit that this was post hoc and the study was not powered or planned for subgroup analysis.
First, metronomic chemotherapy was benefiting the subgroup of patients who had received the drugs for at least 9 weeks. This benefit was not significant for OS but was statistically significant in terms of PFS. Second, another miscellaneous group of patients who did not have a bone sarcoma fared significantly well in terms of PFS and OS. This group had a variety of neuroblastoma, esthesioneuroblastoma, soft-tissue sarcoma, rhabdomyosarcomas, Wilms tumor, and clear cell sarcoma of kidney. Adverse effects were manageable and were an acceptable tradeoff for the modest PFS benefit in the metronomic chemotherapy group (eTable 7 in Supplement 2).
If we delve deeper and try to determine the reasons why metronomic chemotherapy was having different patterns of responses in the above subgroups, one finding is that osteosarcomas and primitive neuroectodermal tumors constituted two-thirds of the population sample studied, and these tumors respond poorly to metronomic chemotherapy. The reason for differences in responses among the different tumors may lie in their biological differences.
Thus, the message is that metronomic antiangiogenic chemotherapy probably does not benefit all classes of pediatric solid tumors and that histologic features matter. Osteosarcomas and primitive neuroectodermal tumors when analyzed separately also revealed that the metronomic chemotherapy and placebo groups were no different. Therefore, we need to choose the patients carefully and wisely before prescribing an antiangiogenic combination as a blanket palliation treatment.
That osteosarcomas and primitive neuroectodermal tumors do not benefit from antiangiogenic therapy was also found in the study by Robison et al. In their single-arm study of 100 patients, the same 4 drugs (thalidomide, celecoxib, etoposide, and cyclophosphamide) were used with the addition of fenofibrate on relapsed pediatric tumors, most being central nervous system tumors; they found that none of their 12 bone tumors had any response. Only low-grade gliomas and miscellaneous brain tumors had fair PFS, whereas 2 of 3 neuroblastomas remained stable.
Because metronomic chemotherapies are being increasingly used in pediatric palliative contexts, their efficacy has become important for the child, parents, and oncologists. Choosing the right therapy at this point not only improves efficacy but also spares unnecessary toxic effects.
Strengths and Limitations
The strength of our study is that it is the first randomized clinical trial, to our knowledge, of pediatric metronomic chemotherapy. The person responsible for randomization and treatment allocation was separately dedicated to that task and could not influence patients or clinicians. This randomization and blinding strategy removes many potential biases from the study and adds to its credibility and impact. The novelty of the study is that it is an endeavor to address the issues of skepticism surrounding metronomic chemotherapy. For the first time since its inception in 2000, oral antiangiogenic chemotherapy has been compared with a placebo in a randomized controlled design. A limitation of the study is its heterogeneity. Prior stratification into homogenous groups based on type of tumor could have refined the study.
Conclusions
This study has given some hints and insights into who might benefit from metronomic chemotherapy. This study provokes a new hypothesis that metronomic antiangiogenic chemotherapy may be beneficial for particular histologic subtypes of cancer only, which needs to be tested in a randomized fashion in homogenous, disease-specific subgroups.
Trial Protocol
eFigure 1. Study Design
eFigure 2. Kaplan-Meier Curves for Progression-Free Survival (A) and Overall Survival (B) in Patients Completing More Than 3 Cycles of Therapy (n = 40) and Kaplan-Meier Curves for Progression-Free Survival (C) and Overall Survival (D) in Patients With Histologic Subtypes Other Than Bone Sarcomas (n = 36)
eTable 1. Drug Schedule Used in the Study
eTable 2. Rationale of the 4-Drug Antiangiogenic Regimen
eTable 3. Comparison of Baseline Characteristics of the 2 Study Groups
eTable 4. Results of the Primary and Secondary End Points of the Study
eTable 5. Toxic Effects Recorded During the Study
eTable 6. Supportive Care Provided in the Study
eTable 7. Comparison of Toxicity Profiles in the Subsets That Benefited From Metronomic Therapy (ie, Patients Tolerating Metronomic Therapy for >3 Cycles and the Nonbone Sarcomas)
eAppendix. Evidence Implications
References
- 1.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol. 2008;9(8):721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nygaard R, Kivivuori S-M. Treatment for recurrent medulloblastoma with intrathecal liposomal cytarabine and systemic metronomic combination therapy. Anticancer Drugs. 2012;23(3):342-346. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Revised June 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed October 1, 2013.
- 5.Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 2002;24(1):39-53. [DOI] [PubMed] [Google Scholar]
- 6.André N, Carré M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11(7):413-431. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson L. Metronomics: an alternative P4 medicine. Nat Rev Clin Oncol. 2016;13(8):461. [DOI] [PubMed] [Google Scholar]
- 8.Traore F, Togo B, Pasquier E, Dembélé A, André N. Preliminary evaluation of children treated with metronomic chemotherapy and valproic acid in a low-income country: Metro-Mali-02. Indian J Cancer. 2013;50(3):250-253. [DOI] [PubMed] [Google Scholar]
- 9.Fousseyni T, Diawara M, Pasquier E, André N. Children treated with metronomic chemotherapy in a low-income country: METRO-MALI-01. J Pediatr Hematol Oncol. 2011;33(1):31-34. [DOI] [PubMed] [Google Scholar]
- 10.Porkholm M, Toiviainen-Salo S, Seuri R, et al. Metronomic therapy can increase quality of life during paediatric palliative cancer care, but careful patient selection is essential. Acta Paediatr. 2016;105(8):946-951. [DOI] [PubMed] [Google Scholar]
- 11.Minturn JE, Janss AJ, Fisher PG, et al. A phase II study of metronomic oral topotecan for recurrent childhood brain tumors. Pediatr Blood Cancer. 2011;56(1):39-44. [DOI] [PubMed] [Google Scholar]
- 12.Robison NJ, Campigotto F, Chi SN, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61(4):636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.André N, Rome A, Coze C, et al. Metronomic etoposide/cyclophosphamide/celecoxib regimen given to children and adolescents with refractory cancer: a preliminary monocentric study. Clin Ther. 2008;30(7):1336-1340. [DOI] [PubMed] [Google Scholar]
- 14.Sterba J, Valik D, Mudry P, et al. Combined biodifferentiating and antiangiogenic oral metronomic therapy is feasible and effective in relapsed solid tumors in children: single-center pilot study. Onkologie. 2006;29(7):308-313. [DOI] [PubMed] [Google Scholar]
- 15.Choi LMR, Rood B, Kamani N, et al. Feasibility of metronomic maintenance chemotherapy following high-dose chemotherapy for malignant central nervous system tumors. Pediatr Blood Cancer. 2008;50(5):970-975. [DOI] [PubMed] [Google Scholar]
- 16.Ali AM, El-Sayed MI. Metronomic chemotherapy and radiotherapy as salvage treatment in refractory or relapsed pediatric solid tumours. Curr Oncol. 2016;23(3):e253-e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.André N, Abed S, Orbach D, et al. Pilot study of a pediatric metronomic 4-drug regimen. Oncotarget. 2011;2(12):960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Study Design
eFigure 2. Kaplan-Meier Curves for Progression-Free Survival (A) and Overall Survival (B) in Patients Completing More Than 3 Cycles of Therapy (n = 40) and Kaplan-Meier Curves for Progression-Free Survival (C) and Overall Survival (D) in Patients With Histologic Subtypes Other Than Bone Sarcomas (n = 36)
eTable 1. Drug Schedule Used in the Study
eTable 2. Rationale of the 4-Drug Antiangiogenic Regimen
eTable 3. Comparison of Baseline Characteristics of the 2 Study Groups
eTable 4. Results of the Primary and Secondary End Points of the Study
eTable 5. Toxic Effects Recorded During the Study
eTable 6. Supportive Care Provided in the Study
eTable 7. Comparison of Toxicity Profiles in the Subsets That Benefited From Metronomic Therapy (ie, Patients Tolerating Metronomic Therapy for >3 Cycles and the Nonbone Sarcomas)
eAppendix. Evidence Implications