Abstract
Importance
Contemporary management of head and neck cancer involves professionals from multiple specializations. Streamlined care that reduces delays yet allows for comprehensive evaluation is needed.
Objective
To evaluate a single-day, single-appointment, multidisciplinary head and neck clinic model for reduction in treatment delay and comprehensiveness of care.
Design, Setting, and Participants
A retrospective cohort analysis was conducted from June 1, 2015, to July 31, 2016, of outpatients at a single, academic medical center.
All eligible outpatients seen in either the multiple-appointment, traditional clinic (n = 73) or the single-day multidisciplinary clinic (MDC) (n = 68) were included. Patients with new squamous cell carcinoma of the oropharynx, hypopharynx, sinonasal tract, and larynx, along with any mucosal site recurrence were eligible for the study.
Main Outcomes and Measures
Primary outcomes were delays between tertiary clinic referral or first appointment and treatment initiation in the MDC compared with the traditional clinic. Secondary outcomes were complete evaluations prior to treatment, enrollment in trials and registries, and rate of patient leak, defined as initiating therapy and then transferring to another center before completion. Outcome selection and hypothesis generation were performed a priori.
Results
Patient factors and tumor characteristics were similar between the traditional clinic cohort (19 women and 54 men; mean [SD] age, 64.0 [10.2] years) and the MDC cohort (8 women and 60 men; mean [SD] age, 61.0 [8.9] years). The MDC cohort had significantly fewer instances of delay greater than 30 days from referral to treatment initiation (28 [41%] vs 43 [59%]) and first appointment to treatment initiation (7 [10%] vs 17 [23%]). Actual median days in these categories were significantly different between the 2 clinic types after the patients in the traditional clinic who saw only a surgeon before treatment initiation were excluded (MDC, 28 days vs traditional, 35 days; median difference, –5 days; 95% CI, –11 to –1).
Conclusions and Relevance
Coordination of the management of head and neck cancer is complex. Treatment is time sensitive, and frequently clinician resources are limited. This MDC model was associated with improved efficiency and completeness of care.
This cohort study evaluates a single-day, single-appointment, multidisciplinary head and neck clinic model for reduction in treatment delay and comprehensiveness of cancer care.
Key Points
Question
Can more efficient and comprehensive head and neck cancer care be accomplished through a single-appointment multidisciplinary clinic than a traditional multiple-appointment clinic?
Findings
In this cohort study, patients seen in the multidisciplinary clinic had fewer instances of treatment delay, and more often and more quickly saw medical oncology, radiation oncology, and speech and audiology clinicians, compared with patients in the traditional clinic cohort.
Meaning
This multidisciplinary clinic model allows for more efficient and comprehensive cancer care.
Introduction
Contemporary management of cancer is multifaceted and relies on the expertise of many uniquely qualified clinicians. Improved understanding of tumor biology and advances in oncology demand increasingly sophisticated treatment strategies. Difficulties arise when coordinating this care effectively to optimize both patient outcomes and clinician resources.
In the United States, a need for standardized integration of health care professionals in cancer care was recognized as early as the 1960s. In Europe, the European Partnership for Action Against Cancer was established in 2009 to define the core elements essential to all tumor-based multidisciplinary teams in an effort to improve outcomes. In the United Kingdom, support for widespread multidisciplinary care was generated with the 1995 Calman-Hine report on the structure and organization of cancer services. The American College of Surgeons’ Commission on Cancer regards multidisciplinary conferences as key aspects of oncology care, and it requires programs to run them to attain accreditation. Continued improvements have more recently stemmed from the development of multidisciplinary clinics (MDCs) in which medical and surgical appointments are streamlined into a single day. Multidisciplinary clinic outcomes in breast and colorectal cancer report improved rates of patient satisfaction, shorter time between diagnosis and initiation of definitive treatment, better access to multimodal therapy, and improved pathologic evaluations. Few studies have been conducted within head and neck oncology to elucidate potential benefits of MDCs, although a recent publication showed a survival advantage for patients with oropharynx squamous cell carcinoma.
The head and neck department at Washington University School of Medicine piloted a head and neck cancer MDC in 2015. Patients who qualify have the opportunity to meet simultaneously with a head and neck surgeon, medical oncologist, and radiation oncologist for an evaluation and discussion of treatments and clinical trials available. They also see audiology and speech pathology clinicians the same day. Their case is then discussed at tumor board to allow for diagnostic input from radiology and pathology clinicians. The purpose of this study is to evaluate the efficacy of the MDC compared with that of a traditional, multiple-appointment approach.
Methods
A retrospective cohort review of patients with head and neck cancer who were referred to Washington University School of Medicine and Siteman Cancer Center from June 1, 2015, to July 31, 2016, was performed. Eligibility criteria for an MDC appointment was patient’s age 18 years or older; a new, biopsy-proven squamous cell carcinoma of the oropharynx, hypopharynx, sinonasal tract, and larynx; or recurrent, biopsy-proven squamous cell carcinoma of any mucosal sites. Oral cavity cancers were excluded on the basis that upfront surgery is recommended as the primary modality for patients with such cancer. Five MDC appointment slots were available per week in the pilot phase. Patients eligible for but unable to be accommodated by the MDC were seen in a traditional clinic model, and they comprise the traditional cohort in this study. Patients with missing documentation, ineligible primary sites, or inpatient workup were excluded. Approval was granted by the Washington University Institutional Review Board. A waiver of informed consent was granted for all patients.
Data Collection
Adult Comorbidity Evaluation-27 scores, age, sex, residential distance from the outpatient clinic, tumor site, and tumor stage at presentation were recorded. Time from tertiary referral to treatment initiation and time from first tertiary clinic appointment to treatment initiation was calculated. Enrollment in a clinical trial or registry and time to consultation with other oncology specialists and ancillary clinicians were also collected. Patient leak, defined as initiating therapy and then transferring to another center before completion, was recorded.
Statistical Analysis
Standard descriptive statistics were used to describe the distribution of demographic and clinical characteristics in both groups. Means and SDs were used for normally distributed, continuous-level variables. Median and range were used to describe variables that violated the assumption of normality. Effect sizes were expressed as mean or median differences for continuous-level variables and percentage differences were calculated for categorical-level variables. Clinically meaningful delay to treatment was defined as more than 30 days between tertiary referral and treatment or first appointment and treatment. Cross-table analysis was used to compare the number of delayed patients in each cohort as well as to investigate which variables were associated with delays greater than 30 days. A linear regression analysis was also used to control for potential confounders in time to treatment comparisons. This analysis was done via a hybrid multivariable linear regression with MDC entered in block 1 and in block 2 the potential confounders of age, sex, tumor site (oropharynx or other), comorbidity (moderate and severe vs none or mild), distance from medical center, tumor stage (early vs late), and treatment type (surgical vs nonsurgical). These confounders were entered using a forward stepwise approach with an α level for model entry set at .05 and for removal from the model set at .10. This analysis was performed for all of the time intervals reported in the Results section. Statistical analysis was performed with IBM SPSS Statistics for Windows, version 24.0 (IBM Corp), and the student version of MINITAB, release 14.11.1 (Minitab Inc) was used to calculate effect sizes and the associated 95% CIs.
Results
Demographics
A total of 163 medical records met inclusion criteria; 22 were excluded on the basis of missing information, patient death during workup, or undergoing an inpatient workup. A total of 141 medical records remained, with 73 patients in the traditional clinic cohort and 68 patients in the MDC cohort (Table 1). Mean (SD) patient age (MDC, 61.0 [8.9] years; traditional, 64.0 [10.2] years) and median residential distance from the clinic (MDC, 54 km; traditional, 40 km) were similar between groups. Both clinic types had more men than women (MDC, 60 men and 8 women; traditional, 54 men and 19 women). The oropharynx was the most common primary site (105 [74.5%]), and the MDC saw 18% more patients (95% CI, 4%-32%) with oropharynx cancer than did the traditional clinic (57 [84%] vs 48 [66%]). Primary treatment was similar between groups. The traditional clinic saw a lower percentage of patients with no comorbidities than did the MDC (7 [10%] vs 18 [26%]; 16% less; 95% CI, –29% to –4%) and moderate comorbidities (14 [19%] vs 21 [31%]; 12% less; 95% CI, –26% to 2%) and a higher percentage of patients with mild comorbidities (42 [58%] vs 23 [34%]; 24% more; 95% CI, 8%-40%). There was no significant difference in the distribution of patients with severe comorbidities. The traditional clinic saw a higher percentage of patients with stage 2 disease than did the MDC (10 [14%] vs 3 [4%]; 10% more; 95% CI, 1%-19%); otherwise stage distribution was similar between groups.
Table 1. Demographics, Tumor Site, Tumor Stage, Treatment, and Comorbidity Data.
| Characteristic | Traditional Clinic (n = 73) |
Multidisciplinary Clinic (n = 68) |
Difference, % (95% CI) |
|---|---|---|---|
| Age, mean (SD), y | 64.0 (10.2) | 61.0 (8.9) | 3.0 (0.1 to 6.5)a |
| Sex, No (%) | |||
| Male | 54 (74) | 60 (88) | –14 (–27 to –1) |
| Female | 19 (26) | 8 (12) | |
| Median distance from clinic (range), kmb | 40 (3 to 1520) | 54 (3 to 477) | 2 (–9 to 15)c |
| Adult Comorbidity Evaluation-27 scores, No. (%) | |||
| None | 7 (10) | 18 (26) | −16 (−29 to −4) |
| Mild | 42 (58) | 23 (34) | 24 (8 to 40) |
| Moderate | 14 (19) | 21 (31) | −12 (−26 to 2) |
| Severe | 10 (14) | 6 (9) | 4 (−6 to 14) |
| Primary tumor site, No. (%) | |||
| Oropharynx | 48 (66) | 57 (84) | −18 (−32 to −4) |
| Otherd | 25 (34) | 11 (16) | |
| Tumor stage, No. (%) | |||
| 1 | 4 (6) | 1 (2) | 4 (−2 to 10) |
| 2 | 10 (14) | 3 (4) | 10 (1 to 19) |
| 3 | 11 (15) | 13 (19) | −4 (−16 to 8) |
| 4 | 48 (66) | 51 (75) | −10 (−25 to 5) |
| Treatment type, No. (%) | |||
| Surgery alone | 13 (18) | 7 (10) | 8 (−4 to 2) |
| Radiotherapy | 0 | 1 (2) | −2 (−7 to 1) |
| Chemotherapy and radiotherapy | 19 (26) | 19 (28) | −2 (−17 to 13) |
| Palliative chemotherapy | 5 (7) | 7 (10) | −3 (−13 to 5) |
| Surgery and adjuvant | 30 (41) | 29 (43) | −2 (−18 to 15) |
| Neoadjuvant and chemotherapy and radiotherapy | 6 (8) | 5 (7) | 1 (−8 to 10) |
Mean difference and 95% CI calculated for age.
To convert to miles, divide by 1.6.
Median difference with 95% CI.
Other sites include 12 cases in the hypopharynx, 7 in the supraglottis, 11 in the glottis, 2 in the subglottis, 2 in the sinonasal tract, and 2 with unknown primary tumor sites.
Days to Treatment Initiation
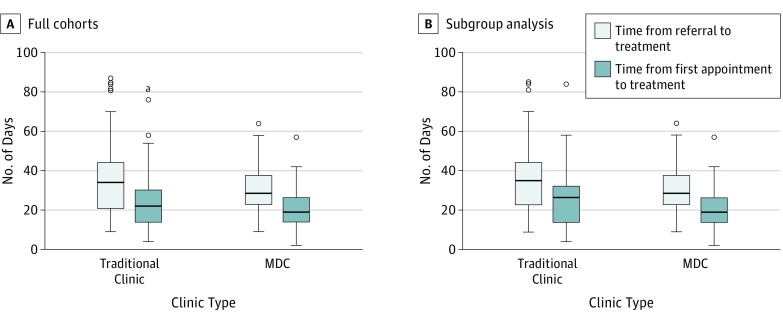
Treatment delay was the primary outcome in this study. Days between clinic referral and treatment initiation in the MDC vs the traditional model are compared. As shown in Figure 1, median days between referral and treatment initiation were fewer in the MDC cohort (29 days; range, 9-64 days) compared with the traditional cohort (34 days; range, 9-87 days), for a median difference of 4 days (95% CI, –1 to 8 days). A linear regression analysis was also performed for this same time interval and, of the demographic data gathered, only site (oropharynx vs other) and tumor stage were found to have a significant association with the MDC. After controlling for these factors, patients in the MDC had an estimated mean of 3.1 days fewer in this time interval (95% CI, –8.2 to 2.0). Similarly, fewer days from the first appointment to treatment initiation were seen in the MDC cohort (19 days; range, 2-57 days) compared with the traditional cohort (22 days; range, 4-84 days), for a median difference of 3 days (95% CI, 0-7). After controlling for tumor stage and treatment modality, patients in the MDC cohort had an estimated mean of 3.6 days fewer in this time interval (95% CI, –7.7 to 0.5). Although a consistent trend was found, these differences did not reach statistical significance.
Figure 1. Median Days From Referral and First Appointment to Treatment Initiation Compared Between Cohorts.
A, Full cohorts. B, Modified traditional cohort and multidisciplinary clinic (MDC). Upper and lower bounds of boxes represent upper and lower quartiles. Horizontal line represents median value, the lower vertical line is the lowest quartile, and the upper vertical line is the highest quartile. Outliers are shown as circles above upper bounds.
It was noted during analysis that a group of 23 patients in the traditional cohort who were treated with upfront surgery did not see medical or radiation oncology clinicians until after surgery, even though nonsurgical management was an alternative primary modality or was indicated in the adjuvant setting. When these 23 patients were excluded, the remaining patients were those who did see all relevant physicians before starting therapy. Demographic characteristics in the traditional group did not significantly change after exclusion of these 23 patients (Table 2). Time delays between the MDC and this modified traditional cohort revealed a significantly shorter median number of days between referral and treatment for the MDC cohort (28 vs 35 days; median difference, –5 days; 95% CI, –11 to –1). After controlling for tumor stage, patients in the MDC cohort were estimated to have a mean of 6 days fewer in this time interval (95% CI, –11.0 to –1.0). Median days between first appointment and treatment were also significantly shorter in the MDC cohort compared with the modified traditional cohort (19 vs 27 days; median difference, –5 days; 95% CI, –9 to –1). After controlling for tumor stage, patients in the MDC cohort were estimated to have a mean of 5.2 days fewer in this time interval (95% CI, –9.5 to –1.0) (Figure 1B).
Table 2. Demographic Data of Modified Traditional Cohort and MDC .
| Characteristic | Traditional Clinic (n = 50) |
Multidisciplinary Clinic (n = 68) |
Difference, % (95% CI) |
|---|---|---|---|
| Age, mean (SD), y | 63.5 (10.2) | 60.8 (8.9) | 2.7 (−0.8 to 6.2)a |
| Sex, No. (%) | |||
| Male | 37 (74) | 60 (88) | −16 (−31 to −1) |
| Female | 13 (26) | 8 (12) | |
| Median distance from clinic (range), kmb | 37 (5 to 1520) | 54 (3 to 477) | −3 (−14 to 10)c |
| Adult Comorbidity Evaluation-27 scores, No. (%) | |||
| None | 5 (10) | 18 (26) | −16 (−29 to −3) |
| Mild | 30 (60) | 23 (34) | 26 (8 to 44) |
| Moderate | 7 (14) | 21 (31) | −17 (−32 to −2) |
| Severe | 8 (16) | 6 (9) | 7 (−5 to 19) |
| Primary tumor site | |||
| Oropharynx | 30 (60) | 57 (84) | −24 (−40 to −8) |
| Otherd | 20 (40) | 11 (16) | |
| Tumor stage | |||
| 1 | 4 (8) | 1 (2) | 6 (−2 to 14) |
| 2 | 3 (6) | 3 (4) | 2 (−6 to 10) |
| 3 | 5 (10) | 13 (19) | −7 (−20 to 6) |
| 4 | 38 (76) | 51 (75) | −1 (−17 to 15) |
| Treatment type | |||
| Surgery alone | 7 (14) | 7 (10) | 4 (−8 to 16) |
| Radiotherapy | 0 | 1 (2) | −1 (−4 to 2) |
| Chemotherapy and radiotherapy | 19 (38) | 19 (28) | 0 (−7 to 27) |
| Palliative chemotherapy | 5 (10) | 7 (10) | 0 (−11 to 11) |
| Surgery and adjuvant | 13 (26) | 29 (43) | −17 (−34 to 0) |
| Neoadjuvant and chemotherapy and radiotherapy | 6 (12) | 5 (7) | 5 (−6 to 16) |
Mean difference and 95% CI calculated for age.
To convert to miles, divide by 1.6.
Median difference with 95% CI.
Other sites include 9 cases in the hypopharynx, 6 in the supraglottis, 8 in the glottis, 2 in the subglottis, 2 in the sinonasal tract, and 2 with unknown primary tumor sites.
Delay in Time to Treatment
A delay of 30 days or more from tertiary care involvement was defined a priori as clinically meaningful based on prior studies. A total of 71 patients were delayed 30 days or more from referral to treatment start. As shown in Figure 2, 43 of the patients in the traditional clinic (59%) had such delays, compared with 28 in the MDC (41%), resulting in a difference of 18% (95% CI, 1%-34%). A total of 24 patients were delayed 30 days or more from their first appointment to treatment start. Seventeen patients (71%) were in the traditional clinic, compared with 7 (29%) in the MDC, for a difference of 42%.
Figure 2. Number of Cases Greater Than 30 Days in the Multidisciplinary Clinic (MDC) vs Traditional Clinic.
A, Delayed patients from referral to treatment initiation. B, Delayed patients from first appointment to treatment initiation.
Delayed patients in both clinic types were further analyzed. Among patients with delays from referral to treatment start, moderate and severe comorbidity scores were seen in 13 of 28 MDC patients (46%) and 12 of 43 traditional patients (28%). In this delayed referral group, 26 MDC patients (93%) and 35 traditional patients (81%) had late-stage disease. Regarding delays from the first appointment to treatment start, moderate and severe comorbidity scores were seen in 9 of 17 traditional patients (53%) and 3 of 7 MDC patients (43%). In this delayed appointment group, 5 MDC patients (71%) and 11 traditional patients (65%) had late-stage disease. Male sex and greater distance from the outpatient clinic were not seen more often in delayed patients. Other factors including tumor site and treatment modality did not have sufficient numbers for this analysis.
Multidisciplinary Evaluation Prior to Treatment Initiation
A comparison between cohorts of pretreatment evaluation by all physicians and ancillary clinicians was performed. Consultations that were refused or not indicated were not counted as missed. A total of 7 of 63 patients (11%) in the traditional group who were eligible for primary nonsurgical therapy were not referred to a medical oncologist at any point during treatment, whereas all patients in the MDC group saw a medical oncologist. Moreover, a median delay of 9 days (range, 0-89 days) to the first medical oncology appointment was found in the traditional group. A total of 6 of 66 patients (9%) in the traditional group who were eligible for primary nonsurgical therapy were not referred to a radiation oncologist at any point during treatment. For those referred, a median delay of 15 days (range, 0-80 days) was found. With 1 exception of a 5-day delay, all patients in the MDC cohort saw these clinicians simultaneously with the surgical evaluation; these differences were statistically significant.
A total of 26 of 70 eligible patients (37%) in the traditional group did not see a speech pathologist before treatment or during the recorded follow-up time. All but 2 patients in the MDC group saw a speech pathologist. The median difference between the cohorts was 35 days (95% CI, 24-75). Likewise, 16 of 62 eligible patients (26%) in the traditional group undergoing potentially ototoxic therapy did not see an audiologist before treatment or in the follow-up time recorded. All patients in the MDC group saw an audiologist. The median difference between the groups was 49 days (95% CI, 41-62). A similar number of patients were enrolled in clinical trials and registries in the traditional group (37) compared with the MDC group (43). Leak rate was measured for both clinics, and was also similar between cohorts, at 12% for the traditional clinic and 10% for the MDC (2% difference; 95% CI, –8% to 12%).
Discussion
Head and neck cancer management is complex and is often routed to tertiary care centers. Studies demonstrate that MDCs help to better orchestrate this complex care. We believe that this MDC approach facilitates improved efficiency and completeness of care via a single-day, single-appointment model. Its design stemmed from a desire to have relevant surgeons and medical oncologists simultaneously present to reduce bias toward a particular primary modality and to facilitate discussion. Appointments were held in the surgeon’s clinic and were scheduled in new patient morning time slots. The surgeons see MDC patients at a designated time and see regular clinic patients around that appointment to maintain workflow. The medical oncologists see the MDC patients back to back with the different surgeons and do not have any waiting time. The tumor board discussion then takes place during a built-in lunch hour. The clinician who received the referral bills as the consultant and the other clinicians bill as an established patient. All clinicians perform and bill for an examination, but the surgeon bills for the nasoendoscopy as the surgery clinic resources are used.
Some of the challenges faced were coordination of many schedules, potential loss of clinic productivity owing to changes in workflow, and management of billing. A design that maintained workflow as well as billing practices and patient volumes for all involved departments was essential. Incorporating clinic administrators and billing specialists into the planning phases facilitated success in these areas and prevented loss of revenue and volume for their departments. Their nurses and study coordinators also traveled with the medical oncologists to the surgery clinic to help with administrative organization.
Of primary interest in this study was whether or not this MDC could reduce treatment delay. Data from large, retrospective studies support an association of decreased survival with longer times to treatment initiation. A study from the Netherlands with 13 000 patients found delays of greater than 37 days to be significantly associated with reduced overall survival in patients with squamous cell carcinoma of all head and neck sites, excluding the lip. National Cancer Database data from more than 50 000 patients with primary tumors of the oral cavity, oropharynx, larynx, and hypopharynx found the lowest risk of mortality predicted to occur at a treatment delay of 30 days, and an increased risk of mortality predicted at 67 days or more. These patients included a mix of individuals treated in the community as well as those referred to academic centers.
With these data in mind, we chose to use a 30-day cutoff as a clinically meaningful marker of delay, as almost all of our patients are referred from outside institutions and thus encounter additional delays before tertiary care. The MDC was associated with significantly fewer patients with greater than 30 days between both referral and first clinic appointment and initiation of therapy. Arguably, a difference between 29 and 31 days is unlikely to have a significant effect on treatment outcome, but we find this improvement to be an overall indication of increased efficiency within the MDC. Analysis of delayed patients in both clinic groups revealed a consistent trend of advanced tumor stage, but causality could not be established with our sample size. A less consistent trend between high comorbidity scores and delay was also seen in both clinic types. Sex, age, distance from the outpatient clinic, tumor site, and treatment modality did not have consistent associations with delay in either clinic type. However, a small sample size limited detection of difference in several of these variables. Delay is likely a multifactorial occurrence that results from multiple patient and disease factors. A larger study with higher power could more readily detect additional factors associated with delays.
The actual number of days between referral or first appointment and initiation of therapy was also analyzed in both clinics. Both the median day analysis and a linear regression model controlling for potential confounding variables were used in this analysis. There was not a statistically significant difference in time intervals between the 2 cohorts, although a trend toward fewer days was seen in the MDC. A closer examination of data from the traditional clinic revealed a distinct subset of patients who moved quickly through the pretreatment workup to therapy. These patients were treated exclusively with upfront surgery and did not see medical or radiation oncology clinicians before surgery. Almost all of these patients required adjuvant therapy and many were candidates for nonsurgical management as the primary modality. Although this pathway is a faster track to treatment, it is arguably less effective at informing patients of all possible treatment options by the appropriate specialty clinicians prior to starting therapy. Comparisons of time intervals between the MDC and the traditional cohort with these patients removed reveal moderately shorter time intervals in the MDC group that do reach statistical significance. We find this outcome to be meaningful in that when patients do see all physicians prior to therapy, time intervals are shorter in the MDC.
Another area of key interest in this study was comprehensive workup prior to treatment initiation, which the MDC unequivocally provided more often. Patients were more often and more quickly evaluated by speech and audiology clinicians. Likewise, they more often and more quickly saw radiation and medical oncologists. There were several instances in the traditional clinic in which nonsurgical therapy was a primary treatment option, but radiation and/or medical oncologists were either not seen or only seen for adjuvant consultation after surgery. All of these clinicians are crucial to provide patients with information about available options, expectations of adverse effects of treatment, and functional outcomes.
There was notable heterogeneity between the 2 cohorts. The MDC cohort had a significantly higher proportion of patients with primary tumors of the oropharynx. There was also a slightly higher proportion of early-stage tumors in the traditional group and late-stage tumors in the MDC group. Finally, there was a slightly higher proportion of patients with no comorbidity scores in the MDC groups and mild comorbidity scores in the traditional group. Multivariate linear regression analysis was performed to account for these potentially confounding variables and results very similar to those calculated by median difference analysis were found, as mentioned above.
In addition, outliers existed in both clinics in terms of days of delay. There were more outliers in the traditional clinic, with 4 patients having delays of greater than 75 days, as opposed to no such delays in the MDC. Three of these patients were delayed by lung nodules requiring workup and 1 patient was delayed by poor follow-up compliance. These outliers were included in the analysis, as the intention was in part to determine if patients in the MDC had fewer instances of prolonged delay despite having similar numbers of late-stage tumors and baseline high comorbidity scores. However, the traditional clinic did have more outliers; this difference could influence results.
Limitations
We acknowledge certain limitations, notably that this is a retrospective comparison of 2 cohorts in which randomization could not be performed. We also did not match individual patients; however, the patients included were all eligible for the MDC and were treated at parallel times. In addition, this investigation is limited by both outliers and by a small sample size. In particular, the numbers of patients with 30 days or more of delay were low, which should be kept in mind when any conclusions are being made. Finally, no survival analysis was able to be performed at this time.
Conclusions
We present a model of a head and neck cancer MDC in which the goals of complete and efficient care are met, and we believe this study provides evidence that this model is superior to a traditional, multiple-appointment approach in this regard. Future studies will investigate whether this clinic approach has a survival effect for patients.
References
- 1.Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2010;102(2):125-134. [DOI] [PubMed] [Google Scholar]
- 2.Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg. 2010;143(5):650-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor C, Munro AJ, Glynne-Jones R, et al. . Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. [DOI] [PubMed] [Google Scholar]
- 4.Gupta T. Multidisciplinary clinics in oncology: the hidden pitfalls. J Oncol Pract. 2007;3(2):72-73. doi: 10.1200/JOP.0722505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN guidelines for head and neck cancer care. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf. Accessed July 2016.
- 6.Rossi E, Vita A, Baccetti S, Di Stefano M, Voller F, Zanobini A. Complementary and alternative medicine for cancer patients: results of the EPAAC survey on integrative oncology centres in Europe. Support Care Cancer. 2015;23(6):1795-1806. [DOI] [PubMed] [Google Scholar]
- 7.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7(11):935-943. [DOI] [PubMed] [Google Scholar]
- 8.American College of Surgeons Commission on cancer: cancer program standards (2016 edition). https://facs.org/cancer/coc/programstandards.html. Accessed July 2016.
- 9.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics: do they work? Cancer. 1997;79(12):2380-2384. [PubMed] [Google Scholar]
- 10.Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timeliness of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631. [DOI] [PubMed] [Google Scholar]
- 11.Levine RA, Chawla B, Bergeron S, Wasvary H. Multidisciplinary management of colorectal cancer enhances access to multimodal therapy and compliance with National Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal Dis. 2012;27(11):1531-1538. [DOI] [PubMed] [Google Scholar]
- 12.Light T, Rassi EE, Maggiore RJ, et al. . Improving outcomes in veterans with oropharyngeal squamous cell carcinoma through implementation of a multidisciplinary clinic. Head Neck. 2017;39(6):1106-1112. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. [DOI] [PubMed] [Google Scholar]
- 14.Murphy CT, Galloway TJ, Handorf EA, et al. . Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ. 2002;17(2):97-100. [DOI] [PubMed] [Google Scholar]
- 16.van Harten MC, Hoebers FJ, Kross KW, van Werkhoven ED, van den Brekel MW, van Dijk BA. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51(3):272-278. [DOI] [PubMed] [Google Scholar]