Key Points
Question
In oral cavity squamous cell carcinoma (OCSCC), is there an association between specific margin distances and local recurrence, and what should be considered a “close” margin?
Findings
In this retrospective cohort study of 432 patients with OCSCC, there was no appreciable difference between local recurrence rates for different margin distances of 1 mm or greater. Regardless of the specific margin distance, resection of additional tissue after a positive frozen section margin did not significantly alter local recurrence rate.
Meaning
A cutoff of less than 1 mm between cut tissue edge and invasive tumor would be appropriate to consider as a close margin at risk for recurrence.
Abstract
Importance
There is a lack of consistency in the literature regarding the definition of “close” resection margins in the surgical treatment of oral cavity squamous cell carcinoma (OCSCC), and the relationship between local recurrence (LR) rates and different distances of invasive tumor from surgical margin is not well characterized.
Objective
To analyze the association between specific distances from invasive tumor to surgical margin and LR in patients with OCSCC.
Design, Setting, and Participants
Retrospective cohort study of 432 patients treated via en bloc resection for OCSCC between 2005 and 2014 at the University of Iowa Hospitals and Clinics. In all cases, permanent margin evaluation was performed on the main tumor specimen and with intraoperative frozen section margin assessment from the tumor bed.
Main Outcomes and Measures
The LR rate based on minimum millimeter distance between invasive tumor and inked main specimen margin.
Results
Of the 432 participants, 252 (58%) were men and 180 (42%) were women (mean age, 62.14 years; range, 19-99 years). In each case, the LR rate was analyzed in relation to each millimeter distance of invasive cancer from the inked main specimen margin, with results showing an exponential inverse relationship. The LR rate for microscopic positive margins was 44% (95% CI, 34%-55%); for margins less than 1 mm, 28% (95% CI, 18%-41%); for 1 mm, 17% (95% CI, 8%-31%); for 2 mm, 13% (95% CI, 6%-27%); for 3 mm, 13% (95% CI, 5%-32%); for 4 mm, 14% (95% CI, 5%-35%); and for 5 mm or greater, 11% (95% CI, 6%-18%). Analysis of the receiver operating characteristic curve identified a cutoff of less than 1 mm as appropriate for classifying higher risk of local recurrence. Regardless of margin distance, resection of additional tissue beyond 1 mm based on intraoperative frozen section was not associated with improved local control.
Conclusions and Relevance
The commonly used cutoff of 5 mm for a close margin lacks an evidential basis in predicting local recurrence. Invasive tumor within 1 mm of the permanent specimen margin is associated with a significantly higher local recurrence rate, though there is no significant difference for greater distances. This study suggests that a cutoff of less than 1 mm identifies patients at increased local recurrence risk who may benefit from additional treatment. Analysis of the tumor specimen rather than the tumor bed is necessary for this determination.
This cohort study of patients with surgically treated oral cavity squamous cell carcinoma evaluates the association between risk of local recurrence and different sizes, in 1-mm increments, of cancer-free surgical margins.
Introduction
Although pathologic margin assessment is widely used in the surgical treatment of head and neck cancers, interpretation of margin status and its prognostic implications are not always straightforward. Achieving clear margins during surgical resection is thought to reduce local recurrence (LR) and improve prognosis; however, what constitutes a clear, close, or involved margin is inconsistent in the literature and in practice. A survey of head and neck surgeons highlights these differences in opinion and practice, with 83% of respondents considering carcinoma in situ (CIS) to be a positive margin, and 17% including any dysplasia at all to be positive. Sixty-nine percent of surveyed head and neck surgeons used a cutoff of less than 5 mm between invasive tumor and resection margin as a close margin; indeed, a cutoff distance of 5 mm or greater of uninvolved tissue around the tumor is most commonly cited as a clear margin, although some reports in the literature suggest that a smaller-distance cutoff constitutes an adequate resection.
To further examine the clinical significance and impact of surgical margins in oral cavity squamous cell carcinoma (OCSCC), we undertook a retrospective assessment of surgical treatment of OCSCC, with specific attention to the assessment of the surgical margin and the outcome of LR.
Methods
Study Population
This study was approved by the University of Iowa Hospitals and Clinics institutional review board, waiving patient written informed consent. For each patient included in the study, clinical notes, operative and pathology reports, and data from the institutional tumor registry were used to conduct a retrospective review. Included were all consecutive patients with primary OCSCC treated with surgical resection at the University of Iowa Hospitals and Clinics between the years of 2005 and 2014. Exclusion criteria included cancer outside of the oral cavity; cancer type other than OCSCC or one of its variants; lack of invasive cancer in the resection specimen; cancer previously treated by either irradiation or surgery; recurrent cancer; resection that was not en bloc or not done with curative intent; or gross residual remaining disease following surgery. Patients who had several separate OCSCCs over the course of the study period were included as distinct cancers, provided the tumors did not occur in adjoining sites. Patients who had multiple OCSCCs with numerous cancer resections over time in whom it was impossible to distinguish a recurrence from a second primary were excluded from the study.
Surgical Resection and Pathologic Analysis
The approach for surgical resection was uniform among patients included in this study, with attempted 1-cm margins around the tumor wherever possible. Conservative bone resection was performed for tumors approaching bone but without clinical evidence of invasion, while more radical bone resection was performed for tumors with identifiable bony invasion. In the overwhelming majority of patients, intraoperative frozen margins were taken from the tumor bed rather than the resection specimen; therefore, the few patients in whom margins were taken from the resection specimen were excluded from further analysis to ensure a homogeneous method of margin assessment in the data set. When an intraoperative margin was positive, we recorded whether further resection was performed in an attempt to obtain an uninvolved margin. We did not count additional frozen section biopsies from the tumor bed as a definitive additional resection. Pathology reports were used to gather information regarding frozen section margins, including intraoperative vs final results, whether any additional resection was performed, the presence of tumor in the additional resection specimen(s), and assessment (by an independent reviewer, such as the tumor board, would interpret the result) of the final margin status based on all available information from frozen margins and additional resections and/or margins.
When intraoperative tumor bed frozen sections were performed, the worst initial intraoperative diagnosis was recorded, as well as whether any additional tissue was subsequently resected in response to the frozen result. Subsequently, a final operative margin result could be recorded, indicating the worst margin status at the conclusion of the surgery. For purposes of assessing the success of the frozen margin technique, we defined a final operative margin without carcinoma present following additional excisions as successful margin clearance. The worst margin assessment from the main tumor specimen following formalin fixation was also recorded, which we designated as the specimen margin; any patients for whom the intraoperative or permanent specimen margin assessment was missing were excluded. For the purposes of this study, a margin was classified as positive if there was invasive cancer present at the inked edge of the specimen; very close if cancer was less than 1 mm from the inked edge; close if 1 to 5 mm; and negative if 5 mm or more distant from the inked edge. Dysplasia and CIS present at the inked edge of the margin were noted; mild and moderate dysplasia were grouped together, as were severe dysplasia and CIS. If available, the exact distance of the closest invasive tumor from any inked specimen edge was recorded. Dysplasia was not considered in the definition of a positive or close margin.
The institutional tumor registry was used to obtain outcome data such as recurrence and survival for patients included in the study. We defined LR as invasive cancer occurring within 5 years of primary surgical resection at a contiguous site. Also recorded were any regional and distant recurrences. Any patient without a recurrence who did not survive or have follow-up 6 months after surgery was not included in the calculation of LR. Survival analyses were developed using time from surgery date to the date of death or last censor (last known follow-up).
All statistical analyses were performed with R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing; https://www.R-project.org).
Results
A total of 547 pathology records were reviewed, 81 (15%) of which were excluded based on the study criteria. Nine patients did not have margin assessment reported from the main specimen, and 21 did not have intraoperative frozen margin sampling, all of which were excluded from further analysis. In 9 patients, the intraoperative frozen sections were taken from the main specimen rather than the tumor bed, and these were excluded to decrease heterogeneity. These exclusions left 432 remaining patients to be included in further analysis, and 422 of these had a documented distance of invasive cancer from the inked main specimen margin. Those 10 patients with an unknown distance measurement all had a finding of CIS at the specimen mucosal margin, and a distance of the closest invasive cancer from the margin was not given (though we know that the margin was not positive).
The study population characteristics are summarized in Table 1. The T-stage distribution was 45% T1 (n = 188), 21% T2 (n = 89), and 34% T3/T4 (n = 145). The N-stage distribution was 70% N0 (n = 296), 11% N1/N2a (n = 46), 19% N2b/N2c/N3 (n = 79). The overall distribution of subsites included oral tongue (45%; n = 190), alveolus (21%; n = 89), floor of the mouth (18%; n = 78), and other oral cavity subsite (15% (n = 65).
Table 1. Patient Characteristics by Specimen Margin Resulta.
| Characteristic | Overall | Specimen Margin, mm | RR (95% CI)b | |||
|---|---|---|---|---|---|---|
| 0 (Positive) (n = 100) |
<1 (n = 66) |
1-5 (n = 146) |
≥5 (n = 110) |
|||
| Age at diagnosis, median (quartiles), y (n = 422) | 61 (53-71)c | 63 (52-70) | 58 (52-70) | 60 (53-69) | 62 (54-73) | 1.01 (0.99-1.02)d |
| Sex (n = 422) | ||||||
| Male | 248 (59) | 57 (57) | 43 (65) | 86 (59) | 62 (56) | 1 [Reference] |
| Female | 174 (41) | 43 (43) | 23 (35) | 60 (41) | 48 (44) | 0.96 (0.75-1.2) |
| Subsite (n = 422) | ||||||
| Oral tongue | 190 (45) | 36 (36) | 36 (55) | 69 (47) | 49 (45) | 1 [Reference] |
| Alveolus | 89 (21) | 26 (26) | 8 (12) | 21 (14) | 34 (31) | 1.01 (0.73-1.4) |
| Floor of mouth | 78 (18) | 22 (22) | 13 (20) | 28 (19) | 15 (14) | 1.12 (0.81-1.5) |
| Other | 65 (15) | 16 (16) | 9 (14) | 28 (19) | 12 (11) | 0.93 (0.64-1.4) |
| T stage (collapsed) (n = 422) | ||||||
| T1 | 188 (45) | 29 (29) | 23 (35) | 76 (52) | 60 (55) | 1 [Reference] |
| T2 | 89 (21) | 24 (24) | 16 (24) | 25 (17) | 24 (22) | 1.7 (1.2-2.4) |
| T3/T4 | 145 (34) | 47 (47) | 27 (41) | 45 (31) | 26 (24) | 2.0 (1.5-2.6) |
| N stage (collapsed) (n = 421) | ||||||
| N0 | 296 (70) | 59 (60) | 941 (62) | 108 (74) | 88 (80) | 1 [Reference] |
| N1/2a | 46 (11) | 10 (10) | 9 (14) | 17 (12) | 10 (9) | 1.3 (0.86-1.8) |
| N2b/2c/3 | 79 (19) | 30 (30) | 16 (24) | 21 (14) | 12 (11) | 1.7 (1.35-2.2) |
| Neck dissection (n = 421) | ||||||
| Yes | 290 (69) | 69 (69) | 47 (71) | 105 (72) | 69 (63) | 1 [Reference] |
| No | 131 (31) | 31 (31) | 19 (29) | 41 (28) | 40 (37) | 0.9 (0.69-1.2) |
| Reconstruction (n = 422) | ||||||
| None | 210 (50) | 42 (42) | 27 (41) | 76 (52) | 65 (59) | 1 [Reference] |
| Pedicled flap | 34 (8) | 10 (10) | 7 (11) | 12 (8) | 5 (5) | 1.6 (1.09-2.4) |
| Soft-tissue free flap | 104 (25) | 30 (30) | 19 (29) | 35 (24) | 20 (18) | 1.5 (1.14-2.0) |
| Osteocutaneous free | 74 (18) | 18 (18) | 13 (20) | 23 (16) | 20 (18) | 1.4 (0.97-1.9) |
| Mandibulectomy (n = 420) | ||||||
| None | 280 (67) | 51 (51) | 48 (73) | 106 (74) | 75 (68) | 1 [Reference] |
| Marginal | 55 (13) | 24 (24) | 3 (5) | 15 (10) | 13 (12) | 1.4 (1.1-2.0) |
| Segmental | 85 (20) | 25 (25) | 15 (23) | 23 (16) | 22 (20) | 1.4 (1.0-1.8) |
| ECE (n = 291) | ||||||
| Yes | 74 (25) | 28 (40) | 14 (30) | 19 (18) | 74 (19) | 1 [Reference] |
| No | 217 (75) | 42 (60) | 33 (70) | 85 (82) | 57 (81) | 0.62 (0.47-0.82) |
| PNI (n = 417) | ||||||
| Yes | 151 (36) | 51 (53) | 37 (56) | 45 (31) | 18 (17) | 1 [Reference] |
| No | 266 (64) | 46 (47) | 29 (44) | 101 (69) | 90 (83) | 0.48 (0.38-0.61) |
| LVI (n = 418) | ||||||
| Yes | 117 (28) | 39 (40) | 24 (36) | 37 (25) | 17 (16) | 1 [Reference] |
| No | 301 (72) | 59 (60) | 42 (64) | 109 (75) | 91 (84) | 0.61 (0.49-0.78) |
| Grade (n = 410) | ||||||
| Well | 73 (18) | 13 (13) | 7 (11) | 27 (19) | 26 (25) | 1 [Reference] |
| Moderate | 267 (65) | 63 (64) | 46 (71) | 90 (62) | 68 (67) | 1.6 (1.1-2.5) |
| Poor | 70 (17) | 22 (22) | 12 (18) | 28 (19) | 8 (8) | 1.9 (1.2-3.1) |
| Initial frozen margin (n = 422) | ||||||
| Negative | 242 (57) | 42 (42) | 37 (56) | 87 (60) | 76 (69) | 1 [Reference] |
| Positive | 71 (17) | 35 (35) | 13 (20) | 12 (8) | 11 (10) | 2.1 (1.6-2.6) |
| Severe dysplasia or CIS | 73 (17) | 15 (15) | 11 (17) | 36 (25) | 11 (10) | 1.0 (0.7-1.5) |
| Mild or moderate dysplasia | 36 (9) | 8 (8) | 5 (8) | 11 (8) | 12 (11) | 1.1 (0.7-1.8) |
| Final operative margin (n = 422) | ||||||
| Negative | 339 (80) | 75 (75) | 55 (83) | 116 (79) | 93 (85) | 1 [Reference] |
| Positive | 10 (2) | 9 (9) | 1 (2) | 0 | 0 | 2.65 (2.31-3.0) |
| Severe dysplasia or CIS | 29 (7) | 7 (7) | 5 (8) | 10 (7) | 7 (6) | 1.00 (0.62-1.6) |
| Mild or moderate dysplasia | 44 (10) | 9 (9) | 5 (8) | 20 (14) | 10 (9) | 0.78 (0.49-1.3) |
| Additional excision (n = 422) | ||||||
| Yes | 138 (33) | 51 (51) | 26 (39) | 42 (29) | 19 (17) | 1 [Reference] |
| No | 284 (67) | 49 (49) | 40 (61) | 104 (71) | 91 (83) | 0.58 (0.46-0.73) |
| Adjuvant treatment (n = 422) | ||||||
| None | 249 (59) | 51 (51) | 31 (47) | 90 (62) | 77 (70) | 1 [Reference] |
| Radiation | 120 (28) | 27 (27) | 24 (36) | 41 (28) | 28 (25) | 1.3 (0.98-1.7) |
| Chemotherapy + radiation | 53 (13) | 22 (22) | 11 (17) | 15 (10) | 5 (5) | 1.9 (1.42-2.5) |
Abbreviations: CIS, carcinoma in situ; ECE, extracapsular extension; LVI lymphovascular invasion; PNI, perineural invasion; RR, relative risk.
Unless otherwise noted, data are reported as number (percentage) of patients.
Relative risk based on dichotomous outcome of positive or less than 1-mm close specimen margin vs 1-mm or greater specimen margin.
Numbers provided represent median (quartiles) for continuous variables.
Odds ratio (95% CI) for age predicting specimen margin in a linear regression model.
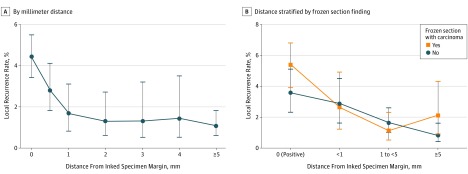
The LR rate was examined based on the millimeter distance of invasive cancer from the inked edge of the main specimen and is summarized in Table 2. The LR rate for positive margins was 44% (95% CI, 34%-55%); for less than 1 mm, 28% (95% CI, 18%-41%); for 1 mm, 17% (95% CI, 8%-31%); for 2 mm, 13% (95% CI, 6%-27%); for 3 mm, 13% (95% CI, 5%-32%); for 4 mm, 14% (95% CI, 5%-35%); and for greater than 5 mm, 11% (95% CI, 6%-18%). This was plotted for all patients with an available measurement, regardless of the presence of dysplasia or CIS at the margin. These data demonstrated an exponential inverse relationship between distance and LR, with no appreciable difference in LR for distances greater than 1 mm (Figure 1A). The relationship of LR and specific distance of invasive cancer from the margin was also stratified based on intraoperative frozen section assessment from tumor bed sampling (Figure 1B). This demonstrated similar LR rates for close margin distances between those with involved and those with negative frozen sections. In patients with a positive main specimen margin, those with an involved intraoperative frozen margin had the highest LR rate of 54% (95% CI, 39%-68%), with an absolute rate difference compared with a negative frozen margin of 18% (95% CI, −6% to 42%). For each specific distance from the inked margin, the relative risk (RR) of LR was also calculated, as summarized in Table 2.
Table 2. Local Recurrence Rate by Distance to the Specimen Margin.
| Specimen Margin, mm | Tumors, No. | LR Rate (95% CI), % | RR (95% CI) |
|---|---|---|---|
| 0 (Positive) | 81 | 44 (34-55) | 4.18 (2.22-7.88) |
| <1 | 54 | 28 (18-41) | 2.61 (1.26-5.40) |
| 1 | 42 | 17 (8-31) | 1.57 (64-3.83) |
| 2 | 39 | 13 (6-27) | 1.21 (0.44-3.30) |
| 3 | 23 | 13 (5-32) | 1.23 (0.37-4.10) |
| 4 | 21 | 14 (5-35) | 1.34 (0.40-4.46) |
| ≥5 | 94 | 11 (6-18) | 1 [Reference] |
Abbreviations: LR, local recurrence; RR, relative risk.
Figure 1. Local Recurrence Rates by Specimen Margin Size (Distance From Invasive Cancer).
A, Local recurrence rate vs the distance from the cut specimen edge to invasive tumor in millimeters. B, Local recurrence rate vs the distance from the cut specimen edge to invasive tumor stratified by the intraoperative frozen section result.
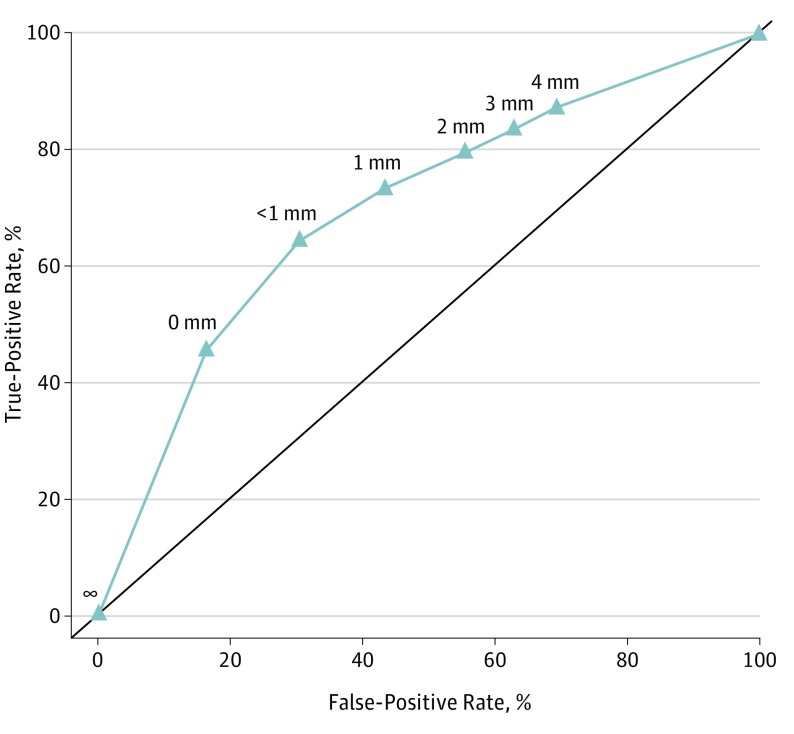
A receiver operating characteristic (ROC) curve was developed to identify a cutoff appropriate for classifying a higher risk of LR (Figure 2). The calculated area under the ROC curve was 0.7.
Figure 2. Receiver Operating Characteristic Curve for Margin Distance in Relation to Local Recurrence.
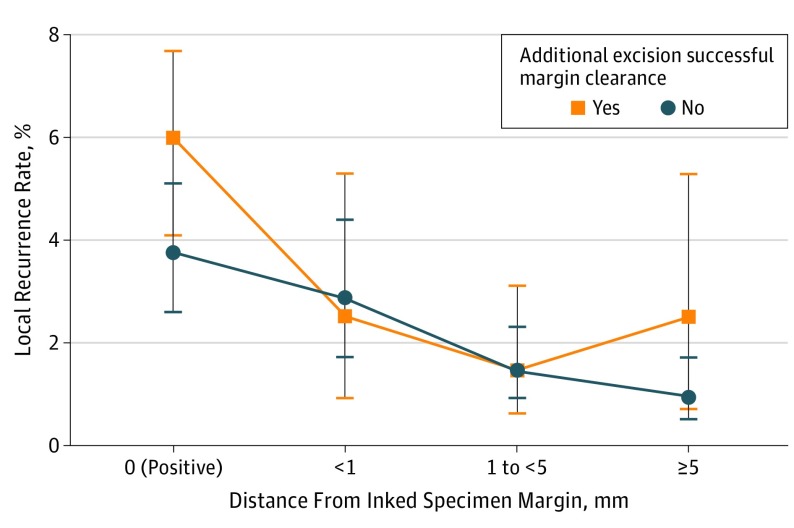
With the LR stratified by specific margin distance from invasive cancer, the results were further examined based on whether additional tissue was resected to achieve a negative margin after initial frozen section indicated carcinoma. The purpose of this further stratification was to determine a relationship between these additional operative steps and LR in specific margin distance categories (eTable 1 in the Supplement; Figure 3). The specimen margin categories were collapsed to 5 mm or greater, 1 to 5 mm, less than 1 mm, and positive. Successful additional resection was defined as cases in which after further resection the final margin was uninvolved with either invasive carcinoma or CIS. For patients with a positive main specimen margin, a successful additional resection was not associated with improved local control and appeared to have a higher LR rate (absolute rate difference, 22%; 95% CI, −03% to 48%). For patients with final margin distances greater than 0 mm, the LR rate appeared to be the same whether a successful additional resection of the margin was performed or not.
Figure 3. Local Recurrence Based on Margin Distance and Stratified by Performance of Additional Tissue Resection.
The LR outcomes were also stratified based on adjuvant treatments after surgical resection. A total of 175 patients (41%) were treated with radiation, and 53 (30%) of these also had chemotherapy. The comparison of LR for main specimen margin categories stratified by adjuvant radiation treatment can be seen graphically in the eFigure and eTable 2 in the Supplement. For patients with a positive main specimen margin, radiation treatment was not associated with improved local control and appeared to have a higher LR rate (absolute rate difference, 15%; 95% CI, −9% to 39%). For each of the other main specimen margins categories, the LR rate was the same whether adjuvant radiation therapy was used or not.
Discussion
This study examines the relationship between LR rate and the specific distance between cut tissue edge and invasive tumor in patients with OCSCC treated surgically. This study benefits from its large sample size and broad sampling of patients with OCSCC as well as a relatively standardized approach to surgical resection and margin assessment technique.
As noted, there is variation in opinion, practice, and published reports regarding the definition of an involved surgical margin, sometimes including noninvasive dysplasia or CIS at the cut tissue edge, and sometimes including invasive carcinoma if located within a certain distance of the cut edge. A strict definition of positive would include only invasive carcinoma at the cut tissue edge, which is the definition used in this study; carcinoma located within some defined distance of the cut edge would be considered a close margin. A review of articles that report outcomes specifically for patients with close margins demonstrates a broad range of local recurrence rates, from 6% to 74%, but these have generally placed patients with margins of less than 5 mm into 1 group and have not considered individual margin distances (eTable 3 in the Supplement). In the present study, we have sought to gain clarity on what should be considered a clinically significant close margin.
Most commonly, a margin of less than 5 mm has been considered close; however, this determination appears to have been arbitrary and has persisted despite a lack of supporting evidence. Studies examining the best cutoff for margin distance report a linear relationship of increasing margin distance and improved outcomes. In the present analysis, we report an exponential relationship. Certainly, a positive margin is different than any close margin distance in terms of outcome; however, the choice of a cutoff close margin distance on which to base prognosis assessments for the patient and potentially influence adjuvant treatment decisions in clinical care and future trials is not clear. Overall, we consider the close margin distance to be a weak predictor of local recurrence, based on the area under the ROC curve. Increasing the cutoff distance improves the sensitivity of the test at the expense of specificity, which leads to an increasing number of patients being identified as high risk who are in actuality low risk, potentially leading to overtreatment and unnecessary toxic effects. Based on the ROC in this study, a distance of less than 1 mm would be appropriate as the optimum point on the curve for such a classification test of close margin distance.
The resection of additional tissue in response to a positive margin is the basis for performing intraoperative margin assessment in oral cancer surgery, but it is unclear if this practice leads to improved outcomes for patients. In examining the use of frozen sections in surgical resection of head and neck cancers, Byers et al reported that 23% of cases had positive margins, allowing further resection to obtain ultimately negative margins. They found that those with initially negative margins and those with negative margins after additional resection had similar LR and 2-year survival rates; however, those with positive margins at the conclusion of surgery had significantly higher LR rates and much poorer survival. They went on to calculate that 30% of patients derived a benefit from intraoperative margin sampling, and they suggested its routine use. Other retrospective studies since then have looked at the benefit of additional resection, and a few found no benefit despite clearance of a positive margin. Our research group, using the same data set analyzed for the present study, found that achieving a negative margin from additional tissue resection was not associated with improved LR rate or overall prognosis; rather, the main specimen margin was most strongly predictive of this regardless of whether the margin was ultimately cleared with additional resection.
Importantly, the present study differs from that of Byers et al and certain other studies in that frozen sections were obtained intraoperatively from the tumor bed and not from the surgical specimen. Surveys suggest that obtaining frozen sections from the tumor bed is a common practice in the United States. The assessment of a positive or close margin was not made until the final pathology report; therefore, any decision about additional tissue resection was made based on the intraoperative sampling of the tumor bed and not based on a close margin distance. The data from our study do not show any improvement in LR rate with resection of additional tissue to a negative margin regardless of the distance between invasive tumor and cut tissue edge on the main specimen. Indeed, patients with a positive main specimen margin had a higher LR rate than those with negative margins, despite additional resection. This argues against the practice of routine intraoperative sampling of the tumor bed for margin assessment. However, we did not examine whether intraoperative assessment of the main surgical specimen has benefit if further margin clearance is carried out. The hypothesis from our data is that patients with invasive cancer less than 1 mm from an inked margin might benefit from additional resection, if the at-risk tissue can be reliably relocated and is amenable to resection. Determining the closeness of a margin intraoperatively would require examination of the tumor specimen itself, though, and not the tumor bed.
Limitations
Some limitations of the study include a relatively small cohort of surgeons performing the majority of surgical procedures, as well as inability to compare results based on different methods of intraoperative margin evaluation, ie, tumor bed vs main specimen sampling.
Conclusions
This retrospective analysis of a series of patients with OCSCC treated with surgery with intraoperative frozen margin assessment from the tumor bed demonstrates several important points regarding the close margin distance. The risk of LR decreases rapidly with at least a 1 mm distance from the cut tissue edge. The close margin distance is a weak predictor of local recurrence, and our data suggest that a distance of less than 1 mm would be an appropriate cutoff.
eTable 1. Local recurrence rate by successful intraoperative additional tissue excision
eTable 2. Local recurrence rate by adjuvant radiation treatment
eTable 3. Literature review of local recurrence and survival based on margin
eTable 4. Local recurrence based on margin distance, stratified by use of adjuvant radiation
eFigure. Local recurrence based on margin distance, stratified by use of adjuvant radiation
References
- 1.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg. 1990;160(4):410-414. [DOI] [PubMed] [Google Scholar]
- 2.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30-34. doi: 10.1054/ijom.2002.0313 [DOI] [PubMed] [Google Scholar]
- 3.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125(10):2298-2307. doi: 10.1002/lary.25397 [DOI] [PubMed] [Google Scholar]
- 4.Binahmed A, Nason RW, Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol. 2007;43(8):780-784. doi: 10.1016/j.oraloncology.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sándor GKB. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):625-629. doi: 10.1016/j.tripleo.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice: the results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27(11):952-958. doi: 10.1002/hed.20269 [DOI] [PubMed] [Google Scholar]
- 7.Looser KG, Shah JP, Strong EW. The significance of “positive” margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1978;1(2):107-111. doi: 10.1002/hed.2890010203 [DOI] [PubMed] [Google Scholar]
- 8.Vikram B, Strong EW, Shah JP, Spiro R. Failure at the primary site following multimodality treatment in advanced head and neck cancer. Head Neck Surg. 1984;6(3):720-723. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Guillamondegui O Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21(5):408-413. [DOI] [PubMed] [Google Scholar]
- 10.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. . Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167-178. [DOI] [PubMed] [Google Scholar]
- 11.Wong LS, McMahon J, Devine J, et al. . Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg. 2012;50(2):102-108. doi: 10.1016/j.bjoms.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 12.Ch’ng S, Corbett-Burns S, Stanton N, et al. . Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer. 2013;119(13):2427-2437. doi: 10.1002/cncr.28081 [DOI] [PubMed] [Google Scholar]
- 13.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue: prognostic and therapeutic implications. Am J Surg. 1986;152(4):354-360. doi: 10.1016/0002-9610(86)90304-1 [DOI] [PubMed] [Google Scholar]
- 14.Ravasz LA, Slootweg PJ, Hordijk GJ, Smit F, van der Tweel I. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J Craniomaxillofac Surg. 1991;19(7):314-318. doi: 10.1016/S1010-5182(05)80339-7 [DOI] [PubMed] [Google Scholar]
- 15.Yuen AP, Lam KY, Wei WI, et al. . A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000;180(2):139-143. [DOI] [PubMed] [Google Scholar]
- 16.Kurita H, Nakanishi Y, Nishizawa R, et al. . Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010;46(11):814-817. doi: 10.1016/j.oraloncology.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Woolgar JA, Rogers S, West CR, Errington RD, Brown JS, Vaughan ED. Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. 1999;35(3):257-265. [DOI] [PubMed] [Google Scholar]
- 18.Byers RM, Bland KI, Borlase B, Luna M. The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978;136(4):525-528. doi: 10.1016/0002-9610(78)90275-1 [DOI] [PubMed] [Google Scholar]
- 19.Pathak KA, Nason RW, Penner C, Viallet NR. Impact of use of frozen section assessment of operative margins on survival in oral cancer. Oral Surgery; 2009;35(3):257-265. [DOI] [PubMed] [Google Scholar]
- 20.Guillemaud JP, Patel RS, Goldstein DP, Higgins KM, Enepekides DJ. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2010;39(4):370-377. [PubMed] [Google Scholar]
- 21.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1191-1198. doi: 10.1001/jamaoto.2016.2329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Local recurrence rate by successful intraoperative additional tissue excision
eTable 2. Local recurrence rate by adjuvant radiation treatment
eTable 3. Literature review of local recurrence and survival based on margin
eTable 4. Local recurrence based on margin distance, stratified by use of adjuvant radiation
eFigure. Local recurrence based on margin distance, stratified by use of adjuvant radiation