Abstract
Using data from a prostate cancer registry, this study correlated HSD3B1 genotype with response to androgen-deprivation therapy in patients with prostate cancer.
Substantial advances have been made in the development of therapeutic biomarkers in various cancers, but not in prostate cancer. A germline inherited polymorphic variant (1245A→C) in the HSD3B1 gene was recently reported to correlate with shorter duration of response to androgen-deprivation therapy (ADT) in hormone-sensitive prostate cancer (HSPC). The HSD3B1 gene encodes the enzyme 3β-hydroxysteroid dehydrogenase-1 (3βHSD1), which catalyzes adrenal androgen precursors into dihydrotestosterone, the most potent androgen. In the study by Hearn et al, presence of 1 or more variant alleles of the HSD3B1 (1245C) was associated with decreased progression-free survival (PFS) compared with the absence of any variant alleles in 3 cohorts of men with prostate cancer treated with ADT: 2 cohorts with post-prostatectomy biochemical recurrence, and 1 cohort with metastatic HSPC (mHSPC) (total, n = 443). In the present analysis, we provide, to our knowledge, the first independent validation of these results, which have the potential to introduce the first predictive biomarker of response to therapy for this patient population.
Methods
We analyzed data from a prospectively maintained prostate cancer registry to determine HSD3B1 genotype retrospectively in men treated with ADT for newly diagnosed mHSPC and to correlate this genotype with response to ADT. The study was approved by the University of Utah institutional review board. All included patients have provided their written informed consent. Genotyping was performed as described by Hearn at al. The primary end point was PFS on ADT. Progression was defined as 2 consecutive increases in the level of prostate-specific antigen (PSA) meeting the following criteria: 2 ng/mL or greater and 25% increase or more from the nadir, as defined by the Prostate Cancer Working Group 2, and/or radiographic or clinical findings of progression. We performed prespecified multivariate analyses to assess the independent predictive value of HSD3B1 genotype on PFS on ADT (Table).
Table. Baseline Characteristics of the Primary Cohort by HSD3B1 Genotype.
| Characteristic | Homozygous Wild Type (n = 48) |
Heterozygous (n = 44) |
Homozygous Variant (n = 10) |
P Valuea |
|---|---|---|---|---|
| Log PSA at ADT initiation | 1.60 (0.76) | 1.67 (0.71) | 1.52 (1.39) | .85 |
| Gleason score, No. (%) | ||||
| 6 | 6 (12) | 8 (18) | 2 (20) | .39 |
| 7 | 9 (18) | 14 (33) | 3 (30) | |
| 8-10 | 33 (70) | 22 (48) | 5 (50) | |
| Prior definitive therapy (surgery or radiation) | ||||
| No | 28 | 27 | 4 | .47 |
| Yes | 20 | 17 | 6 | |
| Neoadjuvant or adjuvant ADT | ||||
| No | 44 | 40 | 9 | >.99 |
| Yes | 4 | 4 | 1 | |
| Cox regression results | ||||
| Median PFS, mo | 21 | 19 | 11 | NA |
| Hazard ratio (95% CI) | 1 [Reference] | 1.04 (0.64-1.07) | 2.16 (1.01-4.58) | NA |
| P value | NA | .86 | .046 | NA |
Abbreviations: ADT, androgen-deprivation therapy; PFS, progression free survival; NA, not applicable; PSA, prostate-specific antigen.
These listed P values are for comparisons across the 3 genotypes.
Results
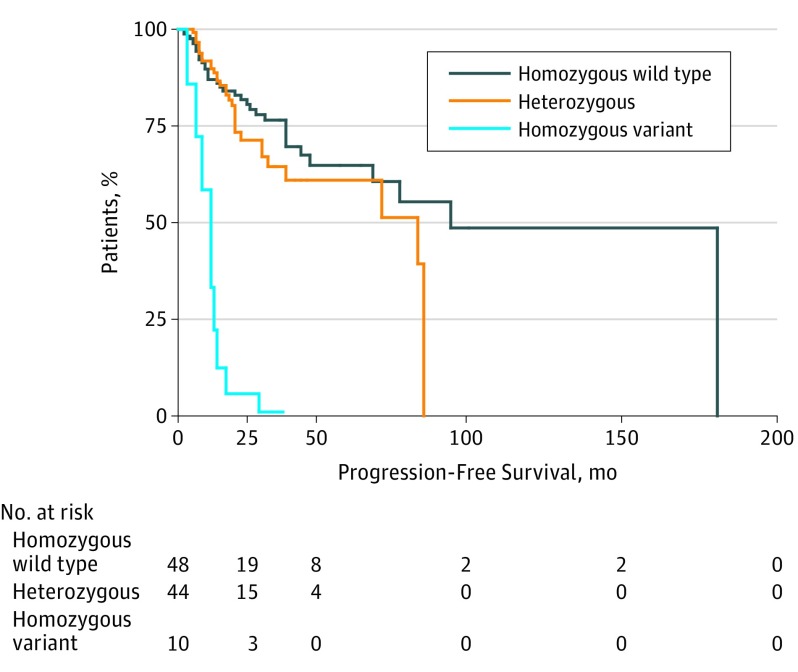
A total of 102 men diagnosed with new-onset mHSPC had all of the required clinical and genotype data available and were eligible to be included in this study. Median follow-up for this study was 35.5 months. The allelic frequency of the HSD3B1 (1245C) variant in our cohort was 31%, which is similar to that reported by Hearn et al (26%-36% in their 3 cohorts). In multivariate analysis controlling for Gleason score and baseline log PSA, those men in the homozygous variant group had significantly shorter median PFS than those in the homozygous wild-type group (11 vs 21 months; HR, 2.16; 95% CI, 1.01-4.58; P = .046). No significant difference in median PFS was observed between those men in the heterozygous group and those in the homozygous wild-type group (19 vs 21 months; HR, 1.04; 95% CI, 0.64-1.07; P = .86) (Table). The Figure shows a Kaplan-Meier curve of PFS by the number of HSD3B1 variants present while controlling for Gleason score and log PSA. In comparison with the metastatic cohort reported by Hearn et al, the prevalence of homozygosity for the variant allele was higher in the present study (9.8% in 102 patients vs 5.8% in 188 patients). The median PFS on ADT for the homozygous variants cohort in the present study (11.0 months) was similar to that found by Hearn et al (9.8 months). One limitation of the present study is that PFS was determined by PSA progression alone in most patients because imaging studies were not consistently performed in this real world population.
Figure. Kaplan-Meier Curve of Progression-Free Survival by Number of HSD3B1 Variants.
Data adjusted for Gleason score and log prostate-specific antigen level.
Conclusions
In this study, we independently validate that the HSD3B1 (1245C) variant allele is associated with a shorter PFS on ADT in men with mHSPC, and we confirm that the allele frequency of HSD3B1 is about 30% in this population. The 10% of men homozygous for variant allele HSD3B1 (1245C) are likely to have suboptimal response to ADT alone. These men may benefit more from up-front docetaxel treatment or from enrollment in trials investigating up-front deeper androgen blockade with novel androgen-signaling inhibitors.
References
- 1.Hearn JW, AbuAli G, Reichard CA, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17(10):1435-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26(4):525-582. [DOI] [PubMed] [Google Scholar]
- 3.Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. doi: 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]