Key Points
Question
Has the epidemiology of neuroendocrine tumors changed over time?
Findings
In this population-based study that included 64 971 patients with neuroendocrine tumors, age-adjusted incidence rates increased 6.4-fold between 1973 and 2012, mostly for early-stage tumors. Survival for all neuroendocrine tumors has improved, especially for distant-stage gastrointestinal and pancreatic neuroendocrine tumors.
Meaning
Neuroendocrine tumors are increasing in incidence and prevalence owing to increased diagnosis of early-stage tumors. Survival of patients with distant-stage tumors has improved, reflecting improvements in therapies.
Abstract
Importance
The incidence and prevalence of neuroendocrine tumors (NETs) are thought to be rising, but updated epidemiologic data are lacking.
Objective
To explore the evolving epidemiology and investigate the effect of therapeutic advances on survival of patients with NETs.
Design, Setting, and Participants
A retrospective, population-based study using nationally representative data from the Surveillance, Epidemiology, and End Results (SEER) program was conducted to evaluate 64 971 patients with NETs from 1973 to 2012. Associated population data were used to determine annual age-adjusted incidence, limited-duration prevalence, and 5-year overall survival (OS) rates. Trends in survival from 2000 to 2012 were evaluated for the entire cohort as well as specific subgroups, including distant-stage gastrointestinal NETs and pancreatic NETs. Analyses were conducted between December 2015, and February 2017.
Main Outcomes and Measures
Neuroendocrine tumor incidence, prevalence, and OS rates.
Results
Of the 64 971 cases of NETs, 34 233 (52.7%) were women. The age-adjusted incidence rate increased 6.4-fold from 1973 (1.09 per 100 000) to 2012 (6.98 per 100 000). This increase occurred across all sites, stages, and grades. In the SEER 18 registry grouping (2000-2012), the highest incidence rates were 1.49 per 100 000 in the lung, 3.56 per 100 000 in gastroenteropancreatic sites, and 0.84 per 100 000 in NETs with an unknown primary site. The estimated 20-year limited-duration prevalence of NETs in the United States on January 1, 2014, was 171 321. On multivariable analyses, the median 5-year OS rate varied significantly by stage, grade, age at diagnosis, primary site, and time period of diagnosis. The OS rate for all NETs improved from the 2000-2004 period to the 2009-2012 period (hazard ratio [HR], 0.79; 95% CI, 0.73-0.85). Even larger increases in OS between these periods were noted in distant-stage gastrointestinal NETs (HR, 0.71; 95% CI, 0.62-0.81) and distant-stage pancreatic NETs (HR, 0.56; 95% CI, 0.44-0.70).
Conclusions and Relevance
The incidence and prevalence of NETs are steadily rising, possibly owing to detection of early-stage disease and stage migration. Survival for all NETs has improved over time, especially for distant-stage gastrointestinal NETs and pancreatic NETs in particular, reflecting improvement in therapies. These data will help to prioritize future research directions.
This population-based study evaluates SEER data on the incidence and prevalence of neuroendocrine tumors over 20 years.
Introduction
Given the rarity and indolent clinical course of neuroendocrine tumors (NETs), the epidemiology of these tumors is best studied in large, population-based registries with considerable longitudinal follow-up. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program is a comprehensive source of population-based information initiated in 1973 that is updated annually. Previously, the most comprehensive population-based study of NETs in the United States had been performed by our group using the November 2006 submission of SEER data with cases diagnosed up to 2004 that showed increasing incidence. Since then, the SEER Program has expanded and the current (SEER 18) registry grouping now includes approximately 30% of the US population. Diagnostic techniques for NETs, such as computed tomography and endoscopy, have improved and have likely increased NET diagnosis rates and accuracy of staging. In addition, updated staging and grading classifications for NETs have been proposed and more universally adopted, possibly further increasing the recognition of NETs and improving their pathologic classification. Based on these observations, we hypothesized that the increased incidence of NETs is associated mainly with the rise in detection of early-stage disease.
The somatostatin analogue octreotide acetate was initially introduced in 1987 and as a long-acting release form in 1998 for management of carcinoid syndrome given their ability to inhibit hormone secretion by NETs. With the lack of adequate treatment options for NETs until recently, these agents were likely used for tumor control even before the completion of randomized trials (octreotide long-acting release in mid-gut NETs in 2008 and lanreotide acetate for gastroenteropancreatic NETs in 2011) establishing their efficacy. In addition, for pancreatic NETs, the alkylating agent streptozocin was the first drug approved in 1982; further research in the mid-2000s showed that another alkylating agent, temozolomide, also had antitumor activity. Given the rise in early-stage disease and improvements in systemic therapies, we also hypothesized that the limited-duration prevalence (the proportion of patients alive on a certain day and diagnosed within a limited duration prior to that date) would also be increasing. Therefore, in the present study, we attempted to comprehensively evaluate the demographic, clinical, and prognostic features of NETs using data from the SEER Program.
Methods
Data Source
The SEER database on the November 2014 submissions was used for our study. The SEER Program is a coordinated system of population-based state cancer registries collecting incidence and survival data on cases reported from the target geographic areas. Since its inception in 1973 (SEER 9 registry), the program has undergone 2 major expansions to include additional areas (SEER 13 in 1992 and SEER 18 in 2000) and currently includes 20 geographic areas with demographics representative of the entire US population. The pertinent population data are obtained from the US Census Bureau and mortality data are obtained from the US National Center for Health Statistics. Strict quality control is maintained by the SEER Quality Improvement program that establishes standards for cancer registries and maintains them through continual monitoring, assessment, and education. We used histologic codes from the International Classification of Diseases for Oncology,3rd Edition, to identify patients with NETs, as detailed in a prior publication. Per policy of The University of Texas MD Anderson Cancer Center, no institutional review board approval was required for the study. Data analysis was conducted between December 2015, and February 2017.
NET Classification
We used SEER histologic grade information to classify cases as grade (G) 1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; and G4, undifferentiated or anaplastic. G3 and G4 were combined into 1 category for all analyses.
Statistical Analysis
Given that there are 3 SEER registry groupings, to maximize the representativeness of our study, we calculated the 1973-2012 incidences using SEER 9, the 1992-1999 incidences using SEER 13, and the 2000-2012 incidences using SEER 18 databases. Limited-duration prevalence rates were calculated for 10 and 20 years. We examined the 15-year survival by site and stage using the Kaplan-Meier method and log-rank test. Furthermore, we provided the median overall survival (OS) by site, stage, and grade with a maximum follow-up time of 30 years using data from the SEER 9 registry. Finally, we provided the most recent median, 3-year, and 5-year survival rates for distant-stage G1 and G2 NETs from the SEER 18 cohort. Time of follow-up for all analyses was from the date of diagnosis until death, date of last contact, or end of study period.
To evaluate the most recent trends in survival, we conducted multivariable survival analyses of the SEER 18 data (2000-2012). Three cohorts were identified for multivariable survival analyses: the total SEER 18 NET cohort, which comprised all patients with NETs in SEER 18; the distant-stage gastrointestinal NET cohort (liver was excluded since it had a high probability of being a metastatic rather than primary site); and the distant-stage pancreatic NET cohort. The latter 2 cohorts were chosen to evaluate the effect of advances in systemic therapies for these sites on survival. Distant-stage pancreatic NETs were analyzed separately given the unique biology and clinical behavior of this subgroup. Five-year OS and the Cox proportional hazards model were used in the multivariable analysis, with censoring applied at 5 years. Covariates for this analysis included factors known to influence prognosis of NETs, including grade, race, age, stage, site, and time interval from diagnosis. The overall model was significant at P < .001.
Incidence (including annual percentage change) and limited-duration prevalence rates (10-year and 20-year) were calculated using SEER*Stat software, version 8.2.1 (Surveillance Research Program, National Cancer Institute). In this software, annual percentage change is calculated by fitting a least-squares regression to the natural logarithm of the rates, using the calendar year as a regressor variable, and age-adjusted incidence rates are computed using weighted proportions of corresponding age groups in the 2000 US standard population. The projected prevalence of NETs in the US population on January 1, 2014, matched by age, sex, and race, was calculated using ProjPrev, version 1.0.4 (Data Modeling Branch, National Cancer Institute).
All other statistical analyses were performed using SAS, version 9.3 (SAS Institute). Comparative differences were considered significant at P < .05.
Results
The data set that we used contained a total of 64 971 patients, including 7294, 10 631, and 64 971 in SEER 9, 13, and 18 registries, respectively. Of these patients, 34 233 (52.7%) were women. The annual number of NET cases and the numbers at risk are detailed in eTable 1 in the Supplement. Of 45 318 NETs with a known grade, 23 126 were G1, 7416 were G2, and 14 766 were G3 and G4. Of 53 465 NETs with a known stage, 28 031 were localized, 10 777 were regional, and 14 657 were distant at the time of diagnosis.
Annual Incidence
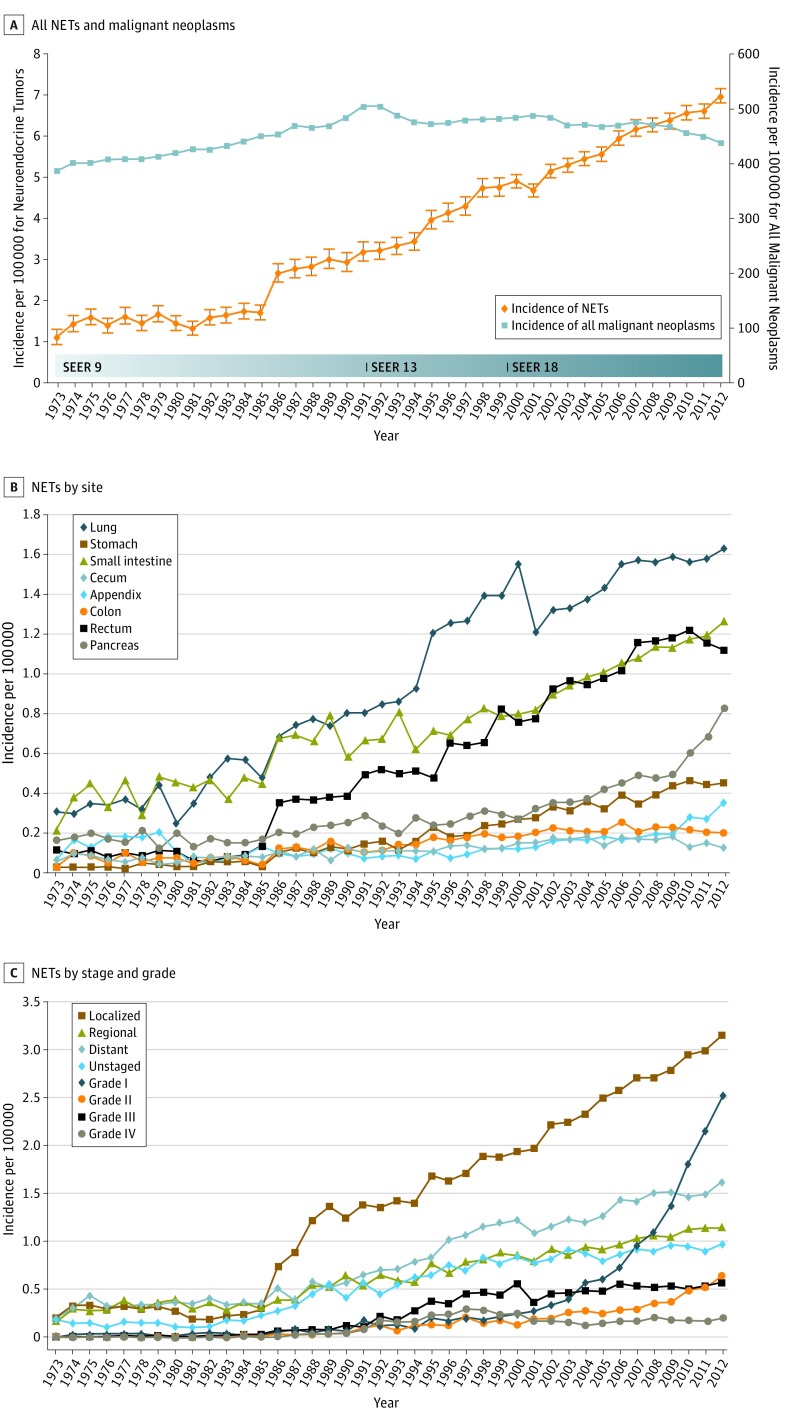
The annual age-adjusted incidence of NETs was 1.09 per 100 000 persons in 1973 and increased to 6.98 per 100 000 persons by 2012 as shown in Figure 1A (and contrasted with annual age-adjusted incidence of all malignant neoplasms). Age-specific incidence rates were calculated for 3 age groups: younger than 50 years, 50 to 64 years, and 65 years or older. The most dramatic rise in incidence was noted in patients 65 years or older with a more than 8-fold rise to 25.3 per 100 000 persons and, in those 50 to 64 years, to 14.3 per 100 000 persons; those younger than 50 years had a more modest 3-fold rise to 1.75 per 100 000 persons (eTable 1 in the Supplement). The annual percentage change for age-adjusted incidence from 2000 to 2012 in SEER 18 was 3.2 per 100 000 persons (P < .001).
Figure 1. Incidence Trends of Neuroendocrine Tumors (NETs) From 1973 to 2012.
A, Annual age-adjusted incidence of all neuroendocrine tumors and all malignant neoplasms. B, Annual age-adjusted incidence of NETs by site. C, Annual age-adjusted incidence of NETs by stage and grade.
The increase in the incidence of NETs from 1973 to 2012 occurred across all sites, stages, and grades. The increases in incidence for various sites ranged from 15-fold in the stomach to 2-fold in the cecum (Figure 1B). Among stage groups, the incidence increased the most in localized NETs, from 0.21 per 100 000 persons in 1973 to 3.15 per 100 000 persons in 2012 (P < .001) (Figure 1C). Among grade groups, incidence increased the most in G1 NETs, from 0.01 per 100 000 persons in 1973 to 2.53 per 100 000 persons in 2012 (P < .001) (Figure 1C). In SEER 18 (2000-2012), the highest incidences were 1.49 per 100 000 persons in the lung, 3.56 per 100 000 persons in gastroenteropancreatic sites (including 1.05 per 100 000 persons in the small intestine, 1.04 per 100 000 persons in the rectum, and 0.48 per 100 000 persons in the pancreas), and 0.84 per 100 000 persons in NETs with an unknown primary site of origin.
Prevalence
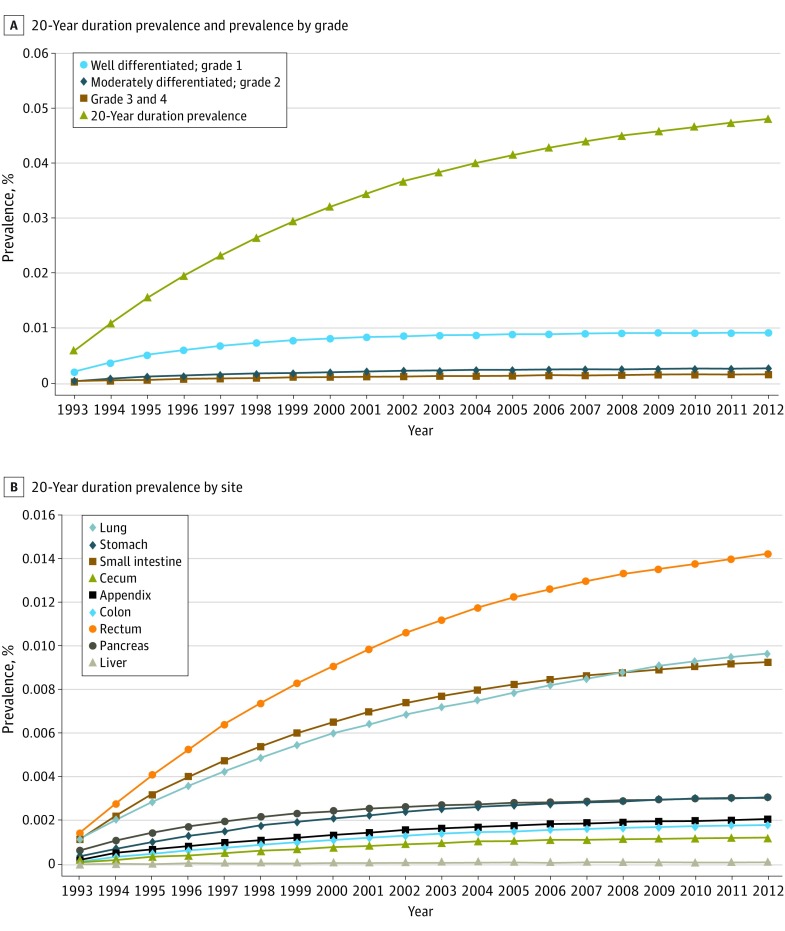
Reflecting the rising incidence and indolent nature of NETs, the 20-year limited-duration prevalence increased substantially, from 0.006% in 1993 to 0.048% in 2012 (P < .001) (Figure 2A). Ten-year limited-duration prevalence and absolute counts for both time periods are detailed in eTable 2 in the Supplement. Among grade groups, prevalence increased the most in G1 NETs and, among sites, prevalence was highest in the rectum, followed by the lung and small intestine (Figure 2). The projected prevalence of NETs in the US population on January 1, 2014, matched by age, sex, and race, was 171 321 per 100 000 persons.
Figure 2. Limited Duration Prevalence of Neuroendocrine Tumors (NETs).
A, 20-Year limited duration prevalence of all neuroendocrine tumors and according to grade. B, 20-Year limited duration prevalence of neuroendocrine tumors by site.
Survival
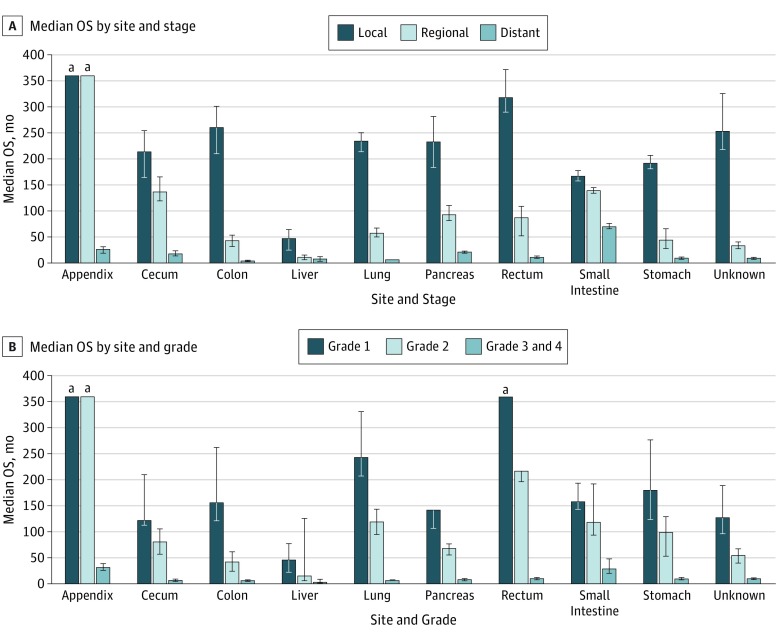
The median OS time for all patients was 9.3 years (112 months). Localized NETs had better median OS (>30 years) compared with regional NETs (10.2 years) and distant NETs (12 months) (P < .001). Of those with known grades, G1 NETs had the highest median OS (16.2 years) among grade groups, G2 NETs had the worse OS (8.3 years), and G3 and G4 NETs had the worst OS (10 months). NETs in the rectum (24.6 years) and appendix (>30.0 years) had the best median OS among site groups, while NETs in the pancreas (3.6 years) and lung (5.5 years) had the worst median OS. All these differences in survival were significant (log-rank P < .001).
We then evaluated survival patterns according to site and stage (Figure 3). In localized NETs, median OS ranged from 14 years in the small intestine to more than 30 years in the appendix. In regional NETs, median OS ranged from 33 months for NETs with an unknown primary to more than 30 years in the appendix. For distant NETs, those in the small intestine had the best median OS (5.83 years); NETs in the lung (6 months) and colon (4 months) had the worst median OS. All of these differences in OS were significant (log-rank P < .001).
Figure 3. Median Overall Survival (OS) of Neuroendocrine Tumors.
A, Median OS of all patients included in study according to stage. B, Median OS of all patients included in study according to grade. Error bars indicate 95% CI.
aMaximum follow-up time was 360 months.
Next, we evaluated OS according to site and grade. Patients with G1 or G2 appendiceal NETs or G1 rectal NETs had the longest median OS (>30 years). Irrespective of site, patients with G3 and G4 NETs had poor OS, ranging from 30 to 33 months for the small intestine and appendix, respectively, to 8 months for the cecum and colon (P < .001) (Figure 3).
Finally, we evaluated the median, 3-year, and 5-year survival rates for well-differentiated to moderately differentiated distant stage NETs in the SEER 18 cohort (2000-2012) since we believed that this information would be most helpful for practicing clinicians (eTable 3 in the Supplement).
Multivariable Analysis of OS
We next performed multivariable analysis with hazard ratios (HRs) calculated for 5-year mortality hazard rates (Table). We found that patients with G2 NETs (HR, 1.76; 95% CI, 1.59-1.94) and G3 and G4 NETs (HR, 5.26; 95% CI, 4.85-5.71) had worse OS than did those with G1 NETs. Race, age, stage, and site were all found to have significant correlation with survival. Overall survival was worse in regional NETs (HR, 1.73; 95% CI, 1.57-1.90) and distant NETs (HR, 5.05; 95% CI, 4.64-5.50) than in localized NETs after adjustment for other covariates. NETs in the liver had the worst OS (HR, 1.85; 95% CI, 1.46-2.36) and NETs in the stomach had the second-worst OS (HR, 1.20; 95% CI, 1.07-1.34) compared with NETs in the lung.
Table. Multivariable Survival Analysis of Patients With NETs Diagnosed From 2000 to 2012.
| Covariate | HR (95% CI) | ||
|---|---|---|---|
| Total SEER 18 NET Cohort (n = 14 757) |
Distant GI NET (n = 2681) |
Distant Pancreatic NET (n = 850) |
|
| Year | |||
| 2000-2004 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2005-2008 | 0.83 (0.78-0.89) | 0.76 (0.67-0.86) | 0.76 (0.61-0.96) |
| 2009-2012 | 0.79 (0.73-0.85) | 0.71 (0.62-0.81) | 0.56 (0.44-0.70) |
| Grade | |||
| 1: Well differentiated | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2: Moderately differentiated | 1.76 (1.59-1.94) | 1.81 (1.52-2.14) | 1.36 (1.04-1.77) |
| 3 and 4: Poorly differentiated and undifferentiated; anaplastic | 5.26 (4.85-5.71) | 6.72 (5.89-7.67) | 4.81 (3.85-6.02) |
| Race | |||
| White | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| American Indian/Alaska Native | 1.45 (1.00-2.11) | 1.73 (0.86-3.47) | 2.07 (0.66-6.50) |
| Asian or Pacific Islander | 1.03 (0.91-1.17) | 1.40 (1.11-1.76) | 1.00 (0.69-1.46) |
| Black | 1.23 (1.13-1.34) | 1.31 (1.12-1.52) | 1.28 (0.98-1.68) |
| Age, y | |||
| ≤30 | 0.23 (0.17-0.33) | 0.46 (0.28-0.76) | 0.44 (0.23-0.86) |
| 31-60 | 0.54 (0.51-0.57) | 0.62 (0.56-0.69) | 0.58 (0.48-0.70) |
| ≥61 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Stage | NA | NA | |
| Localized | 1 [Reference] | ||
| Regional | 1.73 (1.57-1.90) | ||
| Distant | 5.05 (4.64-5.50) | ||
| Site | NA | NA | |
| Lung | [Reference] | ||
| Appendix | 0.53 (0.43-0.65) | ||
| Cecum | 0.81 (0.72-0.91) | ||
| Colon | 0.99 (0.88-1.12) | ||
| Liver | 1.85 (1.46-2.36) | ||
| Pancreas | 0.86 (0.78-0.94) | ||
| Rectum | 0.71 (0.62-0.82) | ||
| Small intestine | 0.53 (0.48-0.59) | ||
| Stomach | 1.20 (1.07-1.34) | ||
Abbreviations: HR, hazard ratio; NA, not applicable; NET, neuroendocrine tumor; SEER, Surveillance, Epidemiology, and End Results.
We then focused on the SEER 18 cohort to evaluate the most recent trends in OS over 3 time periods: 2000-2004, 2005-2008, and 2009-2012. In the overall SEER 18 cohort, compared with 2000-2004, patients who received the NET diagnosis between 2005 and 2008 had a 17.1% lower risk of death (HR, 0.83; 95% CI, 0.78-0.89) and those diagnosed in 2009-2012 had a 21.3% lower risk of death (HR, 0.79; 95% CI, 0.74-0.85). In these 2 subcohorts, we also found better survival in recent years compared with previous years. The improvement in survival over the same time intervals was more pronounced in the subgroup with distant GI NETs (HR, 0.76; 95% CI, 0.67-0.86 for 2005-2008 and HR, 0.71; 95% CI, 0.63-0.82 for 2009-2012 compared with 2000-2004). The subgroup with distant pancreatic NET saw the biggest improvements. Compared with patients who received the NET diagnosis in 2000-2004, those diagnosed in 2005-2008 had a 24% reduction in risk of death (HR, 0.76; 95% CI, 0.61-0.96) and those diagnosed in 2009-2012 had a 44% reduction in risk of death (HR, 0.56; 95% CI, 0.44-0.71). All of the above comparisons were significant at P < .001.
Discussion
In this population-based study, we found that the age-adjusted annual incidence of NETs increased from 1.09 per 100 000 in 1973 to 6.98 per 100 000 in 2012, a 6.4-fold increase. Survival of patients with NET has improved over time, and this increase was especially pronounced in distant gastrointestinal NETs and in distant pancreatic NETs, reflecting improvements in therapies for those sites.
Although prior studies done across the world have also shown a rise in the incidence of NETs, this elevation has been most marked in North American studies. Whether these differences are due to underlying biologic factors, environmental factors, health care patterns, and/or data capture by registries is unknown. Although the increase in incidence occurred across all sites and all stages during this period, it was markedly greater for the localized stage, possibly due to an increased diagnosis of asymptomatic, early-stage disease. This finding is supported by a Canadian population-based study that showed that, despite the overall increase in the incidence of NETs, the proportion of patients with metastatic disease has remained constant over time. In the present study, we provide extensive details regarding the trends at each site during a much longer time period and show that the rise in incidence was greatest in the stomach (15-fold) and rectum (9-fold). The trends at these sites may be associated with the increased use of endoscopic procedures. The steady rise in the incidence of NETs at other common sites, including the lung and small intestine, is probably related to increased use of imaging procedures in clinical practice. Similarly, the steep rise in G1 NETs is possibly related to increased recognition and widespread adoption of the formalization of the nomenclature, grading, and staging of these tumors. To highlight the burden of NETs, we evaluated the rising prevalence in the form of 20-year and 10-year limited-duration prevalence rates. Since these prevalence rates include patients irrespective of whether they are under treatment or considered cured, they are a composite of the incidence and survival rates. The age-, sex-, and race-adjusted 20-year limited duration prevalence for the US population for January 1, 2014, was estimated to be 171 321, which is significantly higher than the previously reported prevalence of 103 312 in 2004.
Survival analyses using SEER 18 confirmed prior findings of the prognostic significance of age, sex, histologic grade, primary site, and stage at diagnosis. Most cases that were coded as liver likely represented metastatic disease from other primary sites, thus making for a very heterogeneous group but with poor outcomes overall. The improvements in survival for the entire cohort over time were likely driven largely by factors pertaining to nonmetastatic disease and may be due in part to the changes in the incidence discussed above, including a higher proportion of relatively more indolent NETs, such as gastric and rectal carcinoids, being discovered that would have otherwise gone undetected. Stage migration (also known as the “Will Rogers phenomenon”) also may have occurred affecting survival owing to improvements in general radiology techniques, such as more sensitive computed tomography and magnetic resonance imaging. Improvements in the management of NETs, including development of octreoscans in the late 1980s, and the adoption of standardized staging and pathology guidelines may also have contributed.
To evaluate the effect of the evolution of systemic therapies on survival, we evaluated OS trends of distant-stage NETs in the SEER 18 registry grouping, with subgroup analyses in distant gastrointestinal NETs and distant pancreatic NETs. We found improvements in OS in all distant NETs in SEER 18 over time, with pronounced improvements in OS in distant gastrointestinal NETs and distant pancreatic NETs. It is likely that these trends are an underestimation of the true impact of recent advances in systemic therapies for these subtypes, given the data’s inability to account for more recent drug approvals. Furthermore, these favorable trends in the survival of patients with metastatic NETs will likely continue as data on newer agents, such as peptide receptor radionuclide therapy, become integrated into routine clinical care. A large volume of retrospective data from Europe supports the efficacy of peptide receptor radionuclide therapy in well-differentiated NETs that show adequate expression of somatostatin receptors as demonstrated by activity on somatostatin receptor scintigraphy. The recently completed phase 3 Neuroendocrine Tumors Therapy-1 trial, although limited to small-bowel NETs, provides the first randomized data and firmly establishes the activity of this modality. It is likely that peptide receptor radionuclide therapy and other peptide radionuclide conjugate therapies targeted toward somatostatin receptors currently in development will have a significant effect on the natural history of NETs arising at sites other than the small bowel.
Limitations
Our study has several limitations. First, given that NETs may not have been reported to cancer registries unless considered malignant, it is likely that we have underestimated their true incidence and prevalence. Second, several known prognostic indicators are not captured by the SEER database. In addition, the database does not provide information regarding the functional status of the NETs that may also affect treatment decisions and survival. Finally, treatment factors, such as quality of surgery, time to diagnosis, and systemic therapy, were unavailable and may have confounded the results. Such drawbacks are inherent to any retrospective, population-based study and may raise concerns about the generalizability of the findings. However, the size of the present study, which we believe to be the largest to date, and the long duration of follow up compensate to a great extent and provide a comprehensive epidemiologic picture of NETs.
Conclusions
The incidence and prevalence of NETs have continued to rise in the United States, owing to the increased diagnosis of early-stage disease and possibly stage migration. The survival of patients with NETs has improved, and this improvement has been greater for those with distant gastrointestinal NETs and, in particular, distant pancreatic NETs.
eTable 1. Incidence of Neuroendocrine Tumors (NETs) Over Time
eTable 2. 10-Year and 20-Year Prevalence of NET
eTable 3. Median Survival of Distant Stage G1/G2 NETs Diagnosed From 2000-2012
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-3072. [DOI] [PubMed] [Google Scholar]
- 2.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589-597. [DOI] [PubMed] [Google Scholar]
- 3.Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork . Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21(3):R153-R163. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Surveillance, Epidemiology, and End Results Program: SEER*Stat Database: SEER 17 Registries; November 2014 submission (1973-2012). Accessed December 3, 2015.
- 5.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/. Accessed February 1, 2017.
- 6.Hess EP, Haas LR, Shah ND, Stroebel RJ, Denham CR, Swensen SJ. Trends in computed tomography utilization rates: a longitudinal practice-based study. J Patient Saf. 2014;10(1):52-58. [DOI] [PubMed] [Google Scholar]
- 7.Pelc NJ. Recent and future directions in CT imaging. Ann Biomed Eng. 2014;42(2):260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rindi G, Klöppel G, Alhman H, et al. ; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS) . TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451(4):757-762. [DOI] [PubMed] [Google Scholar]
- 11.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707-712. [DOI] [PubMed] [Google Scholar]
- 12.Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23(12):R551-R566. [DOI] [PubMed] [Google Scholar]
- 13.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19(10):1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinke A, Müller HH, Schade-Brittinger C, et al. ; PROMID Study Group . Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656-4663. [DOI] [PubMed] [Google Scholar]
- 15.Caplin ME, Pavel M, Ćwikła JB, et al. ; CLARINET Investigators . Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224-233. [DOI] [PubMed] [Google Scholar]
- 16.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24(3):401-406. [DOI] [PubMed] [Google Scholar]
- 17.Kerr C. Oral regimen for metastatic neuroendocrine tumours. Lancet Oncol. 2006;7(3):197. [DOI] [PubMed] [Google Scholar]
- 18.Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 19.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604-1608. [DOI] [PubMed] [Google Scholar]
- 20.Yao JC, Shah MH, Ito T, et al. ; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501-513. [DOI] [PubMed] [Google Scholar]
- 22.Yao JC, Fazio N, Singh S, et al. ; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group . Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators . Phase 3 trial of (177)lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26(13):2124-2130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Incidence of Neuroendocrine Tumors (NETs) Over Time
eTable 2. 10-Year and 20-Year Prevalence of NET
eTable 3. Median Survival of Distant Stage G1/G2 NETs Diagnosed From 2000-2012