Abstract
Importance
Capecitabine is an oral cytotoxic chemotherapeutic commonly used across cancer subtypes. As with other oral medications though, it may suffer from drug interactions that could impair its absorption.
Objective
To determine if gastric acid suppressants such as proton pump inhibitors (PPIs) may impair capecitabine efficacy.
Design, Setting, and Participants
This secondary analysis of TRIO-013, a phase III randomized trial, compares capecitabine and oxaliplatin (CapeOx) with or without lapatinib in 545 patients with ERBB2/HER2-positive metastatic gastroesophageal cancer (GEC); patients were randomized 1:1 between CapeOx with or without lapatinib. Proton pump inhibitor use was identified by medication records. Progression-free survival (PFS) and overall survival (OS) were compared between patients treated with PPIs vs patients who were not. Specific subgroups were accounted for, such as younger age (<60 years), Asian ethnicity, female sex, and disease stage (metastatic/advanced) in multivariate Cox proportional hazards modeling. The TRIO-013 trial accrued and randomized patients between June 2008 and January 2012; this analysis took place in January 2014.
Interventions
Patients were divided based on PPI exposure.
Main Outcomes and Measures
Primary study outcome was PFS and OS between patients treated with PPIs vs patients who were not. Secondary outcomes included disease response rates and toxicities.
Results
Of the 545 patients with GEC (median age, 60 years; 406 men [74%]) included in the study, 229 received PPIs (42.0%) and were evenly distributed between arms. In the placebo arm, PPI-treated patients had poorer median PFS, 4.2 vs 5.7 months (hazard ratio [HR], 1.55; 95% CI, 1.29-1.81, P < .001); OS, 9.2 vs 11.3 months (HR, 1.34; 95% CI, 1.06-1.62; P = .04); and disease control rate (72% vs 83%; P = .02) vs patients not treated with PPIs. In multivariate analysis considering age, race, disease stage, and sex, PPI-treated patients had poorer PFS (HR, 1.68; 95% CI, 1.42-1.94; P < .001) and OS (HR, 1.41; 95% CI, 1.11-1.71; P = .001). In patients treated with CapeOx and lapatinib, PPIs had less effect on PFS (HR, 1.08; P = .54) and OS (HR, 1.26; P = .10); however, multivariate analysis in this group demonstrated a significant difference in OS (HR, 1.38; 95% CI, 1.06-1.66; P = .03).
Conclusions and Relevance
Proton pump inhibitors negatively effected capecitabine efficacy by possibly raising gastric pH levels, leading to altered dissolution and absorption. These results are consistent with previous erlotinib and sunitinib studies. Whether PPIs affected lapatinib is unclear given concurrent capecitabine. Given capecitabine’s prevalence in treatment breast cancer and colon cancer, further studies are under way.
Trial Registration
clinicaltrials.gov Identifier: NCT00680901
This secondary analysis of the TRIO-013 phase 3 randomized clinical trial attempts to determine if gastric acid suppressants such as proton pump inhibitors may impair capecitabine efficacy.
Key Points
Question
Do proton pump inhibitors (PPIs) impair the activity of capecitabine in patients with metastatic gastroesophageal cancer (GEC)?
Findings
In this secondary analysis of TRIO-013, a phase III randomized trial of capecitabine and oxaliplatin (CapeOx) with or without lapatinib in patients with metastatic GEC receiving CapeOx, PPIs significantly and negatively affected progression-free survival (4.2 vs 5.7 months), overall survival (9.2 vs 11.3 months), and disease control rate (72% vs 83%).
Meaning
Oncologists treating patients with capecitabine should be aware of the potential negative interaction with gastric acid suppressants such as PPIs and possibly histamine antagonists.
Introduction
Prognosis of advanced or metastatic gastric cancer has remained consistently poor over time.1 Amplification or overexpression of human epidermal growth factor receptor 2 (ERBB2/HER2) occurs in 7% to 34% of gastric cancers, making trastuzumab, an anti-ERBB2/HER2 monoclonal antibody, a viable therapy for ERBB2/HER2-positive gastroesophageal cancer (GEC).2 The ToGA trial3 found an improvement in both progression-free survival (PFS) and overall survival (OS) when trastuzumab was combined with cisplatin and capecitabine or fluorouracil in comparison with the chemotherapy alone. The clinical superiority of a doublet chemotherapy plus a biologic drug is questionable, however, owing to nearly simultaneous reporting of improved outcomes of combined next-generation cytotoxic chemotherapies, such as oxaliplatin and capecitabine.4
Subsequent development of lapatinib, a small molecule tyrosine kinase inhibitor (TKI) of ERBB2/HER2, led to further investigations of newer combinations. Given lapatinib’s single-agent activity in ERBB2/HER2 amplified disease,5 TRIO-013/LOGiC investigated capecitabine and oxaliplatin (CapeOx) with or without lapatinib in a phase III setting for patients with advanced and/or metastatic GEC whose disease overexpressed ERBB2/HER2.6 A total of 545 patients were accrued and randomized equally among experimental and control arms. Though the lapatinib-containing arm had better response rates, the study missed its primary and secondary end points of detecting statistically significant OS and PFS improvements with lapatinib use.
The focus in oncology therapeutics has intensified toward targeted and oral options in hopes of improving efficacy while limiting toxic effects. Oral therapeutics are popular with patients owing to convenience and an association with less stress for patients,7 as well as potentially less institutional costs as infusion time is lessend. An increasing body of literature has developed that questions pharmacokinetic variability between parenteral and oral administration. Specifically, a large number of TKIs rely on pH-dependent solubility to dissolve within the stomach and be subsequently absorbed.8 Much of these data stem from preclinical data wherein gastric pH level elevations can impair a TKI’s ability to dissolve and ultimately reach systemic circulation. In fact, recent retrospective data have found that coadministration of gastric acid suppressants such as proton pump inhibitors (PPIs) or histamine receptor antagonists (H2RAs) with TKIs can lead to poorer efficacy of erlotinib and sunitinib in advanced and/or metastatic non–small-cell lung cancer and renal cell cancer, respectively.9,10 This interaction with gastric acid suppressants may not be limited to TKIs given that many oral drugs require a sufficiently acidic environment to properly dissolve for systemic absorption. Therefore, this analysis of TRIO-013/LOGiC was set out to determine if the orally administered drugs capecitabine and/or lapatinib were hampered by concomitant PPI administration.
Methods
Patients
All patients included in the intention-to-treat assessment of TRIO-013/LOGiC were included in this ad hoc analysis. Patients’ concomitant medication records were reviewed to identify patients who were treated with PPIs. Coadministration of PPIs was defined by 20% or more overlap between PPI prescription and trial treatment duration as identified previously.9 Other pertinent factors that may have factored into outcomes were collected, including age, sex, histological subtype (intestinal vs diffuse), disease stage (locally advanced vs metastatic), and race (Asian vs non-Asian). The original TRIO-013/LOGiC trial is a clinical trial conducted according to current ethical principles as set out by the Declaration of Helsinki and Good Clinical Practice; written informed consent was obtained from participating patients prior to any study procedures; and approval by local ethics review boards at individual participating sites was obtained, which subsequently allows for secondary analyses. Procedures followed for this secondary analysis were in accordance with the ethical standards as set forth by the 1964 Helsinki Declaration of the World Medical Association.
Post Hoc Analysis
After dividing patients based on coadministration of PPIs and arm of study, PFS and OS outcomes were compared by Kaplan-Meier methods. Multivariate analysis by Cox proportional hazards modeling took into consideration demographics (age, sex, race), disease stage at diagnosis (locally advanced vs metastatic), and histologic subtype. Differences in median survival were considered significant if P was less than .05.
Secondary Outcomes
Given that the hypothesis of this post hoc analysis is ultimately rooted in a difference of drug exposure between patients receiving PPIs vs no PPIs, comparison of secondary outcomes between these 2 groups included objective response rate (ORR), disease control rate (DCR), incidence of grade 3 or 4 diarrhea, incidence of any rash, incidence of hand-foot syndrome, number of patients requiring capecitabine dose reductions (any cause), and number of patients requiring lapatinib dose reductions (any cause).
Statistical Considerations
Statistical analysis was performed with SAS version 9.3 (SAS Institute Inc). All P values were calculated using 2-sided statistical testing and Cox proportional hazards ratios with 95% CIs.
Results
Patients
Of 545 patients in the intention-to-treat population on study, 229 (42%) patients received PPIs with 110 (40%) and 119 (43%) in the lapatinib and control arms, respectively. All patients identified as having received any PPI therapy in fact received nearly complete overlap between PPI prescription and study treatment. Consequently, no further distinction is made regarding PPI treatment duration.
As a cohort, 406 patients (74%) were male, predominantly with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1 (n = 332 [61%]), presenting with metastatic disease (n = 521 [96%]) and/or poorly differentiated disease (n = 212 [39%]). There was even distribution of those patients with intestinal histologic subtype (n = 481 [88%]) between PPI-treated patients vs patients who were not treated with PPIs. Of the prognostic factors collected, there was a significantly higher proportion of Asians (n = 126 [55%]) and patients with an ECOG PS of 0 or 1 in the PPI-treated group. While there was a higher proportion of women and locally advanced disease in the PPI group, these differences did not meet statistical significance. Details of patient demographics and characteristics can be found in Table 1.
Table 1. Demographics of Patients Treated With or Without PPIs Divided by Treatment Arm.
| Characteristic | No. (%) | P Value for No PPI vs PPI | ||||
|---|---|---|---|---|---|---|
| All (545) | No PPI (316) | PPI (229) | ||||
| CapeOx (155) | CapeOx + Lapatinib (161) | CapeOx (119) | CapeOx + Lapatinib (110) | |||
| Age, median, y | 60 | 59 | 61 | 58 | 60 | .41 |
| Sex | .09 | |||||
| Male | 406 (74) | 123 (79) | 124 (77) | 77 (65) | 82 (75) | |
| Female | 139 (26) | 32 (21) | 37 (23) | 42 (35) | 28 (25) | |
| ECOG PS | .04 | |||||
| 0 | 166 (30) | 39 (25) | 56 (35) | 36 (30) | 35 (32) | |
| 1 | 332 (61) | 101 (65) | 94 (58) | 72 (61) | 65 (59) | |
| 2 | 47 (9) | 15 (10) | 11 (7) | 11 (9) | 10 (9) | |
| Histological grade | .60 | |||||
| Well differentiated | 37 (7) | 13 (8) | 11 (7) | 3 (3) | 10 (9) | |
| Moderately differentiated | 176 (32) | 54 (35) | 57 (35) | 39 (33) | 26 (24) | |
| Poorly differentiated | 212 (39) | 60 (39) | 56 (35) | 52 (44) | 44 (40) | |
| Not assessed | 120 (22) | 28 (18) | 37 (23) | 25 (21) | 30 (27) | |
| Histological subtype | .97 | |||||
| Intestinal | 481 (88) | 137 (88) | 144 (89) | 98 (82) | 102 (93) | |
| Diffuse | 27 (5) | 8 (5) | 8 (5) | 9 (8) | 2 (2) | |
| Not assessed/other | 37 (7) | 10 (7) | 9 (6) | 12 (10) | 6 (5) | |
| Stage | .06 | |||||
| Locally advanced | 24 (4) | 9 (6) | 6 (4) | 2 (2) | 7 (6) | |
| Metastatic | 521 (96) | 146 (94) | 155 (96) | 117 (98) | 103 (94) | |
| Race | <.001 | |||||
| Asian | 240 (44) | 56 (36) | 59 (37) | 62 (52) | 64 (58) | |
| Non-Asian | 305 (56) | 99 (64) | 102 (63) | 57 (48) | 46 (42) | |
Abbreviations: CapeOx, capecitabine and oxaliplatin; PPI, proton pump inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status.
PPIs vs No PPIs
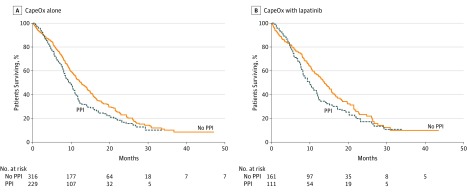
Within the control (CapeOx + placebo) arm, patients not treated with PPIs were found to have an improved median PFS (5.7 vs 4.2 months; hazard ratio [HR], 1.55; 95% CI, 1.29-1.81; P < .001 (Figure 1A) and median OS (11.3 vs 9.2 months; 95% CI, 1.06-1.62; HR, 1.34; P = .04) (Figure 2A) compared with PPI-treated patients.
Figure 1. Progression-Free Survival.
Kaplan-Meier curves show progression-free survival betweens patients treated with or without proton pump inhibitors (PPIs) and (A) capecitabine and oxaliplatin (CapeOx) alone or (B) CapeOx with lapatinib.
Figure 2. Overall Survival.
Kaplan-Meier curves show overall survival betweens patients treated with or without proton pump inhibitors (PPIs) and (A) capecitabine and oxaliplatin (CapeOx) alone or (B) CapeOx with lapatinib.
In contrast, patients assigned to the lapatinib arm (CapeOx + lapatinib) did not have a significant difference in PFS (6.8 vs 5.7 months; HR, 1.08; 95% CI, 0.82-1.34; P = .54) (Figure 1B) or median OS (13.8 vs 9.6 months; HR, 1.26; 95% CI, 0.97-1.55; P = .10) (Figure 2B) between patients not treated with PPIs vs PPI-treated patients in univariate analysis.
Effect of Other Prognostic Factors and Multivariate Analysis
Prognostic factors that have previously been reported to affect patient outcomes were included in Cox proportional hazards multivariate modeling. Specifically age (≥60 years), male sex, diffuse subtype, metastatic disease at presentation, and non-Asian race/ethnicity were considered poor prognostic factors.11 In patients treated with CapeOx alone, effects of PPIs on both PFS and OS was still significant on multivariate analysis (HR, 1.68; P < .001 and HR, 1.41; P = .001, respectively) (Table 2). When the multivariate analysis was applied to patients treated on the lapatinib arm, PPIs affected OS (HR, 1.38; P = .03) but not PFS (HR, 1.14; P = .33).
Table 2. Cox Proportional Hazards Modeling of Other Prognostic Factors Weighed Against PPI Effect in Control (CapeOx + Placebo) Arm.
| Variable | Analysis, Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|
| Univariate | P Value | Multivariate | ||
| PFS | ||||
| Age (>60 y) | 0.71 (0.45-0.97) | .01 | ||
| Female sex | 0.85 (0.55-1.15) | .26 | ||
| Intestinal subtype | 0.67 (0.25-1.19) | .06 | 0.72 (0.48-0.96) | .12 |
| Metastatic | 1.00 (0.67-1.33) | >.99 | ||
| Asian | 0.81 (0.53-1.09) | .25 | ||
| PPI | 1.55 (1.29-1.81) | <.001 | 1.68 (1.42-1.94) | <.001 |
| OS | ||||
| Age (>60 y) | 0.73 (0.43-0.98) | .03 | ||
| Female sex | 0.96 (0.62-1.30) | .81 | ||
| Intestinal subtype | 0.68 (0.26-1.20) | .07 | 0.70 (0.22-1.18) | .14 |
| Metastatic | 1.06 (0.34-1.82) | .88 | ||
| Asian | 0.74 (0.44-0.99) | .05 | ||
| PPI | 1.34 (1.06-1.62) | .04 | 1.41 (1.11-1.71) | .001 |
Abbreviations: CapeOx, capecitabine and oxaliplatin; OS, overall survival; PFS, progression-free survival; PPI, proton pump inhibitors.
Secondary Outcomes
Patients treated without PPIs had a slightly higher DCR (81.0% vs 76.8%; P = .24) in which this difference was largely owing to control arm patients where DCR was significantly improved (82.5% vs 71.2%; P = .02). Overall response rate and DCR in the experimental arm with lapatinib was not significant (Table 3).
Table 3. Secondary Outcomes Between PPI vs No PPI Treated Within Trial Study Arm.
| Secondary End Point | No. (%) | P Value | ||||
|---|---|---|---|---|---|---|
| Control Arm (CapeOx + Placebo) | P Value | Lapatinib Arm (CapeOx + Lapatinib) | ||||
| No PPI (155) | PPI (119) | No PPI (161) | PPI (110) | |||
| Objective response rate | 65 (42) | 43 (36) | .33 | 84 (52) | 60 (54) | .70 |
| Disease control rate | 128 (83) | 84 (71) | .02 | 128 (80) | 92 (84) | .24 |
| Capecitabine reductions | 47 (30) | 42 (36) | .38 | 72 (45) | 54 (49) | .48 |
| Lapatinib reductions | NA | NA | NA | 39 (24) | 39 (35) | .06 |
| Hand-foot syndrome | 22 (14) | 12 (10) | .32 | 33 (21) | 20 (18) | .43 |
| Rash | 9 (6) | 7 (6) | .99 | 29 (18) | 16 (14) | .42 |
| Diarrhea | 4 (3) | 5 (4) | .46 | 20 (12) | 13 (12) | .88 |
Abbreviations: CapeOx, capecitabine and oxaliplatin; NA, not applicable; PPI, proton pump inhibitors.
There was no statistically significant difference in the incidence of capecitabine or lapatinib dose reductions, grade 3 or 4 diarrhea, or any rash between PPI-treated patients vs patients treated without PPIs. The largest difference in toxic effects was seen in the control arm where there was a numerical higher incidence of hand-foot syndrome in the population treated without PPIs within both control and lapatinib arms, though neither group met statistical significance (14.2% vs 10.2%; P = .32 and 20.8% vs 18.0%; P = .43, respectively).
Discussion
This secondary analysis of the TRIO-013/LOGiC trial demonstrates an association between poorer outcomes with concomitant PPI use and capecitabine therapy in patients with ERBB2/HER2-positive metastatic GEC. An improved PFS and OS favoring patients treated without PPIs suggests that a drug interaction may exist. Further, the significant difference in DCR between PPI-treated patients vs patients treated without PPIs in the control arm suggests that the survival difference stems from treatment efficacy, lending support to a drug exposure explanation. Unfortunately, the original study did not collect plasma drug levels from patients and therefore a direct pharmacokinetic explanation cannot be drawn. These observations are in line with a previous retrospective study in which patients with colorectal cancer receiving PPI treatment and adjuvant capecitabine also experienced poorer relapse-free survival compared with patients not receiving a PPI.12 Taken together, this ad hoc analysis gives further support that a negative interaction exists between capecitabine and PPIs and warrants further investigation.
While a numerical difference was present including a trend toward an OS disadvantage, no significant detriment was seen in lapatinib-treated patients who were exposed to PPIs. Drawing conclusions from these patients is difficult given that they received 2 oral therapeutics of which both may have been affected by coadministration of gastric acid suppression therapy. Further, though the primary outcomes of TRIO-013 were negative, preplanned subgroup analyses found younger patients and Asian patients benefited from adding lapatinib to CapeOx.6 Consequently, in light of a PPI-related effect on outcomes in the control arm, the lack of poorer outcomes in lapatinib-treated patients suggests that PPIs impair capecitabine absorption. Whether or not there is a substantial interaction with lapatinib cannot be answered from this analysis due to these patients receiving 2 oral therapeutics.
A negative interaction between capecitabine and gastric acid suppressants has not previously been reported except in a single retrospective study.12 Consideration of capecitabine’s pharmacokinetic properties may explain what this analysis has observed. Capecitabine reaches its peak plasma concentration approximately 1.5 hours after ingestion suggesting that it likely dissolves quickly and is absorbed predominantly in the upper gastrointestinal tract.13 With its dissociation constant of 1.92, it is sensitive to gastric pH level changes in which substantial reductions in gastric acidity can lead to less drug dissolution and subsequent less absorption into systemic circulation.14 Only 1 study has previously examined effects of coadministration of an antacid, aluminum hydroxide and magnesium hydroxide (Maalox; Novartis), and capecitabine.15 Twelve patients were randomly divided equally between receiving Maalox either immediately following a single dose of capecitabine or 2 hours after. Investigators failed to find a difference in peak plasma concentration and overall time exposure (area under time–concentration curve) between both groups. Whether Maalox raises gastric pH sufficiently to impair capecitabine’s ability to dissolve is questionable.16 Alternatively, median gastric pH levels rise above 5 in patients receiving PPIs.17 As a result, PPIs are more likely to have a stronger interaction with capecitabine than Maalox. Lapatinib, on the other hand, does not appear to be as sensitive to gastric acidity with its dissociation constant of 7.2, which might partly explain why less of a detriment is seen in PPI-treated patients on that arm of TRIO-013.18 While many PPIs undergo liver cytochrome P450 metabolization like capecitabine, specific enzymatic steps are thought to be separate and unlikely to interact in this way.19
We have previously reported on negative interactions between gastric acid suppressants and oral TKIs. In a retrospective study10 of nearly 200 patients with advanced renal cell cancer treated with sunitinb, coadministration of both PPIs was associated with poorer PFS and OS in sunitinib-treated patients. A similar finding was demonstrated in over 500 patients with advanced non–small-cell lung cancer treated with erlotinib.9 Furthermore, patients with non–small-cell lung cancer concomitantly receiving gastric acid suppression with erlotinib also had significantly less TKI-related toxic effects and lower ORR. These associations mirror similar findings of poorer outcomes and lower DCR in patients cotreated with PPIs and capecitabine in the control arm of TRIO-013. For both studies, PPI effects were weighed against other known prognostic indices in multivariate analyses and were found not to be contributing significantly to PPI-treated patient outcomes. In fact, patients treated without PPIs in TRIO-013 had a significantly higher proportion of patients carrying better prognostic factors such as Asian descent and female sex (Table 1).
Drug interactions are challenging to manage, particularly in light of a population of patients with GEC in which many will receive gastric acid suppressants for symptom management, leading to a higher prevalence of PPI therapy. Coupled with increasing rates of obesity and gastroesophageal reflux disease (GERD), further evidence of interactions between PPIs and other oral, antineoplastic therapeutics is likely to surface.20 Sequential administration of capecitabine and PPIs is unlikely to overcome their interaction. Proton pump inhibitors have short half-lives, but this is likely owing to their ability to quickly, covalently, and irreversibly bind to gastric parietal cells’ proton pumps.21 Consequently, the duration of effect on overall gastric pH levels is long lasting. Given that data exists to demonstrate a lack of interaction between Maalox and capecitabine, perhaps similar GERD treatments may serve as an alternative. Theoretically, H2RAs do not raise gastric pH levels to levels seen with PPIs and may have less interaction with oral therapeutics, but data are lacking in this regard.17 More recently, erlotinib absorption in patients receiving PPIs was shown to be improved when administering the drug with an acidic beverage, such as cola.22 Whether this reversal can be also done for capecitabine remains to be investigated.
Limitations
As stated previously, the largest limitation of this analysis is the lack of pharmacokinetic data to fully corroborate an explanation of lower drug exposure and differences in outcomes between PPI-treated patients vs patients treated without PPIs. It should also be noted that inaccuracies regarding concomitant medication may occur; and further, over-the-counter gastric acid suppressants may also not be captured during the study’s implementation. However, this ad hoc analysis of prospective trial evidence builds upon previous retrospective work. Future directions include proper pharmacokinetic studies and assessment of other oral, antineoplastic therapeutics.
Conclusions
This secondary analysis of TRIO-013/LOGiC confirms previous retrospective data that concomitant use of gastric acid suppressants such as PPIs with capecitabine may lower the antitumor efficacy of capecitabine. This negative association is strongest when taking into consideration reduced DCR coupled with lower PFS and OS in CapeOx-treated patients. However, this trial did not examine for pharmacokinetics and circulating drug levels so a direct link with potential reduced capecitabine absorption cannot be made in this analysis.
What becomes more concerning is the increasing tendency toward development of oral chemotherapeutics. Other oral drugs such as erlotinib and sunitinib may suffer from the same drug interaction phenomenon.9,10 While stopping gastric acid suppressants may prove difficult, it may be possible to temporarily counteract a lowered absorption by using an acidic beverage for drug administration.22 Overall, more food-effect studies are warranted in the development of oral therapeutics, and our study draws light to the possibility that such an interaction may also affect more commonly used cytotoxic chemotherapeutics, such as capecitabine, used across tumor subtypes.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [DOI] [PubMed] [Google Scholar]
- 2.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523-1529. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362(9):858-859. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22(12):2610-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol. 2015;34(5):443-451. [DOI] [PubMed] [Google Scholar]
- 7.McLeod HL, Evans WE. Oral cancer chemotherapy: the promise and the pitfalls. Clin Cancer Res. 1999;5(10):2669-2671. [PubMed] [Google Scholar]
- 8.Budha NR, Frymoyer A, Smelick GS, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203-213. [DOI] [PubMed] [Google Scholar]
- 9.Chu MP, Ghosh S, Chambers CR, et al. Gastric acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clin Lung Cancer. 2014;16(1):33-39. [DOI] [PubMed] [Google Scholar]
- 10.Ha VH, Ngo M, Chu MP, Ghosh S, Sawyer MB, Chambers CR. Does gastric acid suppression affect sunitinib efficacy in patients with advanced or metastatic renal cell cancer? J Oncol Pharm Pract. 2014;21(3):194-200. [DOI] [PubMed] [Google Scholar]
- 11.Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2(2):77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Ilich AI, Kim CA, et al. Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin Colorectal Cancer. 2016;15(3):257-263. [DOI] [PubMed] [Google Scholar]
- 13.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85-104. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Biotechnology Information PubChem Compound Database; CID=60953, https://pubchem.ncbi.nlm.nih.gov/compound/capecitabine#section=Top. Accessed November 28, 2014.
- 15.Reigner B, Clive S, Cassidy J, et al. Influence of the antacid Maalox on the pharmacokinetics of capecitabine in cancer patients. Cancer Chemother Pharmacol. 1999;43(4):309-315. [DOI] [PubMed] [Google Scholar]
- 16.Hürlimann S, Michel K, Inauen W, Halter F. Effect of Rennie Liquid versus Maalox Liquid on intragastric pH in a double-blind, randomized, placebo-controlled, triple cross-over study in healthy volunteers. Am J Gastroenterol. 1996;91(6):1173-1180. [PubMed] [Google Scholar]
- 17.Gursoy O, Memiş D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig. 2008;28(12):777-782. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information PubChem Compound Database; CID=208908, https://pubchem.ncbi.nlm.nih.gov/compound/Lapatinib#section=Top. Accessed September 7, 2016.
- 19.McColl KE, Kennerley P. Proton pump inhibitors—differences emerge in hepatic metabolism. Dig Liver Dis. 2002;34(7):461-467. [DOI] [PubMed] [Google Scholar]
- 20.Morgen CS, Sørensen TI. Obesity: global trends in the prevalence of overweight and obesity. Nat Rev Endocrinol. 2014;10(9):513-514. [DOI] [PubMed] [Google Scholar]
- 21.Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: what the practising physician needs to know. Drugs. 2003;63(24):2739-2754. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen RW, Peric R, Hussaarts KG, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2016;34(12):1309-1314. [DOI] [PubMed] [Google Scholar]