TO THE EDITOR:
Myeloid sarcoma (MS) is a subtype of acute myeloid leukemia (AML), in which leukemic cells invade extramedullary tissues and form solid tumors.1-3 MS may manifest as an isolated event or with concomitant involvement of leukemic bone marrow (BM), the latter affecting up to 20% to 30% of all AML cases.2,4-8 Clinical data about the prognostic relevance of MS are still conflicting, mainly because of variable clearing of extramedullary leukemic blasts by conventional chemotherapy.2,7,9 Hence, knowledge of the pathogenetic mechanisms, which endow leukemic blasts with an invasive potential and thereby cause the formation of MS, will help to improve therapeutic regimens for MS patients. RAF kinase inhibitor protein (RKIP) is a negative regulator of RAS-MAPK/ERK signaling.10-12 A somatic loss of RKIP has recently been described as a frequent event in AML. It has been shown to be associated with monocytic AML phenotypes and proven to be of functional relevance for leukemogenesis.13-15 It is interesting to note that RKIP loss has also been observed in a variety of solid cancers and has been described as a bona fide metastasis suppressor in these entities.10,16,17 Because of the similarities between metastasis formation and tissue infiltration of AML cells in the development of MS, we now aimed to identify the role of RKIP in the pathogenesis of this AML subtype.
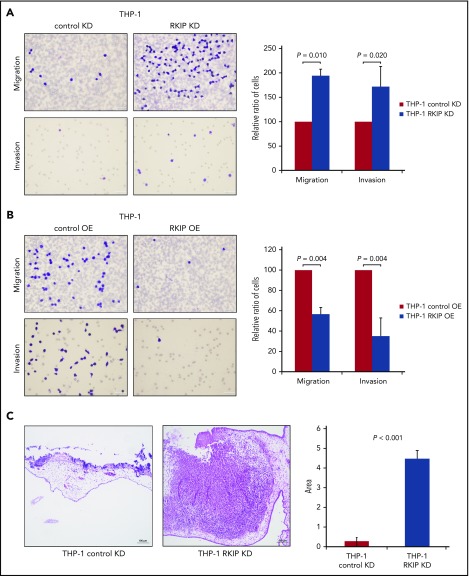
Initially, we studied the functional role of RKIP in leukemic tissue infiltration in vitro by assessing the migration and invasion potential of THP-1 AML cells with stable RKIP knockdown (THP-1 RKIP KD) and overexpression (THP-1 RKIP OE), respectively (supplemental Methods; supplemental Figure 1A-B, available on the Blood Web site).15,18 In these experiments, RKIP KD significantly increased both migration and invasion of THP-1 cells (P = .010 and P = .020, respectively; Figure 1A), whereas RKIP OE caused the opposite effects (P = .004 and P = .004, respectively; Figure 1B). The same phenomenon was observed in U937, an additional AML cell line (supplemental Methods; supplemental Figure 2 and 3). To further corroborate these findings in vivo, we performed a chorioallantoic membrane (CAM) assay (supplemental Methods). In this assay, we evaluated the potential of THP-1 cells with and without RKIP KD to invade the CAM of chicken embryos and, consequently, to form solid tumor masses. In agreement with our in vitro data, this was the case in all RKIP KD experiments, whereas only occasional and sparse infiltrations of single THP-1 cells were observed in control transfected cells (P < .001; Figure 1C). Taken together, these data demonstrate that RKIP is indeed involved in the tissue infiltration of leukemic blasts and, consequently, in the formation of MS.
Figure 1.
RKIP loss is involved in the development of MS. (A-B) Results of migration and invasion experiments using THP-1 AML cells with RKIP short hairpin RNA KD (A) and FLAG-RKIP OE (B). Representative images (40× magnification) of PET membranes with Giemsa-stained cells are displayed. The number of cells was counted with an inverted microscope. In all cases, cells carrying the empty control vectors (control KD and control OE, respectively) have been arbitrarily set to a value of 100, and the x-fold change in cells transduced with either RKIP short hairpin RNA (THP-1 RKIP KD) or FLAG-RKIP (THP-1 RKIP OE) was calculated using the ratio “number of cells RKIP KD/number of cells control KD” and “number of cells RKIP OE/number of cells control OE,” respectively. Graphs summarize the results of at least 3 independent experiments. Data are expressed as mean values ± standard deviation, and P values have been calculated using the Student t test. (C) Representative hematoxylin and eosin staining of chicken CAMs after seeding of THP-1 AML cells with RKIP KD (THP-1 RKIP KD) and without (THP-1 control KD) showing invasion and tumor formation in the THP-1 RKIP KD condition (10× magnification). The graph displays the area of invading cells/tumors as assessed by ImageJ and summarizes the results of 4 independent experiments. Data are expressed as mean values ± standard deviation, and P values have been calculated using the Student t test.
Next, we aimed to shed more light on the mechanisms by which RKIP loss promotes MS development. Unexpectedly, although RKIP is an inhibitor of RAS-MAPK/ERK signaling, inhibition of MEK with U0126 failed to rescue the effects of RKIP KD (P = .889; supplemental Methods; supplemental Figure 4), indicating that MS formation in AML with RKIP loss is mediated via other RAS-MAPK/ERK–independent effectors. To identify potential candidate genes, we performed messenger RNA (mRNA) microarrays (supplemental Methods) in THP-1 cells with and without RKIP KD. It is interesting to note that KD of RKIP thereby induced a distinct gene expression profile, which included the prominent deregulation of networks involved in degradation of connective tissues and migration as well as in the interaction, binding, and engulfment of hematopoietic cells (supplemental Figure 5; supplemental Table 1).
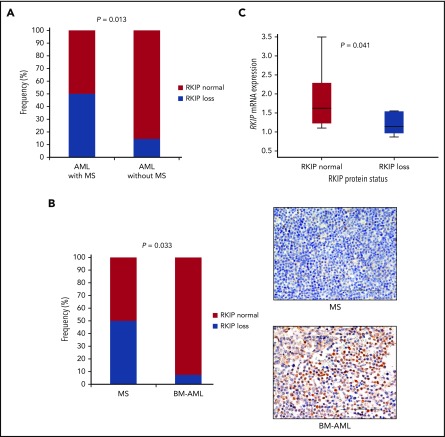
Having proven that decreased expression of RKIP indeed plays a role in tissue infiltration of leukemic blasts, we then aimed to clarify the clinical relevance of this finding (supplemental Methods; supplemental Figure 6). Therefore, we initially screened the medical records of 103 patients with AML, whose BM specimens have previously been characterized in respect to RKIP expression,15 for clinical evidence of MS as previously described7,19 (“cohort I”). This retrospective clinical evaluation was possible in 58 patients, with MS being present in 16 (28%). In agreement with our functional data, specimens with RKIP loss were significantly enriched in AML cases with concomitant occurrence of MS (8/16 [50%] vs 6/42 [14%]; P = .013; Figure 2A; supplemental Table 2). We then tried to additionally corroborate our findings in an independent cohort, where the presence of MS was confirmed by biopsy (“cohort II”; supplemental Table 3 and 4). Therefore, we studied RKIP protein expression by immunohistochemistry in a series of formalin-fixed, paraffin-embedded patient specimens of MS (n = 14) and corresponding leukemic BM samples (n = 6). In brief, we classified RKIP expression as either “RKIP normal” or “RKIP loss” by implementing a previously described scoring system that incorporates both the amount and intensity of positively stained cells.20 Loss of RKIP was observed in 7 of 14 cases (50%) with no differences in RKIP expression between MS and corresponding leukemic BM specimens, as well as between AML/MS diagnostic and relapse material (supplemental Figure 7). It is important to realize that RKIP loss was detected in only 1 of 14 BM specimens from AML patients without any evidence of MS (7%; P = .033; Figure 2B; supplemental Figure 8), which further supports the association between RKIP loss and MS. These data also suggest that RKIP expression could serve as a potential AML biomarker, which can easily be detected in leukemic BM and which aids in identifying cases with additional extramedullary manifestations. As a limitation of our study, it has to be noted that the small sample size of the cohort analyzed did not enable us to test whether MS, and particularly its RKIP expression status, might be of prognostic relevance for AML. Therefore, analysis of larger, preferably prospective clinical cohorts in future studies will be necessary.
Figure 2.
Loss of RKIP is a frequent event in MS, both at the protein and mRNA levels. (A) Clinical evaluation of extramedullary involvement in 58 AML patients, who have been analyzed in respect to RKIP protein expression in the leukemic BM previously (cohort I).15 The frequency of specimens defined as RKIP loss was significantly increased in AML patients with MS compared with those without, as assessed by Fisher’s exact test. (B) RKIP loss was also associated with MS in an independent cohort, where MS was confirmed by biopsy (cohort II). The frequency of RKIP losses was significantly increased in 14 samples of MS compared with 14 specimens of BM from AML patients without any evidence of MS (BM-AML), as calculated by Fisher's exact test. A representative immunohistochemical analysis showing loss of RKIP expression in a specimen of MS and normal RKIP expression in BM-AML is also shown (40× magnification). (C) RKIP mRNA expression analysis by quantitative polymerase chain reaction demonstrating that samples with RKIP loss at the protein level also exhibit decreased levels of RKIP mRNA. NB4 AML cells served as a calibrator, and statistical significance was calculated using the Mann-Whitney-Wilcoxon test.
To gain more insight into the molecular landscape of MS patients with RKIP loss, we performed next-generation sequencing of 39 leukemia-associated genes in all formalin-fixed, paraffin-embedded MS specimens with sufficient DNA quality for this analysis (n = 11; supplemental Figure 9) as previously described.21 Mutations in RAS signaling were enriched in samples with RKIP loss, with 5 of 6 cases (83%) carrying 1 or more of these substitutions, whereas only 2 of 5 cases (40%) with normal RKIP expression were affected. Unfortunately, statistical analysis was precluded by the small sample size. However, because a functional synergism in leukemogenesis between mutant RAS and RKIP loss has been shown previously,15 these data might suggest a potential relevance of this interaction for MS formation as well. It is interesting to note that in accordance with this hypothesis, mutations in NPM1 and DNMT3A were almost exclusively detected in cases with normal RKIP expression. Both aberrations are frequently detected in MS and have been shown to promote RAS-independent MS formation in vitro and in vivo.2,21-25 Finally, when examining the mechanisms that cause RKIP loss in MS, we observed that quantitative polymerase chain reaction analyses delineated that samples harboring RKIP loss at the protein level also showed decreased levels of RKIP mRNA (P = .041; Figure 2C). This finding is in agreement with previous data of our own group, where we did show that RKIP loss in myeloid malignancies is caused by miR-23a–induced downregulation of its mRNA.13,15,18
In conclusion, we show that loss of the metastasis-suppressor RKIP is a frequent event in MS and that decreased expression of RKIP in leukemic BM of AML patients might serve as a biomarker for the occurrence of additional extramedullary manifestations. Finally, we demonstrate that RKIP is functionally involved in tissue infiltration of leukemic cells and in the development of MS, which makes it an attractive target for future MS-directed therapies.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors would like to thank Karin Wagner for excellent technical support at microarray experiments. The authors also thank SFL Technologies (Stallhofen, Austria) for providing Olympus microscopes used in this study.
This study was supported by research funding from the Austrian Science Fund (grant P26619-B19) (A.Z.). Work in the laboratories of A.Z., A.W., and H. Sill is further supported by Leukämiehilfe Steiermark. PhD candidate V.C. received funding from the Austrian Science Fund (grant P26619-B19 [A.Z.]) and was trained within the frame of the PhD Program Molecular Medicine of the Medical University of Graz. PhD candidate J.L.B. received funding from the Medical University of Graz within the PhD Program Molecular Medicine.
Authorship
Contribution: A.Z. designed and supervised the study; V.C., B.P., J.L.B., S.S., G.H., H. Strobl, K.K., N.G.-T.-W., H. Sill, and A.Z. acquired data; B.U., G.H., H. Sill, A.W., and A.Z. provided patient samples and clinical data; V.C., J.L.B., K.K., G.H., H. Strobl, A.W., H. Sill, and A.Z. analyzed and interpreted the data; V.C. and A.Z. wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Armin Zebisch, Division of Hematology, Medical University of Graz, Auenbruggerplatz 38, 8036 Graz, Austria; e-mail: armin.zebisch@medunigraz.at.
References
- 1.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. [DOI] [PubMed] [Google Scholar]
- 2.Ohanian M, Faderl S, Ravandi F, et al. Is acute myeloid leukemia a liquid tumor? Int J Cancer. 2013;133(3):534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 4.Chang H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28(10):1007-1011. [DOI] [PubMed] [Google Scholar]
- 5.Klco JM, Welch JS, Nguyen TT, et al. State of the art in myeloid sarcoma. Int J Lab Hematol. 2011;33(6):555-565. [DOI] [PubMed] [Google Scholar]
- 6.Zebisch A, Cerroni L, Beham-Schmid C, Sill H. Therapy-related leukemia cutis: case study of an aggressive disorder. Ann Hematol. 2003;82(11):705-707. [DOI] [PubMed] [Google Scholar]
- 7.Ganzel C, Manola J, Douer D, et al. Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: analysis of patients in ECOG-ACRIN cancer research group trials, 1980-2008. J Clin Oncol. 2016;34(29):3544-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gornicec M, Wölfler A, Stanzel S, Sill H, Zebisch A. Evidence for a role of decitabine in the treatment of myeloid sarcoma. Ann Hematol. 2017;96(3):505-506. [DOI] [PubMed] [Google Scholar]
- 9.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785-3793. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mulla F, Bitar MS, Taqi Z, Yeung KC. RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol. 2013;228(8):1688-1702. [DOI] [PubMed] [Google Scholar]
- 11.Zebisch A, Troppmair J. Back to the roots: the remarkable RAF oncogene story. Cell Mol Life Sci. 2006;63(11):1314-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung K, Seitz T, Li S, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401(6749):173-177. [DOI] [PubMed] [Google Scholar]
- 13.Zebisch A, Haller M, Hiden K, et al. Loss of RAF kinase inhibitor protein is a somatic event in the pathogenesis of therapy-related acute myeloid leukemias with C-RAF germline mutations. Leukemia. 2009;23(6):1049-1053. [DOI] [PubMed] [Google Scholar]
- 14.Zebisch A, Staber PB, Delavar A, et al. Two transforming C-RAF germ-line mutations identified in patients with therapy-related acute myeloid leukemia. Cancer Res. 2006;66(7):3401-3408. [DOI] [PubMed] [Google Scholar]
- 15.Zebisch A, Wölfler A, Fried I, et al. Frequent loss of RAF kinase inhibitor protein expression in acute myeloid leukemia. Leukemia. 2012;26(8):1842-1849. [DOI] [PubMed] [Google Scholar]
- 16.Escara-Wilke J, Keller JM, Ignatoski KM, et al. Raf kinase inhibitor protein (RKIP) deficiency decreases latency of tumorigenesis and increases metastasis in a murine genetic model of prostate cancer. Prostate. 2015;75(3):292-302. [DOI] [PubMed] [Google Scholar]
- 17.Lamiman K, Keller JM, Mizokami A, Zhang J, Keller ET. Survey of Raf kinase inhibitor protein (RKIP) in multiple cancer types. Crit Rev Oncog. 2014;19(6):455-468. [DOI] [PubMed] [Google Scholar]
- 18.Hatzl S, Geiger O, Kuepper MK, et al. Increased expression of miR-23a mediates a loss of expression in the RAF kinase inhibitor protein RKIP. Cancer Res. 2016;76(12):3644-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer C, Marcucci G, Holland KB, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2009;27(19):3198-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee D, Sabo E, Tavares R, Resnick MB. Inverse association between Raf Kinase Inhibitory Protein and signal transducers and activators of transcription 3 expression in gastric adenocarcinoma patients: implications for clinical outcome. Clin Cancer Res. 2008;14(10):2994-3001. [DOI] [PubMed] [Google Scholar]
- 21.Kashofer K, Gornicec M, Lind K, et al. Detection of prognostically relevant mutations and translocations in myeloid sarcoma by next generation sequencing. Leuk Lymphoma. 2018;59(2):501-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Stölzel F, Onel K, et al. Next-generation sequencing reveals clinically actionable molecular markers in myeloid sarcoma. Leukemia. 2015;29(10):2113-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xian J, Shao H, Chen X, et al. Nucleophosmin mutants promote adhesion, migration and invasion of human leukemia THP-1 cells through MMPs up-regulation via Ras/ERK MAPK signaling. Int J Biol Sci. 2016;12(2):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Zhang W, Yan XJ, et al. DNMT3A mutation leads to leukemic extramedullary infiltration mediated by TWIST1. J Hematol Oncol. 2016;9(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastoret C, Houot R, Llamas-Gutierrez F, et al. Detection of clonal heterogeneity and targetable mutations in myeloid sarcoma by high-throughput sequencing. Leuk Lymphoma. 2017;58(4):1008-1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.