Abstract
Radiolabeled bombesin (BBN) analogs have long been used for developing gastrin-releasing peptide receptor (GRPR) targeted imaging probes, and tracers with excellent in vivo performance including high tumor uptake, high contrast, and favorable pharmacokinetics are highly desired. In this study, we compared the 68Ga-labeled GRPR agonist (Gln–Trp–Ala–Val–Gly–His–Leu–Met–NH2, BBN7–14) and antagonist (d-Phe–Gln–Trp–Ala–Val–Gly–His–Sta–Leu–NH2, RM26) for the positron emission tomography (PET) imaging of prostate cancer. The in vitro stabilities, receptor binding, cell uptake, internalization, and efflux properties of the probes 68Ga–1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)–Aca–BBN7–14 and 68Ga–NOTA–poly(ethylene glycol)3 (PEG3)–RM26 were studied in PC-3 cells, and the in vivo GRPR targeting abilities and kinetics were investigated using PC-3 tumor xenografted mice. BBN7–14, PEG3-RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 showed similar binding affinity to GRPR. In PC-3 tumor-bearing mice, the tumor uptake of 68Ga–NOTA–PEG3–RM26 remained at around 3.00 percentage of injected dose per gram of tissue within 1 h after injection, in contrast with 68Ga–NOTA–Aca–BBN7–14, which demonstrated rapid elimination and high background signal. Additionally, the majority of the 68Ga–NOTA–PEG3–RM26 remained intact in mouse serum at 5 min after injection, while almost all of the 68Ga–NOTA–Aca–BBN7–14 was degraded under the same conditions, demonstrating more-favorable in vivo pharmacokinetic properties and metabolic stabilities of the antagonist probe relative to its agonist counterpart. Overall, the antagonistic GRPR targeted probe 68Ga–NOTA–PEG3–RM26 is a more-promising candidate than the agonist 68Ga–NOTA–Aca–BBN7–14 for the PET imaging of prostate cancer patients.
Introduction
Prostate cancer (PCa) accounts for almost 20% of the newly diagnosed cancers among men in the United States in 2017 and remains the third-leading cause of cancer related male death.1 A typical diagnosis of PCa relies on the histopathological examination of suspected prostate biopsy tissues or specimens from benign prostatic enlargement surgeries or transurethral resection of the prostate following the detection of elevated prostate-specific antigen (PSA) levels, abnormal digital rectal examination (DRE), bone scanning, or a combination of all three. X-ray computed tomography and magnetic resonance imaging (MRI) are currently the major imaging techniques for further identification of PCa.2 However, the capacity of conventional diagnostic techniques for primary lesion detection, staging, or relapse monitoring of PCa is limited.3 For example, the PSA test can be interfered by noncancerous factors such as prostate enlargement, old age, and prostatitis, and low levels of PSA do not necessarily rule out the incidence of PCa.4 The sensitivity and specificity of either ultrasound or MRI is also limited by abnormal signals confounded by prostatitis or benign prostatic hyperplasia (BPH).5,6 The notable multiparametric MRI (MP-MRI) remains imperfect as well, with a pooled sensitivity of up to 89% and a specificity of up to 73%.7
Interest in applying molecular imaging to positron emission tomography (PET) has grown, and a plethora of radiotracers have been developed and investigated actively for PCa. The classical 2-deoxy-2-18F-fluoro-d-glucose (18F-FDG) has been used for evaluating late-stage or recurrent PCa but is not particularly avid.8,9 Other promising agents targeting metabolites such as fatty acids and amino acids (e.g., 11C- and 18F-choline, 11C-acetate, and 18F-FACBC) have been further introduced3,10 as well as agents targeting specific PCa antigens such as prostate-specific membrane antigen (PSMA).11,12 These tracers are proven beneficial for recurrent PC diagnosis and staging. The PSMA targeted tracers have also been applied specially for predicting the optimal timing of PSMA-based therapies.13 However, almost all these tracers show limited diagnostic accuracy for primary lesions,3,10,14 and few of those tracers have been sufficiently investigated and clinically validated to date.
The gastrin-releasing peptide receptor (GRPR) is a G protein-coupled receptor expressed in various organs of mammals, especially in the gastrointestinal tract and the pancreas. Upon binding with the ligand gastrin-releasing peptide (GRP), GRPR can be activated and elicit certain exocrine or endocrine secretions to regulate multiple physiological processes.15 Notably, GRPR over-expression is presented in several types of tumors such as prostate, urinary tract, gastrointestinal stromal, breast, and lung and is related to proliferation and growth of these malignancies.16,17 Especially, GRPR is almost 100% expressed in clinical PCa samples investigated by PCR, immunohistochemistry, or radionuclide binding assays,16 which makes GRPR an attractive target for PCa imaging and therapy.
As an amphibian homologue of GRP, bombesin (BBN) was found to bind to GRPR with a high affinity. For decades, the BBN motifs have been used extensively in radioactive imaging or in radionuclide therapy for GRPR-over-expressing cancers.18,19 For example, the GRPR agonist BBN7–14, a truncated form of BBN with the sequence of Gln–Trp–Ala–Val–Gly–His–Leu–Met–NH2, has been studied as PET or single photon emission computed tomography (SPECT) tracers in both preclinical and clinical research.20−23 In the meantime, numerous clinical trials have been performed using antagonistic GRPR targeting PET radiopharmaceuticals including 68Ga–RM2,24,2568Ga–SB3,2668Ga–BAY86–7458,27 and 64Cu–CB–TE2A–AR06.28 Recently, a nine-amino-acid analog of nonapeptide BBN6–14, d-Phe–Gln–Trp–Ala–Val–Gly–His–Sta–Leu–NH2 (RM26) has been developed as an antagonist against GRPR and has been applied actively in preclinical studies.29−32 Based on these observations, both BBN7–14 and RM26 are considered high-quality candidates for further clinical translation.
Despite the outstanding tumor targeting potential, BBN related research is accompanied by a debate on the superiority of GRPR antagonist- versus agonist-based tracers.33−36 It is generally claimed that even though antagonists are not internalized, radiolabeled antagonists may depict clearer images and pharmacokinetic profiles than agonists. More data are expected to emerge for direct comparison of specific radiolabeled agonist and antagonist tracers to address this controversy, especially among tracers that are promising for clinical translation.
Herein, we establish the distinction of GRPR targeted agonist and antagonist with similar sequences by applying 68Ga-labeled BBN7–14 and RM26 for side-by-side comparative studies, including in vitro receptor binding, cell uptake, internalization, and efflux studies on PC-3 cells and in vivo microPET imaging study of PC-3 tumor bearing mice. The in vitro and in vivo stabilities of both radio conjugates were presented and compared as well.
Results
Synthesis and Radiolabeling
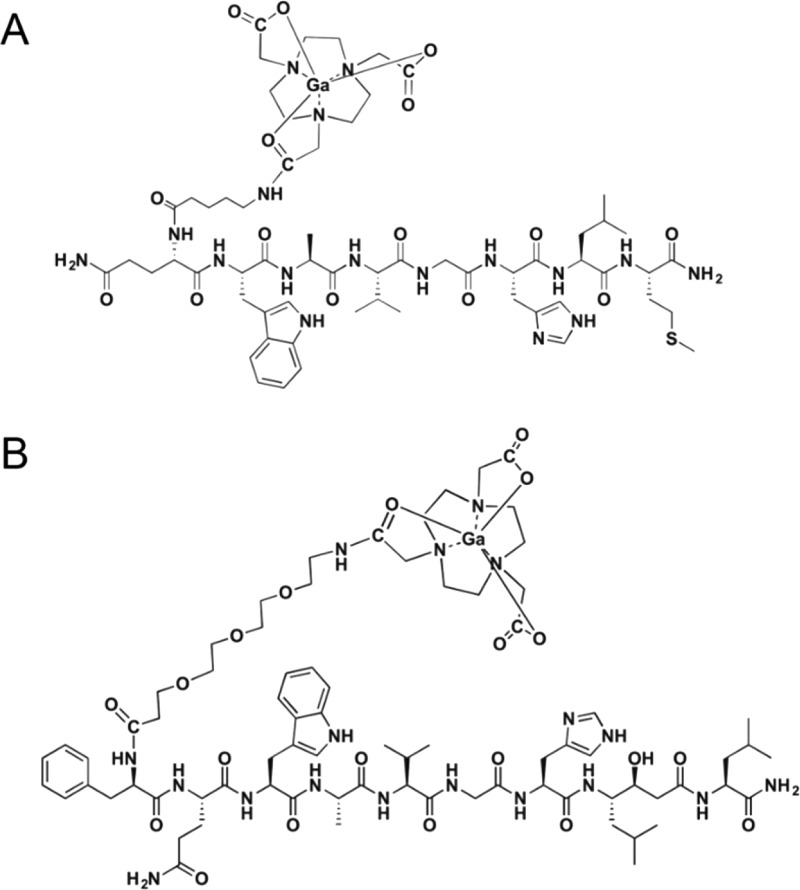
With excess amounts of 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)–N-hydroxysuccinimide (NHS), the NOTA–Aca–BBN7–14 and NOTA–poly(ethylene glycol)3 (PEG3)–RM26 conjugate were produced in >95% yield. A m/z of 1338 for [M + H+] was identified for NOTA–Aca–BBN7–14 using matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI–TOF MS). NOTA–PEG3–RM26 was synthesized and characterized by the same method (m/z = 1601 for [M + H+]). Both conjugates were labeled with 68Ga within 20 min, with specific activities of 21.6–40.01 and 26.7–53.33 MBq/nmol, respectively, for 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26, and for both, radiochemical yield was >90–95% and radiochemical purity was >98%. The chemical structures of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 were presented in Figure 1.
Figure 1.
Schematic structures of (A) GRPR agonist 68Ga–NOTA–Aca–BBN7–14 and (B) antagonist 68Ga–NOTA–PEG3–RM26.
In Vitro Stability
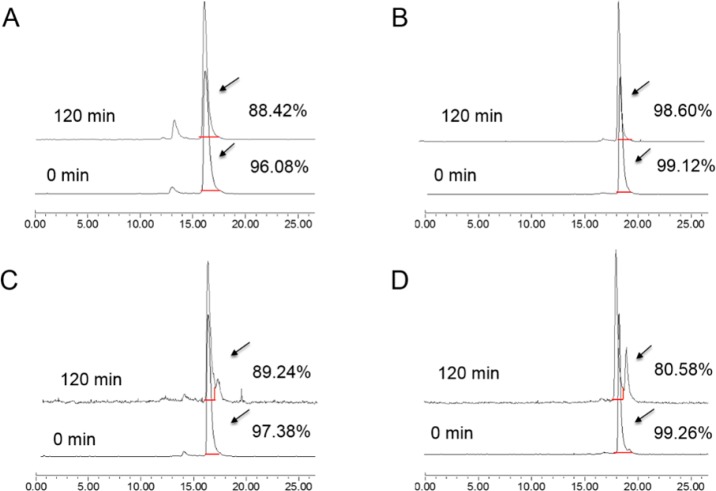
In vitro stabilities of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 in saline and nonheat-inactivated fetal bovine serum (FBS) (Gibco) were determined according to peak integration of analytical high-performance liquid chromatography (HPLC). At 0 min of the incubation, the radiochemical purities of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 were all >95% in both saline and FBS (Figure 2). After 2 h of incubation, the parent compound of 68Ga–NOTA–Aca–BBN7–14 in saline dropped to 88.42% along with a more-hydrophilic peak of 11.58%, while this metabolism for 68Ga–NOTA–Aca–BBN7–14 incubated in FBS was not as obvious. Metabolites represented by radio peaks of slightly higher lipophilicity than the parent compounds were observed for both after 2 h incubation in FBS, accompanied by the percentages of the parent compounds dropping to 89.24% and 80.58%, respectively, for 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26.
Figure 2.
In vitro radioactive stabilities of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 in saline and fetal bovine serum (FBS) for 0 and 120 min after incubation. (A) In vitro radioactive stabilities of 68Ga–NOTA–Aca–BBN7–14 in saline at 0 and 120 min after incubation. (B) In vitro radioactive stabilities of 68Ga–NOTA–PEG3–RM26 in saline at 0 and 120 min after incubation. (C) In vitro radioactive stabilities of 68Ga–NOTA–Aca–BBN7–14 in FBS at 0 and 120 min after incubation. (D) In vitro radioactive stabilities of 68Ga–NOTA–PEG3–RM26 in FBS at 0 and 120 min after incubation.
Competitive Binding Assay
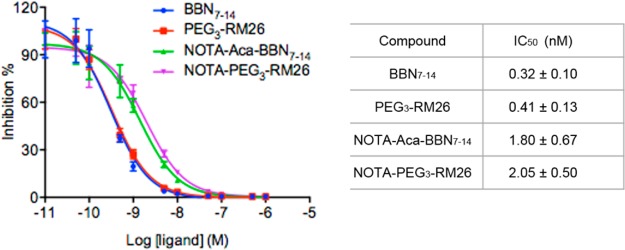
The GRPR-binding affinities of BBN7–14, PEG3–RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 were assessed by competitive binding assay using 125I––Tyr4]BBN as the radioligand. The results of these assays were shown in Figure 3. The binding of 125I–[Tyr4]BBN to GRPR was displaced by the cold analogs in a concentration-dependent manner. The half maximal inhibitory concentration (IC50) values of BBN7–14, PEG3–RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 were 0.32 ± 0.10, 0. 41 ± 0.13, 1.80 ± 0.67, and 2.05 ± 0.50 nM, respectively. The results indicated that the intermolecular targeting abilities of BBN7–14 and PEG3–RM26 for GRPR were comparable. After the NOTA conjugation, the affinities of both compounds decreased to some extent. However, there were no distinct disparities discovered between NOTA–Aca–BBN7–14 and NOTA–PEG3–RM26, either.
Figure 3.
Inhibition of 125I–[Tyr4]BBN binding to GRPR on PC-3 cells by BBN7–14, PEG3-RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 (n = 3/group, mean ± SD).
Cell Uptake, Internalization, and Efflux
A time-dependent cellular-uptake pattern in GRPR positive PC-3 cells was observed for both 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26. The uptake of 68Ga–NOTA–Aca–BBN7–14 increased rapidly to nearly 27% within 1 h of incubation, and that of 68Ga–NOTA–PEG3–RM26 was slightly lower (Figure 4A). The agonist 68Ga–NOTA–Aca–BBN7–14 showed distinctively high internalization, and around 74% of the radioactivity uptake was internalized within 1 h of incubation. In contrast, 68Ga–NOTA–PEG3–RM26 showed very low internalization (<15% of total uptake; Figure 4A). After washing and medium replacement, both of the tracers showed efflux with a similar pattern (Figure 4B). At 60 min, 50% of radioactivity uptake was still retained with the cells.
Figure 4.
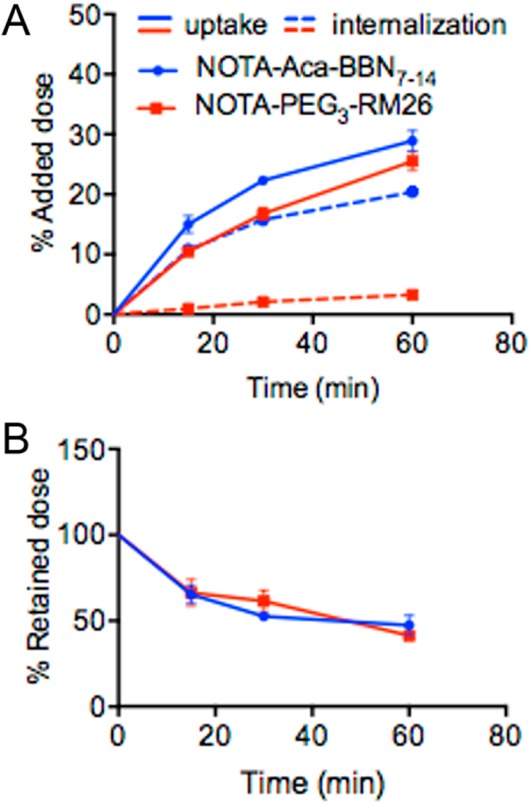
In vitro cell uptake, internalization, and efflux studies of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3-RM26 on PC-3 cells. (A) Cell uptake and internalization assay of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 on PC-3 tumor cells (n = 3, mean ± SD). (B) Cell efflux assay of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 on PC-3 tumor cells (n = 3, mean ± SD).
In Vivo PET Imaging
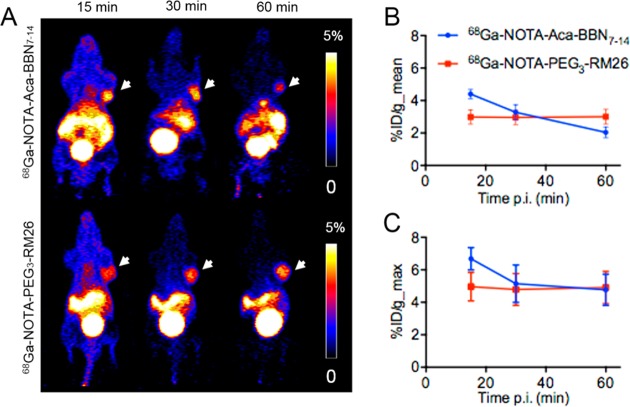
Representative coronal PET images of PC-3 tumor-bearing mice at different time points are shown in Figure 5. The tumors were clearly visualized with high contrast at all the time points for 68Ga–NOTA–PEG3–RM26 (n = 3) as well as at 15 and 30 min for 68Ga–NOTA–Aca–BBN7–14 (n = 4). However, at 60 min post-injection, the tumor became much less visible in the mice administered with 68Ga–NOTA–Aca–BBN7–14 (Figure 5A). Meanwhile, both of the tracers showed considerable accumulation and retention in the abdominal regions including pancreas and intestines, although less was observed for 68Ga–NOTA–PEG3–RM26 than for 68Ga–NOTA–Aca–BBN7–14. At these early time points, relatively high kidney radioactivity was observed while the radioactivity in the bladder was constantly high for these two probes, suggesting that the tracers were excreted mainly by the renal system.
Figure 5.
GRPR-specific imaging in PC-3 xenografted mouse model using 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26. (A) Representative microcoronal PET scans of mice bearing PC-3 xenografts 15, 30, and 60 min after injection of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26. (B) PET quantification of tumor uptake in PC-3 tumors 15, 30, and 60 min after injection of 68Ga–NOTA–Aca–BBN7–14 (n = 4, mean ± SD) and 68Ga–NOTA–PEG3–RM26 (n = 3, mean ± SD), expressed as %ID/g_mean. (C) PET quantification of tumor uptake in PC-3 tumors 15, 30, and 60 min after the injection of 68Ga–NOTA–Aca–BBN7–14 (n = 4, mean ± SD) and 68Ga–NOTA–PEG3–RM26 (n = 3, mean ± SD), expressed as %ID/g_max.
Activity accumulation in the tumor was quantified by measuring the regions of interest (ROIs) on the coronal images (Figure 5B,C). The mean tumor uptake was determined to be 4.40 ± 0.29, 3.28 ± 0.47, and 2.04 ± 0.34 percentage of injected dose per gram of tissue (%ID/g) for 68Ga–NOTA–Aca–BBN7–14, and 2.99 ± 0.44, 2.96 ± 0.45, and 3.01 ± 0.45%ID/g for 68Ga–NOTA–PEG3–RM26 at 15, 30, and 60 min, with the corresponding P values of <0.01, 0.39, and 0.02, respectively, when comparing tumor uptake of the two tracers at the same time points post-injection.
However, the maximum tumor uptake was determined to be 6.68 ± 0.69, 5.14 ± 1.15, and 4.77 ± 0.96%ID/g for 68Ga–NOTA–Aca–BBN7–14, and 4.96 ± 0.87, 4.79 ± 0.98, and 4.91 ± 1.00%ID/g for 68Ga–NOTA–PEG3–RM26 at 15, 30, and 60 min, with the corresponding P values of 0.03, 0.68, and 0.85, respectively, when comparing the tumor uptake of the two tracers at the same time points post-injection.
Biodistribution of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26
The biodistribution of 68Ga–NOTA–Aca–BBN7–14 (n = 4) and 68Ga–NOTA–PEG3–RM26 (n = 3) in tumor and normal tissues determined by γ-counting was presented in Figure 6A. In good agreement with the PET images, high uptake and long retention of the radioactivity were observed for the GRPR-positive tissues including pancreas, intestine, and PC-3 tumor at 60 min post-injection, which were 17.99 ± 1.48, 4.33 ± 1.90, and 2.40 ± 0.38 ID/g for 68Ga–NOTA–Aca–BBN7–14 and 9.51 ± 0.73, 0.93 ± 0.12, and 3.31 ± 0.68%ID/g for 68Ga–NOTA–PEG3–RM26.
Figure 6.
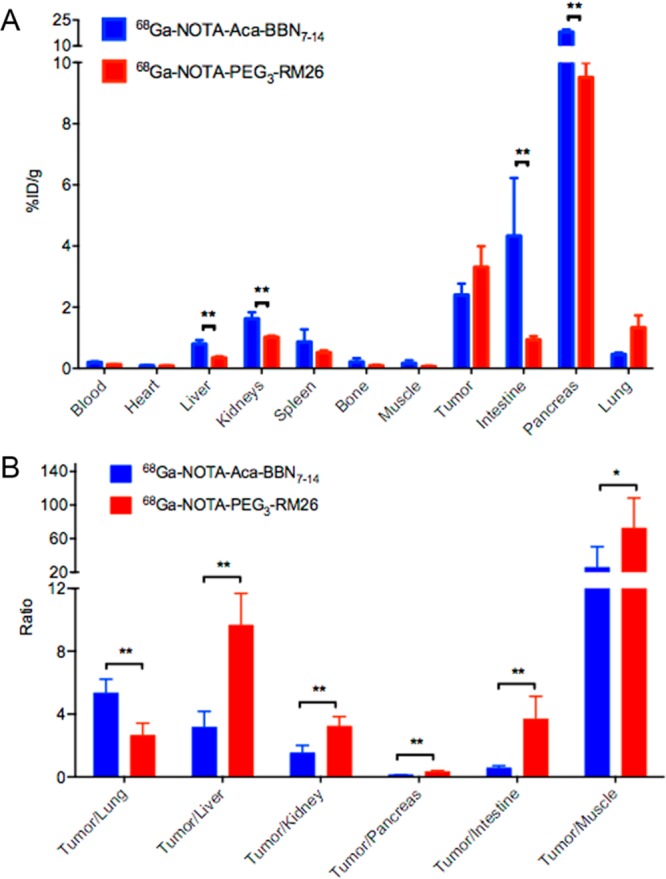
Biodistribution of 68Ga–NOTA–Aca–BBN7–14 (blue bars, n = 4) and 68Ga–NOTA–PEG3–RM26 (red bars, n = 3) in PC-3 tumor bearing nude mice at 60 min after administration (mean ± SD). (A) Biodistribution of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 in multiple organs of PC-3 tumor bearing nude mice. Significant differences (P < 0.05) in biodistribution between probes were marked with asterisks. (B) Comparative ratios of the tumor uptake to major organs for 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 at 1 h post-injection. Significant differences (P values of <0.01 and <0.05) in tumor-to-organ ratios between probes were marked with asterisks.
However, the tumor-to-lung, tumor-to-liver, tumor-to-kidney, tumor-to-pancreas, tumor-to-intestine, and tumor-to-muscle ratios for the radiopharmaceuticals at 1 h post-injection were shown in Figure 6B, which were 2.66, 9.66, 3.22, 0.34, 3.69, and 72.5 for 68Ga–NOTA–PEG3–RM26 and 5.34, 3.15, 1.52, 0.13. 0.56, and 25.57 for 68Ga–NOTA–Aca–BBN7–14, respectively. Except for the tumor-to-lung uptake ratios, 68Ga–NOTA–PEG3–RM26 showed much better tumor-to-organ contrast than that of 68Ga–NOTA–Aca–BBN7–14 (P values of <0.01 and <0.05). Specially, the tumor-to-muscle ratio of 68Ga–NOTA–PEG3–RM26 was 2.88-fold of that of the radio-agonist.
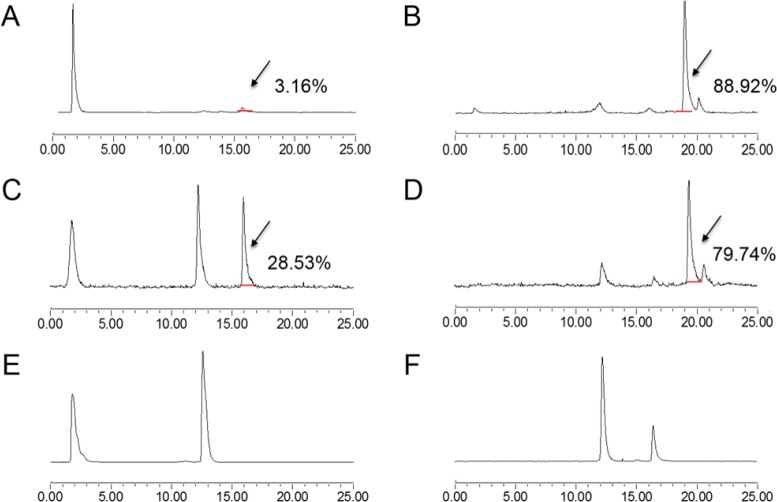
In Vivo Stability of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26
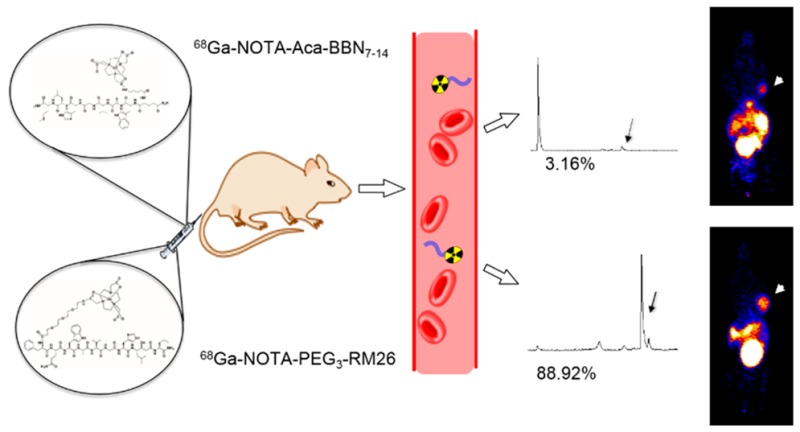
The in vivo metabolic stabilities of the radio conjugates were determined in each sample of mouse serum at 5 min after injection and in urine and tumor homogenates at 30 min after injection (Figure 7). The average extraction efficiencies were 89.3%, 97%, and 84.2% for serum, urine, and tumor, respectively. The composition of the metabolites was not identified, and almost all of the metabolites came earlier off the HPLC column than the parent compound for 68Ga–NOTA–Aca–BBN7–14, but the 68Ga-NOTA-PEG3-RM26 profile revealed a radio-metabolite with slightly higher lipophilicity that came off of the column after the parent compound. At 5 min post-injection, almost no parent form of 68Ga–NOTA–Aca–BBN7–14 was found in serum (Figure 7A); however, 88.92% of 68Ga–NOTA–PEG3–RM26 remained intact at the same time point (Figure 7B). In tumor, about 28.53% for 68Ga–NOTA–Aca–BBN7–14 (Figure 7C) and 79.74% for 68Ga–NOTA–PEG3–RM26 (Figure 7D) were intact. In urine, no parent form of either probe was observed (Figure 7E,F).
Figure 7.
In vivo metabolic stabilities of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26, respectively, in serum at 5 min after injection and urine and tumor at 30 min after injection. (A) Stability of 68Ga–NOTA–Aca–BBN7–14 in serum at 5 min post-injection. (B) Stability of 68Ga–NOTA–PEG3–RM26 in serum at 5 min post-injection. (C) Stability of 68Ga–NOTA–Aca–BBN7–14 in tumor at 30 min post-injection. (D) Stability of 68Ga–NOTA–PEG3–RM26 in tumor at 30 min post-injection. (E) Stability of 68Ga–NOTA–Aca–BBN7–14 in urine at 30 min post-injection. (F) Stability of 68Ga–NOTA–PEG3–RM26 in urine at 30 min post-injection.
Discussion
BBN analogues have been widely labeled with radionuclides such as 68Ga, 64Cu, 18F, and 177Lu.8,20,22,23,37 In particular, 68Ga can be easily produced due to the availability and commercialization of the in-house 68Ge and 68Ga generators (68Ge, t1/2 = 270.8 days). The 68Ga-labeling of BBN derivatives conjugates come with high labeling yield, satisfactory radiochemical purity, and specific activity, and the clinical implementation of 68Ga radiopharmaceuticals is preferred in the routine clinical setup of nuclear medicine. In this study, both BBN-based probes were prepared with high radiochemical purity and specific activity within 20 min, demonstrating ideal radiochemical processing for clinical translation.
In this study, we synthesized the NOTA-conjugated GRPR agonist BBN7–14 and antagonist RM26; PEG3 was used as a linker to conjugate NOTA to the antagonist peptide. The IC50 values of NOTA–Aca–BBN7–14 and NOTA–PEG3–RM26 were similar to each other and also close to analogs reported from other laboratories previously.31,33 Despite similar binding affinities, the tumor uptake of 68Ga–NOTA–Aca–BBN7–14 dropped rapidly from 4.40%ID/g at 15 min to 2.04%ID/g at 60 min post-injection in our study, and that of 68Ga–NOTA–PEG3–RM26 remained at a plateau of around 3.00%ID/g. The declining pattern of tumor uptake for 64Cu-NOTA–Aca–BBN7–14 in PC-3 tumor mice from 1 to 24 h post-injection has also been reported previously, and the authors speculate that the signal decrease was primarily due to rapid clearance from the bloodstream and excretion via the renal-urinary pathway.22 Both 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 were mainly excreted through the urinary tract, so high accumulation of radioactivity in the bladder was observed. However, the kidney uptake was moderate compared with other peptide-based tracers.38 Consistently, we observed most of 68Ga–NOTA–PEG3–RM26 in mouse serum remained intact at 5 min after injection, in comparison to 3.16% of 68Ga–NOTA–Aca–BBN7–14, indicating much greater metabolic stability in vivo for the antagonist and better suitability for GRPR imaging of 68Ga–NOTA–PEG3–RM26.
In agreement with the PET imaging study, the ex vivo biodistribution results further validated higher tumor-to-organ ratios for 68Ga–NOTA–PEG3–RM26 in comparison with those of 68Ga–NOTA–Aca–BBN7–14 at 60 min after injection. Hence, the higher tumor uptake and lower background offer 68Ga–NOTA–PEG3–RM26 more sensitivity in detecting indistinct lesions, such as metastasis distributed in the abdominal area in clinical settings.
It is well-known that introduction of different linkers between the metal chelator and targeting peptide sequence results in different tumor uptake and distinct normal organ distribution.39,40 Indeed, we have included 68Ga–NOTA–PEG3–Aca-BBN7–14 in the experimental design for this comparison study. However, both PET imaging and biodistribution results revealed that 68Ga–NOTA–PEG3–Aca-BBN7–14 was the worst probe compared with the other two probes. The results indicated that the PEG3 modification itself did not substantially alter the in vivo pharmacokinetics of BBN7–14, and the difference between 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 mainly came from the inherent distinction of the peptide sequences and the agonism and antagonism of the two probes.
For in vitro stability assay, a minor portion of relatively hydrophilic radio peak was observed for 68Ga–NOTA–Aca–BBN7–14 incubated in saline, which could result from radiolysis of the Met residue of 68Ga–NOTA–Aca–BBN7–14, while in FBS incubation, this kind of decomposition was possibly suppressed by the presence of serum proteins.41,42 Moreover, a slightly more lipophilic radio peak was observed after 120 min incubation in nonheat inactivated FBS for both 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26, each consisting 10.76% and 19.42% of the radiopharmaceuticals. Formation of these metabolites may have been derived from the action of serum proteases in nonheat inactivated FBS such as carboxamidase, which could catalyze the hydrolysis of C-terminal carboxamides to the corresponding carboxy acid.43
In vivo stability assay via radio-HPLC also indicated better stability of 68Ga–NOTA–PEG3–RM26 compared to 68Ga–NOTA–Aca–BBN7–14 in tumor homogenates. However, the structure of the radioactive fragments was not identified because the mass amount for the injection was limited. In a previous study, we observed a rapid degradation of BBN7–14 during incubation in rat hepatocytes (t1/2 = 4 min), and the metabolites of BBN7–14 were found to be derived from peptide bond hydrolysis between amino acids Trp and Ala and between Ala and Val and within the C-terminal amide by high-resolution mass spectrometry coupled with HPLC (LC–MS).44 Nevertheless, in vitro study does not necessarily represent the situation encountered by intravenously administered radiopeptides in vivo. Indeed, using in vivo mouse plasma metabolized fragment of 177Lu–AMBA, the weak sites were revealed within the backbone of BBN7–14 between amino acids Gln and Trp, Trp and Ala, and His and Leu, as well as the terminal amide of 177Lu–AMBA.43 It has been proposed and confirmed that neutral endopeptidase (NEP) acts as the major protease in the degradation of bombesin-like radiopeptides in vivo and that the co-injection of NEP inhibitors could enhance stability and tumor uptake of those radiopeptides.45−47
It is worth pointing out that a lack of stability is not an inherent issue for all GRPR agonists. Through structural interventions including peptide chain-length modification, amino acid substitutions, application of an amide-to-triazole replacement strategy, or the introduction of different lengths of spacer bridging the chelator to the peptide receptor-recognition site, the biological profiles (especially of radioligand pharmacokinetics in vivo) could be significantly improved.45,48−50 The major hindrance of GRPR agonists is the possible biological effect induced upon receptor binding when a large amount of the agonists is administered for therapeutic purpose. A more-stable GRPR agonist may be still meaningful as an imaging probe.
Conclusions
The antagonist-based probe 68Ga–NOTA–PEG3–RM26 showed higher tumor uptake with lower background in the PC-3 tumor bearing mouse model at 1 h post-injection and displayed more-favorable in vivo pharmacokinetic properties as well as metabolic stabilities. 68Ga–NOTA–PEG3–RM26 is, therefore, a more-promising candidate for clinical translation of PET imaging for PCa compared to the agonist 68Ga–NOTA–Aca–BBN7–14.
Materials and Methods
Chemistry
Aminocaproic acid (Aca)–BBN7–14 and RM26 were synthesized using solid-phase Fmoc chemistry by Peptides International Inc. and CSBio. 1,4,7-Triazacyclononane-1,4,7-triacetic acid N-hydroxysuccinimide (NOTA–NHS) ester was purchased from CheMatech (Dijon, France). All other chemicals were obtained from Sigma-Aldrich. NOTA–Aca–BBN7–14 and NOTA–PEG3–RM26 were prepared according to a procedure published previously.37
68Ga was eluted from a 68Ge and 68Ga generator (ITG) with 0.6 M HCl at 0.5 mL per fraction. A total of 0.5 mL of 1 M HEPES buffer was added to the fraction containing the most amount of 68Ga radioactivity (185–222 MBq) to a final pH of around 6.0. A total of 10 μg of NOTA–Aca–BBN7–14 or NOTA–PEG3–RM26 in 10 μL of water was added to the above solution. After shaking, the mixture was incubated at 80 °C for 10 min. The reaction mixture was trapped on an activated Varian Bond Elut C18 column (100 mg) by a syringe. Another 10 mL of water was used to wash the column, followed by 0.3 mL of 1 mM HCl ethanol solution to elute off the trapped radioactivity. Next, the product was diluted with saline for further use. An analytical reverse-phase radio HPLC (Waters Symmetry C-18 column, 3.9 × 150 mm, 5 μm) running a linear gradient starting from 5% A (0.1% TFA in acetonitrile) and 95% B (0.1% TFA in water) for 5 min and A increased to 65% at 2% per min, with the flow rate of 1 mL/min used for characterization of both tracers and in vitro and in vivo stability analysis.
In Vitro stability Test
The in vitro stability of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 was examined by radio HPLC according to a procedure reported previously.51 Briefly, about 37 kilobecquerel (KBq) of 68Ga–NOTA–Aca–BBN7–14 (40.0 MBq/nmol) or 68Ga–NOTA–PEG3–RM26 (39.8 MBq/nmol) in 50 μL was incubated in 450 μL of normal saline or FBS at 37 °C. At 0 and 120 min incubation, 25 μL of the saline-incubated sample was taken out of the tube and subjected to HPLC analysis. However, 25 μL of the serum sample was taken out at the same time points, added to an equal volume of acetonitrile, and centrifuged at 6000 rpm for 10 min. The supernatant was then extracted and submitted for radio-HPLC analysis.
Cell Culture and Animal Models
The PC-3 human prostate carcinoma cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The cells were grown in Roswell Park Memorial Institute (RPMI) 1640 Medium supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 mg/mL) (Invitrogen, Carlsbad, CA) and cultured at 37 °C in a humidified atmosphere containing 5% CO2. Cells were passaged three times per week.
All animal studies were conducted according to the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Clinical Center, National Institutes of Health (NIH). Female BALB/c mice (age, 5–7 weeks; weights of 18–20 g) and female athymic nude/nude mice (age, 5–7 weeks; weights of 18–20 g) were purchased from Harlan Laboratories.
The PC-3 tumor models were generated by subcutaneous injection of 5 × 106 cells into the right shoulder of nude mice. Tumor sizes were measured using a digital caliper. Tumor volume (mm3) was calculated according to the formula 0.5 × length × width2. The mice were subjected to micro PET studies when the tumor volume reached 100–300 mm3 (3–4 weeks after inoculation).
In Vitro Cell Receptor Binding Assay
In vitro GRPR-binding affinities and specificities of BBN7–14, PEG3-RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 were determined by displacement PC-3 cell-binding assays using 125I–[Tyr4]BBN as the radioligand. 125I–[Tyr4]BBN (81 400 gigabecquerel (GBq)/mmol) was purchased from American Radiolabeled Chemicals, Inc. Experiments were performed on PC-3 cells by modifying a previously described method.52 Briefly, PC-3 cells were freshly harvested and seeded in 96-well plates at 105 cells/50 μL per well in RMPI 1640 binding buffer (with 0.1% bovine serum albumin, serum free). 125I–Tyr4]BBN was diluted in binding buffer with a specific activity of 2.2 KBq/50 μL. The cells and diluted 125I–[Tyr4]BBN were then incubated, respectively, with increasing concentrations of BBN7–14, PEG3-RM26, NOTA–Aca–BBN7–14, and NOTA–PEG3–RM26 ranging from 0 to 2000 nmol/L in 37 °C up to 60 min. The IC50 values were determined by nonlinear regression analysis using Graph-Pad Prism (GraphPad Software, Inc.). Experiments were performed in triplicate.
Cell Uptake and Internalization Studies
The uptake and internalization of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26 in PC-3 cells were examined according to the following procedures, respectively. For the cell uptake experiment, PC-3 cells were seeded in 24-well plates at a density of 105 cells per well 24 h before the assay. The medium was removed, and the cells were rinsed twice with PBS. Then, 7.4 KBq per well of tracers (with the corresponding specific activities of 40.0 MBq/nmol and 53.3 MBq/nmol for 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26) were added in 0.5 mL of serum-free media (SFM). The cells were incubated at 37 °C for 5, 15, 30, and 60 min. At each indicated time point, the medium was removed, and cells were rinsed twice with cold PBS (1 mL) and lysed by the addition of 0.2 mL of 0.1 M NaOH. The cell lysate was collected for γ-counting. For internalization study, after the removal of the medium at the same indicated time point as the cell uptake study, the cells were incubated for 1 min with 0.5 mL of acid buffer (50 mM glycine and 100 mM NaCl at pH 2.8). Next, the acid buffer was removed, and the cells were washed twice with 1 mL of PBS, followed by addition of 0.2 mL of 0.1 M NaOH. Cell lysate was collected, and the radioactivity was measured by a γ-counter. The cell uptake and internalization values were normalized to the amount of added radioactivity. Each experiment was performed in triplicate.
Cell Efflux Studies
For a cell-efflux study of 68Ga–NOTA–Aca–BBN7–14 (39.8 MBq/nmol)and 68Ga–NOTA–PEG3–RM26 (52.5 MBq/nmol), 7.4 KBq per well of tracers were added to PC-3 cells in a 24-well plate and incubated for 1 h at 37 °C. Next, cells were washed twice with cold PBS and incubated with SFM for 5, 15, 30, and 60 min. After being washed twice with PBS, cells were harvested by the addition of 0.2 mL of 0.1 mol/L NaOH. Cell lysate was collected and the radioactivity measured by a γ-counter. Efflux values were calculated by subtracting retention at different time points from 0 min retention and normalized by dividing the total counts at 0 min.
PET of Tumor-Bearing Mice
PET scans were obtained using an Inveon small animal PET scanner (Siemens Medical Solutions). Under isoflurane anesthesia, 5 min of static PET scanning was performed at 15, 30, and 60 min after the PC-3 tumor-bearing mice were each intravenously injected with 3.7 MBq of 68Ga–NOTA–Aca–BBN7–14 (28.2 MBq/nmol) (n = 4) or 68Ga–NOTA–PEG3–RM26 (32.6 MBq/nmol) (n = 3) in a volume of 100 μL of PBS.
The PET images were reconstructed using 3-dimensional ordered-subsets expectation maximum (3D OSEM) followed by maximum a posteriori (MAP) algorithm with a smoothing parameter (OSEM-3D-MAP) of 0.1. For each scan, regions of interest (ROIs) were drawn over the tumor on whole-body decay-corrected coronal images using vendor software (ASI Pro 5.2.4.0; Siemens Medical Solutions). The radioactivity accumulation within the tumor was calculated from mean pixel values of the multiple ROI volumes. These values were converted to MBq/mL and then further divided by the administered activity to obtain an image-ROI-derived %ID/g value (assuming a tissue density of 1 g/mL). No correction was applied in this study.
Biodistribution of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26
Each mouse was intravenously injected with 3.7 MBq of 68Ga–NOTA–Aca–BBN7–14 (28.2 MBq/nmol) (n = 4) or 68Ga–NOTA–PEG3–RM26 (32.6 MBq/nmol) (n = 3) in a volume of 100 μL of PBS. At 1 h post-injection, the mice were sacrificed, and blood, heart, liver, kidneys, spleen, bone, muscle, tumor, intestine, pancreas, and lung tissues were collected. The organs were wet-weighed, and the radioactivity was assayed using a γ-counter.
In Vivo Stability of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26
For serum metabolic stability studies, each healthy Balb/c mouse was injected intravenously with 37 MBq of 68Ga–NOTA–Aca–BBN7–14 (34.9 MBq/nmol) or 68Ga–NOTA–PEG3–RM26 (46.8 MBq/nmol). At 5 min after injection, the mice were anesthetized, and 100 μL of blood was collected. The blood sample was immediately centrifuged at 13 200 rpm for 5 min. A total of 25 μL of the supernatant was removed and mixed with an equal volume of acetonitrile, with centrifugation at 6000 rpm for 10 min. Next, the extracted solution was injected into an HPLC device for analysis. For urine analysis, the urine was collected at 30 min post-injection, and then 25 μL of the urine was mixed with an equal volume of acetonitrile and centrifuged at 13 200 rpm for 5 min. The supernatant was moved and subjected to HPLC analysis.
For tumor metabolism analysis, a pair of PC-3 tumor bearing mice were injected with 37 MBq of each tracer (with the specific activities of 32.9 and 31.6 MBq/nmol for of 68Ga–NOTA–Aca–BBN7–14 and 68Ga–NOTA–PEG3–RM26, respectively). At 30 min after injection, the mice were sacrificed under anesthesia, and the tumors were removed and weighed. With an equal weight of acetonitrile, the tumor was homogenized on ice and centrifuged at 13 200 rpm for 5 min. A total of 50 μL of the supernatant was removed for HPLC analysis.
The radioactivity of in vivo collected blood, urine, and tumor samples was measured with a γ-counter each time before and after the samples were homogenized or centrifuged to calculate the extraction efficiency. All samples were collected in pre-chilled vials, and all further manipulations were conducted on ice or at 4 °C for centrifugation to prevent further degradation during sample workup.
Statistical Analysis
All quantitative data are presented as mean ± SD. Mean values were compared using one-way ANOVA and the Student t test. P values of <0.05 were considered statistically significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 81671718 and 81271600), Natural Science Foundation of Hubei Province of China (grant no. 2016CFB687), and the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. S.C. is a Ph.D. candidate of Tongji Medical College, Huazhong University of Science and Technology, and is partially funded by the China Scholarship Council (CSC) as a visiting student of NIH.
The authors declare no competing financial interest.
References
- Siegel R. L.; Miller K. D.; Jemal A. (2017) Cancer Statistics, 2017. Ca-Cancer J. Clin. 67, 7–30. 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Mottet N.; Bellmunt J.; Bolla M.; Briers E.; Cumberbatch M. G.; De Santis M.; Fossati N.; Gross T.; Henry A. M.; Joniau S.; et al. (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 71, 618–629. 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Schuster D. M.; Nanni C.; Fanti S. (2016) PET Tracers Beyond FDG in Prostate Cancer. Semin. Nucl. Med. 46, 507–521. 10.1053/j.semnuclmed.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barentsz J. O.; Richenberg J.; Clements R.; Choyke P.; Verma S.; Villeirs G.; Rouviere O.; Logager V.; Futterer J. J. (2012) ESUR prostate MR guidelines 2012. Eur. Radiol. 22, 746–757. 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.; Rajesh A.; Futterer J. J.; Turkbey B.; Scheenen T. W.; Pang Y.; Choyke P. L.; Kurhanewicz J. (2010) Prostate MRI and 3D MR Spectroscopy: How We Do It. AJR, Am. J. Roentgenol. 194, 1414–1426. 10.2214/AJR.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G.; Haider M.; Ghai S.; Sreeharsha B. (2013) The Expanding Role of MRI in Prostate Cancer. AJR, Am. J. Roentgenol. 201, 1229–1238. 10.2214/AJR.12.10178. [DOI] [PubMed] [Google Scholar]
- Woo S.; Suh C. H.; Kim S. Y.; Cho J. Y.; Kim S. H. (2017) Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-analysis. Eur. Urol. 72, 177–188. 10.1016/j.eururo.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Schoder H.; Herrmann K.; Gonen M.; Hricak H.; Eberhard S.; Scardino P.; Scher H. I.; Larson S. M. (2005) 2-[18F]Fluoro-2-deoxyglucose Positron Emission Tomography for the Detection of Disease in Patients with Prostate-Specific Antigen Relapse After Radical Prostatectomy. Clin. Cancer Res. 11, 4761–4769. 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- Wibmer A. G.; Burger I. A.; Sala E.; Hricak H.; Weber W. A.; Vargas H. A. (2016) Molecular Imaging of Prostate Cancer. Radiographics 36, 142–159. 10.1148/rg.2016150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster D. M.; Nanni C.; Fanti S. (2016) Evaluation of Prostate Cancer with Radiolabeled Amino Acid Analogs. J. Nucl. Med. 57, 61S–66S. 10.2967/jnumed.115.170209. [DOI] [PubMed] [Google Scholar]
- Jadvar H. (2011) Prostate Cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J. Nucl. Med. 52, 81–89. 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbock S. M.; Rauscher I.; Bluemel C.; Fendler W. P.; Rowe S. P.; Pomper M. G.; Asfhar-Oromieh A.; Herrmann K.; Eiber M. (2017) PSMA Ligands for PET-Imaging of Prostate Cancer. J. Nucl. Med. 58, 1545–1552. 10.2967/jnumed.117.191031. [DOI] [PubMed] [Google Scholar]
- Ceci F.; Herrmann K.; Hadaschik B.; Castellucci P.; Fanti S. (2017) Therapy Assessment in Prostate Cancer Using Choline and PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 44 (1), 78–83. 10.1007/s00259-017-3723-3. [DOI] [PubMed] [Google Scholar]
- Bach-Gansmo T.; Nanni C.; Nieh P. T.; Zanoni L.; Bogsrud T. V.; Sletten H.; Korsan K. A.; Kieboom J.; Tade F. I.; Odewole O.; et al. (2017) Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 197, 676–683. 10.1016/j.juro.2016.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.; Qu X.; Qin X. Q. (2015) Bombesin-Like Peptides and Their Receptors: Recent Findings in Pharmacology and Physiology. Curr. Opin. Endocrinol., Diabetes Obes. 22, 3–8. 10.1097/MED.0000000000000126. [DOI] [PubMed] [Google Scholar]
- Patel O.; Shulkes A.; Baldwin G. S. (2006) Gastrin-Releasing Peptide and Cancer. Biochim. Biophys. Acta, Rev. Cancer 1766, 23–41. 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Morgat C.; MacGrogan G.; Brouste V.; Velasco V.; Sevenet N.; Bonnefoi H.; Fernandez P.; Debled M.; Hindie E. (2017) Expression of Gastrin-Releasing Peptide Receptor (GRPR) in Breast Cancer and its Association with Pathologic, Biologic and Clinical Parameters: A Study of 1432 Primary Tumors. J. Nucl. Med. 58, 1401–1407. 10.2967/jnumed.116.188011. [DOI] [PubMed] [Google Scholar]
- Sancho V.; Di Florio A.; Moody T. W.; Jensen R. T. (2011) Bombesin Receptor-Mediated Imaging and Cytotoxicity: Review and Current Status. Curr. Drug Delivery 8, 79–134. 10.2174/156720111793663624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C. A.; Fuscaldi L. L.; Townsend D. M.; Rubello D.; Barros A. L. B. (2017) Radiolabeled Bombesin Derivatives for Preclinical Oncological Imaging. Biomed. Pharmacother. 87, 58–72. 10.1016/j.biopha.2016.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J.; Gali H.; Sieckman G. L.; Hayes D. L.; Owen N. K.; Mazuru D. G.; Volkert W. A.; Hoffman T. J. (2003) Radiochemical Investigations of 177Lu-DOTA-8-Aoc-BBN[7–14]NH2: An In Vitro/In Vivo Assessment of the Targeting Ability of This New Radiopharmaceutical for PC-3 Human Prostate Cancer Cells. Nucl. Med. Biol. 30, 101–109. 10.1016/S0969-8051(02)00391-8. [DOI] [PubMed] [Google Scholar]
- Smith C. J.; Gali H.; Sieckman G. L.; Higginbotham C.; Volkert W. A.; Hoffman T. J. (2003) Radiochemical Investigations of 99mTc-N3S-X-BBN[7–14]NH2: An In Vitro/In Vivo Structure-Activity Relationship Study Where X = 0-, 3-, 5-, 8-, and 11-Carbon Tethering Moieties. Bioconjugate Chem. 14, 93–102. 10.1021/bc020034r. [DOI] [PubMed] [Google Scholar]
- Prasanphanich A. F.; Nanda P. K.; Rold T. L.; Ma L.; Lewis M. R.; Garrison J. C.; Hoffman T. J.; Sieckman G. L.; Figueroa S. D.; Smith C. J. (2007) [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] Targeting Vector for Positron-Emission Tomography Imaging of Gastrin-Releasing Peptide Receptor-Expressing Tissues. Proc. Natl. Acad. Sci. U. S. A. 104, 12462–12467. 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Li D.; Lang L.; Zhu Z.; Wang L.; Wu P.; Niu G.; Li F.; Chen X. (2016) 68Ga-NOTA–Aca–BBN(7–14) PET/CT in Healthy Volunteers and Glioma Patients. J. Nucl. Med. 57, 9–14. 10.2967/jnumed.115.165316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamimoto R.; Hancock S.; Schneider B.; Chin F. T.; Jamali M.; Loening A.; Vasanawala S.; Gambhir S. S.; Iagaru A. (2016) Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 57, 557–562. 10.2967/jnumed.115.168393. [DOI] [PubMed] [Google Scholar]
- Wieser G.; Popp I.; Christian Rischke H.; Drendel V.; Grosu A. L.; Bartholoma M.; Weber W. A.; Mansi R.; Wetterauer U.; Schultze-Seemann W.; et al. (2017) Diagnosis of Recurrent Prostate Cancer with PET/CT Imaging Using the Gastrin-Releasing Peptide Receptor Antagonist 68Ga-RM2: Preliminary Results in Patients with Negative or Inconclusive [18F]Fluoroethylcholine-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 44, 1463–1472. 10.1007/s00259-017-3702-8. [DOI] [PubMed] [Google Scholar]
- Maina T.; Bergsma H.; Kulkarni H. R.; Mueller D.; Charalambidis D.; Krenning E. P.; Nock B. A.; de Jong M.; Baum R. P. (2016) Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 43, 964–973. 10.1007/s00259-015-3232-1. [DOI] [PubMed] [Google Scholar]
- Kahkonen E.; Jambor I.; Kemppainen J.; Lehtio K.; Gronroos T. J.; Kuisma A.; Luoto P.; Sipila H. J.; Tolvanen T.; Alanen K.; et al. (2013) In Vivo Imaging of Prostate Cancer Using [68Ga]-Labeled Bombesin Analog BAY86–7548. Clin. Cancer Res. 19, 5434–5443. 10.1158/1078-0432.CCR-12-3490. [DOI] [PubMed] [Google Scholar]
- Wieser G.; Mansi R.; Grosu A. L.; Schultze-Seemann W.; Dumont-Walter R. A.; Meyer P. T.; Maecke H. R.; Reubi J. C.; Weber W. A. (2014) Positron Emission Tomography (PET) Imaging of Prostate Cancer with a Gastrin Releasing Peptide Receptor Antagonist--From Mice to Men. Theranostics 4, 412–419. 10.7150/thno.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinares M.; Devin C.; Chaloin O.; Azay J.; Noel-Artis A. M.; Bernad N.; Fehrentz J. A.; Martinez J. (1999) Syntheses and Biological Activities of Potent Bombesin Receptor Antagonists. J. Pept. Res. 53, 275–283. 10.1034/j.1399-3011.1999.00028.x. [DOI] [PubMed] [Google Scholar]
- Varasteh Z.; Velikyan I.; Lindeberg G.; Sorensen J.; Larhed M.; Sandstrom M.; Selvaraju R. K.; Malmberg J.; Tolmachev V.; Orlova A. (2013) Synthesis and Characterization of a High-Affinity NOTA-Conjugated Bombesin Antagonist for GRPR-Targeted Tumor Imaging. Bioconjugate Chem. 24, 1144–1153. 10.1021/bc300659k. [DOI] [PubMed] [Google Scholar]
- Varasteh Z.; Mitran B.; Rosenstrom U.; Velikyan I.; Rosestedt M.; Lindeberg G.; Sorensen J.; Larhed M.; Tolmachev V.; Orlova A. (2015) The Effect of Macrocyclic Chelators on the Targeting Properties of the 68Ga-Labeled Gastrin Releasing Peptide Receptor Antagonist PEG2-RM26. Nucl. Med. Biol. 42, 446–454. 10.1016/j.nucmedbio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Mitran B.; Varasteh Z.; Selvaraju R. K.; Lindeberg G.; Sorensen J.; Larhed M.; Tolmachev V.; Rosenstrom U.; Orlova A. (2016) Selection of Optimal Chelator Improves the Contrast of GRPR Imaging Using Bombesin Analogue RM26. Int. J. Oncol. 48, 2124–2134. 10.3892/ijo.2016.3429. [DOI] [PubMed] [Google Scholar]
- Ginj M.; Zhang H.; Waser B.; Cescato R.; Wild D.; Wang X.; Erchegyi J.; Rivier J.; Macke H. R.; Reubi J. C. (2006) Radiolabeled Somatostatin Receptor Antagonists Are Preferable to Agonists for In Vivo Peptide Receptor Targeting of Tumors. Proc. Natl. Acad. Sci. U. S. A. 103, 16436–16441. 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescato R.; Maina T.; Nock B.; Nikolopoulou A.; Charalambidis D.; Piccand V.; Reubi J. C. (2008) Bombesin Receptor Antagonists May Be Preferable to Agonists for Tumor Targeting. J. Nucl. Med. 49, 318–326. 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- Yang M.; Gao H.; Zhou Y.; Ma Y.; Quan Q.; Lang L.; Chen K.; Niu G.; Yan Y.; Chen X. (2011) F-Labeled GRPR Agonists and Antagonists: A Comparative Study in Prostate Cancer Imaging. Theranostics 1, 220–229. 10.7150/thno/v01p0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Ananias H. J.; Carlucci G.; Hoving H. D.; Helfrich W.; Dierckx R. A.; Wang F.; de Jong I. J.; Elsinga P. H. (2013) An Update of Radiolabeled Bombesin Analogs for Gastrin-Releasing Peptide Receptor Targeting. Curr. Pharm. Des. 19, 3329–3341. 10.2174/1381612811319180015. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Niu G.; Wang F.; Chen X. (2009) 68Ga-Labeled NOTA-RGD-BBN Peptide for Dual Integrin and GRPR-Targeted Tumor Imaging. Eur. J. Nucl. Med. Mol. Imaging 36, 1483–1494. 10.1007/s00259-009-1123-z. [DOI] [PubMed] [Google Scholar]
- Lin M.; Welch M. J.; Lapi S. E. (2013) Effects of Chelator Modifications on 68Ga-Labeled [Tyr3]Octreotide Conjugates. Mol. Imaging Biol. 15, 606–613. 10.1007/s11307-013-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.; Yang J.; Gallazzi F.; Miao Y. (2011) Effects of the Amino Acid Linkers on the Melanoma-Targeting and Pharmacokinetic Properties of 111In-Labeled Lactam Bridge-Cyclized Alpha-MSH Peptides. J. Nucl. Med. 52, 608–616. 10.2967/jnumed.110.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi R.; Wang X.; Forrer F.; Waser B.; Cescato R.; Graham K.; Borkowski S.; Reubi J. C.; Maecke H. R. (2011) Development of a Potent DOTA-Conjugated Bombesin Antagonist for Targeting GRPr-Positive Tumours. Eur. J. Nucl. Med. Mol. Imaging 38, 97–107. 10.1007/s00259-010-1596-9. [DOI] [PubMed] [Google Scholar]
- Chen J.; Linder K. E.; Cagnolini A.; Metcalfe E.; Raju N.; Tweedle M. F.; Swenson R. E. (2008) Synthesis, Stabilization and Formulation of [177Lu]Lu-AMBA, a Systemic Radiotherapeutic Agent for Gastrin Releasing Peptide Receptor Positive Tumors. Appl. Radiat. Isot. 66, 497–505. 10.1016/j.apradiso.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Cagnolini A.; Chen J.; Ramos K.; Skedzielewski T. M.; Lantry L. E.; Nunn A. D.; Swenson R. E.; Linder K. E. (2010) Automated Synthesis, Characterization and Biological Evaluation of [68Ga]Ga-AMBA, and the Synthesis and Characterization of natGa-AMBA and [67Ga]Ga-AMBA. Appl. Radiat. Isot. 68, 2285–2292. 10.1016/j.apradiso.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Linder K. E.; Metcalfe E.; Arunachalam T.; Chen J.; Eaton S. M.; Feng W.; Fan H.; Raju N.; Cagnolini A.; Lantry L. E.; et al. (2009) In Vitro and In Vivo Metabolism of Lu-AMBA, a GRP-Receptor Binding Compound, and the Synthesis and Characterization of Its Metabolites. Bioconjugate Chem. 20, 1171–1178. 10.1021/bc9000189. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Yang M.; Gao H.; Niu G.; Yan Y.; Lang L.; Kiesewetter D. O.; Chen X. (2012) Evaluation of Fluorine-Labeled Gastrin-Releasing Peptide Receptor (GRPR) Agonists and Antagonists by LC/MS. Amino Acids 43, 1625–1632. 10.1007/s00726-012-1238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina T.; Kaloudi A.; Valverde I. E.; Mindt T. L.; Nock B. A. (2017) Amide-to-Triazole Switch vs. In Vivo NEP-Inhibition Approaches to Promote Radiopeptide Targeting of GRPR-Positive Tumors. Nucl. Med. Biol. 52, 57–62. 10.1016/j.nucmedbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Nock B. A.; Maina T.; Krenning E. P.; de Jong M. (2014) ″To Serve and Protect″: Enzyme Inhibitors as Radiopeptide Escorts Promote Tumor Targeting. J. Nucl. Med. 55, 121–127. 10.2967/jnumed.113.129411. [DOI] [PubMed] [Google Scholar]
- Shipp M. A.; Tarr G. E.; Chen C. Y.; Switzer S. N.; Hersh L. B.; Stein H.; Sunday M. E.; Reinherz E. L. (1991) CD10/Neutral Endopeptidase 24.11 Hydrolyzes Bombesin-Like Peptides and Regulates the Growth of Small Cell Carcinomas of the Lung. Proc. Natl. Acad. Sci. U. S. A. 88, 10662–10666. 10.1073/pnas.88.23.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani M.; Maecke H. R. (2012) Radiopharmaceutical Development of Radiolabelled Peptides. Eur. J. Nucl. Med. Mol. Imaging 39 (1), 11–30. 10.1007/s00259-011-2001-z. [DOI] [PubMed] [Google Scholar]
- Mascarin A.; Valverde I. E.; Vomstein S.; Mindt T. L. (2015) 1,2,3-Triazole Stabilized Neurotensin-Based Radiopeptidomimetics for Improved Tumor Targeting. Bioconjugate Chem. 26, 2143–2152. 10.1021/acs.bioconjchem.5b00444. [DOI] [PubMed] [Google Scholar]
- Valverde I. E.; Vomstein S.; Mindt T. L. (2016) Toward the Optimization of Bombesin-Based Radiotracers for Tumor Targeting. J. Med. Chem. 59, 3867–3877. 10.1021/acs.jmedchem.6b00025. [DOI] [PubMed] [Google Scholar]
- Lang L.; Ma Y.; Kiesewetter D. O.; Chen X. (2014) Stability Analysis of Glutamic Acid Linked Peptides Coupled to NOTA Through Different Chemical Linkages. Mol. Pharmaceutics 11, 3867–3874. 10.1021/mp400706q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Park R.; Hou Y.; Tohme M.; Shahinian A. H.; Bading J. R.; Conti P. S. (2004) MicroPET and Autoradiographic Imaging of GRP Receptor Expression with 64Cu-DOTA-[Lys3]Bombesin in Human Prostate Adenocarcinoma Xenografts. J. Nucl. Med. 45, 1390–1397. [PubMed] [Google Scholar]