Abstract
The MEK/ERK pathway is found to be important in regulating different biological processes such as proliferation, differentiation and survival in a wide variety of cells. However, its role in self‐renewal of haematopoietic stem cells is controversial and remains to be clarified. The aim of this study was to understand the role of MEK/ERK pathway in ex vivo expansion of mononuclear cells (MNCs) and purified CD34+ cells, both derived from human umbilical cord blood (hUCB). Based on our results, culturing the cells in the presence of an inhibitor of MEK/ERK pathway—PD0325901 (PD)—significantly reduces the expansion of CD34+ and CD34+ CD38− cells, while there is no change in the expression of stemness‐related genes (HOXB4, BMI1). Moreover, in vivo analysis demonstrates that PD reduces engraftment capacity of ex vivo expanded CD34+ cells. Notably, when ERK pathway is blocked in UCB‐MNCs, spontaneous erythroid differentiation is promoted, found in concomitant with increasing number of burst‐forming unit‐erythroid colony (BFU‐E) as well as enhancement of erythroid glycophorin‐A marker. These results are in total conformity with up‐regulation of some erythroid enhancer genes (TAL1, GATA2, LMO2) and down‐regulation of some erythroid repressor genes (JUN, PU1) as well. Taken together, our results support the idea that MEK/ERK pathway has a critical role in achieving the correct balance between self‐renewal and differentiation of UCB cells. Also, we suggest that inhibition of ERK signalling could likely be a new key for erythroid induction of UCB‐haematopoietic progenitor cells.
Keywords: MEK/ERK pathway, human umbilical cord blood (hUCB), haematopoietic stem cells (HSCs), ex vivo expansion, erythroid differentiation
Introduction
Haematopoietic stem cells (HSCs), the most recognized stem cells in the field of cell therapy, have been used in clinic for the last three decades 1. These cells are responsible for populating and sustaining the blood system through highly coordinated self‐renewal and differentiation process 2, 3. So far, extensive efforts have been made to identify the mechanisms controlling self‐renewal, differentiation and homing of HSCs 4. However, the key signalling molecules involved in determining the fate of these cells are not fully understood.
The extracellular signal‐regulated protein kinases 1 and 2 (ERK1/2) belong to the mitogen‐activated protein kinase (MAPK) super family that transmit signals from various cell surface receptors to cytosolic and nuclear targets 5. In a variety of cell types, the activation of RAS/MEK/ERK cascade leads to promoting the cell proliferation and survival 6, 7, 8. However, this is not the case for all cell types. Remarkably, the ERK1/2 signalling is dispensable for proliferation and self‐renewal of embryonic stem cells, whereas there is dependency on ERK upon lineage commitment 9, 10.
In haematopoietic system, in vivo analysis of ERK1−/− mice has revealed an essential function of ERK1 through thymocyte maturation 11. In addition, based on ex vivo studies, ERK pathway plays a critical role in regulating differentiation of megakaryocyte 12, erythrocyte 13, 14, macrophage 15, as well as granulocyte and monocyte 16, 17. Indeed, it seems that activation of ERK pathway may somehow act as a stimulus for HSCs to exit from the self‐renewal programme and enter into differentiation phase 18. Furthermore, there is more evidence that ERK1/2 signalling pathway may also be involved in regulation of other cellular processes of haematopoietic system 19.
The HSCs fate can be affected by time and duration of ERK activation as well as paracrine stimulations from other cells in developmental milieu. To understand more about the precise role of ERK signalling in HSCs fate determination, we used PD0325901 (PD) to block the MEK/ERK pathway in purified UCB‐CD34+ cells and their more commitment progenitors in UCB‐MNCs. The effect of ERK inhibition on cord blood cells was assessed after 10 days in serum‐free liquid cultures containing stem cell factor (SCF), Fms‐like tyrosine kinase 3 ligand (Flt3L) and thrombopoietin (TPO), in which the cells are in active expansion phase through proliferation and self‐renewal (Fig. S1).
Here, we show that ERK1/2 activation is required for the maintenance of HSCs self‐renewal and engraftment capacities. Further, according to our results, ERK inhibition by PD and consequently JUN hampering promotes the path of erythroid differentiation of MNCs.
Materials and methods
Cell culture
Cells were obtained from UCB samples of consenting mothers. Only cord blood samples were used which do not meet the criteria for banking at Royan Cord Blood Bank. Institutional human research ethics approval was also obtained from Royan institute ethic committee (IR.ACECR.ROYAN.REC.1394.175).
MNCs were isolated using 6% hydroxyethyl starch (HES) followed by LymphoprepTM (Stem cell Technology Inc., Canada) density gradient centrifugation. The purity of CD34+ cells was enriched to greater than 85% using CD34 immunomagnetic selection kit (Miltenyi Biotec, Germany). To ex vivo expansion of the cells, 106 MNCs or 104 CD34+ cells/well were cultured for 10 days in the StemSpanTM medium (Stem cell Technology Inc.) supplemented with 100 ng/ml SCF, 100 ng/ml Flt3L, 50 ng/ml TPO all from R&D Systems. To inhibit MEK1/2 pathway, PD0325901 (stemgent, USA) was added to expansion medium at the concentration of 0.25 μM. Every 3 days, half of the medium was replaced by the freshly prepared expansion medium.
Immunophenotyping
Phenotypic analysis of ex vivo expanded UCB cells was performed using flow cytometry. Cells were blocked with PBS‐BSA 1% and were stained with PE‐conjugated anti‐human CD34 and Percp anti‐human CD38 antibodies (BD Pharmingen, Germany). The negative population was determined using relevant isotype control antibodies. To remove blood cells, ammonium chloride lysing solution was added to stained leucocyte suspension. At least 104 events were acquired on a Partec PAS system (Germany), and data were analysed with FloMax software.
Colony‐forming assay
Colony‐forming unit (CFU) assays were performed using MethoCult (H4434, Stem Cell Technologies). Briefly, 2 × 103 MNCs or 300 CD34+ cells re‐suspended in 1.1 ml MethoCult diluted with IMDM + 2% FBS at a ratio of 1/10 and were plated in 35‐mm bacterial dishes. CFUs were scored 14–16 days following plating at 4× magnification and scored into three categories: granulocyte–monocyte (GM), granulocyte–erythrocyte–macrophage–megakaryocyte (GEMM), burst‐forming unit‐erythroid colony (BFU‐E).
Gene expression analysis
Cells were collected and preserved at −70°C until RNA extraction. Total RNA was isolated with QIAzol lysis reagent. Integrity and quality of RNA samples were checked using a Nanodrop (ND‐1000) spectrophotometer. 1 μg of the total RNA was subjected to reverse transcription using oligo‐dT and PrimeScript™ 1st strand cDNA kit (Takara, Japan). Transcript levels were determined using the SYBR Green master mix and Corbett Rotor‐Gene 6000. Gene expression level was normalized to the human GAPDH housekeeping gene. Relative quantification of gene expression relative to the positive control group was calculated using the ΔΔCt method. Primer sequences for qRT–PCR are listed in Table S1.
In‐utero mouse transplantation model
NMRI mice strains (8–10 weeks old) were obtained from the animal facility of Royan Institute. All mice were maintained in accord with guidelines approved by the animal welfare committee of the Royan Institute. Mice were mated under controlled conditions, and formation of vaginal plugs was used to assess pregnancy and gestational age. Pregnant mice were anaesthetized with isoflurane on gestational days 11.5‐13.5, and the uterine horns were exteriorized. Each embryo was injected intraperitoneally with 3–5 × 104 cells in 50 μl PBS. Control animals received only 50 μl PBS. The uterine horns were replaced in the abdomen, followed by abdominal closure. The mothers were kept warm until they recovered from anaesthesia. The ethical committee of Royan Institute approved all procedures (Fig. S2).
Identification of donor cell engraftment
Newborn mice were expanded with subcutaneous injections of human IL3 (4 ng/g), SCF (4 ng/g) and G‐CSF (50 ng/g), three times a week beginning at 2 weeks of age. For analysing haematopoietic engraftment, 1 week later, the peripheral blood samples of newborn mice were labelled with PE Anti‐Human CD45 (BD Pharmingen™). To delete non‐specific background signal, the cells were stained with the appropriate isotype‐matched control and at least 105 events were acquired on a Partec PAS system. Data were analysed with FloMax software. Moreover, bone marrow smears were prepared, air‐dried and fixed with ice‐cold acetone and subjected for human nuclear antigen staining using specific antibody.
In vitro migration assay
StemSpan medium and 100 ng/ml stromal cell‐derived factor‐1 (SDF‐1; R&D Systems) were placed into the lower chamber of a 24‐well Transwell (Corning, Corning). Freshly isolated or 10‐day expanded CD34+ cells (105) in 100 μl medium were loaded into the upper chamber over a porous membrane (pore size, 5 μm). After 4‐hr incubation at 37°C, the upper chamber was removed, and the cells in the bottom were collected and counted. Control experiments were performed without SDF‐1 in the lower chamber.
Protein–protein interaction analysis
To identify protein–protein interaction (PPI) and gene correlation, Expression2Kinases (X2K) software packages including Gene2Network and Gene2List (Version 1.6.1207, Mount Sinai School of Medicine, http://www.maayanlab.net/X2K) were used. The methods and databases were in compliance with the software protocol. The output results were optimized by means of yED works Software (yWorks GmbH, Germany, version 3.16.2.1). Then, the correlation and interaction between selected proteins were analysed by Pathway common‐PCvis software (http://www.pathwaycommons.org/pcviz). The linked proteins to CD34 in visualized network were mapped in detail in terms of their role in erythroid differentiation by applying protein–protein interaction software STRING (http://string-db.org/).
Statistical analysis
All the data were presented as mean ± S.D. of at least three different biological replicates. Statistical comparisons between the groups were examined by two‐tailed Student's t‐test assuming unequal variances. P < 0.05 was considered statistically significant difference.
Results
ERK1/2 pathway is critical for in vitro proliferation of UCB cells
To determine whether the MEK/ERK signalling pathway has an essential function in regulating the proliferation process of cord blood cells, an ex vivo expansion system was used. UCB‐CD34+ cells and UCB‐MNCs were cultured for 10 days in the presence of SCF, TPO and FLT3L (STF medium) to induce cell proliferation. To determine the cytotoxic effect of PD0325901 on UCB cells, the cells were cultured in the presence of different concentrations of PD (0–4 μM) for 48 hrs. As shown in Figure 1A, PD reduced dose‐dependently cell viability. Therefore, we chose the concentration of 0.25 μM that was not toxic for the cells. In the presence of selected dose of PD, the number of UCB‐MNCs and UCB‐CD34+ cells significantly reduced (1.4‐fold in UCB‐MNCs, P < 0.05; and 2.2‐fold in UCB‐CD34+ cells; P < 0.01) (Fig. 1B and C). As shown in Figure 1D, although the expansion of CD34+ cells was superior as we expected, the modest effect of PD was observed in MNCs, which include the more commitment cells. Moreover, PD‐expanded cells changed morphologically. As seen in Figure 1E, the large cytoplasm with side nucleus suggested the myeloblast differentiation of PD‐expanded MNCs as well as PD‐expanded CD34+ cells.
Figure 1.
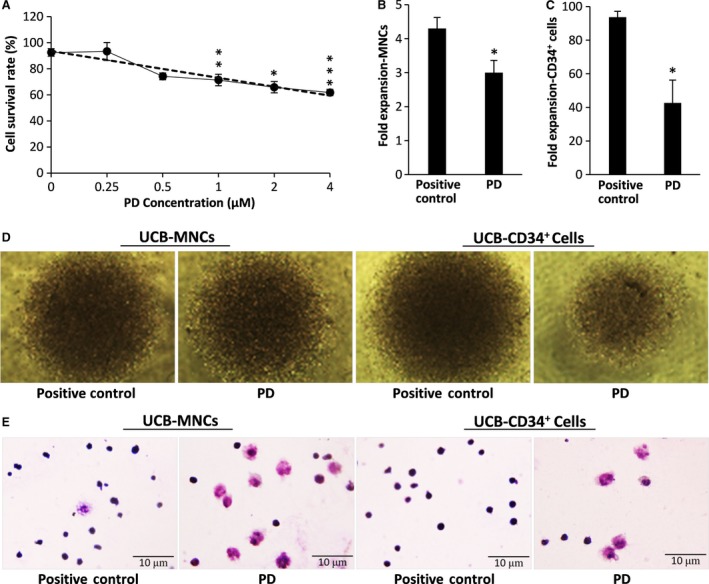
(A) UCB‐MNC cells were cultured in cytokine containing medium in the presence of indicated concentrations of PD0325901 for 48 hrs. Then, cell viability was analysed by MTS assay. Results indicated that PD decreased viable cells, dose dependently. (B) Effect of PD on the fold expansion of UCB‐MNCs. (C) Effect of PD on the fold expansion of UCB‐CD34+ cells. Fold expansion was determined by dividing the total number of viable cells after 10 days of culture with cytokines in the presence or absence of PD, by the number of viable cells in the first day of culture. Each bar represents the fold expansion of total nucleated cells (TNCs) per well. n = 3, statistically significant difference compared with positive control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (D) Phase‐contrast microscopy of proliferating cells cultured in 96‐well plate for 10 days (described in B and C). (E) Giemsa staining images of proliferating cells cultured for 10 days (described in B and C).
ERK1/2 pathway tunes self‐renewal and differentiation of UCB cells
In the next step, we sought the effect of PD0325901 on the self‐renewal and differentiation potential of UCB cells. The percentage of CD34+ and CD34+ CD38− cells were analysed either in the presence or absence of PD. In first day of experiment, the percentage of CD34+ cells was 1.4% in UCB‐MNCs and 90% in UCB‐CD34 purified cells (Fig. S3). Our results showed that under identical culture condition, 10.9 ± 4% of expanded UCB‐MNCs (Fig. 2A) and 18.3 ± 2% of expanded UCB‐CD34+ cells (Fig. 2E) were positive for CD34. These numbers significantly declined to 3.9 ± 1.3% and 8 ± 1.8% in the PD‐expanded respective cells. Similarly, cultures containing PD had a lower percentage of CD34+ CD38− cells compared with control (6.6 ± 3.6% versus 3.0 ± 0.8% in MNCs and 17.5 ± 1.8% versus 7.0 ± 1.5% in CD34+ cells). Furthermore, the fold expansion of primitive CD34+ cells and CD34+CD38‐ cells analysed in comparison with their initial seeding numbers, confirmed that PD0325901 resulted in huge reduction in numbers of UCB cells either in MNCs or CD34+ cells (Fig. 2B and F).
Figure 2.
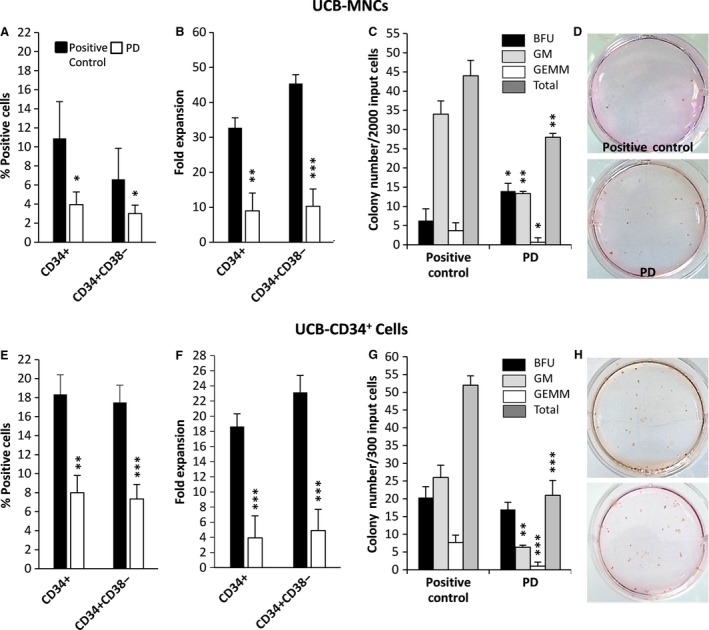
(A) The percentage of CD34+ and CD34+ CD38− cells in UCB‐MNCs and (E) UCB‐CD34+ cells, expanded for 10 days by cytokines in the presence or absence of PD. (B) The fold expansion of CD34+ and CD34+ CD38− cells in UCB‐MNCs and (F) UCB‐CD34+ cells expanded for 10 days by cytokines in the presence or absence of PD. Fold expansion was determined by dividing the total number of viable cells expressing the phenotype at the end of the culture by the input number of viable cells expressing the same phenotype (n = 3). Classification and number of colony‐forming units (CFUs) in (C) 2000 progeny of UCB‐MNCs and (G) 300 progeny of UCB‐CD34+ cells expanded for 10 days by cytokines in the presence or absence of PD, summarized from three separate samples (n = 6), statistically significant difference compared with positive control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (D, H) Photomicrograph of colonies developed in six‐well plates.
To assess the differentiation potential of cultured cells, the live cells were plated separately in CFU assays and haematopoietic colonies were counted after 14 days. PD0325901 significantly reduced colony‐forming efficiency either in UCB‐MNCs (Fig. 2C and D) or UCB‐CD34+ cells (Fig. 2G and H), mainly in CFU‐GM and CFU‐GEMM. However, the number of erythroid (BFU) colonies was increased, especially in UCB‐MNCs population (Fig. 2C). The increase in the expression of erythroid surface marker glycophorin‐A (CD235) also confirmed our data for promotion of erythroid differentiation in UCB‐MNCs (Fig. 3).
Figure 3.
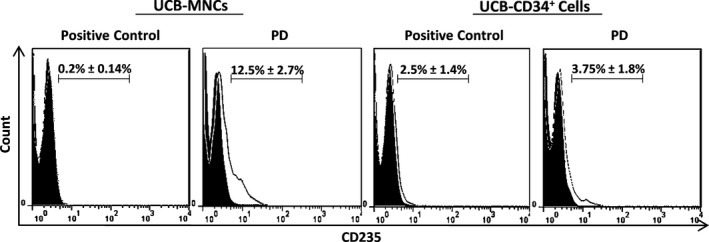
Representative flow cytometric analysis of expanded UCB‐MNCs and UCB‐CD34+ cells using monoclonal antibody for glycophorin‐A (CD235). The unfilled curve shows the percentage of CD235 positive cells and the grey filled represents the isotype control.
MEK/ERK inhibition interferes with the UCB cells development
The colony assay data revealed that inhibition of ERK pathway results in induction of erythroid differentiation which is dominant in MNCs as well as reduction of myeloid differentiation which is dominant in CD34+ cells. Therefore, we checked the key transcription factors involving in HSCs/HPCs development. As shown in Figure 4, the expression levels of HOXB4 and BMI1 genes—generally implicated in HSC self‐renewal 20, 21—were not significantly differ in purified CD34+ cells after treatment with PD, but significantly up‐regulated in PD‐expanded MNCs (Fig. 4A and C). However, OCT3/4—known recently as a gene involving in HSCs self‐renewal 22—was significantly under expressed in PD‐expanded mononuclear and CD34+ cells (Fig. 4B and D). Interestingly, TAL1, GATA1, LMO2 and FOG—the core factors of erythroid transcription complex 23—up‐regulated significantly (Fig. 4A and C) in PD‐expanded cells. Conversely, PU1 and JUN—the important repressors of erythroid differentiation programme 24, 25—significantly down‐regulated in PD‐expanded cells. However, RUNX1 was under expressed in CD34+ group and did not change in UCB‐MNCs (Fig. 4B and D).
Figure 4.
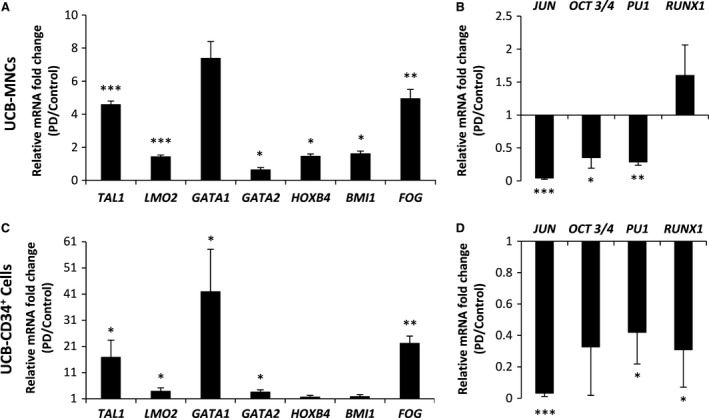
Treatment by PD modifies the gene expression of UCB‐MNCs (A, B) and UCB‐CD34+ cells (C, D). Bars represent the mean fold changes of gene expression in the PD‐expanded cells relative to the positive control group detected by quantitative real‐time PCR (n = 3), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus positive control.
Inhibition of MEK/ERK pathway reduces the engraftment of ex vivo expanded UCB‐CD34+ cells in immunodeficient mouse embryos
To find the effect of ERK inhibition on engraftment ability of cultured CD34+ cells, the short‐term engraftment capacity of fresh and cultured CD34+ cells was evaluated through in‐utero transplantation of immunodeficient NMRI embryos 26, 27, 28. Embryos (E11.5–E13.5) were transplanted with 30–50 × 103 primary non‐cultured CD34+ cells or their entire progeny following 10‐day culture in the presence or absence of PD. Human haematopoietic cell engraftment was assessed in three‐week age mice after one‐week subcutaneous injection of recombinant human growth factors SCF (4 ng/g), IL3 (4 ng/g) and G‐CSF (50 ng/g). As shown in Figure 5A, the average human cell engraftment in peripheral blood of the mice transplanted with freshly isolated CD34+ cells was about 0.75 ± 0.22%. The engraftment reduced to less than 0.3 ± 0.2% in mice transplanted with PD‐expanded CD34+ cells. In contrast, treatment of CD34+ cells with cytokine alone resulted in more than twofold (1.7 ± 0.4%) increase in human cell engraftment compared with the freshly isolated CD34+ cells.
Figure 5.
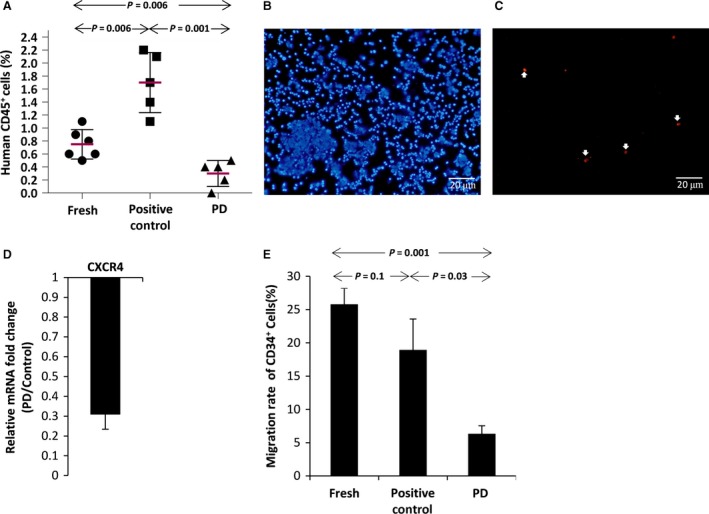
(A) Percentage of human CD45 positive cells in peripheral blood of newborn mice after one‐week treatment with human haematopoietic growth factors [SCF (4 ng/g), IL3 (4 ng/g) and G‐CSF (50 ng/g)]. (B) Immunocytochemistry to identify human cells in the bone marrow of newborn mice, DAPI stains nucleus (Blue), (C) human nuclear antibody (Red). (D) CXCR4 gene expression in PD‐expanded CD34+ cells relative to the positive control group detected by quantitative real‐time PCR (n = 3). (E) Percentage of CD34+ expanded cells migrated through the transwell in response to SDF‐1 as a chemoattractant factor.
Next, to determine the possible cause of poor engraftment ability of PD‐expanded CD34+ cells, we examined the effect of MEK/ERK inhibition on CXCR4 gene expression. The result showed significant down‐regulation of CXCR4 gene in the cells expanded with PD (Fig. 5D). Moreover, such cells showed less ability to migration towards SDF‐1 medium in comparison with the cells treated only with cytokines (Fig. 5E). So, based on our results, inhibition of MEK/ERK pathway can down‐regulate the CXCR4 expression in UCB CD34+ cells during ex vivo expansion, which may also contribute to the reduced engraftment ability of PD‐expanded cells.
Discussion
The vital role of MEK/ERK signalling in de novo generation of foetal hematopoietic stem cells as well as regulation of haematopoietic niche has been revealed before 19, 29. Also, ex vivo studies using differentiation‐competent cell lines have shown the importance of ERK signalling in the regulation of myeloid, erythroid, megakaryocyte and thymocyte differentiation 30. However, the precise function of MEK/ERK signalling in ex vivo self‐renewal and differentiation of haematopoietic stem cells remains to be understood. To explore this question, we evaluated the function of ERK pathway, during ex vivo expansion of purified UCB‐CD34+ cells and their more commitment progenitors in UCB‐MNCs. Based on our results, PD could significantly inhibit ex vivo proliferation and self‐renewal of both MNCs and CD34+ cells. Also, inhibition of ERK signalling by PD leads to spontaneous erythroid differentiation of UCB‐MNCs, which is in concomitant with overexpression of erythroid lineage transcription factors, the presence of more CD235+ cells and increasing number of BFU‐E colony in the population of PD‐expanded MNCs. This occurred despite using the effective combination of cytokines (SCF, FLT3L and TPO) to maintain the self‐renewal of HSCs 31. Therefore, it seems that ERK activation may play an important role in achieving the correct balance between the self‐renewal and differentiation of UCB‐MNCs.
In vivo studies have shown before that ERK1/2 activity is required for the maintenance of HSCs in a cell‐autonomous manner; and deletion of ERK1/2 leads to leucopenia, anaemia, and early lethality in mice 32, 33. Moreover, promotion of erythroid differentiation upon ERK inhibition has also been observed in avian immature erythroid progenitor cells. Likewise, constitutive activation of the ERK pathway prevents the cells exiting from the self‐renewal and entering a differentiation process 34. Some articles reported that an active form of MEK1 skews HSCs towards the granulocyte/macrophage lineage, specifically during myeloid commitment 16, 35, 36. Also, selective requirement for ERK activation through thymocyte differentiation has been shown before 37, 38. The activation of ERK signalling by GM‐CSF is also considered to be important for differentiation and maturation of macrophage and megakaryocytes 16, 17, 39, 40, 41, 42, 43. Otherwise, it has been reported that survival and proliferation of neutrophil progenitors but not their maturation are dependent to MEK/ERK activation 44. Similarly, ERK1/2 activation is not an essential requirement for growth and differentiation of leukaemic cell lines 45 and even can repress erythroid differentiation of several established cell lines 46, 47. These controversial functions of ERK function may be depend to the source of the target cells “including defined cell lines or purified HSCs derived from bone marrow/cord blood,” the exogenous cytokines in culture medium, the inactivation approach “gene modification or chemical inhibitors,” duration and level of ERK modification 48, 49.
Here, addition of PD0325901 to the culture was associated with increased transcript levels of GATA1, LMO2, TAL1 and FOG which are known to be the key transcription factors for normal erythroid differentiation. These proteins cooperate with each other to form a transcriptional complex which facilitates the GATA1‐mediated development of erythroid cells 50. An interesting question raised for us is how PD can promote erythroid differentiation of UCB‐MNCs. As GATA1 function is required for erythroid differentiation of human haematopoietic stem cells, the c‐Jun ability to repress GATA1 is most likely responsible for inhibition of erythroid differentiation by c‐Jun observed in HSCs 24. On the other hand, c‐Jun has long been recognized as a major downstream target of ERK signalling 51. Here, it seems that inhibition of ERK pathway by PD reversed the inhibition of erythroid differentiation by c‐Jun.
To further understand the correlation between the transcription factors and the pathways regulated by them, we used data processing software. The PPI network analysis emphasized on the role of c‐Jun and its correlation with other proteins contributed in haematopoiesis (Fig. S4). Unexpected PPI network results showed that JUN, indirectly through IL‐8 (Fig 6A) and HIF‐1A (Fig. 6B), plays an important role in the regulation of SDF‐1/CXCR4 signalling (Fig. 6C) 52, 53. It is well known that, the interaction between CXCR4 and SDF‐1 is a key signal, governing the homing of HSCs after transplantation 54. In this study, down‐regulation of CXCR4 gene upon ERK inhibition, may also contribute to the decreased engraftment ability of PD‐expanded CD34+ cells. This suggestion is partially supported by the finding that significantly less PD‐expanded CD34+ cells migrated in response to SDF‐1 as compared with the cells cultured without PD. Furthermore, JUN controls the expression of KLF4, STAT3 and c‐MYC genes which are involved in the cell cycle regulation 55, 56, 57. In summary, based on our results, JUN is most likely a key node that controls self‐renewal, differentiation and engraftment of UCB‐haematopoietic stem cells (Fig. 6D).
Figure 6.
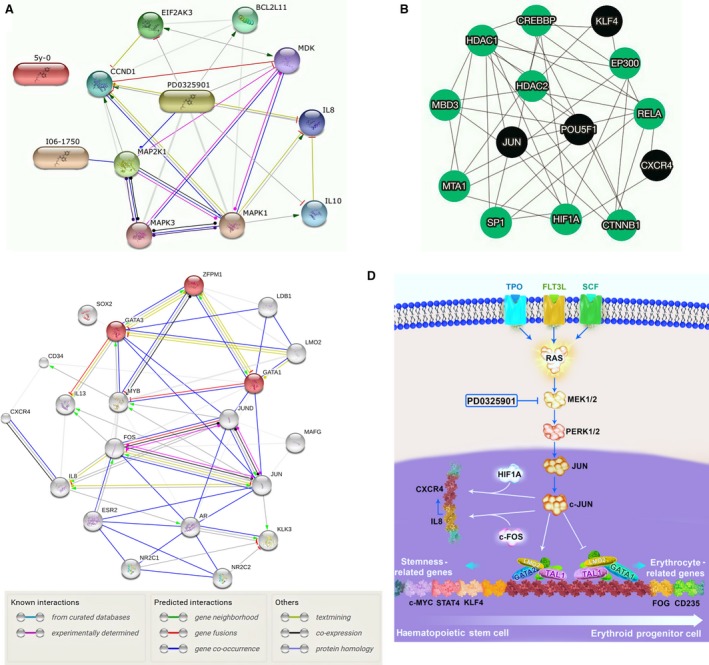
(A) String pathway to show the node of PD effects. (B, C) Visualization of protein–protein interaction using selected transcription factors in the present study and those which contribute in erythropoiesis. (D) Schematic illustration of PD effects on maintenance, differentiation and homing of HSCs.
Conclusion
Our results showed that the ERK1/2 signalling pathway plays an important role in the maintenance, proliferation and engraftment of UCB‐mononuclear and UCB‐CD34+ cells. Furthermore, according to our result, the cross‐talk between HSCs with other commitment cells in UCB‐MNC population, coupled with inhibition of ERK signalling could promote erythroid differentiation of the UCB‐haematopoietic progenitor cells. Also we suggest that erythroid induction of haematopoietic progenitor cells is primarily attributable to PD‐mediated inhibition of Jun activation.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting information
Figure S1 (A) Experimental design for investigation of ERk1/2 activity in ex vivo expansion of cord blood derived HSCs/PCs.
Figure S2 In‐utero transplantation of CD34+ cells into fetal peritoneal cavity as an immunodeficiency model. The handmade glass micropipette was used to inject cells into fetuses. (A) Preparation of the surgery site. (B) The uterine horns were exteriorized. (C) Each embryo was injected intra‐peritoneally with 30–50 × 103 cells in 50 μl PBS. (D) The uterine horns were replaced in the abdomen, followed by abdominal closure.
Figure S3 Flow cytometry analysis of CD34+ cells and CD34+ CD38− cells in UCB‐MNCs and UCB‐CD34+ cells at the first day of isolation and after 10 days of culture (PD‐expanded cells versus positive control group). Just one study selected for data presentation.
Figure S4 (A) Protein‐protein interaction of selected transcription factors obtained in this study and (B) suggested correlation by string.
Table S1 List of primers sequences used in this study
Acknowledgement
Funding source: This study has been funded by Royan Institute (Code: 91000597), and granted partly by Royan Stem Cell Technology Company. We thank the collaboration and the valuable help of Zahra Pour‐Safavi for flow cytometry experiment, Vahid Asghari for language assistance, Seyed Mehdi Hosseini and Scientific Illustration Service (http://sciellus.com/) for graphical picturing.
M.Z. and E.A. performed all in vitro experiments, analysed the data and wrote the manuscript; M.H.A. performed in vivo experiments; M.M. and H.A.E. analysed the data; M.E. designed the study, analysed the data and wrote the manuscript.
References
- 1. Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013; 122: 491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiao W, Wang W, Laurenti E, et al Intercellular network structure and regulatory motifs in the human hematopoietic system. Mol Syst Biol. 2014; 10: 741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Csaszar E, Kirouac DC, Yu M, et al Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012; 10: 218–29. [DOI] [PubMed] [Google Scholar]
- 4. Seita J, Weissman IL. Hematopoietic stem cell: self‐renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010; 2: 640–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wortzel I, Seger R. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer. 2011; 2: 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li B, Qu C, Chen C, et al Basic fibroblast growth factor inhibits osteogenic differentiation of stem cells from human exfoliated deciduous teeth through ERK signaling. Oral Dis. 2012; 18: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006; 58: 621–31. [DOI] [PubMed] [Google Scholar]
- 8. Tripathi P, Sahoo N, Ullah U, et al A novel mechanism for ERK‐dependent regulation of IL4 transcription during human Th2‐cell differentiation. Immunol Cell Biol. 2012; 90: 676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim MO, Kim SH, Cho YY, et al ERK1 and ERK2 regulate embryonic stem cell self‐renewal through phosphorylation of Klf4. Nat Struct Mol Biol. 2012; 19: 283–90. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton WB, Brickman JM. Erk signaling suppresses embryonic stem cell self‐renewal to specify endoderm. Cell Rep. 2014; 9: 2056–70. [DOI] [PubMed] [Google Scholar]
- 11. Pages G, Guerin S, Grall D, et al Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999; 286: 1374–7. [DOI] [PubMed] [Google Scholar]
- 12. Sevinsky JR, Whalen AM, Ahn NG. Extracellular signal‐regulated kinase induces the megakaryocyte GPIIb/CD41 gene through MafB/Kreisler. Mol Cell Biol. 2004; 24: 4534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woessmann W, Zwanzger D, Borkhardt A. ERK signaling pathway is differentially involved in erythroid differentiation of K562 cells depending on time and the inducing agent. Cell Biol Int. 2004; 28: 403–10. [DOI] [PubMed] [Google Scholar]
- 14. Matsuzaki T, Aisaki K, Yamamura Y, et al Induction of erythroid differentiation by inhibition of Ras/ERK pathway in a friend murine leukemia cell line. Oncogene. 2000; 19: 1500–8. [DOI] [PubMed] [Google Scholar]
- 15. Richardson ET, Shukla S, Nagy N, et al ERK signaling is essential for macrophage development. PLoS One. 2015; 10: e0140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miranda MB, Xu H, Torchia JA, et al Cytokine‐induced myeloid differentiation is dependent on activation of the MEK/ERK pathway. Leuk Res. 2005; 29: 1293–306. [DOI] [PubMed] [Google Scholar]
- 17. Miranda MB, McGuire TF, Johnson DE. Importance of MEK‐1/‐2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002; 16: 683–92. [DOI] [PubMed] [Google Scholar]
- 18. Bonati A, Lunghi P, Gammaitoni L, et al MEK‐ERK pathway is expressed but not activated in high proliferating, self‐renewing cord blood hematopoietic progenitors. Hematol J. 2002; 3: 105–113. [DOI] [PubMed] [Google Scholar]
- 19. Saulnier N, Guihard S, Holy X, et al ERK1 regulates the hematopoietic stem cell niches. PLoS One. 2012; 7: e30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwama A, Oguro H, Negishi M, et al Enhanced self‐renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi‐1. Immunity. 2004; 21: 843–51. [DOI] [PubMed] [Google Scholar]
- 21. Antonchuk J, Sauvageau G, Humphries RK. HOXB4‐induced expansion of adult hematopoietic stem cells ex vivo . Cell. 2002; 109: 39–45. [DOI] [PubMed] [Google Scholar]
- 22. Huang X, Lee MR, Cooper S, et al Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression. Leukemia. 2016; 30: 144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Love PE, Warzecha C, Li L. Ldb1 complexes: the new master regulators of erythroid gene transcription. Trends Genet. 2014; 30: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elagib KE, Xiao M, Hussaini IM, et al Jun blockade of erythropoiesis: role for repression of GATA‐1 by HERP2. Mol Cell Biol. 2004; 24: 7779–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang P, Zhang X, Iwama A, et al PU.1 inhibits GATA‐1 function and erythroid differentiation by blocking GATA‐1 DNA binding. Blood. 2000; 96: 2641–8. [PubMed] [Google Scholar]
- 26. Pixley JS, Zanjani ED, Shaft DM, et al Prolonged hematopoietic chimerism in normal mice transplanted in utero with human hematopoietic stem cells. Pathobiology. 1998; 66: 230–9. [DOI] [PubMed] [Google Scholar]
- 27. Pixley JS, Tavassoli M, Zanjani ED, et al Transplantation in utero of fetal human hematopoietic stem cells into mice results in hematopoietic chimerism. Pathobiology. 1994; 62: 238–44. [DOI] [PubMed] [Google Scholar]
- 28. Zanjani ED, Almeida‐Porada G, Ascensao JL, et al Transplantation of hematopoietic stem cells in utero. Stem Cells. 1997; 15(Suppl 1): 79–92; discussion 3. [DOI] [PubMed] [Google Scholar]
- 29. Kim AD, Stachura DL, Traver D. Cell signaling pathways involved in hematopoietic stem cell specification. Exp Cell Res. 2014; 329: 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009; 86: 237–50. [DOI] [PubMed] [Google Scholar]
- 31. Ema H, Takano H, Sudo K, et al In vitro self‐renewal division of hematopoietic stem cells. J Exp Med. 2000; 192: 1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan G, Gu S, Neel BG. Erk1 and Erk2 are required for maintenance of hematopoietic stem cells and adult hematopoiesis. Blood. 2013; 121: 3594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staser K, Park SJ, Rhodes SD, et al Normal hematopoiesis and neurofibromin‐deficient myeloproliferative disease require Erk. J Clin Invest. 2013; 123: 329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dazy S, Damiola F, Parisey N, et al The MEK‐1/ERKs signalling pathway is differentially involved in the self‐renewal of early and late avian erythroid progenitor cells. Oncogene. 2003; 22: 9205–16. [DOI] [PubMed] [Google Scholar]
- 35. Hsu CL, Kikuchi K, Kondo M. Activation of mitogen‐activated protein kinase kinase (MEK)/extracellular signal regulated kinase (ERK) signaling pathway is involved in myeloid lineage commitment. Blood. 2007; 110: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kondo M, Scherer DC, Miyamoto T, et al Cell‐fate conversion of lymphoid‐committed progenitors by instructive actions of cytokines. Nature. 2000; 407: 383–6. [DOI] [PubMed] [Google Scholar]
- 37. Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double‐negative to double‐positive thymocyte. Cell. 1996; 86: 243–51. [DOI] [PubMed] [Google Scholar]
- 38. Alberola‐Lla J, Forbush KA, Seger R, et al Selective requirement for MAP kinase activation in thymocyte differentiation. Nat Struct Mol Biol. 1995; 373: 620–3. [DOI] [PubMed] [Google Scholar]
- 39. Hu X, Moscinski LC, Valkov NI, et al Prolonged activation of the mitogen‐activated protein kinase pathway is required for macrophage‐like differentiation of a human myeloid leukemic cell line. Cell Growth Differ. 2000; 11: 191–200. [PubMed] [Google Scholar]
- 40. Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011; 49: 248–68. [DOI] [PubMed] [Google Scholar]
- 41. Rouyez MC, Boucheron C, Gisselbrecht S, et al Control of thrombopoietin‐induced megakaryocytic differentiation by the mitogen‐activated protein kinase pathway. Mol Cell Biol. 1997; 17: 4991–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hara T, Miyajima A. Function and signal transduction mediated by the interleukin 3 receptor system in hematopoiesis. Stem Cells. 1996; 14: 605–18. [DOI] [PubMed] [Google Scholar]
- 43. Fichelson S, Freyssinier JM, Picard F, et al Megakaryocyte growth and development factor‐induced proliferation and differentiation are regulated by the mitogen‐activated protein kinase pathway in primitive cord blood hematopoietic progenitors. Blood. 1999; 94: 1601–13. [PubMed] [Google Scholar]
- 44. Geest CR, Buitenhuis M, Groot Koerkamp MJ, et al Tight control of MEK‐ERK activation is essential in regulating proliferation, survival, and cytokine production of CD34+ ‐derived neutrophil progenitors. Blood. 2009; 114: 3402–12. [DOI] [PubMed] [Google Scholar]
- 45. Ajenjo N, Aaronson DS, Ceballos E, et al Myeloid leukemia cell growth and differentiation are independent of mitogen‐activated protein kinase ERK1/2 activation. J Biol Chem. 2000; 275: 7189–97. [DOI] [PubMed] [Google Scholar]
- 46. Miyazaki R, Ogata H, Kobayashi Y. Requirement of thrombopoietin‐induced activation of ERK for megakaryocyte differentiation and of p38 for erythroid differentiation. Ann Hematol. 2001; 80: 284–91. [DOI] [PubMed] [Google Scholar]
- 47. Racke FK, Lewandowska K, Goueli S, et al Sustained activation of the extracellular signal‐regulated kinase/mitogen‐activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem. 1997; 272: 23366–70. [DOI] [PubMed] [Google Scholar]
- 48. McCubrey JA, Steelman LS, Chappell WH, et al Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007; 1773: 1263–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009; 8: 1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki M, Moriguchi T, Ohneda K, et al Differential contribution of the Gata1 gene hematopoietic enhancer to erythroid differentiation. Mol Cell Biol. 2009; 29: 1163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lopez‐Bergami P, Huang C, Goydos JS, et al Rewired ERK‐JNK signaling pathways in melanoma. Cancer Cell. 2007; 11: 447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Asri A, Sabour J, Atashi A, et al Homing in hematopoietic stem cells: focus on regulatory role of CXCR7 on SDF1a/CXCR4 axis. EXCLI J. 2016; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rajagopal S, Kim J, Ahn S, et al β‐arrestin‐but not G protein‐mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci. 2010; 107: 628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma M, Afrin F, Satija N, et al Stromal‐derived factor‐1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2011; 20: 933–46. [DOI] [PubMed] [Google Scholar]
- 55. Chappell J, Dalton S. Roles for MYC in the establishment and maintenance of pluripotency. Cold Spring Harb Perspect Med. 2013; 3: a014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kidder BL, Yang J, Palmer S. Stat3 and c‐Myc genome‐wide promoter occupancy in embryonic stem cells. PLoS One. 2008; 3: e3932–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmidt R, Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012; 13: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) Experimental design for investigation of ERk1/2 activity in ex vivo expansion of cord blood derived HSCs/PCs.
Figure S2 In‐utero transplantation of CD34+ cells into fetal peritoneal cavity as an immunodeficiency model. The handmade glass micropipette was used to inject cells into fetuses. (A) Preparation of the surgery site. (B) The uterine horns were exteriorized. (C) Each embryo was injected intra‐peritoneally with 30–50 × 103 cells in 50 μl PBS. (D) The uterine horns were replaced in the abdomen, followed by abdominal closure.
Figure S3 Flow cytometry analysis of CD34+ cells and CD34+ CD38− cells in UCB‐MNCs and UCB‐CD34+ cells at the first day of isolation and after 10 days of culture (PD‐expanded cells versus positive control group). Just one study selected for data presentation.
Figure S4 (A) Protein‐protein interaction of selected transcription factors obtained in this study and (B) suggested correlation by string.
Table S1 List of primers sequences used in this study


