Abstract
Streptococcus mutans contributes significantly to dental caries, which arises from homoeostasic imbalance between host and microbiota. We hypothesized that Lactobacillus sp. inhibits growth, biofilm formation and gene expression of Streptococcus mutans. Antibacterial (agar diffusion method) and antibiofilm (crystal violet assay) characteristics of probiotic Lactobacillus sp. against Streptococcus mutans (ATCC 25175) were evaluated. We investigated whether Lactobacillus casei (ATCC 393), Lactobacillus reuteri (ATCC 23272), Lactobacillus plantarum (ATCC 14917) or Lactobacillus salivarius (ATCC 11741) inhibit expression of Streptococcus mutans genes involved in biofilm formation, quorum sensing or stress survival using quantitative real‐time polymerase chain reaction (qPCR). Growth changes (OD600) in the presence of pH‐neutralized, catalase‐treated or trypsin‐treated Lactobacillus sp. supernatants were assessed to identify roles of organic acids, peroxides and bacteriocin. Susceptibility testing indicated antibacterial (pH‐dependent) and antibiofilm activities of Lactobacillus sp. against Streptococcus mutans. Scanning electron microscopy revealed reduction in microcolony formation and exopolysaccharide structural changes. Of the oral normal flora, L. salivarius exhibited the highest antibiofilm and peroxide‐dependent antimicrobial activities. All biofilm‐forming cells treated with Lactobacillus sp. supernatants showed reduced expression of genes involved in exopolysaccharide production, acid tolerance and quorum sensing. Thus, Lactobacillus sp. can inhibit tooth decay by limiting growth and virulence properties of Streptococcus mutans.
Keywords: probiotic Lactobacillus, Streptococcus mutans, biofilm, dental caries
Introduction
Dental caries is a common chronic oral disease that can affect the health of adults and children 1. A number of studies demonstrated correlations between poor oral health and heart diseases 2, 3. Dental caries is an endogenous disease that results from homoeostatic imbalance between the host and microbiota 4. The shift of non‐pathogenic micro‐organism from commensalism to parasitism resulted from changes in the oral environment due to poor hygiene, smoking, systemic diseases and decrease in saliva flow 1, 5. Streptococcus mutans has been identified as a main contributor to dental caries 6.
The oral cariogenic biofilm formation occurs through phases that start by early colonization of pellicle by non‐mutans Streptococci. This phase creates a favourable area for the growth of Streptococcus mutans and initial biofilm formation 7. Streptococcus mutans possesses virulence factors that contribute to caries formation such as:
Production of acid that damages dental hard tissues 8;
An agmatine deiminase system and F‐ATPase encoded by the aguBDAC operon 9 and atpD gene 10, which are major components in acid‐adaptive response that contribute to the aciduric characteristics.
The ability to synthesize exopolysaccharides (EPS) from sucrose by the action of multiple glucosyltransferases (Gtfs) encoded by the genes gtfb, gtfc, gtfd, in addition to fructosyltransferase encoded by sacB (ftf) gene. The glucosyltransferase and fructosyltransferase enzymes catalyse the synthesis of extracellular glucan and fructan polymers from sucrose, respectively 11. The EPS formed is thought to play dual roles in promoting microbial adherence to surface in addition to protecting embedded bacteria 12. These virulence factors work under the control of quorum‐sensing systems. The two‐component signal transduction systems (TCSTS), including comCDE and vicRKX, are among the regulatory networks that regulate gene expression in response to stimuli from the surrounding environment and are thus essential for bacterial survival and virulence modulation 13, 14.
Caries management strategies include the use of conventional physical removal of plaque and the reduction of bacterial population by chlorhexidine. Other interventions include maintaining the oral ecosystem by probiotics 15. Probiotic bacteria are live micro‐organisms that can confer health benefits to the host when administered in sufficient amounts 16. Most probiotics are Gram‐positive bacteria that belong to the genera Lactobacillus or Bifidobacterium 17.
Lactobacilli (LB) constitute part of the oral microbiota and can be linked to the oral health status of the individual 18. They comprise about 1% of the cultivable oral microbiota. The most common LB strains isolated from oral microbiota include L. casei, L. paracasei, L. plantarum, L. rhamnosus, L. fermentum, L. acidophilus and L. salivarius 19. Isolated LB strains from subjects without dental caries have a significantly increased capacity to inhibit the growth of Streptococcus mutans compared with the strains isolated from subjects with active caries. Thus, probiotic LB does have a therapeutic anticaries potential 20, 21, 22. Stamatova and Meurman 23.
There could be universal mechanisms by which probiotics impact oral pathogens. Generally, probiotics are believed to compete with pathogens for space and nutrients but have mostly unknown mechanisms of action. These may include impacts on the production of lactic acid, peroxide or bacteriocin in addition to possible immunomodulatory activities 24. We hypothesized that Lactobacillus sp. inhibits the growth, biofilm formation and gene expression of Streptococcus mutans. We then studied the mechanisms by which the probiotic Lactobacillus sp. antagonizes Streptococcus mutans.
Materials and methods
Bacterial strains, media and growth conditions
Four Lactobacillus sp. namely: Lactobacillus casei subspecies casei (ATCC 393), Lactobacillus reuteri (ATCC 23272), Lactobacillus plantarum subspecies plantarum (ATCC 14917) and Lactobacillus salivarius (ATCC 11741) were selected to study their effect on Streptococcus mutans (ATCC 25175) isolated from carious dentine. Lactobacillus sp. and Streptococcus mutans were cultured in deMan, Rogosa and Sharpe (MRS) and brain–heart infusion (BHI) media (Oxoid, Hampshire, Thermo Fisher Scientific, UK), respectively, at 37°C under anaerobic conditions using Oxoid Anaerogen® sachets (Thermo Fisher Scientific, UK).
Preparation of spent culture supernatant (SCS)
The spent culture supernatant (SCS) for each Lactobacillus sp. strain was prepared according to Lin et al. 25, and then the supernatant was filtered using 0.45‐μm filters (Millipore, Bedford, MA, USA). The supernatant was divided into four portions. One portion was left untreated, and the other three portions were treated to eliminate the effect of organic acids, hydrogen peroxide and bacteriocin. The effect of organic acid was neutralized by adjusting the pH of SCS to 6.5 with 1 N NaOH. The other two portions were treated with 1 mg/ml trypsin (Sigma‐Aldrich, USA) and 0.5 mg/ml catalase (Sigma‐Aldrich, C1345, USA) to eliminate the effect of bacteriocin and hydrogen peroxide, respectively 26. Treated and untreated supernatants were stored at −20°C.
The agar diffusion method for antimicrobial screening of Lactobacillus sp
The antibacterial activity of Lactobacillus sp. on Streptococcus mutans was assessed using an agar diffusion method adapted from the one used by Cadirci and Citak 27. Streptococcus mutans was incubated in Brain–Heart Infusion (BHI) at 37°C for 24 hrs. Melted BHI agar medium held at 45°C was inoculated with Streptococcus mutans at a concentration equivalent to McFarland 0.5 standard (1.5 × 108 CFU/ml). Wells of 7 mm diameter were filled by 100 μl of SCS. Inhibition zones were measured in millimetres after incubating the plates anaerobically at 37°C for 24 hrs. The same test was performed using Lactobacillus sp. whole bacterial culture (WBC) instead of SCS, with a turbidity equivalent to McFarland 0.5.
Antibacterial testing of treated and untreated SCS
To determine the antibacterial activity of the SCS, Streptococcus mutans was grown overnight at 37°C in BHI broth. The Streptococcus mutans culture was diluted with BHI broth medium to a turbidity equivalent to McFarland 0.5 (1.5 × 108 cells/ml). Then, 100 μl of the Streptococcus mutans suspension and 100 μl of untreated supernatants were added to the wells of 96‐well microtitre plate in eight replicates for each Lactobacillus SCS (Greiner Bio‐One, KremsmÜnster, Austria). The plates were then incubated anaerobically at 37°C for 24 hrs. In control wells, the SCS was replaced by sterile MRS broth. The OD600 nm was recorded after incubation using microplate reader (Stat Fax®2100) 28. The same steps were repeated with treated supernatants to determine the change in antimicrobial activity after removing the effect of acidic pH, peroxides and bacteriocin.
The effect of Lactobacillus sp. SCS on Streptococcus mutans adherence
This test was performed in a similar manner as the antimicrobial test using BHI medium supplemented with 0.2% sucrose. After incubation, supernatants were removed, plates were stained, and reduction in biofilm formation was evaluated by crystal violet assay as previously described 29.
The effect of Lactobacillus sp. SCS on Streptococcus mutans preformed biofilm
An overnight culture of Streptococcus mutans was diluted to McFarland 0.5 in BHI supplemented with 0.2% sucrose. This culture was distributed in the 96‐well microtitre plate by the volume of 100 μl and incubated at 37°C for 24 hrs. Culture supernatant was removed, and wells were washed with sterile saline. A volume of 100 μl of untreated supernatant was added in each well and incubated at 37°C for 24 hrs. Reduction in biofilm formation was determined as previously described 29.
Scanning electron microscopy (SEM) observation of dual‐Streptococcus mutans–Lactobacillus sp. biofilm
Streptococcus mutans and Lactobacillus sp. were cocultured overnight at 37°C in BHI and MRS broth respectively followed by dilution to a concentration equivalent to McFarland 0.5. A clean sterile cover slide was added to the wells of the six‐well plate (Greiner Bio‐One, KremsmÜnster, Austria). In each well, 250 μl of the Streptococcus mutans suspension and 250 μl of one of the Lactobacillus sp. suspension were added to 1.5 ml of BHI broth (supplemented with 0.2% sucrose) and incubated anaerobically at 37°C for 24 hrs.
A monospecies culture of Streptococcus mutans biofilm was similarly prepared except that we replaced the Lactobacillus sp. culture with uncultured MRS medium. Cover slides were gently washed with phosphate‐buffered saline (PBS) once, fixed and prepared for SEM observation (JSM‐7600F, JEOL) according to a previously published protocol 30.
Extraction of total bacterial RNA
We studied the effect of Lactobacillus sp. filtered supernatant on Streptococcus mutans in the planktonic form and the biofilm form. Streptococcus mutans was grown overnight at 37°C in BHI broth and was diluted to McFarland 0.5. A volume of 250 μl Streptococcus mutans suspension and 250 μl of the SCS were added to 1.5 ml of BHI broth and were incubated anaerobically at 37°C for 24 hrs. In control wells, the Lactobacillus sp. supernatant was replaced by MRS broth 25. After incubation, culture suspension was removed from wells for RNA extraction from planktonic bacteria. Cells adhering to the plate wells were washed twice by sterile saline and then dislodged and suspended in saline by scraping into a centrifuge tube. The total RNA was isolated from Streptococcus mutans planktonic and adherent cells using Direct‐Zol RNA MiniPrep kit (Zymo Research, CA, USA) according to the manufacturer's instructions. The remaining DNA in RNA samples was treated by RNase‐free DNase I (New England Biolab, MA, USA) to eliminate DNA contamination. Agarose gel electrophoresis of RNA samples verified its integrity. RNA concentration and purity were determined by the ND‐1000 spectrophotometer (NanoDrop Technology; Wilmington, DE, USA). Finally, the SensiFast™ cDNA synthesis kit (Bioline, MA, USA) was used to reverse transcribe 1 μg of total RNA sample into cDNA.
Quantitative real‐time polymerase chain reaction (qRT‐PCR) and data analysis
Using qPCR, we examined the effect of Lactobacillus sp. spent supernatant on the expression levels of ten target genes [gtfb, gtfc, gtfd, sacB, comC, comD, vick, vicR, aguD and atpD] involved in glucan production, fructan production, quorum sensing and acid tolerance in Streptococcus mutans. The primers used for amplification of comC, comD and sacB (ftf) genes were designed using the complete genome sequence of Streptococcus mutans ATCC 25175 obtained from the NCBI database (GenBank accession no. PRJNA179256) and used as the base for primer design. Primers for the qPCR used in the current study (Table 1) were synthesized by Invitrogen (Massachusetts, USA). Quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR) was performed by Applied Biosystems StepOne™ Instrument using SensiFast™ SYBR Hi‐Rox Master (Bioline, Massachusetts. USA). All reactions (20 μl) were performed using three technical replicates. Each reaction mixture contained 100 ng cDNA and 400 nM primers per reaction. The RT‐PCR cycling conditions were as follows: one cycle with 95°C for 2 min.; then 40 cycles of denaturation at 95°C for 5 sec., annealing at 52–62°C (depending on primers used) for 10 sec., and extension and fluorescent data collection at 72°C for 20 sec. A dissociation curve was generated at the end of each reaction. In all qPCR runs, negative controls without template were run in parallel. The 16s rRNA gene (housekeeping gene) was selected as the internal control based on the results of BestKeeper® software tool 31. The relative mRNA levels of genes of interest were determined and normalized to the expression of the housekeeping gene using the ∆∆CT value analysis 32. The qPCR data were expressed as the fold change in expression levels of genes in Streptococcus mutans ATCC 25175 cells exposed to SCS of the four tested Lactobacillus sp. as compared to their levels in the untreated cells (calibrators). The changes in gene expression were tested in the Streptococcus mutans cells in the planktonic form and the biofilm‐forming state.
Table 1.
List of oligonucleotide sequences, their annealing temperature and amplicon size
| Target genea | Oligonucleotide sequence 5′–3′ | Ta (°C) | Amplicon size (bp) | References |
|---|---|---|---|---|
| gtfb |
For. ACGAACTTTGCCGTTATTGTCA Rev. AGCAATGCAGCCAATCTACAA |
52 | 96 | 74 |
| gtfc |
For. CTCAACCAACCGCCACTGTT Rev. GGTTTAACGTCAAAATTAGCTGTATTAG |
52 | 136 | 74 |
| gtfd |
For. TGTCTTGGTGGCCAGATAAAC Rev. GAACGGTTTGTGCAGCAAGG |
62 | 132 | 74 |
| sacB (ftf) |
For. CCTGCGACTTCATTACGATTGGTC Rev. ATTGGCGAACGGCGACTTACTC |
62 | 103 | This study |
| comC |
For. TATCATTGGCGGAAGCGGAA Rev. TCCCCAAAGCTTGTGTAAAACT |
56 | 74 | This study |
| comD |
For. CGCGATTGGAGCCTTTAG Rev. CCTGAAATTCAGTTAGCCTTT |
52 | 133 | This study |
| vicK |
For. CACTTTACGCATTCGTTTTGCC Rev. CGTTCTTCTTTTTCCTGTTCGGTC |
56 | 102 | 65 |
| vicR |
For. CGCAGTGGCTGAGGAAAATG Rev. ACCTGTGTGTGTCGCTAAGTGATG |
56 | 157 | 65 |
| aguD |
For. ATCCCGTGAGTGATAGTATTTG Rev. CAAGCCACCAACAAGTAAGG |
56 | 80 | 63 |
| atpD |
For. CGTGCTCTCTCGCCTGAAATAG Rev. ACTCACGATAACGCTGCAAGAC |
62 | 85 | 63 |
| 16s rRNAb |
For. CCTACGGGAGGCAGCAGTAG Rev. CAACAGAGCTTTACGATCCGAAA |
52 | 101 | 75 |
Ta: annealing temperature.
gtfb, encoding glucosyltransferase I; gtfC, glucosyltransferase SI; gtfD, glucosyltransferase S; sacB(ftf), encoding levansucrase enzyme (fructosyltransferase); comC, competence stimulating peptide; comD, Putative histidine kinase of the competence regulon; vicK, Putative histidine kinase CovS VicK‐like protein; vicR, Putative response regulator CovR VicR‐like protein; aguD, Agmatine: putrescine antiporter; atpD, F‐ATPase beta‐subunit; 16s rRNA, 16s ribosomal RNA gene sequence.
16s gene was used as an internal control.
Immunomodulatory effect of probiotic Lactobacillus sp
Human peripheral blood mononuclear cells (hPBMCs) from healthy volunteers were treated with SCS of Lactobacillus sp. as previously described by Wu et al. 30. The concentrations of IFN‐γ and IL‐10 were determined using enzyme‐linked immunosorbent assay (ELISA) according to the manufacturer's instructions (CUSABIO, BIOTECH CO, USA). A written consent was obtained from each subject. The protocol was approved by the Ethics Committee of the Faculty of Pharmacy, October University for Modern Sciences and Arts.
Statistics
Experimental results were analysed for statistical significance using GraphPad Prism (GraphPad, San Diego, CA, USA). A one‐way analysis of variance (ANOVA) was performed. Data comparisons were performed using either Dunnett's multiple comparison test or Tukey's multiple comparison test.
Results
Agar diffusion assay
The zone of inhibition produced by whole bacterial culture (WBC) (concentration 1.5 × 108 cells/ml) was larger than that produced by spent culture supernatant (SCS) produced by equivalent concentration of cells. This indicates the higher antimicrobial effect of WBC as compared to the cell‐free filtered supernatant. According to the zone of inhibition diameter, the highest antimicrobial activities of Lactobacillus sp. were observed with L. casei and L. reuteri, whereas the lowest antimicrobial activities were observed with L. plantarum and L. salivarius (Table 2).
Table 2.
Antimicrobial effect of Lactobacillus sp. whole bacterial culture and filtered supernatant on the growth of Streptococcus mutans
| Strain | Zone of inhibitiona (mm) | |
|---|---|---|
| Whole bacterial culture (WBC)b | Spent culture supernatant (SCS)b | |
| Lactobacillus casei | 23 ± 1 | 18 ± 1 |
| Lactobacillus reuteri | 23 ± 3 | 18 ± 2 |
| Lactobacillus plantarum | 19 ± 1 | 14 ± 1 |
| Lactobacillus salivarius | 19 ± 2 | 14 ± 1 |
The values are arithmetic means ± S.D. of inhibition zones (mm).
All results were significantly different from control (P < 0.01).
Antimicrobial effect of treated and untreated Lactobacillus sp. supernatant against Streptococcus mutans
The untreated supernatants of the four Lactobacillus sp. showed strong significant inhibitory effect (Fig. 1) on the growth of Streptococcus mutans (P < 0.01). There was no significant difference in the potency of the inhibitory effect between the four samples (P > 0.05). After neutralizing the supernatant acidity, the antimicrobial effect was significantly reduced (P < 0.01) compared with untreated supernatant, yet still showing significant reduction (P < 0.05) in Streptococcus mutans growth (Fig. 2A, B, C and D). Lactobacillus salivarius was the only tested strain that showed significant reduction (P < 0.05) in its antimicrobial effect on Streptococcus mutans after addition of catalase (Fig. 2D) indicating that peroxides contribute in its antimicrobial effect against Streptococcus mutans.
Figure 1.
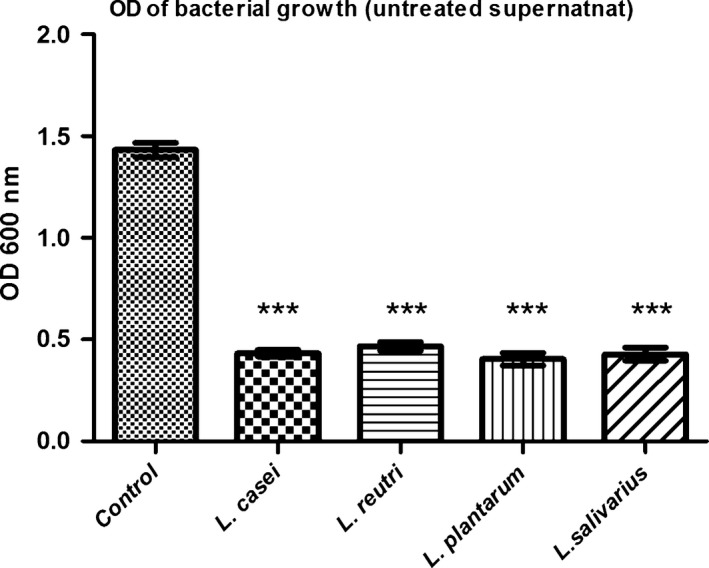
Streptococcus mutans growth in the presence of untreated Lactobacillus sp. supernatant. Optical density (OD) of Streptococcus mutans growth in the presence of untreated Lactobacillus sp. supernatants (L. casei, L. reuteri, L. plantarum and L. salivarius). Control: Streptococcus mutans growth in BHI broth. Untreated: spent culture supernatant (SCS). Data are expressed as the mean ± S.D., ***P < 0.01 compared with Streptococcus mutans growth in BHI broth as control (Dunnett's multiple comparison test).
Figure 2.
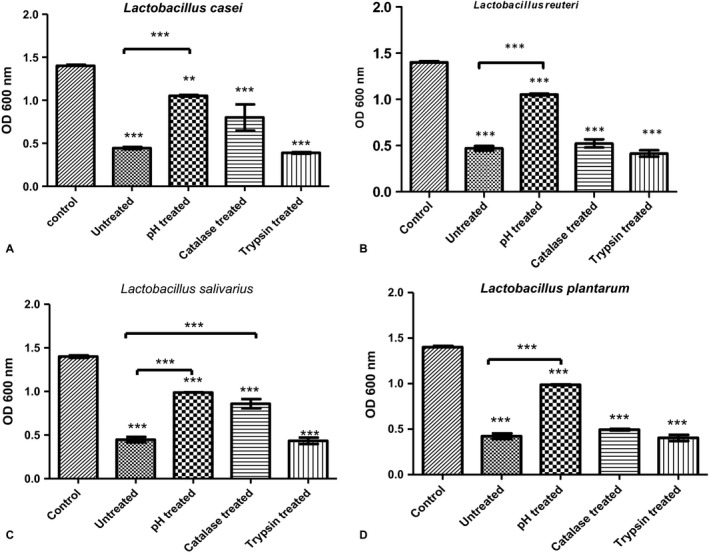
Streptococcus mutans growth in the presence of treated and untreated Lactobacillus sp. supernatant. Optical density (OD) of Streptococcus mutans growth in the presence of treated and untreated Lactobacillus sp. supernatants (L. casei, L. reuteri, L. plantarum and L. salivarius). Control: Streptococcus mutans grown in BHI broth. Untreated: Spent Culture Supernatant (SCS) of each strain supernatant, pH treated: supernatant with adjusted pH 6.5, catalase treated: supernatant after addition of 0.5 mg/ml catalase enzyme and trypsin treated: supernatant after addition of 1 mg/ml trypsin enzyme. Data are expressed as the mean ± S.D., **P < 0.05 and ***P < 0.01 compared with Streptococcus mutans grown in BHI broth as control (Tukey's multiple comparison test).
Effect of Lactobacillus sp. filtered supernatants on Streptococcus mutans adherence and preformed biofilm
Lactobacillus salivarius supernatant caused significant reduction (P < 0.01) in Streptococcus mutans adherence and preformed biofilm. Reduction percentages were 87% and 47%, respectively. The effect of L. casei supernatant was the least among tested supernatants on adherence as it showed no significant effect on the preformed biofilm. The L. plantarum and L. reuteri supernatant caused reduction in adherence with percentages of 81.7–80.5% and reduction in preformed biofilm with percentage of 26.5–24.7% (Fig. 3).
Figure 3.
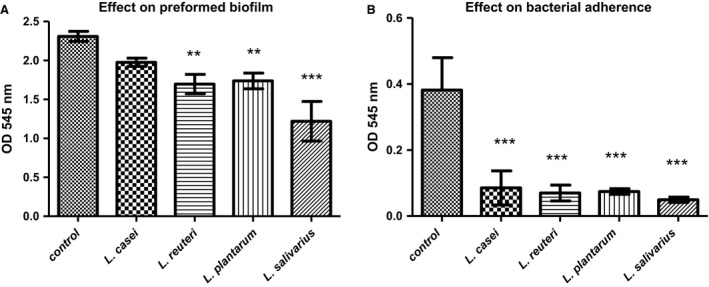
Effect of untreated supernatant on Streptococcus mutans (A) Effect of untreated supernatant on Streptococcus mutans adherence. (B) Effect of untreated supernatant on Streptococcus mutans preformed biofilm Optical density (OD 545 nm) of Streptococcus mutans biofilm in the presence of untreated Lactobacillus sp. supernatants (L. casei, L. reuteri, L. plantarum and L. salivarius). Control: Streptococcus mutans grown in BHI broth. Data are expressed as the mean ± S.D. **P < 0.05, ***P < 0.01 compared with control (Dunnett's multiple comparison test).
Scanning electron microscope
As shown in Figure 4, the Streptococcus mutans appeared to form a compact, island‐like biofilm covered by large amounts of slime or network‐like structures. Changes in exopolysaccharides (EPS) matrix structure and quantity were observed in biofilm formed by coculture of Streptococcus mutans and different Lactobacillus sp. strains. Moreover, we observed fewer bacteria and smaller microcolonies attached to the surface.
Figure 4.
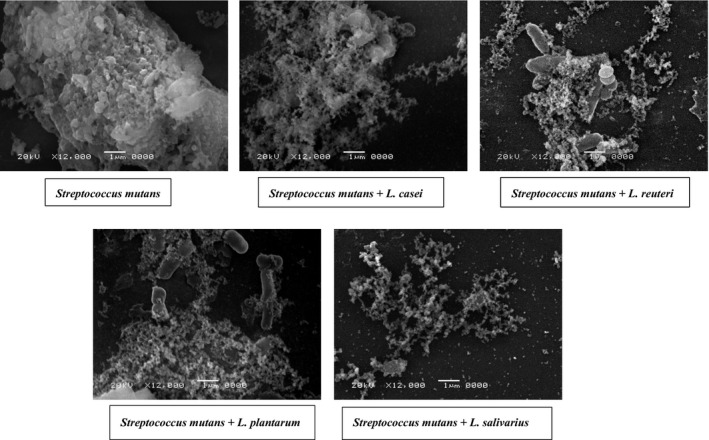
Scanning electron microscopy (SEM) of the biofilms. Streptococcus mutans was cocultured with Lactobacillus sp. as compared to Streptococcus mutans monoculture. The resulting biofilms were observed by SEM at 12,000× magnification.
Analysis of qPCR results
We used qPCR to evaluate and compare the impact on Streptococcus mutans ATCC 25175 cells after exposure to four Lactobacillus sp. SCS (diluted 1:8 in BHI) overnight. The levels of expression of ten genes, that have been previously shown to be involved in virulence of the S. mutans in the planktonic and biofilm‐forming cells, were compared to the control untreated cells prepared under the same conditions without tested SCS. The selected genes included four genes involved in the two‐component signal transduction systems (TCSTS) [comC, comD, vicK, vicR], four genes involved in EPS formation [three of which are involved in glucan formation (gtfB, gtfC and gtfD), one gene is involved in fructan formation (sacB (ftf))], and two genes associated with stress survival (aguD, and atpD).
As revealed by the one‐way ANOVA, there was an overall significant reduction (P < 0.01) in the expression of most of the tested genes among the different groups, in both planktonic forms and biofilm‐forming cells. Dunnett's multiple comparison test was used to assess the significance of the difference between gene expression levels in target genes of exposed and control groups. As shown in Figure 5, few genes showed no significant difference (P > 0.01) in expression as compared to the control under certain conditions. These genes are comC and gtfD in planktonic cells exposed to L. plantarum SCS, gtfC gene in planktonic cells exposed to L. salivarius SCS, and comC gene in the biofilm‐forming cells exposed to L. reuteri SCS.
Figure 5.
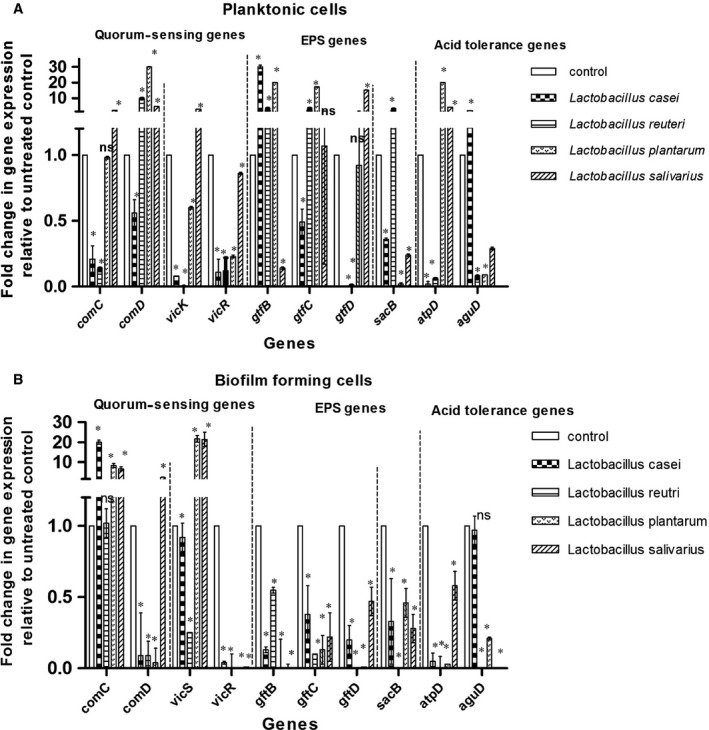
Alterations in gene expression profiles associated with exposure of Streptococcus mutans (ATCC 25175), in (A) planktonic form and (B) biofilm‐forming state, to the tested Spent culture supernatant (SCS) of Lactobacillus casei (ATCC 393), Lactobacillus reuteri (ATCC 23272), Lactobacillus plantarum (ATCC 14917) and Lactobacillus salivarius (ATCC 11741) as determined by qPCR. In each panel, fold change refers to the mean levels of gene expression across replicates, calculated using the ΔΔCt method relative to untreated control. Fold change = 2−ΔΔCt. Fold change (>1) indicates up‐regulation, (<1) indicates down‐regulation and fold change (~1) means insignificant change. Asterisks indicate statistically significant differences in the expression of each gene between treated samples and control, as analysed using the one‐way ANOVA with Dunnett's post‐testing for multiple testing (*P ≤ 0.01; ns, no significant difference). Error bars indicate standard deviation
The effect of SCS of different Lactobacillus sp. was variable on all tested TCSTS system genes. L. salivarius supernatant caused up‐regulation of the vicK gene by threefold to 21‐fold, in the planktonic and adherent cell forms, respectively. In planktonic cells, the expression of the comC gene, coding for competence‐stimulating peptide, was up‐regulated in the presence of L. salivarius supernatant only. Up‐regulation of the same gene was observed in biofilm‐forming cells treated with L. casei, L. plantarum and L. salivarius. The comD gene, coding for cognate histidine kinase receptor, was up‐regulated in the planktonic cells exposed to SCS of tested Lactobacillus sp. except for L. casei. On the other hand, it was down‐regulated in biofilm‐forming cells except those exposed to L. salivarius supernatant.
Significant reduction in gene expression of glucan (gtfB, gtfC, gtfD) and fructan (sacB) forming genes was observed in the adherent Streptococcus mutans cells in the presence of all tested SCS. The effects of the same supernatants were variable on the planktonic cells, as they showed significant up‐regulation (P < 0.01) in gtfB and gtfC genes in the following cases: high up‐regulation in gene expression levels in presence of the supernatants of L. casei (30‐fold change in gtfB gene expression), and L. plantarum (20‐fold and 17‐fold change in gtfB and gtfC gene expression, respectively); moderate up‐regulation in gene expression of gtfB and gtfC genes in the presence of L. reuteri (2.5‐fold) supernatant. Significant up‐regulation (P < 0.01) of the gtfD gene was observed in the presence of L. salivarius supernatant (15‐fold). Similarly, significant up‐regulation of the sacB (ftf) gene (2.5‐fold) was observed in the presence of L. reuteri supernatant.
Stress response genes (atpD and aguD) were down‐regulated in biofilm‐forming cells in the presence of all tested SCS. In planktonic forms, these two genes showed significant reduction (P < 0.01) in expression except in two cases: The first is the atpD gene in the presence of L. plantarum and L. salivarius supernatants, and second is the aguD gene in the presence of Lactobacillus casei.
Immunomodulatory activities of Lactobacillus sp
The SCS of Lactobacillus sp. was incubated with hPBMCs isolated from healthy volunteers for 48 hrs. The production levels of the immunostimulatory IFN‐γ and immunoregulatory IL‐10 cytokines were measured by ELISA. All Lactobacillus sp. standard strains stimulated hPBMCs to produce IFN‐γ higher than untreated controls. In contrast, IL‐10 concentrations were reduced after treating hPBMC with Lactobacillus sp. supernatants (Table 3).
Table 3.
Effect of filtered Lactobacillus supernatant on interferon‐γ (IFN‐γ) production and interleukin‐10 (IL‐10) production in human peripheral blood mononuclear cells (hPBMCs) using enzyme‐linked immunosorbent assay (ELISA)
| Sample | Cytokine concentration (pg/ml)a | |
|---|---|---|
| IFN‐γ Mean ± S.D. | IL‐10 Mean ± S.D. | |
| Control | 15 ± 1 | 78 ± 1 |
| L. casei | 23.1 ± 0.5 | 63.5 ± 0.4 |
| L. reuteri | 31.2 ± 0.3 | 51.4 ± 0.5 |
| L. plantarum | 54.2 ± 0.5 | 27.3 ± 0.64 |
| L. salivarius | 49.3 ± 0.4 | 38.7 ± 0.6 |
All results showed significant difference from control (P < 0.01).
Discussion
Dental caries is one of the most common diseases worldwide. The oral microbiota is composed of over 700 bacterial taxa 33. Under certain conditions, bacteria like Streptococcus mutans can be pathogenic and cause dental caries. Streptococcus mutans is a major contributor to dental caries development due to its virulence factors including the ability to synthesize extracellular polysaccharide and the ability to produce acidic metabolites 8.
Lactobacillus sp. constitute a main constituent of the microbiota in our oral cavity 34. Lactobacillus sp. probiotics have been proven to be efficient in treating certain gastrointestinal disorders 35. Lactobacillus sp. probiotics could possibly control dental caries using similar mechanisms that can play against Streptococcus mutans invasion strategies 8. This is because Lactobacillus sp. were shown to be able to produce organic acids, hydrogen peroxide, bacteriocins and adhesion inhibitors 36.
The Lactobacillus sp. used in this study were L. casei subspecies casei (ATCC 393), L. reuteri (ATCC 23272), L. plantarum subsp. Plantarum (ATCC 14917) and L. salivarius (ATCC 11741). These strains were chosen because they caused reduction in dental caries in previous studies including: Lactobacillus casei 37, 38, Lactobacillus reuteri 39, 40, 41, Lactobacillus plantarum 42 and Lactobacillus salivarius 43, 44. The precise mechanisms by which this happens are still unclear. Thus, the aim of the study was to assess mechanisms by which Lactobacillus sp. can control dental caries. We tested the effect of these four Lactobacillus sp. on the growth, adherence, biofilm formation and gene expression of Streptococcus mutans (ATCC 25175), in addition to the immunomodulatory effect.
The antimicrobial screening of the four tested strains of Lactobacillus using the agar diffusion method revealed differences in antimicrobial activity between different strains as determined by the size of the zone of inhibition. The highest effect was detected by L. casei, and L. reuteri, followed by L. salivarius and L. plantarum. Lactobacillus WBC caused higher antimicrobial effect on Streptococcus mutans than their corresponding SCS of the same Lactobacillus species. The difference in zone of inhibition caused by WBC compared to SCS may suggest that the presence of living metabolically active Lactobacillus sp. cells in WBC could result in the production of active antimicrobial agents in response to stimuli 45. The tested Lactobacillus sp. strains caused significant reduction in the microbial growth of Streptococcus mutans in BHI broth, as determined by the change in OD 600. At the same time, there was no significant difference between different Lactobacillus species regardless of their metabolic pattern. Strict homofermentative organisms such as Lactobacillus salivarius, facultative heterofermentative organisms such as Lactobactobacillus casei and Lactobacillus plantarum, and obligate heterofermentative organisms such as Lactobacillus reuteri showed similar antimicrobial effects.
To determine the effect of organic acids, hydrogen peroxide and bacteriocin produced by tested Lactobacillus sp., their effect was demolished by neutralization, catalase and trypsin addition, respectively. Neutralization of SCS to pH 6.5 significantly reduced the antimicrobial effect of the tested SCS. The low pH is an important factor for growth inhibition, and it is important for the production of bacteriocin 46. Streptococcus mutans is an acidogenic bacteria, that is produce organic acid as end product for sugar fermentation, and it is an aciduric bacteria, that is can tolerate acid in the plaque environment, hence, it can survive under acidic conditions 47. The acid tolerance genes, such as atpD and aguD genes, allow Streptococcus mutans to carry out metabolic processes at low‐pH values 48. The observed reduction in gene expression of atpD, aguD, in Streptococcus mutans, can decrease its acid tolerance, which can lead to bacteriostasis and eventual death 49. Anticaries agents such as the natural compounds α‐mangostin and catechin epigallocatechin gallate can down‐regulate the atpD and aguD genes 10, 50.
It was observed that neutralized SCS caused lower reduction in microbial growth than untreated SCS, but yet neutralized supernatant still showed significant reduction in Streptococcus mutans growth when compared to control. This suggests the influence of other antimicrobial agents such as hydrogen peroxide, bacteriocin, 51 and biosurfactant 52 that contribute with acid to growth inhibition.
Some Lactobacillus sp. have the ability to produce hydrogen peroxide, which can be toxic to organisms lacking hydrogen peroxide‐scavenging enzymes such as Streptococcus mutans 53. Adding catalase to Lactobacillus sp. supernatant caused reduction in the antimicrobial effect against Streptococcus mutans, but the significant reduction was observed only with Lactobacillus salivarius (ATCC 11741). This indicates that hydrogen peroxide contribution in antimicrobial activity of the tested Lactobacillus sp. is low except for L. salivarius supernatant.
Streptococcus mutans has been shown to initiate a response to various adverse environmental stressors, including oxidative stress, and acidic pH, by actively producing competence‐stimulating peptide (CSP) encoded by the comC gene 54. In our study, the expression of the comC was up‐regulated in biofilm‐forming cells compared with the untreated control. This was in contrast to comD which was down‐regulated in biofilm‐forming cells treated with L. casei, L. reuteri or L. plantarum. The antimicrobial testing of L. salivarius supernatant on Streptococcus mutans demonstrated the influence of supernatant pH and peroxide in the antimicrobial activity. Thus, the production of these stress factors by this strain might explain the significant up‐regulation in comC and comD genes in both the planktonic and biofilm‐forming Streptococcus mutans cells treated with this supernatant. Biofilm formed of Streptococcus mutans having single mutation in comC, comD and comE, or the triple mutation of comCDE showed different biofilm architecture in comparison with the wild‐type strain 55. This might explain the difference in biofilm formed by the coculture of Streptococcus mutans and Lactobacillus sp. as observed by SEM due to difference in comCDE expression.
Lactobacillus sp. can produce bacteriocin or bacteriocin‐like polypeptides that have a small molecular weight of <10 kD. In our study, trypsin‐treated supernatant showed no significant difference from the untreated SCS on Streptococcus mutans growth. This result indicates the low production of bacteriocin by Lactobacillus sp. This may not be the only explanation though because bacteriocin production by Lactobacillus sp. has been reported in several previous studies carried on L. casei 56, L. reuteri 57, L. plantarum 58 and L. salivarius 59.
Streptococcus mutans contributing to dental caries usually exists in the biofilm form inside the oral cavity. EPS of Streptococcus mutans contribute to dental caries by helping develop an oral biofilm in addition to forming a barrier against chemical agents. Therefore, therapeutic agents that target the biofilm can be the most suitable for dental caries prevention.
The cariogenic properties of Streptococcus mutans biofilms are regulated by various essential genes 60. Thus, the expression of representative biofilm‐associated genes was investigated. The genes studied included genes for sucrose‐dependent adhesion such as gtfb, gtfC 52 and sacB (ftf) 60. It also included two systems for controlling biofilms: (i) The vicRKX operon regulating the expression of virulence‐associated genes responsible for regulating the synthesis of polysaccharides, including gtfBCD, sacB, and polysaccharide‐binding sites as gbpB 61, (ii) comCDE quorum‐sensing system 48. In addition, it included genes for synthesis of insoluble glucan (gtfB, gtfC), soluble glucan (gtfD) 62 and fructan polymers (sacB) 61. Finally, it included genes responsible for acid tolerance (aguD and atpD) 63, 64.
The SCS of the four tested Lactobacillus sp. caused reduction in Streptococcus mutans biofilm with variable degrees. The highest reduction observed was with the supernatants of L. salivarius (87% for Streptococcus mutans adherence, and 47% for Streptococcus mutans preformed biofilm). The vicR gene was down‐regulated in planktonic forms and biofilm‐forming Streptococcus mutans exposed to all tested SCS. On the other hand, the vicK gene was down‐regulated upon the exposure of Streptococcus mutans to L. casei and L. reuteri supernatants. This down‐regulation might explain the reduction in Streptococcus mutans adherence and preformed biofilm as demonstrated by the SEM results. The vicKRX system has a significant influence on biofilm formation, and the null mutation in the vicK and vicR genes can cause aberrant biofilms which are easily removed 65. The vicRKX system regulates the glucosyltransferase‐encoding genes, and thus, mutation in this system can cause a significant decrease in gtfD gene expression, as well as increased expression of the gtfB gene 66. Changes in the gtfB and gtfD gene expressions, due to a mutated vicRKX system, were observed in planktonic Streptococcus mutans in the presence of L. casei, L. reuteri and L. plantarum supernatants, which caused a reduction in both vicK and vicR genes. In biofilm‐forming cells, both gtfB and gtfD genes were down‐regulated in the presence of all tested Lactobacillus sp. despite reduction in vicK and vicR genes. This could be attributed to the influence of factors other than vicKRX on the gtf genes such as: luxS (AI‐2 autoinducer‐coding synthesis), ropA (encoding for the trigger factor) and RegM (the catabolite‐repression regulator in Streptococcus mutans) 67, in addition to biosurfactants produced by Lactobacillus sp. which could reduce gtfB, gtfC and gtfD expressions in Streptococcus mutans 68.
Gtf genes that code for glucosyltransferase enzyme are primary virulence factors for Streptococcus mutans and thus can be a selective drug target for prevention of cariogenic biofilms. The Lactobacillus sp. supernatant‐induced altered gene expression indicates a promising anticaries effect. In vitro studies indicated that gtfB and gtfC were essential for the sucrose‐dependent attachment of Streptococcus mutans cells to hard surfaces and for microcolonies formation, but gtfD was not essential 52. In the biofilm‐forming bacteria, the expression of the three glucosyltransferase genes (gtfB, gtfC and gtfD) and the fructosyltransferase genes sacB (ftf) showed significant down‐regulation as compared to the control group of untreated biofilm‐forming cells. Glucosyltransferase S, encoded by gtfB, synthesizes insoluble glucan 67 and allows cell clustering 69. Thus, mutant Streptococcus mutans strains, defective in gtfB, are less cariogenic than their parent strains 70. The disruption of insoluble glucans synthesis can induce a reduction in biofilm formation, which can influence the pathogenesis 63. Thus, the gtfB expression reduction could explain the highest antibiofilm effect produced by L. salivarius on Streptococcus mutans adherence (87% reduction) and on preformed biofilm (47% reduction). The promising results of L. salivarius supernatant on Streptococcus mutans biofilms may indicate its possible anticaries effect. Thus, restoring the oral microenvironment with L. salivarius might be effective in preventing the colonization of periodontopathic bacteria 30. The difference in expression of gtfB and gtfC genes, in our study, indicates that there is no common promoter for them. Ullrich reported the potential presence of independent promoters for both genes 71. Low‐pH value increases the expression of the gtfBC gene but reduces sacB gene expression, which can lead to high‐biomass biofilms 48. The reduced Streptococcus mutans adherence and EPS formation in presence of SCS of L. casei, L. reuteri and L. plantarum despite the up‐regulation in the expression of the gtfb genes could be attributed to reduction in enzymatic function rather than reduction in gene expression 63.
The effect of Lactobacillus sp. on the production of IL‐10 and IFN‐γ was studied. IL‐10 is an immunosuppressive cytokine that is normally up‐regulated in inflamed pulp by bacterial infection to prevent the spread of inflammation 72. In our study, all tested Lactobacillus sp. supernatants inhibited IL‐10 production. To the best of our knowledge, this is the first study to show reduced IL‐10 production in response to Lactobacillus sp. This warrants further investigation. IFN‐γ is a pro‐inflammatory cytokine that synergizes with TNFα in increasing the microbicidal capacity of macrophages 73. The tested Lactobacillus sp. induced higher levels of IFN‐γ which can signify more robust innate and potentially adaptive immune responses at the site of infection.
The study showed that Lactobacillus sp. can inhibit tooth decay and control dental caries. This possible anticaries effect could be attributed to: (i) the inhibitory effect on Streptococcus mutans growth which were mainly due to organic acid generation and peroxide production; (ii) reduction in cell adherence and preformed biofilm; (iii) down‐regulation in several Streptococcus mutans virulence genes including acid tolerance genes (atpD and aguD genes), EPS‐producing genes (gtfBCD and sacB) and quorum‐sensing genes (vicKR and comCD); (iv) immunomodulatory effect due to the induction of IFN‐γ production and inhibition of IL‐10 production.
Author contributions
Dr. Reham Wasfi, Dr. Ola A. Abd El‐Rahman, Dr. Mai M. Zafer and Dr. Hossam M. Ashour contributed to the design of the study, performance of experiments, analysis of the results and writing of the manuscript.
Conflict of interest
The authors declare no competing financial interests.
References
- 1. Ben Taheur F, Kouidhi B, Fdhila K, et al Anti‐bacterial and anti‐biofilm activity of probiotic bacteria against oral pathogens. Microb Pathog. 2016; 97: 213–20. [DOI] [PubMed] [Google Scholar]
- 2. Mathews MJ, Mathews EH, Mathews GE. Oral health and coronary heart disease. BMC Oral Health. 2016; 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dietrich T, Webb I, Stenhouse L, et al Evidence summary: the relationship between oral and cardiovascular disease. Br Dent J. 2017; 222: 381–5. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008; 42: 409–18. [DOI] [PubMed] [Google Scholar]
- 5. Tong Z, Zhou L, Li J, et al An in vitro investigation of Lactococcus lactis antagonizing cariogenic bacterium Streptococcus mutans. Arch Oral Biol. 2012; 57: 376–82. [DOI] [PubMed] [Google Scholar]
- 6. Abranches J, Miller JH, Martinez AR, et al The collagen‐binding protein Cnm is required for streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun. 2011; 79: 2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009; 28: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients. 2010; 2: 290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bezerra DS, Stipp RN, Neves BG, et al Insights into the virulence traits of streptococcus mutans in dentine carious lesions of children with early childhood caries. Caries Res. 2016; 50: 279–87. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen PT, Falsetta ML, Hwang G, et al alpha‐Mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS ONE. 2014; 9: e111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koo H, Xiao J, Klein MI, et al Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010; 192: 3024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao J, Klein MI, Falsetta ML, et al The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed‐species oral biofilm. PLoS Pathog. 2012; 8: e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senadheera D, Krastel K, Mair R, et al Inactivation of VicK affects acid production and acid survival of Streptococcus mutans. J Bacteriol. 2009; 191: 6415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith EG, Spatafora GA. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res. 2012; 91: 133–41. [DOI] [PubMed] [Google Scholar]
- 15. Cagetti MG, Mastroberardino S, Milia E, et al The use of probiotic strains in caries prevention: a systematic review. Nutrients. 2013; 5: 2530–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FAO/WHO . Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Ontario, Canada, 2002.
- 17. Vuotto C, Longo F, Donelli G. Probiotics to counteract biofilm‐associated infections: promising and conflicting data. Int J Oral Sci. 2014; 6: 189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badet C, Thebaud NB. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. 2008; 2: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marsh P, Martin MV, Lewis M. Oral Microbiology. China: Churchill Livingstone; 2009. [Google Scholar]
- 20. Twetman S, Stecksen‐Blicks C. Probiotics and oral health effects in children. Int J Paediatr Dent. 2008; 18: 3–10. [DOI] [PubMed] [Google Scholar]
- 21. Teanpaisan R, Piwat S, Tianviwat S, et al Effect of long‐term consumption of Lactobacillus paracasei SD1 on reducing mutans streptococci and caries risk: a randomized placebo‐controlled trial. Dent J. 2015; 3: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sidhu GK, Mantha S, Murthi S, et al Evaluation of Lactobacillus and Streptococcus mutans by addition of Probiotics in the form of curd in the diet. J Int Oral Health. 2015; 7: 85–9. [PMC free article] [PubMed] [Google Scholar]
- 23. Stamatova I, Meurman JH. Probiotics: health benefits in the mouth. Am J Dent. 2009; 22: 329–38. [PubMed] [Google Scholar]
- 24. Compare D, Rocco A, Coccoli P, et al Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex‐vivo organ culture model of post‐infectious irritable bowel syndrome. BMC Gastroenterol. 2017; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin X, Chen X, Chen Y, et al The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015; 21: e128–34. [DOI] [PubMed] [Google Scholar]
- 26. Shokryazdan P, Sieo CC, Kalavathy R, et al Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res Int. 2014; 927268: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cadirci BH, Citak S. A comparison of two methods used for measuring antagonistic activity of lactic acid bacteria. Pak J Nutr. 2005; 4: 237–41. [Google Scholar]
- 28. Chew SY, Cheah YK, Seow HF, et al Probiotic Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 exhibit strong antifungal effects against vulvovaginal candidiasis‐causing Candida glabrata isolates. J Appl Microbiol. 2015; 118: 1180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wasfi R, Abd El‐Rahman OA, Mansour LE, et al Antimicrobial activities against biofilm formed by Proteus mirabilis isolates from wound and urinary tract infections. Indian J Med Microbiol. 2012; 30: 76–80. [DOI] [PubMed] [Google Scholar]
- 30. Wu CC, Lin CT, Wu CY, et al Inhibitory effect of Lactobacillus salivarius on Streptococcus mutans biofilm formation. Mol Oral Microbiol. 2015; 30: 16–26. [DOI] [PubMed] [Google Scholar]
- 31. Pfaffl MW, Tichopad A, Prgomet C, et al Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel‐based tool using pair‐wise correlations. Biotechnol Lett. 2004; 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 32. Pfaffl MW. Relative quantification In: Dorak T, editor. Real‐Time PCR. International University Line 2004. [Google Scholar]
- 33. Kilian M, Chapple ILC, Hannig M, et al The oral microbiome—an update for oral healthcare professionals. Br Dent J. 2016; 221: 657–66. [DOI] [PubMed] [Google Scholar]
- 34. Petrova MI, Lievens E, Malik S, et al Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015; 6: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Floch MH, Walker WA, Madsen K, et al Recommendations for probiotic use‐2011 update. J Clin Gastroenterol. 2011; 45(Suppl.): S168–71. [DOI] [PubMed] [Google Scholar]
- 36. Donelli G. Activity of probiotics on biofilm‐growing pathogens of the oral cavity. Microb Ecol Health Dis. 2013; 24: 6. [Google Scholar]
- 37. Lin Y‐TJ, Chou C‐C, Hsu C‐YS. Effects of Lactobacillus casei Shirota intake on caries risk in children. J Dent Sci. 2017; 12: 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imran F, Das S, Padmanabhan S, et al Evaluation of the efficacy of a probiotic drink containing Lactobacillus casei on the levels of periodontopathic bacteria in periodontitis: a clinico‐microbiologic study. Indian J Dent Res. 2015; 26: 462–8. [DOI] [PubMed] [Google Scholar]
- 39. Caglar E, Cildir SK, Ergeneli S, et al Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws and tablets. Acta Odontol Scand. 2006; 64(5): 314–318. [DOI] [PubMed] [Google Scholar]
- 40. Saha S, Tomaro‐Duchesneau C, Rodes L, et al Investigation of probiotic bacteria as dental caries and periodontal disease biotherapeutics. Benef Microbes. 2014; 5: 447–60. [DOI] [PubMed] [Google Scholar]
- 41. Baca‐Castanon ML, De la Garza‐Ramos MA, Alcazar‐Pizana AG, et al Antimicrobial effect of Lactobacillus reuteri on cariogenic bacteria Streptococcus gordonii, Streptococcus mutans, and periodontal diseases Actinomyces naeslundii and Tannerella forsythia. Probiotics Antimicrob Proteins. 2015; 7: 1–8. [DOI] [PubMed] [Google Scholar]
- 42. Hasslöf P. Probiotic Lactobacilli in the Context of Dental Caries as a Biofilm‐Mediated Disease [Doctoral thesis, comprehensive summary]. Umeå: Umeå universitet; 2013. [Google Scholar]
- 43. Ishikawa H, Aiba Y, Nakanishi M, et al Suppression of periodontal pathogenic bacteria in the saliva of humans by the administration of Lactobacillus salivarius TI 2711. Nihon Shishubyo Gakkai Kaishi (J Japanese Soc Periodontol). 2003; 45: 105–12. [Google Scholar]
- 44. Nishihara T, Suzuki N, Yoneda M, et al Effects of Lactobacillus salivarius‐containing tablets on caries risk factors: a randomized open‐label clinical trial. BMC Oral Health. 2014; 14: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oldak A, Zielinska D, Rzepkowska A, et al Comparison of antibacterial activity of Lactobacillus plantarum strains isolated from two different kinds of regional cheeses from Poland: Oscypek and Korycinski Cheese. Biomed Res Int. 2017; 6820369: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taheri P, Samadi N, Ehsani MR, et al An evaluation and partial characterization of a bacteriocin produced by Lactococcus lactis subsp lactis ST1 isolated from goat milk. Braz J Microbiol. 2012; 43: 1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo L, McLean JS, Lux R, et al The well‐coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 2015; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y, Burne RA. Multiple two‐component systems of Streptococcus mutans regulate agmatine deiminase gene expression and stress tolerance. J Bacteriol. 2009; 191: 7363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dalié DKD, Deschamps AM, Richard‐Forget F. Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Control. 2010; 21: 370–80. [Google Scholar]
- 50. Xu X, Zhou XD, Wu CD. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother. 2011; 55: 1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dasari S, Shouri RND, Wudayagiri R, et al Antimicrobial activity of Lactobacillus against microbial flora of cervicovaginal infections. Asian Pac J Trop Dis. 2014; 4: 18–24. [Google Scholar]
- 52. Tahmourespour A, Salehi R, Kasra KR. Lactobacillus Acidophilus‐derived biosurfactant effect on GTFB and GTFC expression level in Streptococcus mutans biofilm cells. Braz J Microbiol. 2011; 42: 330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hasslöf P, Hedberg M, Twetman S, et al Growth inhibition of oral mutans streptococci and candida by commercial probiotic lactobacilli—an in vitro study. BMC Oral Health. 2010; 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leung V, Dufour D, Lévesque CM. Death and survival in Streptococcus mutans: differing outcomes of a quorum‐sensing signaling peptide. Front Microbiol. 2015; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banu LD. Gene expression in Streptococcus mutans biofilms. University of Zurich, 2010.
- 56. Lü X, Hu P, Dang Y, et al Purification and partial characterization of a novel bacteriocin produced by Lactobacillus casei TN‐2 isolated from fermented camel milk (Shubat) of Xinjiang Uygur Autonomous region, China. Food Control. 2014; 43: 276–83. [Google Scholar]
- 57. Morita H, Toh H, Fukuda S, et al Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008; 15: 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. da Silva SS, Vitolo M, González JMD. Oliveira RPdS. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res Int. 2014; 64: 527–36. [DOI] [PubMed] [Google Scholar]
- 59. O'Shea EF, O'Connor PM, O'Sullivan O, et al Bactofencin A, a new type of cationic bacteriocin with unusual immunity. MBio. 2013; 4: e00498–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Decker E‐M, Klein C, Schwindt D, et al Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int J Oral Sci. 2014; 6: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shemesh M, Tam A, Feldman M, et al Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr Res. 2006; 341: 2090–7. [DOI] [PubMed] [Google Scholar]
- 62. Narisawa N, Kawarai T, Suzuki N, et al Competence‐dependent endogenous DNA rearrangement and uptake of extracellular DNA give a natural variant of Streptococcus mutans without biofilm formation. J Bacteriol. 2011; 193: 5147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeon JG, Klein MI, Xiao J, et al Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol. 2009; 9: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Welin‐Neilands J, Svensater G. Acid tolerance of biofilm cells of Streptococcus mutans. Appl Environ Microbiol. 2007; 73: 5633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Senadheera MD, Guggenheim B, Spatafora GA, et al A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol. 2005; 187: 4064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krzysciak W, Jurczak A, Koscielniak D, et al The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014; 33: 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bowen WH, Koo H. Biology of Streptococcus mutans‐derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011; 45: 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Savabi O, Kazemi M, Kamali S, et al Effects of biosurfactant produced by Lactobacillus casei on gtfB, gtfC, and ftf gene expression level in S. mutans by real‐time RT‐PCR. Adv Biomed Res. 2014; 3: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiao J, Koo H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J Appl Microbiol. 2010; 108: 2103–13. [DOI] [PubMed] [Google Scholar]
- 70. Yamashita Y, Bowen WH, Burne RA, et al Role of the Streptococcus mutans gtf genes in caries induction in the specific‐pathogen‐free rat model. Infect Immun. 1993; 61: 3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ullrich M. Bacterial Polysaccharide: Current Innovations and Future Trends. Norfolk UK: Caister Academic Press; 2009. [Google Scholar]
- 72. Farges JC, Alliot‐Licht B, Renard E, et al Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. 2015; 230251: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Trinchieri G. Interleukin‐12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003; 3: 133–46. [DOI] [PubMed] [Google Scholar]
- 74. Lee SH, Kim YJ. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch Microbiol. 2014; 196: 601–9. [DOI] [PubMed] [Google Scholar]
- 75. Salehi R, Savabi O, Kazemi M, et al Effects of Lactobacillus reuteri‐derived biosurfactant on the gene expression profile of essential adhesion genes (gtfB, gtfC and ftf) of Streptococcus mutans. Adv Biomed Res. 2014; 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]


