Abstract
Several empirically supported treatments have been identified for post-traumatic stress disorder (PTSD), yet a sizable number of patients are either unable to tolerate these approaches or remain symptomatic following treatment. Transcranial direct current stimulation (tDCS) is a well-tolerated method of modulating neuronal excitability that may hold promise as a novel intervention in PTSD and related disorders. The current review summarizes literature on the disrupted neural circuitry in PTSD and discusses the rationale for the commonly targeted prefrontal cortex (PFC) as it relates to PTSD. We then review the few prior (case) studies that have evaluated tDCS in patients with PTSD (1 study) and other anxiety disorders (4 studies). There was considerable variability in both the methods/justification for selecting the targeted brain region(s) and the tDCS montage used, which obscured any clear trends in the data. Finally, we describe the rationale for our ongoing study that specifically targets the lateral temporal cortex as a method of treating the symptoms of hyperarousal and re-experiencing in PTSD. Overall, it is clear that additional work is needed to establish dosing (e.g., intensity and duration of sessions, number of sessions) and optimal treatment targets as well as to identify synergistic effects with existing treatments.
Keywords: Transcranial Direct Current Stimulation, tDCS, Post-traumatic stress disorder, PTSD, Anxiety, Generalized Anxiety Disorder, Panic disorder, Obsessive Compulsive Disorder, brain stimulation, neuromodulation, functional neuroimaging
Introduction
Posttraumatic stress disorder (PTSD) develops in response to a traumatic event and is characterized by an intrusive re-experiencing of that event, hyperarousal, negative cognition and mood, and avoidance [1]. While there is evidence that PTSD can be treated effectively with specific forms of medication and psychotherapy [2–4], 33% to 50% of patients continue to meet diagnostic criteria for PTSD after treatment [5, 6]. Thus, there is a clear need to identify additional treatments that are effective on their own or that act synergistically with existing approaches to enhance their tolerability and efficacy.
The current review focuses on transcranial direct current stimulation (tDCS), which has long been a tool for understanding motor plasticity and motor rehabilitation but is also gaining traction as a treatment of cognitive [7] and emotional [8] disorders. For example, recent meta-analyses revealed medium to large effect sizes for cognition in patient populations (e.g., Cohen’s d = .42; [9]) as well as older adults (d = .44–.89) and AD patients (d =1.35;[10]) and similar findings for symptom reduction in depression (Hedges g = .74; [8]). tDCS uses weak electric currents (typically 1 – 2 mA) to modulate neuronal excitability. tDCS is most commonly delivered using two electrodes (an anode and a cathode) that are placed on the scalp according to the international 10–20 system. The electrical current flows from the anode to the cathode. Neurophysiological evidence suggests that the neuronal populations under the anode become depolarized (“excited”) whereas those underlying the cathode become hyperpolarized (“inhibited”) [11] While actual effects in practice may be more nuanced and may depend on a number of factors at the cellular and systems levels, this traditional description of tDCS effects provides a useful explanatory framework. The available evidence suggests that tDCS has a favorable side-effect profile, with physical sensations being most often reported (e.g., itching, numbness, and tingling under the electrodes), though these are experienced at nearly the same rate by those receiving sham stimulation [12].
tDCS effects are heavily dependent on electrode placement and even relatively slight changes in the position of electrodes can cause large alterations in electrical current flow. Non-cephalic electrode placement alters this flow to an even greater extent. Figure 1 highlights these effects by using magnetic resonance imaging (MRI) based finite element modeling of the electrical current in each of the studies reviewed below. As visualized by this figure, there is a balance between stimulation focality and intensity. More focal, but less intense, stimulation can be obtained by placing electrodes closer together. In contrast, focality decreases but intensity increases as the distance between (cephalic) electrodes increases. These models also demonstrate that the greatest stimulation intensity occurs between, rather than under, the electrodes. Therefore, the neuroanatomical correlates of the targeted symptoms, ability, or disorder must be carefully considered when selecting a tDCS electrode montage. Fortunately, a considerable and growing body of research has examined the neuroanatomy of PTSD and may prove useful for both reviewing existing research and planning new studies in this area.
Figure 1.
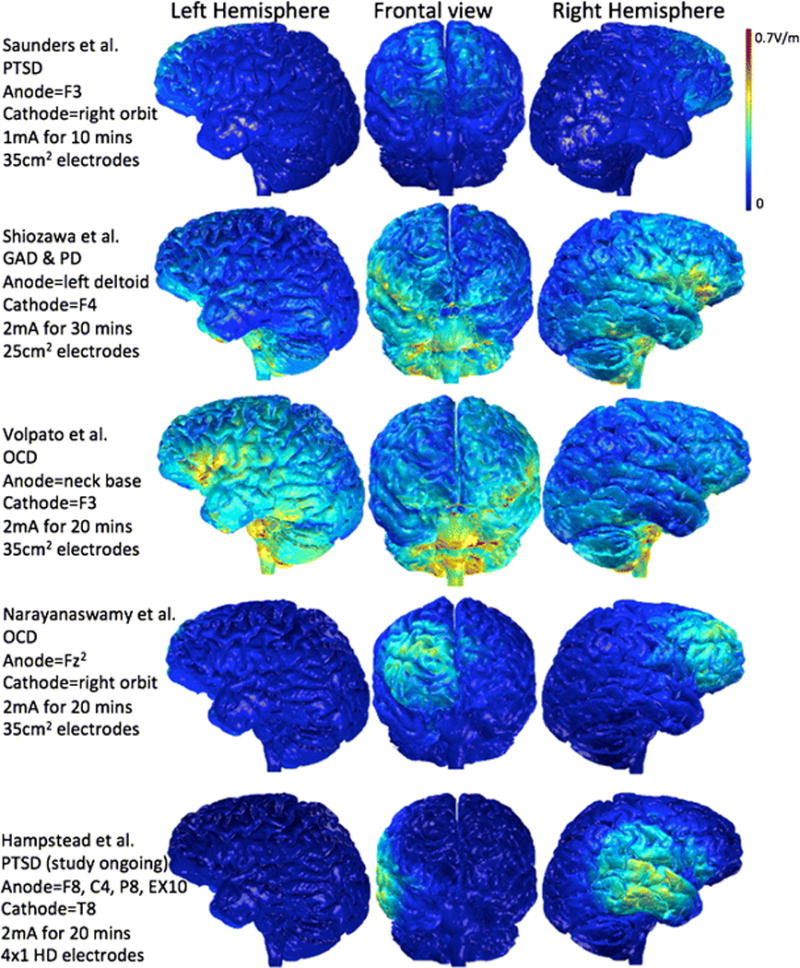
Finite element method models comparing electrical current flow in simulated heads using electrode montages from each of the tDCS studies included in the current review.
Neuroanatomy of PTSD
The “fear circuit” is the most widely recognized neuroanatomical model of PTSD and is comprised of three “core” regions: the amygdala, hippocampus, and ventromedial PFC (see [13]). Meta-analyses of task-based fMRI studies consistently reveal dysfunction of this circuit in patients with PTSD relative to controls [14, 15]. Summarizing the roles of these regions: a hyperactive amygdala is believed to be central to the development of PTSD [13–15]. This up-regulation has adverse effects on the hippocampus [16], which may lead to learning and memory deficits that are typically found in those with PTSD [16, 17]. The ventromedial PFC (including the rostral anterior cingulate) is believed to mediate “reflexive” (or automatic) forms of emotional regulation, which is reduced in those with PTSD [15]. Dysregulation in this region may also contribute to executive dysfunction that is commonly reported in PTSD [18]. The “deep” location of these core structures presents a major challenge for tDCS. As is clear in Figure 1, the electrical current must first pass through lateral brain regions, presumably causing physiologic change, before reaching these deeper structures of the fear circuit. Thus, an ideal solution to this inherent methodological limitation is to select electrode location based on structural and functional relationships with the fear circuit such that the anode could be used to “excite” hypoactive areas whereas the cathode could “inhibit” hyperactive regions.
Within this context, the nearly 30-year-old dimensional model of Heller and Nitschke [19] may be of particular value for PTSD and other anxiety disorders. This model holds that the symptoms of common mood and anxiety disorders are explained along the dimensions of emotional valence (i.e., pleasant vs. unpleasant) and activation (akin to arousal). Specifically, the left cerebral hemisphere preferentially mediates pleasant emotions whereas the right hemisphere is biased toward negative emotions. Posterior brain regions were posited to mediate highly activating (or arousing) emotions whereas anterior regions mediate less salient emotions (or perhaps suppress or modulate such salient effects via top-down control mechanisms as suggested by Etkin and Wagner [15]).
Although oversimplified, this general framework for emotional processing may be particularly useful when we consider the symptoms of PTSD (and possibly other anxiety disorders). Higher-order cognitive processes (i.e., executive abilities like working memory) are commonly impaired in those with PTSD [18, 20]. Such cognitive control abilities are critical for regulating the emotional response to intrusive memories [15, 21, 22]. For example an fMRI study [20] and meta-analysis [14] in PTSD suggest a reduction of cognitive control as evidenced by dorsolateral prefrontal cortex (PFC) hypoactivation during tasks requiring emotional control/processing [14, 20]. The left dorsolateral PFC may be particularly important in the regulation of emotion for the following reasons: 1) its putative role in mediating positive emotions [19], 2) evidence of dysfunction in those with Major Depressive Disorder [23] – a common comorbidity in PTSD [24], and 3) symptomatic relief of depression following non-invasive brain stimulation to the left PFC [25–27]. There is also debate about whether preexisting weaknesses in cognitive control serve as a risk factor for, or consequence of, PTSD (e.g., [28]). Together, these findings raise the possibility that the loss of top-down control increases one’s susceptibility to the intrusive and inordinately distress-laden memories that characterize the re-experiencing symptoms of PTSD. In fact, a recent study revealed that gray matter volume of the left PFC was inversely related to clinical measures of re-experiencing in patients with PTSD [29]. If true, then the data suggest that anodal stimulation over the left PFC may enhance both mood and cognitive control abilities, like working memory, in patients with PTSD – with the end result being symptomatic improvement.
We now turn to a review of the few tDCS studies performed in PTSD and other anxiety disorders, all of which targeted the PFC. We then reconsider the type and target of stimulation by providing theoretical data to support a novel approach that we are currently investigating.
tDCS effects in Anxiety Disorders
PTSD
To date, we are only aware of a single study that investigated effects of tDCS in patients with PTSD. In an uncontrolled case series of 4 patients (55–65 years old), Saunders and colleagues [30] used standard 35cm2 electrodes to provide 10 minutes of tDCS at 1mA during a total of 5 weekly sessions. Here, the anode was placed over the left dorsolateral PFC (DLPFC; site F3) while the cathode was placed over the contralateral orbit (see top row of Figure 1). After these tDCS sessions, the patients underwent in-home working memory training using a computerized program (i.e., CogMed) for 5 days per week (35–45 minute sessions) for 5 weeks. Primary outcome measures included a computerized neuropsychological battery (IntegNeuro) that included 14 subtests that appear to target a number of cognitive domains (e.g., attention, working memory, emotional recognition) as well as a measure of emotional functioning (i.e., Brain Resource for Emotional Intelligence Factors). Neurophysiological change was measured using QEEG at rest (to capture the Alpha Peak Frequency, which was reported as abnormal in patients with PTSD) and event-related potential (ERP) during a visual continuous performance task (to capture P3a, a metric reported as responsive to novelty and abnormal in patients with PTSD).
Following treatment, the authors reported modest evidence of improvement on measures that appear to assess attention and/or memory in each of the patients as well as self-reported emotional functioning (2 patients reported increased empathy/intuition and one reported greater self-esteem). However, certain aspects of attention also declined in three of the four patients. There were a large number of statistical comparisons performed in evaluating treatment effects but correction for multiple comparisons does not appear to have been performed. With regard to QEEG, the authors reported normalization of the P3a in all patients and normalization of the alpha peak frequency in three of the four patients, although the definition of “normal” was unclear. Overall then, the study provides some feasibility data for the use of tDCS in those with PTSD. However, the study appeared to be focused on cognitive rather than emotional symptom change and critical methodological factors (e.g., lack of a control group) limit the conclusions that can be reached.
Generalized Anxiety Disorder (GAD)
A single case study has been reported using tDCS for the symptoms of GAD [31]. Here, a 58 year-old woman with a three-year history of medication resistant GAD underwent 15 consecutive daily sessions of tDCS (5 days per week for 3 weeks) at 2.0 mA for 30 minutes per day using 25 cm2 rubber electrodes. An atypical montage was used where the anode was placed on the left deltoid muscle and the cathode was placed over the right DLPFC (presumably F4) (row 2 of Figure 1). The non-cephalic site on the left (or contralateral) deltoid muscle is a common procedure when trying to avoid modulatory effects on the brain. In this case, the authors wanted to (theoretically) only “inhibit” the right DLPFC and minimize “excitatory” anodal effects. The authors selected this montage based on prior studies that found symptomatic improvement in GAD, panic disorder, and MDD following low-frequency TMS (which decreases cortical excitability) to the right DLPFC. Consistent with our neuroanatomical discussion above, the group hypothesized that cathodal stimulation may serve to modulate other structures critical in the pathogenesis of GAD, such as the medial PFC, amygdala, and insula. Following these 15 sessions, the patient demonstrated a substantial reduction in anxiety and these benefits persisted at a 45-day follow-up. As such, this study provides preliminary (albeit uncontrolled) evidence that cathodal tDCS may correctively modulate extended neural networks responsible for symptoms of GAD (and possibly other anxiety disorders).
Panic Disorder
A single case study applied tDCS to treat a 44-year old female with a 3-year history of medication resistant panic disorder [32]. The same montage was used as in the patient with GAD (anode on left deltoid, cathode over right DLPFC). Stimulation was provided at 2mA for 30 minutes per day over 10 days (5 days per week for 2 weeks) using 25 cm2 electrodes. The patient was reportedly asymptomatic on treatment day 10 and remained so at a 30-day follow-up.
Obsessive Compulsive Disorder (OCD)
Two studies have evaluated efficacy of tDCS in reducing symptoms of OCD. The first study [33] used both tDCS and rTMS in a 35 year old patient with a 23 year history of treatment-resistant OCD. At the time of the study, the patient also met criteria for GAD and a major depressive episode. Prior to tDCS and rTMS, the patient underwent fMRI, which reportedly revealed hyperactivation of the left and hypoactivation of the right PFC relative to a group of 10 demographically comparable controls (the analytic methods appear somewhat atypical as functional runs were collected during the resting state but the magnitude of BOLD signal was examined as in task-based paradigms). Using these findings, the authors elected to provide inhibitory stimulation (i.e., cathodal tDCS; low-frequency rTMS) to the left DLPFC. The patient first completed 10 sessions (5 days per week for 2 weeks) of tDCS (2mA for 20 minutes using 35cm2 electrodes) and then a comparable 10-session course of rTMS. For tDCS, the anode was placed on the posterior neck base (row 3 of Figure 1). Symptoms of OCD did not change following this combined treatment approach. Although symptoms of depression and anxiety showed some decline after tDCS, which were accompanied by complex shifts in resting-state functional connectivity, these symptoms ultimately returned to baseline after rTMS. Thus, it is unclear whether rTMS effects counteracted the initial improvements following tDCS or whether these changes were merely due to non-specific factors (e.g., expectation effects).
In a more recent study, Narayanaswamy and colleagues [34] administered 2mA of stimulation for 20 minutes, twice per day, for 10 days to two patients with OCD. The first case was a 39-year-old female with a 5-year history of treatment resistant OCD. The second case was a 24 year-old male with a 3-year history of medication resistant OCD and comorbid social anxiety disorder and mild symptoms of depression. The authors selected the pre-supplementary motor area/supplementary motor area (pre-SMA/SMA) as a target for tDCS, based on evidence of 1) hypoactivation in OCD and 2) knowledge that pre-SMA inhibits striatal functioning, which is believed to become hyperactive and contribute to the pathogenesis of OCD. Therefore, the anode was placed over Fz2 (a site along the midline of the head) and the cathode over the right supraorbit (row 4 of Figure 1). Both patients showed significant symptom reduction following tDCS, with 40% (Case 1) and 46% (Case 2) reduction in OCD symptoms. This improvement was sustained at follow-ups of 1–2 months. Case 1 demonstrated significant increased blood oxygen level dependent (BOLD) signal in the left pre-SMA/SMA on day 10 relative to pre-treatment during an inhibitory control task, providing support for the hypothesized neocortical-subcortical interactions.
Overall then, we were able to identify a total of 9 patients who suffered from anxiety disorders and who were treated with tDCS to varying levels of success. Treatment parameters varied, especially with regard to stimulation montage and treatment duration. However, there may be a trend in the data where the most beneficial effects were found when the cathode was placed over the right hemisphere (e.g., [31, 32, 34]). These findings are especially intriguing within the context of our theoretical framework presented in the next section.
An Alternative Approach to tDCS in PTSD
The preliminary findings of symptomatic relief after cathodal stimulation of the right PFC are generally consistent with Heller and Nitschke’s [19] model (i.e., that the right hemisphere preferentially mediates negative emotions). PTSD is characterized by hyperarousal and a re-experiencing of traumatic events; symptoms that are consistent with those the model posits are related to the right temporoparietal cortex. Several complementary lines of evidence support this relationship. First, histopathological studies revealed robust and direct reciprocal connections between the amygdala, insula, and lateral temporal cortex (LTC) in macaque monkeys [35]; [36] [37–39]. Likewise, neuroimaging has revealed these structures are part of the same functional network [40] [41]. Taken together, these findings indicate that the amygdala, insula, and LTC form a feedback loop. Second, classic work from over 50 years ago documented that direct electrical stimulation of the LTC during neurosurgery elicited vivid, multisensory, autobiographical “flashbacks” [42]; as would be expected with traumatic memories. A more recent study revealed that the structural integrity of the LTC was related to the frequency of traumatic flashbacks in patients with PTSD [43], suggesting that compromise of the LTC component of the above noted loop may also facilitate the characteristic amygdala hyperactivity. Third, an fMRI study demonstrated significantly greater activation in the LTC as participants viewed traumatic events that were subsequently remembered relative to those later forgotten [44]. Such results are consistent with meta-analytic findings of LTC and insular hyperactivation during task performance in patients with PTSD and other anxiety disorders [15] as well as recent studies showing a right hemisphere bias toward negative/aversive emotional processing in these areas [45, 46]. These findings suggest that the LTC plays a critical role in both the formation and retention/re-experiencing of traumatic memories. Fourth, patients with PTSD demonstrate abnormal functioning in the LTC. For example, resting-state magnetoencephalography revealed hyperactivity within the right LTC in veterans with PTSD relative to veterans without [47]. Important from a treatment standpoint was that activity in this area was attenuated in those who no longer met criteria for PTSD [47]. fMRI studies also report hyperconnectivity within the regions comprising the LTC in trauma survivors with PTSD relative to survivors without PTSD [48]. Additionally, compared to veterans without, veterans with PTSD demonstrate greater connectivity between the LTC and the amygdala [49] as well as a positive relationship between symptoms of re-experiencing and the strength of connectivity between the LTC and insula [50]. Together, these findings support the premise that the LTC is dysfunctional in those with PTSD and that this dysfunction is directly related to the hallmark symptoms of hyperarousal and re-experiencing.
Using this framework, we are currently conducting a study (clinical trial #NCT02442843) that uses cathodal stimulation to inhibit (or otherwise disrupt) the right LTC and associated interactions with the amygdala (and functionally related regions like the insula). In our study, Combat Veterans with PTSD undergo baseline emotional and neuropsychological evaluations as well as resting-state fMRI (among other sequences). Participants then complete up to 10 high definition (HD) tDCS sessions (2mA for 20 minutes) where the center electrode is placed at T8 and the ring electrodes are placed at F8, C4, P8, EX10 (bottom row of Figure 1). HD-tDCS uses a 4×1 ring configuration in which the central electrode is surrounded by four electrodes of the opposite polarity [51, 52]. Practically, this means that the “ring” electrodes each use about ¼ of the electrical current while the central electrode uses the full amount. This approach limits the direct modulation effects to the radius of the 4-electrode ring (see [52]) and presumably minimizes the confounding physiological effects of the ring electrodes. The baseline evaluations, including fMRI, are repeated following HD-tDCS in order to evaluate stimulation effects at both the behavioral and neurophysiological level. We predict that HD-tDCS to the right LTC will specifically reduce symptoms of hyperarousal and re-experiencing of the traumatic event(s), modulate functional connectivity with the amygdala and insula, and have indirect effects on the PFC (perhaps enhancing top-down cognitive control).
Conclusions
The literature evaluating the use of tDCS in treating PTSD and other anxiety disorders is in its infancy. Early studies reviewed above suggest that tDCS holds potential as a treatment, either on its own or one that can complement extant forms of treatment. tDCS has several advantages including its ease of administration, cost-effectiveness, and favorable safety profile,; all of which support its potential in treating these disorders. However, the growing interest in this tool needs to be accompanied by methodologically rigorous studies into the symptomatic and neurophysiological changes associated with its use. Early studies have primarily utilized the PFC as a stimulation site, capitalizing on its convenient geography and its broad role in cognitive and emotional control circuitry. Future studies should continue to explore the efficacy of alternative stimulation sites in addition to factors like the intensity and duration of stimulation and number of sessions needed to induce an effect (i.e., dose-response relationships). It is important to evaluate the effects of individual morphology and physiology on electrode montage since both of these factors may affect electrical current flow and the effects thereof. Finally, it may be worthwhile to integrate dimensional models, like the Research Doman Criteria (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml), when selecting patients and determining electrode placement since this may provide greater flexibility in targeting the patient’s most troubling symptoms relative to traditional diagnostic categories. A symptom-driven approach could also be applied retrospectively once sufficient data have been collected in order to identify those who benefit most from tDCS. Finally, it would be worthwhile to evaluate combined effects of tDCS and existing treatments to determine any synergistic effects. While tDCS holds promise in those with anxiety disorders, it is clear that considerably more work is needed to realize this potential.
Acknowledgments
This work was supported by the National Institute of Mental Health (1R21MH102539-01 to BMH). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
The authors declare no conflicts of interest.
Compliance with Ethics Guidelines
Conflict of Interest
Benjamin M. Hampstead, Emily M. Briceño, Nathan Mascaro, and Andoni Mourdoukoutas declare that they have no conflict of interest. Marom Bikson declares board membership for Soterix Medical Inc., outside of the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Works Cited
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Goodson J, et al. Treatment of posttraumatic stress disorder in US combat veterans: A meta-analytic Review. Psychological Reports. 2011;109(2):573–599. doi: 10.2466/02.09.15.16.PR0.109.5.573-599. [DOI] [PubMed] [Google Scholar]
- 3.Bisson JI, et al. Psychological treatments for chronic post-traumatic stress disorder: Systematic review and meta-analysis. British Journal of Psychiatry. 2007;190:97–104. doi: 10.1192/bjp.bp.106.021402. [DOI] [PubMed] [Google Scholar]
- 4.Watts BV, et al. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2013;74(6):E541–E550. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- 5.Van Etten ML, Taylor S. Comparative efficacy of treatment for posttraumatic stress disorder: A meta-analysis. Clin Psychol Rev. 1998;5:126–144. [Google Scholar]
- 6.Bradley R, et al. A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry. 2005;162:214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 7.Floel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage. 2014;85:934–947. doi: 10.1016/j.neuroimage.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 8.Kalu UG, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychological Medicine. 2012;42(9):1791–1800. doi: 10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- 9.Brunoni AR, Vanderhasselt MA. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain and Cognition. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Hsu WY, et al. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiology of Aging. 2015;36(8):2348–2359. doi: 10.1016/j.neurobiolaging.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Brunoni AR, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology. 2011;14:1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 13.Skelton K, et al. PTSD and gene variants: New pathways and new thinking. Neuropharmacology. 2012;62(2):628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons AN, Matthews SC. Neural circuitry of PTSD with or without mild traumatic brain injury: A meta-analysis. Neuropharmacology. 2012;62:598–606. doi: 10.1016/j.neuropharm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Etkin A, Wager TD. Functional neuroimaging of Anxiety: A meta-analysis of emotional processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD; a meta-analysis. Journal of Affective Disorders. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Aupperle RL, et al. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition and Emotion. 1988;12(3):421–427. [Google Scholar]
- 20.Aupperle RL, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in Posttraumatic stress disorder. Arch Gen Psychiatry. 2012b;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- 21.Anderson MC, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303(5655):232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410(6826):366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 23.Grimm S, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Hankin CS, et al. Mental disorders and mental health treatment among US Department of Veterans Affairs outpatients: The Veterans health study. American Journal of Psychiatry. 1999;156(12):1924–1930. doi: 10.1176/ajp.156.12.1924. [DOI] [PubMed] [Google Scholar]
- 25.O’Reardon JP, Solvason HB, Janicak PGea. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Nitsche MA, Paulus W. Transcranial direct current stimulation – update 2011. Restorative Neurology and Neuroscience. 2011;29:463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 27.Boggio PS, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. International Journal of Neuropsychopharmacology. 2009;11(2):249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbertson MW, et al. Neurocognitive function in monozygotic twins discordant for combat exposure: Relationship to posttraumatic stress disorder. Journal of Abnormal Psychology. 2006;115(3):484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- 29.Tavanti M, et al. Evidence of diffuse damage in frontal and occipital cortex in the brains of patients with post-traumatic stress disorder. Neurol Sci. 2012;33:59–68. doi: 10.1007/s10072-011-0659-4. [DOI] [PubMed] [Google Scholar]
- *30.Saunders N, et al. Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase. 2015;21(3):271–278. doi: 10.1080/13554794.2014.890727. [DOI] [PubMed] [Google Scholar]
- *31.Shiozawa P, et al. Transcranial Direct Current Stimulation for Generalized Anxiety Disorder: A Case Study. Biological Psychiatry. 2014;75(11):E17–E18. doi: 10.1016/j.biopsych.2013.07.014. [DOI] [PubMed] [Google Scholar]
- *32.Shiozawa P, da Silva M, Cordeiro Q. Transcranial Direct Current Stimulation (tDCS) for Panic Disorder: A Case Study. Journal of Depression and Anxiety. 2014;3(3):158. [Google Scholar]
- *33.Volpato C, et al. Modulation of affective symptoms and resting state activity by brain stimulation in a treatment resistant case of obsessive-compulsive disorder. Neurocase. 2013;19(4):360–370. doi: 10.1080/13554794.2012.667131. [DOI] [PubMed] [Google Scholar]
- *34.Narayanaswamy JC, et al. Successful Application of Add-on Transcranial Direct Current Stimulation (tDCS) for Treatment of SSRI Resistant OCD. Brain Stimulation. 2015;8(3):655–657. doi: 10.1016/j.brs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Amaral D, Price J. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 36.Aggleton J, Burton M, Passingham R. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca Mulatta) Brain Research. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- 37.Mufson EJ, Mesulam MM. Insula of the Old-World Monkey .2. Afferent Cortical Input and Comments on the Claustrum. Journal of Comparative Neurology. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 38.Mesulam MM, Mufson EJ. Insula of the Old-World Monkey .1. Architectonics in the Insulo-Orbito-Temporal Component of the Paralimbic Brain. Journal of Comparative Neurology. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 39.Mesulam MM, Mufson EJ. Insula of the Old-World Monkey .3. Efferent Cortical Output and Comments on Function. Journal of Comparative Neurology. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 40.Cauda F, et al. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 41.Cerliani L, et al. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human Brain Mapping. 2012;33(9):2005–2034. doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penfield W, Perot P. The brain’s record of auditory and visual experience. A final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 43.Kroes M, et al. Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. European Psychiatry. 2011;26:525–531. doi: 10.1016/j.eurpsy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Bourne C, Mackay C, Holmes E. The neural basis of flashback formation: the impact of viewing trauma. Psychological Medicine. 2013;43(7):1521–1532. doi: 10.1017/S0033291712002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straube T, Miltner WHR. Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54(3):2534–2538. doi: 10.1016/j.neuroimage.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Duerden EG, et al. Lateralization of affective processing in the insula. Neuroimage. 2013;78:159–175. doi: 10.1016/j.neuroimage.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Engdahl B, et al. Post-traumatic stress disorder: a right temporal lobe syndrome? Journal of Neural Engineering. 2010;7:1–8. doi: 10.1088/1741-2560/7/6/066005. [DOI] [PubMed] [Google Scholar]
- 48.Li BJ, et al. Altered resting-state functional connectivity in post-traumatic stress disorder: a perfusion MRI study. Medical Imaging 2013: Image Perception. Observer Performance, and Technology Assessment. 2013;8673 [Google Scholar]
- 49.Sripada RK, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sripada RK, et al. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic medicine. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cano T, et al. Methods to focalize noninvasive electrical brain stimulation: principles and future clinical development for the treatment of pain. Expert Rev Neurother. 2013;13(5):465–467. doi: 10.1586/ern.13.41. [DOI] [PubMed] [Google Scholar]
- 52.Datta A, et al. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. Journal of Neural Engineering. 2008;5:163–174. doi: 10.1088/1741-2560/5/2/007. [DOI] [PubMed] [Google Scholar]


