Abstract
Objective
To determine the effectiveness of controlling maternal gestational weight gain in the second and third trimesters on neonate body composition.
Methods
210 healthy women with overweight (BMI ≥ 25 < 30) or obesity (BMI ≥30 kg/m2) randomized to a lifestyle intervention (LI) program focused on controlling gestational weight gain through nutrition and activity behaviors or to usual obstetrical care (UC). Infant fat and fat-free mass at birth were measured by air displacement plethysmography (PEAPOD) and by quantitative magnetic resonance (QMR).
Results
At baseline, there were no between group differences in maternal characteristics (mean (±SD)): age 33.8 (±4.3) years, weight 81.9 (±13.7) kg, BMI 30.4 (±4.5) kg/m2, gestational age at randomization 14.9 (±0.8) wk. GWG was less in the LI group by 1.79 kg (p=0.003) or 0.0501 kg/wk (p=0.002). Compared to UC, LI infants had greater weight (131±59 g p=0.03), fat-free mass (98±45 g; p=0.03) by PEAPOD, and lean mass (105±38 g; p=0.006) by QMR. Fat mass and percentage fat were not significantly different.
Conclusion
Intervening in women with overweight and obesity through behaviors promoting healthy diet and physical activity to control gestational weight gain resulted in neonates with similar fat and greater fat-free mass.
Keywords: Neonatal, body composition, lifestyle modifications, pregnancy, perinatal programming
Introduction
Intra-uterine exposures may influence an individual’s long-term risk of developing obesity and related cardiometabolic diseases [1, 2]. The theory is that perturbations early in plastic, developmental stages can lead to worse health trajectories. This implies that strategies for ensuring health of individuals may require early interventions, including prenatally. Mounting evidence in humans suggests that exposure to adverse prenatal environments increases risk of developing obesity, metabolic dysfunction, and related health problems later in life [3, 4, 5, 6]. Fetal exposure to excessive maternal gestational weight gain (GWG) influences long-term adiposity-related disease [4, 7, 8, 9, 10, 11, 12].
Many women with overweight and obesity gain more weight than is recommended during pregnancy [13] and this is a risk factor for obesity in the offspring [14, 15]. A number of randomized clinical trials (RCTs) in the United States have evaluated the effect of behavioral and lifestyle interventions on GWG and glycemic status, with mixed results [16, 17, 18, 19]. A large Australian RCT reported no differences in maternal GWG, neonates large for gestational age (LGA) and birth outcomes except a reduction in neonatal macrosomia in a lifestyle intervention group compared with standard care [20]. A Norwegian study showed less GWG in the intervention group compared to controls (mean difference 1.3 kg; P = 0.009) but no effect on infant birth weight or proportion of LGA newborns [21]. A meta-analysis of antenatal dietary or lifestyle intervention RCTs reported no statistical differences in mean GWG or LGA [22]. It concluded that the uncertainty of both the effect of an antepartum intervention and its optimal intensity, along with inconsistency in maternal and infant outcome reporting, has yielded inadequate findings to assume that limiting GWG improves maternal and infant health. Therefore, whether lifestyle intervention during pregnancy to reduce excessive GWG lowers the risk of childhood obesity remains unknown.
A major limitation of most studies on newborn adiposity is that they have used length and weight based indices rather than actual measures [23] of body composition to infer normal, over-weight or obese status. Such indices assume that a higher weight reflects additional fat mass and fail to consider the non-fat compartment.
This RCT in women with overweight or obesity aimed to investigate whether a lifestyle intervention (LI) to control GWG would affect fat and fat-free mass in their infants at birth.
Methods
Study Design
LIFT (Lifestyle Intervention For Two) is part of the LIFE-Moms consortium [24] which consists of seven independent RCTs, a Research Coordinating Unit, and the NIH as sponsor, collaborating but with different strategies for reducing GWG in women with overweight or obesity. LIFT was a parallel-group, RCT in pregnant women. We compared body composition of newborns of mothers with overweight or obesity randomly assigned at 1:1 ratio at the beginning of the second trimester to LI designed to control GWG or to UC. The primary hypothesis was that percent body fat would be less for neonates from LI than from UC mothers. The study was approved by the IRBs of St. Luke’s-Roosevelt Hospital (SLRH) and Columbia University and registered with ClinicalTrials.gov (NCT01616147).
Participants
Women were recruited from hospital affiliated private and clinic practices from February 2013 to October 2015. Eligibility criteria included age ≥18 years, a BMI ≥25 at baseline measurement, singleton pregnancy and gestational age between 9,0 (week, day) and 15,6 confirmed by dating ultrasound, and intention to deliver at SLRH. Exclusion criteria are listed in Supplement Appendix S1.
Study Treatments
LI continued from randomization to delivery. Its goal was to maintain GWG during the second and third trimesters within IOM recommended limits of 0.32 kg/wk for women with overweight and 0.27 kg/wk for women with obesity [13]. LI focused on diet modification and increased physical activity along with behavioral and social support strategies delivered in individual sessions by study counselors. The intervention program was derived from the Diabetes Prevention Program [25] and Look AHEAD [26] curriculums, with the focus modified from weight loss to GWG control as recommended by 2009 IOM guidelines [13]. The experienced nutritionist providing the counseling, was trained on the nutritional needs of pregnant women to provide counseling appropriate for controlling GWG while assuring adequate nutrition. See details in Supplement Appendix 1.
UC involved a single 20 to 30 minute ‘Introduction’ immediately following randomization. This covered basic nutrition for pregnancy as given in www.choosemyplate.gov and in American Academy of Nutrition guidelines. Thereafter, participants were invited to attend UC group meetings once every 8 weeks through delivery. The topics covered related to health during pregnancy and were similar to those covered in the LI group sessions but with less focus on calorie counting.
Baseline and Follow-Up Assessments for Mothers
There were three core visits for all mothers during pregnancy: the baseline visit that immediately preceded randomization occurred between 12,0 and 15,6 weeks and corresponded approximately to the beginning of the second trimester of pregnancy. The second core visit occurred at 24,0 – 27,6 weeks when participants had a 2-hour 75gm oral glucose tolerance test (OGTT) using the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria [27]. This prenatal visit corresponded to the end of the second trimester. The final prenatal core visit occurred between 35,0 – 36,6 weeks at approximately the end of the third trimester. At all visits, body weight was measured to the nearest 0.1 kg (Tanita BWB-800) and height was measured at baseline to the nearest 0.5 cm using a stadiometer (Seca 222, Seca Deutschland). Baseline weight was measured at or before randomization and no later than 15 weeks 6 days GA. Weight measured in the second trimester was adjusted (−0.45 kg for women at 14 weeks or −0.91 kg for women at 15 weeks). No adjustment was made when weight was measured in the first trimester (on or before 13 weeks 6 days). Baseline BMI was calculated using baseline weight and measured height. All women were asked to complete an automated self-administered 24-hour dietary assessment from which the 2010 Healthy Eating Index (HEI) was calculated, an index of diet quality validated in pregnant women [28].
Infant Study Assessments
Anthropometric and body composition measurements were performed prior to hospital discharge, between 1 and 4 days after birth for term infants, or at 36 weeks post menstrual age for preterm infants. Body weight was measured on a calibrated scale (PEA POD, Cosmed), infant length on a length board (Ellard Instrumentation Ltd), and head circumference using a tape measure with tensiometer (Gulick II, model 67020). Skinfold thicknesses of the triceps, sub-scapula, iliac crest and mid-thigh were measured in duplicate using calipers (Harpenden, model HSB-BI) and the average was reported. When the results of the two duplicate skinfold measures differed by more than a 0.5 mm, a third measurement was acquired. The PEA POD body composition system (COSMED USA, Inc) measures body composition through the measurement of body volume by air displacement plethysmography, as previously described [29]. Infants were undressed and wore a standard tight-fitting hat (Allentown Scientific Associates, Inc, Allentown, PA) to minimize air trapped in the hair. Body volume and mass by PEA POD were used to estimate body density using gender specific equations of Fomon [30] from which fat, the primary outcome and fat-free free mass were derived. In our laboratory, repeated PEA POD tests performed twice on the same day on 29 infants gave CV’s of 6.6% for %fat, 6.5% for fat, and 1.1% for fat-free mass.
Quantitative magnetic resonance (QMR) is a non-imaging technique (EchoMRI-Infants™) that uses an electromagnetic field to detect the hydrogen atoms of fat, lean tissue and water [31, 32]. Once excited by radiofrequency pulses, these protons have different relaxation times relative to the tissue (lipid or fat) or medium (eg, water) in which they are embedded. The processed signal is obtained from the whole body at once. The total water signal by QMR comes from protons primarily found in proteins (lean tissues) and to a lesser degree in fat molecules. The greater absolute value for total water compared to total lean mass for QMR reflects the contribution from fat to the total water measure. In our laboratory, the CV’s in 14 newborns measured 3 times by QMR with repositioning between scans was 5.3%, 2.5% and 1.6% for fat mass, lean mass, and TBW, respectively. Secondary infant outcomes were QMR measures of fat, lean and total body water; skinfold thicknesses at four sites, head circumference, length, and body weight.
Safety monitoring
The safety alerts were maternal weight loss, high blood pressure, contraindications to physical activity, suicidality/depression, incidental findings, and fetal growth. Adverse events included intrauterine growth restriction (IUGR) and small-for-gestational-age (SGA) defined as less than the 10th percentile. Serious adverse events included death, life-threatening condition, hospitalization, disability/permanent change, congenital anomaly, and medical intervention. Serious adverse events were reported within 24 hours of ascertainment by LIFT personnel to a designated NIH monitor for review by the Data Safety Monitoring Committee of LIFE-Moms.
Statistical Analysis
Ninety infants per group has a power of 0.80 to detect a mean difference between groups in infant body fat of 1.8% with a standard deviation (SD) of 4.3% using a t-test. Fifteen additional women were enrolled per group to compensate for drop outs. Group baseline means and SDs were calculated for continuous variables and compared using t-tests. For categorical variables, the number and percentage in each cell were calculated for each group. Chi-squared tests compared the distributions of the two groups. The group means and SDs of the outcome variables were calculated and analysis of covariance compared the adjusted means. The covariates were mother’s age and BMI at baseline, infant’s gestational age and sex. The adjusted difference, standard error of the difference, and P-value for each outcome variable is provided. While randomization balances groups with respect to the distributions of the independent variables (covariates), analysis of covariance is required to calculate the appropriate standard error of the difference between groups. Unadjusted differences are also presented. Statistical analyses were performed using SAS version 9.2 and STATA version 12. Significance was set at P<0.05, two-tailed.
Results
Study Participants
Figure 1 shows enrollment, randomization and retention through infant delivery. Of the 9,412 women who were ineligible, 7,453 (79.2%) were ineligible due to a BMI <25.0 kg/m2. Randomization occurred between 12,0 and 13,6 weeks for 10.5% of women and between 14,0 and 15,6 weeks for 89.5% of women, from February 2013 to October 2015. Baseline group characteristics were similar (Table 1) for all demographic variables. The incidence of gestational diabetes mellitus (GDM) was 10.3% for LI and 6.1% for UC (P=0.28). Mothers who developed GDM were under the care of their obstetrician.
Figure 1.
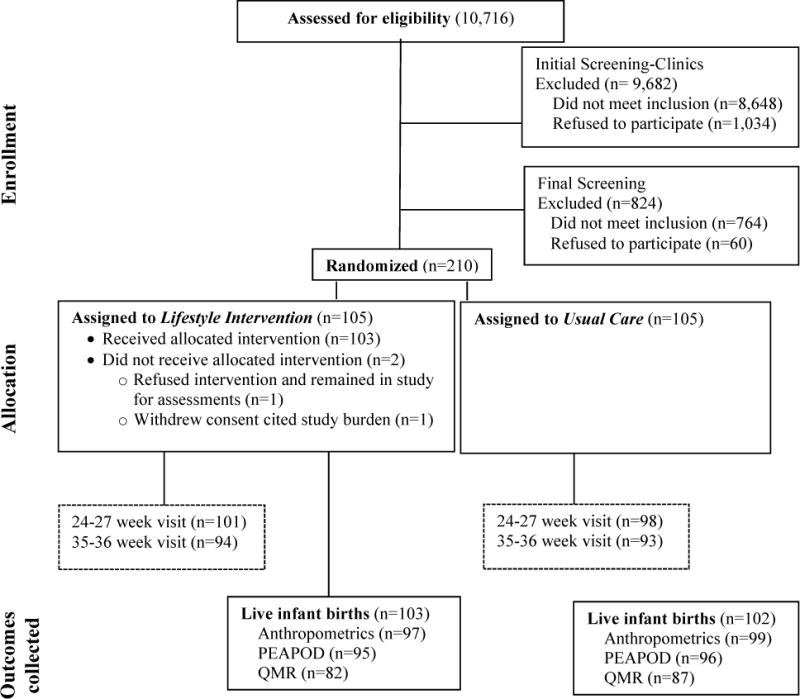
Screening, enrollment, randomization and follow-up of study participants.
Table 1.
Maternal Baseline Characteristics.
| Demographic characteristic1 | Lifestyle Intervention (n = 105) |
Usual Care (n = 105) |
|---|---|---|
| Age (years) | 33.8 ± 4.0 | 33.8 ± 4.7 |
| Race | ||
| White | 48 (46%) | 50 (48%) |
| Black | 25 (24%) | 25 (24%) |
| Other | 26 (25%) | 22 (21%) |
| More than one race | 5 (5%) | 8 (8%) |
| Unknown | 1 (1%) | 0 (0%) |
| Ethnicity | ||
| Not Hispanic/Latina | 72 (69%) | 80 (76%) |
| Hispanic | 32 (30%) | 25 (24%) |
| Unknown | 1 (1%) | 0 (0%) |
| Marital status | ||
| Married | 78 (74%) | 73 (70%) |
| Not married/living with significant | 13 (12%) | 21 (20%) |
| Other | ||
| Separated/divorced/widowed | 3 (3%) | 1 (1%) |
| Not married | 10 (10%) | 10 (10%) |
| Unknown | 1 (1%) | 0 (%) |
| Education-highest level attained Categories | ||
| High school diploma or less | 4 (4%) | 3 (3%) |
| College (1–3 yr)/business/technical | 15 (14%) | 12 (11%) |
| College degree (bachelor) | 46 (44%) | 39 (37%) |
| Postgraduate work | 39 (37%) | 51 (49%) |
| Unknown | 1 (1%) | 0 (%) |
| Annual family income Categories | ||
| ≤$24,999 | 3 (3%) | 7 (7%) |
| $25,000 – $74,999 | 31 (30%) | 29 (28%) |
| $75,000 – $149,000 | 38 (36%) | 35 (33%) |
| ≥$150,000 | 31 (29%) | 34 (32%) |
| Unknown | 2 (2%) | 0 (0%) |
| Pregnancy information | ||
| Gestational age Categories | ||
| 11 – 13 weeks completed (11 to <14) | 10 (10%) | 12 (11%) |
| 14 weeks completed (14 to <15) | 33 (31%) | 37 (35%) |
| 15 weeks completed (15 to <16) | 62 (59%) | 55 (52%) |
| 16 weeks completed (16 to <17) | 0 (0%) | 1 (1%) |
| Gestational age at randomization (wks) | 14.96 ± 0.72 | 14.82 ± 0.78 |
| Height (cm) | 164.3 ± 5.4 | 163.5 ± 7.0 |
| Baseline weight (kg) | 81.5 ± 12.4 | 82.2 ± 15.0 |
| Baseline BMI (kg/m2) | 30.1 ± 4.1 | 30.7 ± 5.0 |
| Baseline BMI Categories | ||
| Overweight (25.0 – 29.9 kg/m2) | 65 (62%) | 60 (57%) |
| Obese (>30.0 kg/m2) | 40 (38%) | 45 (43%) |
| Parity | ||
| 0 | 39 (37%) | 38 (36%) |
| 1 | 30 (29%) | 31 (30%) |
| >2 | 36 (34%0 | 36 (34%) |
Values are n (%) for categorical variables and mean ± SD for continuous variables. Differences in baseline characteristics between the treatment groups were not significant.
Intervention Adherence
Adherence was measured by the participant’s attendance at bi-monthly visits and her weekly food and exercise logs. Attendance of LI women was good with median attendance at 87.5% of visits through end of second trimester (<27 weeks) and 72% of visits through end of pregnancy. Adherence to submitting weekly food logs was moderate, with a median rate of 67.5% in the second trimester and 51.1% overall. Adherence to submitting weekly exercise logs had a median rate of 52.5% in the second trimester and 34.2% overall. Exercise class attendance rate was extremely poor at 9.7%.
Maternal Outcomes
A total of 210 women were randomized, with 97 of 105 women (92.3%) in the LI group and 99 of 105 women (94.3%) in the UC group having body weight measures at baseline and at end of pregnancy and having a live birth with infant weight measured.
Gestational weight gain
The GWG was 1.79 kg less in LI compared to UC (p<0.003) (Table 2). Among the women with overweight (25.0≥BMI≤29.9 kg/m2), the between group difference in GWG did not attain statistical significance (lower in LI group by 1.32 kg, p=0.06). Among the women with obesity (BMI>30.0 kg/m2), the LI group gained 0.17 kg/wk compared to 0.25 kg/wk for the UC group (p=0.01) and the difference in total weight gain was 2.68 kg (p=0.007) less in the LI group. Thirty-eight percent of the UC group gained more than IOM recommended ranges, both in overweight and obese categories while significantly fewer women in the LI group (20% with overweight and 16% with obesity) did so (Table 2).
Table 2.
Maternal gestational weight gain
| Lifestyle Intervention mean (SD) |
Usual Care mean (SD) |
Difference Diff (SE) |
P value |
|
|---|---|---|---|---|
| N=97 | N=99 | |||
| GWG (From baseline to 35–36.6 weeks) (kg) | 7.89 ± 4.07 | 9.67 ± 4.17 | −1.79 ± 0.59 | 0.003 |
| Overweight | N=60 | N=58 | ||
| Weight gain (kg) | 9.01 ± 3.55 | 10.33 ± 4.00 | −1.32 ± 0.70 | 0.06 |
| Per week (kg/wk) | 0.25 ± 0.10 | 0.29 ± 0.11 | −0.04 ± 0.02 | 0.04 |
| Obese | N=37 | N=41 | ||
| Weight gain (kg) | 6.07 ± 4.24 | 8.75 ± 4.27 | −2.68 ± 0.96 | 0.007 |
| Per week (kg/wk) | 0.17 ± 0.12 | 0.25 ± 0.12 | −0.07 ± 0.03 | 0.01 |
| GWG above the IOM guidelines (per week) | 19% | 38% | 0.002 | |
| Overweight | 20% | 38% | 0.03 | |
| Obese | 16% | 39% | 0.03 | |
| N=95 | N=97 | |||
| Second trimester GWG | 3.54 ± 2.24 | 4.99 ± 2.47 | −1.45 ± 0.34 | 0.0001 |
| Overweight | N=59 | N=57 | ||
| Weight gain (kg) | 4.29 ± 2.12 | 5.25 ± 2.28 | −0.96 ± 0.41 | 0.02 |
| Per week (kg/wk) | 0.41 ± 0.19 | 0.52 ± 0.22 | −0.10 ± 0.04 | 0.007 |
| Obese | N=36 | N=40 | ||
| Weight gain (kg) | 2.31 ± 1.89 | 4.62 ± 2.71 | −2.32 ± 0.54 | 0.0001 |
| Per week (kg/wk) | 0.24 ± 0.19 | 0.43 ± 0.23 | −0.19 ± 0.05 | 0.0001 |
| N=91 | N=91 | |||
| Third trimester GWG | 3.98 ± 2.52 | 4.62 ± 2.59 | −0.64 ± 0.38 | 0.09 |
| Overweight | N=58 | N=53 | ||
| Weight gain (kg) | 4.53 ± 2.35 | 5.18 ± 2.48 | −0.65 ± 0.46 | 0.16 |
| Per week (kg/wk) | 0.43 ± 0.22 | 0.48 ± 0.24 | −0.05 ± 0.04 | 0.25 |
| Obese | N=33 | N=38 | ||
| Weight gain (kg) | 3.02 ± 2.54 | 3.85 ± 2.57 | −0.84 ± 0.51 | 0.17 |
| Per week (kg/wk) | 0.28 ± 0.22 | 0.36 ± 0.26 | −0.09 ± 0.06 | 0.13 |
GWG, gestational weight gain; IOM, institute of medicine
Trimester specific GWG
LI women had less second trimester GWG compared to those in UC: approximately 1.0 kg less among women with overweight (p=0.02) and 2.3 kg (p<0.001) less among women with obesity. GWG in the third trimester did not differ (0.64 kg, p=0.09: Table 2) between groups. The greatest impact of LI on controlling GWG occurred during the second trimester. Notably, in women with obesity, GWG was halved in LI compared to UC (2.31 kg versus 4.62 kg, p<0.001; Table 2).
Maternal diet
There were no between group differences for any component of the HEI at baseline (Supplementary Table 1). At 35 weeks, compared to the UC group, the LI had a higher HEI score (p=0.004) reflecting a healthier maternal diet and a higher SOFFAS score (p=0.015) indicating a lower consumption of calories from solid fats, alcoholic beverages, and added sugars. The change in HEI for the LI group (5.33, p=0.0109) was greater (p=0.0301), than the change for UC group (−1.03, p=0.6155).
Infant Birth Characteristics
Births occurred between August 2013 and April 2015. Infant weight, length, and skinfolds were collected on 97 of 103 (94%) live births in LI and 99 of the 102 (97%) live births in UC (Figure 1). Percent fat, fat mass, and fat-free mass by PEAPOD were collected on 95 (93%) of LI and 96 (94%) of UC infants. QMR measures of fat mass, lean mass, and total body water were collected on 82 (81%) of LI and 87 (85%) of UC infants. Poorer compliance with the infant QMR compared to the PEAPOD was due to mothers feeling less comfortable with their newborn having a test involving magnetic resonance compared to air displacement, despite no known risks.
The baseline characteristics of the infant groups were similar for all demographic variables (Table 3). Mean gestational age at birth was 39.4±1.8 weeks. There was a trend for LI compared to UC infants to have a higher birth weight (3373±587 g vs. 3235±532 g, p=0.09) and birth length (51.3±2.7 cm vs. 50.6±2.9 cm, p=0.07).
Table 3.
Infant characteristics at birth
| Lifestyle Intervention (n = 97) |
Usual Care (n = 99) |
P-value |
|
|---|---|---|---|
| Birth mode | |||
| Vaginal | 68 (70%) | 68 (69%) | 0.83 |
| Cesarean | 29 (30%) | 31 (31%) | |
| Preterm birth | |||
| <31w, 6d | 2 (2%) | 0 (0%) | 0.17 |
| 32w,0d – 36w,6d | 3 (3%) | 7 (7%) | |
| Full term birth (>36w,6d) | 92 (95%) | 92 (93%) | |
| Infant sex | |||
| Female | 44 (45%) | 46 (46%) | 0.88 |
| Male | 53 (55%) | 53 (54%) | |
| Small for gestational age | 8 (8%) | 13 (14%) | 0.26 |
| (<10th percentile) | |||
| Large for gestational age | 10 (10%) | 6 (6%) | 0.28 |
| (>90th percentile) | |||
| Gestational age (weeks) | 39.4 ± 1.9 | 39.4 ± 1.7 | 0.89 |
| Birth weight (g) | 3373 ± 587 | 3235±532 | 0.08 |
| Weight for age z-score | 0.09 ± 1.30 | −0.19 ± 1.23 | 0.13 |
| Birth length (cm) | 51.3 ± 2.7 | 50.6 ± 2.9 | 0.07 |
| Length for age z-score | 0.92 ± 1.41 | 0.54 ± 1.55 | 0.07 |
Values are n (%) for categorical variables and mean ± SD for continuous variables.
Infant Outcomes
Infant Body Composition
measurements were collected between 2 and 4 days of age in 89.3%, prior to hospital discharge. Infant body composition measures are in Table 4. The LI group had a greater measured body weight of 131 g (p=0.03), a larger ponderal index (p=0.02), and a larger head circumference (p=0.03) compared to the UC group. Percentage fat and absolute fat mass by PEAPOD did not differ between groups while the LI infants had greater fat-free mass (2871±404 g vs. 2786±405, p=0.03; adjusted difference 98 g). Similarly, lean mass by QMR was greater in the LI infants (2327±325 g vs. 2211±314, p=0.006; adjusted difference 105 g). Total body water by QMR was greater in the LI infants (2452±334 g vs. 2342±320, p=0.02; adjusted difference 97 g) but total fat mass did not differ between the groups. Skinfold thickness did not differ between groups for any of the four sites. In secondary analyses, the infant body composition variables were reanalyzed 1) excluding preterm infants, 2) substituting premenstrual age for GA, and 3) using infant’s age at test as an additional covariate. The infant’s age (days) was a significant covariate for QMR-total water, PEAPOD-fat mass, and PEAPOD-fat free mass suggesting that these body composition values increase with increasing age. For all three analyses, the results and the statistical interpretation of the results were identical to the primary analyses.
Table 4.
Infant body composition at study assessment
| Lifestyle Intervention mean ± SD |
Usual Care mean ± SD |
Unadjusted Differences diff ± SE |
P-value1 |
Adjusted Differences diff ± SE6 |
P-value2 |
|
|---|---|---|---|---|---|---|
| N=97 | N=99 | |||||
| Study weight (g) | 3229 ± 526 | 3108 ± 500 | 121 ± 73 | 0.10 | 131 ± 59 | 0.03 |
| Study length (cm) | 49.6 ± 2.5 | 49.4 ± 2.3 | 0.2 ± 0.3 | 0.53 | 0.3 ± 0.3 | 0.33 |
| Ponderal index (kg/m3) | 26.2 ± 1.9 | 25.6 ± 2.1 | 0.6 ± 0.3 | 0.02 | 0.7 ± 0.3 | 0.02 |
| Head circumference (cm) | 34.33 ± 1.52 | 33.97 ± 1.49 | 0.36 ± 0.22 | 0.10 | 0.38 ± 0.17 | 0.03 |
| Skinfolds | N=97 | N=99 | ||||
| Triceps (mm) | 4.83 ± 1.35 | 4.65 ± 1.11 | 0.18 ± 0.18 | 0.31 | 0.20 ± 0.17 | 0.26 |
| Subscapular (mm) | 4.55 ± 1.27 | 4.32 ± 1.17 | 0.23 ± 0.17 | 0.19 | 0.25 ± 0.17 | 0.14 |
| Iliac crest (mm) | 3.97 ± 0.95 | 3.91 ± 0.99 | 0.06 ± 0.14 | 0.64 | 0.08 ± 0.13 | 0.54 |
| Thigh (mm) | 5.93 ± 1.89 | 5.66 ± 1.57 | 0.27 ± 0.25 | 0.28 | 0.29 ± 0.24 | 0.22 |
| Central3 (mm) | 8.52 ± 2.10 | 8.23 ± 2.01 | 0.29 ± 0.29 | 0.32 | 0.33 ± 0.28 | 0.24 |
| Peripheral4 (mm) | 10.76 ± 3.09 | 10.32 ± 2.54 | 0.45 ± 0.40 | 0.27 | 0.49 ± 0.39 | 0.21 |
| Sum of skinfolds5 (mm) | 19.29 ± 5.03 | 18.55 ± 4.41 | 0.74 ± 0.68 | 0.27 | 0.82 ± 0.65 | 0.20 |
| Outcomes | ||||||
| PEAPOD | N=95 | N=96 | ||||
| PEAPOD age (days) | 2.86 ± 5.34 | 3.32 ± 6.26 | −0.46 ± 0.84 | 0.59 | −0.35 ± 0.70 | 0.62 |
| Percentage fat (%) | 10.86 ± 4.34 | 10.10 ± 3.90 | 0.76 ± 0.60 | 0.20 | 0.79 ± 0.55 | 0.16 |
| Fat mass (g) | 360 ± 173 | 324 ± 157 | 36 ± 24 | 0.13 | 38 ± 22 | 0.08 |
| Fat-free mass (g) | 2871 ± 404 | 2786 ± 405 | 85 ± 59 | 0.15 | 98 ± 45 | 0.03 |
| QMR (g) | N=82 | N=87 | ||||
| QMR age (days) | 2.63 ± 4.95 | 3.13 ± 5.17 | −0.49 ± 0.78 | 0.53 | −0.49 ± 0.60 | 0.42 |
| Total fat mass (g) | 542 ± 189 | 509 ± 179 | 33 ± 28 | 0.25 | 32 ± 24 | 0.19 |
| Total lean mass (g) | 2327 ± 325 | 2211 ± 314 | 116 ± 49 | 0.02 | 105 ± 38 | 0.006 |
| Total body water (g) | 2452 ± 334 | 2342 ± 320 | 109 ± 50 | 0.03 | 97 ± 40 | 0.02 |
p-value for unadjusted differences;
p-value for adjusted differences;
Central is the sum of subscapular and iliac crest.
Peripheral is the sum of triceps and thigh;
Sum of skinfolds is triceps and subscapular and iliac crest and thigh.
The covariates used in the analyses were mother’s age and BMI at baseline, infant’s gestational age and sex.
Relationship with GWG
The relationship between infant’s body composition variables at birth and total GWG was analyzed, with GWG included as an additional variable in the regression models. The correlations between GWG and the infant body composition variables were: PEAPOD: FFM (r=0.03, p=0.67), fat (r=0.04, p=0.57), QMR: FFM (r=0.02, p=0.79), fat (r=0.13, p=0.09) and TBW (r=0.02, p=0.84). The correlations were also calculated within each group. For the UC group only, there was a statistical trend suggesting a positive relationship between GWG and QMR fat (r=0.20, p=0.07). The partial correlation between GWG and the infant body composition variables were calculated adjusting for mother’s baseline age and BMI, gestational age, infant gender, and group. Only the partial correlation between GWG and QMR fat was significant (r=0.20, p=0.01). A similar analysis with mothers classified by GWG category (excessive or within guidelines) found no association with infant body composition. The only significant partial correlation was between GWG and QMR fat within the UC group (r=0.24, p=0.03).
Relationship with HEI
The relationship between infant’s body composition variables at birth and the HEI was analyzed, with HEI included as an additional variable in the regression models. The HEI coefficient was not significant for any of the infant’s body composition variables (p>0.40).
Safety Events
Through end of pregnancy, 13.3% of women in LI (n=14) and 14.3% (n=15) in UC (p=0.84) reported serious adverse events. None were considered related to the study intervention. Maternal hospitalizations accounted for 79.3% of these. Among infants, serious adverse events were reported in 7.6% (n=8) of infants in LI and 9.5% (n=10) in UC (p=0.62). None were considered related to the study intervention. SGA occurred in 4 LI and 11 UC infants, and IUGR occurred in 0 LI and 2 UC infants.
Discussion
This RCT reports that a lifestyle intervention promoting healthy diet and physical activity to control GWG in women with overweight or obesity resulted in neonates who weighed more and had greater fat-free mass, while not differing on amount of fat. Our data show that the intervention impacted fetal development although the exact mechanisms or mediators leading to the observed effects on infant body composition are unknown and were not a target of this investigation. We are unaware of any previous RCT demonstrating the efficacy of a lifestyle intervention on neonatal body composition.
Our methods specifically measured infant body composition. For infants in this study, 10.5% of an infant’s body mass at birth is fat mass (approximately 340 gm of fat) and 89.5% is fat-free (Table 4). While we hypothesized that the intervention would result in less percent infant body fat, no effect was found. Body weight and its lean component were significantly increased in LI measured by two independent methods. Head circumference, an index of greater brain growth, was similarly greater in LI. Fat-free mass by PEAPOD is the non-fat component of body weight and was 105 g higher in LI infants. Lean mass by QMR was 97 g higher in LI infants. The QMR also measured greater total body water in the LI group. By comparison, these data show that a lifestyle intervention had a similar degree of impact on infant FFM (105 gm) as not smoking during pregnancy (113.8 g) [33].
Our trial demonstrates that reliance only on infant body weight, length, and ponderal index would have resulted in an interpretation that LI produced larger and heavier babies, unequivocally assuming them to have greater adiposity and less lean mass per body weight [34]. Our rigorously controlled RCT shows this assumption to be misguided, highlighting the importance of designing newborn trials with sensitive body composition measurement methods for adiposity and lean tissues.
This intervention targeting a healthier pregnancy lifestyle had an important measurable impact on neonate lean tissue. Our data were collected in the first few days of life and are largely devoid of confounding influences of the post-natal environment on body composition, such as mode of feeding (breast or formula). Whether these neonate measureable effects are sustainable into childhood requires further investigation. We cannot attest to the clinical significance of this greater neonate lean mass, but clearly this could be of high relevance given the strong epidemiology linking birth weight to adult health.
The effect of LI on infant body composition could not be explained by baseline maternal characteristics, which were well balanced by randomization (Table 1). Previous RCTs examined effects of interventions to reduce excessive GWG on infant outcomes. The most effective interventions appear to be those that comprehensively target maternal dietary intake and physical activity [35] as conducted here in LIFT [24]. One study [36] found that the intervention group had a significantly lower median GWG compared with the control group (7.0 vs. 8.6 kg; p=0.01) while the intervention group offspring had a higher birth weight compared to the control (median 3742g vs. 3596). This is consistent with our finding of a higher infant weight in the LI group.
A few lifestyle interventions targeting GWG [16] have demonstrated an effect on neonatal weight. Healthy Moms found that an intensive intervention encouraging minimal GWG (0 ± 3 kg) in obese women resulted in lower GWG (mean difference = −3.4 kg) and a lower prevalence of LGA infants at birth (9 vs. 26%, odds ratio = 0.28, 95% CI [0.09–0.84]) [37]. The randomized trial LIMIT found that a prenatal intervention targeting eating, activity, and behaviors and no prescribed calorie goals had no effect on reducing GWG but reduced birth weight >4000 grams (15% versus 19%; RR=.81 [0.67–0.98]) [20]. By contrast, UPBEAT found that a prenatal intervention designed to improve glucose tolerance but not GWG reduced GWG but had no effect on offspring LGA [38].
LIFT was not designed to identify specific mechanisms through which intervention affects neonate body composition. The LIFT hypothesis was that by targeting GWG, maternal pregnancy physiology including glucose-insulin status, lipids, inflammation, blood pressure, and other metabolomics [39] could be improved, producing an intrauterine environment leading to healthier neonatal body composition. The absence of an association of infant lean mass with GWG category (excessive or within guidelines), or between degree of GWG and infant lean mass, suggests that intervention aspects other than limiting GWG may be important in development of lean mass. Prenatal behaviors (physical activity, diet) employed in LIFT could be mediators in the pathway(s) leading to the observed infant body composition, independent of GWG. However, no association was found between any infant body composition variable and HEI. Other postulated mediators for the greater mean mass in LI neonates include possible changes in maternal or intra-amniotic or placental metabolomic and inflammatory substances (secondary to the healthier diet) impacting the transference of macronutrients to the fetus, recently reported to affect placental lipid transfer in women with obesity [40]. Our intervention did not involve the first trimester of pregnancy, possibly missing an important period for fetal and neonatal fat accretion [41] and placental functioning [42]. Our study highlights the need for further research to elucidate potential molecular, epigenetic, and/or biological mechanisms involved in determining fetal body composition. The demonstrated effect on neonatal body composition raises questions as to its persistence into and beyond the post-natal period.
A potential limitation of this study is that we did not measure nutritional intake (breast milk and infant formula) between birth and study measurement which could have differed by group, thereby influencing the body composition measurements. We ensured a high degree of internal validity, with staff involved in collection of measurements blind to group assignment. We employed high quality infant measurements with results consistent between the PEAPOD and QMR, methods employing very different technology, thereby maximizing our confidence in study findings. The generalizability of these findings to the wider patient population (external validity) is unknown. Our trial involved women with overweight and obesity only, and did not include women with lower weight. The women enrolled into LIFT included a racially/ethnically diverse cohort including a large proportion with high income.
Conclusion
This trial demonstrates the efficacy and safety of a lifestyle intervention in pregnant women with overweight and obesity that targeted the control of GWG during a highly malleable period of fetal development. It resulted in no differences in fat mass but greater lean mass in the infant at birth.
Supplementary Material
What is already known about this subject.
Excessive gestational weight gain is a risk factor for obesity in the offspring
The extent to which lifestyle intervention during pregnancy to reduce excessive gestational weight gain lowers the risk of childhood obesity remains unknown
What is known about body composition of neonate at birth is largely derived from anthropometric measures of weight, length, skinfolds and air displacement plethysmography.
What this study adds.
A lifestyle intervention in women with overweight and obesity that targeted the control of GWG resulted in no differences in fat mass but greater lean mass in the infant at birth using state-of-the-art measurement methods.
The intervention delivered during the second and third trimesters promoted a healthier pregnancy lifestyle that had an important measurable impact on neonate lean tissue.
The absence of an association of infant lean mass with gestational weight gain suggests that aspects of the intervention other than limiting gestational weight gain may be important in the development of lean mass.
Acknowledgments
We thank: LIFE-Moms consortium members for their contributions to the development and oversight of common measures and procedures across the trials; the LIFT study participants (women and infants) for enrolling in this study; the LIFT staff for their herculean efforts: Kasey Faulkner, Maryanne Holowaty, Isaiah Janumala, Jill Johnson, Kim Kelly, Rachel Koletsky, Jennifer Patricio, Julie Roman, Elizabeth Widen, and Wen Yu; Rebecca Gersnoviez Clifton, Ph.D., at The George Washington University Biostatistics Center for guidance specific to LIFE-Moms consortium outcomes and definitions.
Funding: National Institutes of Health Grants U01-DK094463; U01-DK094463-Supplement (Supplement to promote diversity, T. Toro-Ramos, PhD); P30-DK026687; T32-DK007559. The content is the responsibility of the authors and does not necessarily represent the official views of the NIH. LIFE-Moms is supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, U01 DK094418, U01 DK094463, U01DK094416, 5U01DK094466 (RCU)), the National Heart, Lung, and Blood Institute (NHLBI, U01HL114344, U01HL114377), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U01 HD072834), the National Center for Complementary and Integrative Health (NCCIH), the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the Indian Health Service, and the Intramural Research Program of the NIDDK.
Footnotes
Disclosure: The authors declare no conflict of interest.
Author contributions: DG, XP, JCT and BR designed the study; DG and XP are co-principal investigators of the LIFT trial and were responsible for supervision of data collection; BR was responsible for obstetrical care; TTR, CP and SG were responsible for newborn outcome measures; MH was the primary project coordinator; JC was the primary lifestyle interventionist; SL was responsible for data management; JCT was responsible for data analysis; DG and XP had primary responsibility for final content. All authors read and approved the final manuscript.
Clinical Trial Registry NCT01616147; www.ClinicalTrials.gov
References
- 1.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88(8):3505–6. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- 3.Lemas DJ, Brinton JT, Shapiro AL, Glueck DH, Friedman JE, Dabalea D. Associations of maternal weight status prior and during pregnancy with neonatal cardiometabolic markers at birth: the Healthy Start study. Int J Obes (Lond) 2015;39(10):1437–42. doi: 10.1038/ijo.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142(10):1851–58. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and metaanalyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019–26. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 7.Haire-Joshu D, Tabak R. Preventing Obesity Across Generations: Evidence for Early Life Intervention. Annu Rev Public Health. 2016;37:253–71. doi: 10.1146/annurev-publhealth-032315-021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourtakos SP, Tambalis KD, Panagiotakos DB, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC Pregnancy Childbirth. 2015;15(66) doi: 10.1186/s12884-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Lichtenstein P, Fraser A, Langstrom N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr. 2011;94(1):142–8. doi: 10.3945/ajcn.110.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91(6):1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557–64. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010;34(1):67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- 13.IOM (Institute of Medicine) and NRC (National research Council) Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academy Press; 2009. [Google Scholar]
- 14.Mamum AA, Mannan M, Doi SAR. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obesity Reviews. 2014;15(4):338–47. doi: 10.1111/obr.12132. [DOI] [PubMed] [Google Scholar]
- 15.Nehrin I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes. 2013;8(3):218–24. doi: 10.1111/j.2047-6310.2012.00110.x. [DOI] [PubMed] [Google Scholar]
- 16.Flynn AC, Dalrymple K, Barr S, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74(5):312–28. doi: 10.1093/nutrit/nuw005. [DOI] [PubMed] [Google Scholar]
- 17.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol. 2009;113:305–12. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- 18.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord. 2002;26(11):1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- 19.Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009;101:569–77. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- 20.Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. doi: 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagedal LR, Sanda B, Øverby NC, et al. The effect of prenatal lifestyle intervention on weight retention 12 months postpartum: results of the Norwegian Fit for Delivery randomised controlled trial. BJOG. 2017;124(1):111–21. doi: 10.1111/1471-0528.13863. [DOI] [PubMed] [Google Scholar]
- 22.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010;117(11):1316–26. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 23.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr. 2015;69(12):1279–89. doi: 10.1038/ejcn.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifton RG, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) 2016;24(2):305–13. doi: 10.1002/oby.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pick ME, Edwards M, Moreau D, Ryan EA. Assessment of diet quality in pregnant women using the Healthy Eating Index. J Am Diet Assoc. 2005;105(2):240–6. doi: 10.1016/j.jada.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Hull HR, Thornton JC, Ji Y, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205(3):e211–7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 31.Kovner I, Taicher GZ, Mitchell AD. Calibration and validation of EchoMRI™ whole body composition analysis based on chemical analysis of piglets, in comparison with the same for DXA. Int J Body Compos Res. 2010;8(1):17–29. [PMC free article] [PubMed] [Google Scholar]
- 32.Toro-Ramos T, Paley C, Wong W, et al. Reliability of the EchoMRI-Infant system for water and fat measurements in newborns. Obesity (Silver Spring) 2017 Jul 16; doi: 10.1002/oby.21918. [Epub ahead of print] PubMed PMID: 28712143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrod CS, Fingerlin TE, Chasan-Taber L, Reynolds RM, Glueck DH, Dabelea D. Exposure to prenatal smoking and early-life body composition: the healthy start study. Obesity (Silver Spring) 2015;23(1):234–41. doi: 10.1002/oby.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16(6):1215–23. doi: 10.1007/s10995-011-0846-1. [DOI] [PubMed] [Google Scholar]
- 35.Phelan S, Jankovitz K, Hagobian T, Abrams B. Reducing excessive gestational weight gain: lessons from the weight control literature and avenues for future research. Womens Health (Lond) 2011;7(6):641–61. doi: 10.2217/whe.11.70. [DOI] [PubMed] [Google Scholar]
- 36.Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jorgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34(12):2502–07. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vesco KK, Karanja N, King JC, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity (Silver Spring) 2014;22(9):1989–96. doi: 10.1002/oby.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–77. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 39.Hivert MF, Perng W, Watkins SM, et al. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. J Dev Orig Health Dis. 2015;6(2):65–78. doi: 10.1017/S204017441500001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, Hauguel de-Mouzon S, Catalano P, O’Tierney-Ginn P. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158(8):2543–2555. doi: 10.1210/en.2017-00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015;100(4):1672–80. doi: 10.1210/jc.2014-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan OR, Rosario FJ, Powell TL, Jansson T. Regulation of placental amino acid transport and fetal growth. Prog Mol Biol Transl Sci. 2017;145:217–51. doi: 10.1016/bs.pmbts.2016.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


