Abstract
Background
Hypercatecholaminemia and bone marrow dysfunction have been implicated in the pathophysiology of persistent-injury associated anemia. The elderly may be more vulnerable to bone marrow dysfunction due to high basal and peak catecholamine levels and impaired hematopoietic progenitor growth. We hypothesized that aging would adversely affect persistent injury-associated anemia.
Methods
Male Sprague Dawley rats age 8-9 weeks and F344-BN rats age 25 months were randomized to: naïve controls, lung contusion plus hemorrhagic shock (LCHS), and LCHS plus daily chronic restraint stress (LCHS/CS). Urine norepinephrine was measured on days one and seven. Mobilization of hematopoietic progenitor cells (HPC), bone marrow colony forming units-erythroid (CFU-E) growth, and peripheral blood hemoglobin, mean corpuscular volume (MCV), and red cell distribution width (RDW) were assessed on day seven. **p <0.05 young vs. aged counterpart by one-way analysis of variance.
Results
Aged rats had higher norepinephrine levels at naïve baseline (97** vs. 27 ng/mL) and seven days following LCHS/CS when compared to young (359** vs. 127 ng/mL). Following LCHS/CS, HPC mobilization was greater among young rats when compared to aged (5.4 vs. 2.5%). CFU-E growth was lower among aged animals for each group (naïve: 47** vs. 65; LCHS: 40** vs. 50; LCHS/CS: 38** vs. 44 cells/plate). Aged naïve rats had higher initial hemoglobin (15.2** vs. 14.3 g/dL), but lower MCV (48** vs. 59 fL/cell) and larger RDW at baseline, and greater differences seven days after LCHS/CS (MCV: 46** vs. 60 fL/cell; RDW: 17.4** vs. 16.3%).
Conclusions
Compared to young rats, aged rats had less HPC mobilization despite elevated basal and peak norepinephrine. Aged rats were disproportionately affected by impaired hematopoietic progenitor growth and an iron-restricted red blood cell phenotype at baseline which persisted seven days after injury. Further research is needed to assess how the clinical approach to persistent-injury-associated anemia should differ for elderly trauma patients.
Level of Evidence
Prognostic study, level III
Keywords: Anemia, erythropoiesis, aging, trauma, injury
Introduction
Trauma patients are often affected by anemia due to acute blood loss and high levels of circulating catecholamines and inflammatory cytokines, which suppress iron availability and inhibit erythropoiesis in the bone marrow, leading to persistent injury-associated anemia (1-5). The elderly may be especially vulnerable to this phenomenon due to baseline anemia, an exaggerated neuroendocrine stress response, and impaired bone marrow function. Anemia affects approximately 10% of subjects age ≥65, and is associated with two-fold increased all-cause mortality among the elderly (6, 7). Basal plasma norepinephrine levels and peak levels in response to stress each rise with increasing age (8-11). Although bone marrow cellularity appears to be preserved among the elderly, aging has been associated with reduced hematopoietic stem cell proliferative and regenerative capacity (12-14).
Preclinical investigation of persistent injury-associated anemia has been performed using young (age 8-9 week) Sprague-Dawley rats (15-17). Like many strains, Sprague-Dawley rats are not suitable for aging studies because they are afflicted by age-dependent renal insufficiency, and typically do not survive to old age (18). In contrast, Fischer-Brown Norway (F344-BN) rats do not appear to be afflicted by age-dependent renal insufficiency, and are less affected by age-related cardiac and hematopoietic dysfunction than other commonly used rat strains (19-21). Importantly, under normal physiologic conditions, F344-BN rats maintain the capacity for erythropoiesis throughout their lifespan, with average hemoglobin 15.3 g/dL at 30 months of age (22). We chose to use 25 month-old F344-BN rats in these experiments, roughly corresponding to 65 year-old humans (23).
The purpose of this study was to compare persistent injury-associated anemia in young versus aged rats. We hypothesized that aging would be associated with an exaggerated post-injury catecholamine surge, decreased bone marrow growth of hematopoietic progenitor cells, and iron-restricted persistent anemia seven days after injury and daily restraint stress.
Methods
Animals
Male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 300-400g and F344-BN rats age 25 months (generously donated by the National Institute of Aging) weighing 450-600g were housed in pairs and fed ad lib with Teklad Diet #7912 (Harlan Laboratories Inc., Tampa, FL) and water during a one week acclimation period. Light and dark cycles were 12 hours each throughout acclamation and experimental periods. All animal care was conducted in accordance with University of Florida Institutional Animal Care and Use Committee standards. Young and aged animals were randomly allocated to naïve (young: n=10, aged: n=8), lung contusion (LC) followed immediately by hemorrhagic shock (LCHS) (young: n=10, aged: n=8), and LCHS followed by daily restraint stress (LCHS/CS) (young: n=10, aged: n=8). Chronic restraint stress was incorporated into the injury models to simulate stressors associated with the intensive care unit environment. Animals were sacrificed on post-injury day seven by cardiac puncture following intraperitoneal injection of ketamine (80-100 mg/kg) and xylazine (5-10 mg/kg) on day seven.
Lung contusion
Animals randomized to LCHS or LCHS/CS were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg). LC was performed by applying a percussive staple gun (PowerShot Model 5700M, Saddle Brook, NJ) to a 12 mm metal plate applied to the right lateral chest wall 1-2 cm below the axillary crease. This model has been shown to produce a clinically significant and reproducible pulmonary contusion based on histologic findings (24-26).
Hemorrhagic shock
Animals randomized to LCHS or LCHS/CS were placed on a heating pad, and PE-50 tubing was inserted into the right internal jugular vein and right femoral artery under direct visualization. The arterial catheter was applied to a BP-2 Digital Blood Pressure Monitor (Columbus Instruments, Columbus, OH) for continuous blood pressure monitoring. Blood was withdrawn through the venous catheter into a heparinized syringe until a mean arterial pressure of 30-35 mm Hg was reached. This blood pressure range was maintained for 45 minutes by withdrawing or reinfusing blood as needed. After 45 minutes, shed blood was reinfused at 1 mL/min.
Chronic stress
For animals randomized to LCHS/CS, CS was performed by placing animals in a restraint cylinder (Kent Scientific, Torrington, CT) for two hours per day. To prevent acclimation, the cylinders were rotated 180 degrees every 30 minutes, and alarms (80 dB) were transmitted by speakers placed immediately adjacent to the cylinders for two minutes each time the cylinders were rotated. Because animals undergoing CS had no access to food or water in the restraint cylinder, all other groups were subjected to a two hour daily fast while CS was being performed.
Norepinephrine
Norepinephrine levels were measured in the urine as a surrogate for catecholamine levels in the blood because blood levels tend to be erratic with labile peaks and troughs, whereas urine norepinephrine is more stable over time (27, 28). Norepinephrine was assessed rather than epinephrine because previous work has demonstrated that post-injury anemia is primarily mediated by norepinephrine (29). On post-injury day one, spontaneous urine samples were obtained during daily handling for non-CS groups, and were obtained during CS for CS groups. Spontaneous urine samples were also obtained on post-injury day seven prior to sacrifice. Samples were stored at −80°C. Urine norepinephrine was measured by enzyme linked immunosorbent assay (Labor Diagnostika Nord, Nordhorn, Germany).
Bone marrow cellularity
Bone marrow samples were obtained at the time of sacrifice by removing the left femoral epiphysis and flushing the femur with a 5 mL syringe containing Iscove’s modified Dulbecco’s Medium and 10% fetal bovine serum. The resulting suspension was passed through a 40 μm sterile nylon strainer, stained with 0.4% Trypan blue, and placed on a hemocytometer plate. Total viable cell counts were assessed by light microscopy.
Hematopoietic progenitor growth
Bone marrow cells were plated and incubated with a stock solution as previously described (30). Hematopoietic progenitor cell growth was assessed at three stages: colony forming unit – granulocyte, erythrocyte, monocyte, megakaryocyte (GEMM, early hematopoietic progenitors, counted fourteen day after plating), blast forming unit – erythroid (BFU-E, occurring between GEMM and CFU-E in the erythrocyte progenitor lineage, counted fourteen days after plating), and colony forming unit – erythroid (CFU-E, late hematopoietic progenitors, counted seven days after plating). Colony growth was assessed by light microscopy to identify colonies by morphology.
Hematopoietic progenitor mobilization
Mobilization of hematopoietic progenitor cells from the bone marrow to the peripheral blood was assessed by flow cytometry. Peripheral blood was stained with CD71 antibodies (BD Biosciences, San Jose, CA) and c-kit antibodies (Southern Biotech, Birmingham, AL) conjugated to phycoerythrin (BD Biosciences) to identify hematopoietic progenitors as CD71+/c-kit+. The percentage of hematopoietic progenitors in peripheral blood was calculated by a BD LSR II flow cytometer equipped with FACSDiva software (BD Biosciences).
Hemoglobin
Peripheral blood hemoglobin levels (g/dL) were measured on the day of sacrifice by analyzing heparinized whole blood samples in a hematology analyzer (Abaxis, Union City, CA).
Erythrocyte phenotype
Erythrocyte mean corpuscular volume (fL/cell) and red cell distribution width (%) were each measured on the day of sacrifice by analyzing heparinized whole blood samples in a hematology analyzer (Abaxis, Union City, CA). These parameters may be used to differentiate among different categories of anemia, e.g. iron restricted anemia is often manifest as reduced mean corpuscular volume with increased red cell distribution width.
Statistical analysis
Statistical analysis and figure production were performed using GraphPad Prism version 6.05 (GraphPad Software, La Jolla, CA) to calculate one-way analysis of variance for continuous variables. Significance was set at α =0.05 and data were reported as mean ±standard deviation.
Results
Norepinephrine
Urine norepinephrine levels are illustrated in Figure 1. Compared to young rats, aged rats had significantly higher urine norepinephrine levels (ng/mL) at baseline (97 ±71 vs. 27 ±32, p =0.023) and one day after LCHS (420 ±239 vs. 23 ±16, p =0.007). For aged and young rats, urine norepinephrine remained persistently elevated seven days after LCHS when animals were also subjected to CS (359 ±99 vs. 127 ±103, p =0.004), and aged animals had significantly greater norepinephrine levels under these conditions (359 ±99 vs. 212 ±130, p =0.004). Aged rats had higher urine norepinephrine than young rats seven days following LCHS as well, though the difference was not statistically significant (212 ±130 vs. 61 ±9, p =0.090).
Figure 1.

Aged rats had significantly higher urine norepinephrine levels at baseline and one day after lung contusion and hemorrhagic shock (LCHS). For young and aged rats, urine norepinephrine remained persistently elevated seven days after LCHS when animals are also subjected to daily restraint stress (CS), and aged animals had significantly greater norepinephrine levels under these conditions (*p <0.05 vs. age-matched Naïve, **p <0.05 Young vs. Aged).
Bone marrow cellularity
Bone marrow cellularity values are illustrated in Figure 2. At naïve baseline, aged animals had bone marrow cellularity (cells ×106/mL) similar to that of young naïve animals (231 ±55 vs. 218 ±46, p =0.511). Aged animals had bone marrow cellularity similar to that of young animals seven days after LCHS (181 ±37 vs. 202 ±40, p =0.237) and LCHS/CS (168 ±38 vs. 189 ±41, p =0.224). For aged and young animals, bone marrow cellularity after LCHS/CS was significantly lower than age-matched naïve animals.
Figure 2.

Young and aged rats each had significantly decreased bone marrow (BM) cellularity seven days following lung contusion, hemorrhagic shock, and daily restraint stress (LCHS/CS, *p <0.05 vs. age-matched Naïve).
Hematopoietic progenitor growth
Hematopoietic progenitor colony growth is illustrated in Figure 3. Compared to young animals, at naïve baseline, aged animals had decreased growth (colonies/plate) of GEMM colonies (23 ±1 vs. 35 ± 4, p <0.001), BFU-E colonies (44 ±4 vs. 56 ±6, p <0.001), and CFU-E colonies (47 ±4 vs. 65 ±5, p <0.001). CFU-E growth, representing late hematopoietic progenitor growth, remained significantly lower among aged animals following LCHS (40 ±1 vs. 50 ±5, p <0.001) and LCHS/CS (38 ± 3 vs. 44 ±5, p =0.013).
Figure 3.

Aged naïve animals had decreased growth of granulocyte, erythrocyte, monocyte, megakaryocyte (GEMM) (A), blast forming unit – erythroid (BFU-E) (B), and colony forming unit – erythroid (CFU-E) (C) colonies compared to young naïve animals. These differences persisted seven days after lung contusion and hemorrhagic shock (LCHS) and LCHS followed by daily restraint stress (LCHS/CS, *p <0.05 vs. age-matched Naïve, **p <0.05 Young vs. Aged).
Hematopoietic progenitor mobilization
Hematopoietic progenitor mobilization results are illustrated in Figure 4. For both aged and young naïve animals, approximately 1.2% of all peripheral blood cells carried surface makers representing hematopoietic progenitors. Progenitor mobilization was similar between aged and young animals seven days after LCHS (2.2 ±1.2 vs. 2.8 ±1.9, p =0.597) and was significantly lower among aged animals seven days after LCHS/CS (2.5 ±2.4 vs. 5.4 ±1.8, p =0.034).
Figure 4.
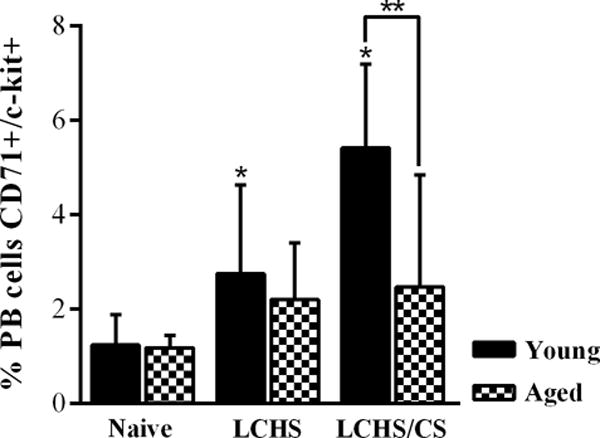
In both young and aged naïve animals, approximately 1-2% of peripheral blood (PB) carried hematopoietic progenitor surface markers. Mobilization of hematopoietic progenitors from the bone marrow to the peripheral blood was significantly increase seven days after lung contusion and hemorrhagic shock (LCHS) and LCHS followed by daily restraint stress (LCHS/CS) for young animals, but not significantly increased among aged animals (*p <0.05 vs. age-matched Naïve, **p <0.05 Young vs. Aged).
Hemoglobin
Hemoglobin levels are illustrated in Figure 5. Aged naïve animals had baseline hemoglobin levels that were significantly higher than young naïve animals (15.2 ±0.9 vs. 14.3 ±0.4, p =0.023), but not significantly higher seven days after LCHS (14.3 ±1.0 vs. 13.4 ±1.2, p =0.096) and LCHS/CS (13.3 ±1.3 vs. 12.3 ±1.2, p =0.0667). For aged and young animals, hemoglobin levels after LCHS/CS was significantly lower than age-matched naïve animals.
Figure 5.

Aged naïve animals had significantly higher baseline hemoglobin levels than young naïve animals, but similar hemoglobin levels seven days after lung contusion and hemorrhagic shock (LCHS) and LCHS followed by daily restraint stress (LCHS/CS). Young and aged animals each had significantly lower hemoglobin levels following LCHS/CS compared to age-matched naïve animals (*p <0.05 vs. age-matched Naïve, **p <0.05 Young vs. Aged).
Erythrocyte phenotype
Erythrocyte phenotype parameters are illustrated in Figure 6. Aged naïve animals had lower erythrocyte mean corpuscular volume compared to young naïve animals (48 ±3 vs. 59 ±5, p <0.001), and this difference persisted levels seven days after LCHS (47 ±1 vs. 59 ±3, p <0.001) and LCHS/CS (46 ±1 vs. 60 ±4, p <0.001). Red cell distribution width was similar between aged and young animals at naïve baseline (17.0 ±0.6 vs. 16.6 ±1.3, p =0.466), but was significantly greater among aged animals seven days after LCHS (16.9 ±0.3 vs. 16.2 ±0.8, p =0.038) and LCHS/CS (17.4 ±0.2 vs. 16.3 ±1.1, p =0.013).
Figure 6.

Aged naïve animals had lower erythrocyte mean corpuscular volume (MCV) compared to young naïve animals, and this difference persisted levels seven days after lung contusion and hemorrhagic shock (LCHS) and LCHS followed by daily restraint stress (LCHS/CS) (A). Red cell distribution width (RDW) was similar between young and aged animals at baseline, but was significantly greater among aged animals seven days after LCHS and LCHS/CS (B) (*p <0.05 vs. age-matched Naïve, **p <0.05 Young vs. Aged).
Discussion
Our results indicate that aged rats are disproportionately affected by post-injury hypercatecholaminemia and bone marrow dysfunction. At naïve baseline, aged animals had higher urine norepinephrine, decreased hematopoietic progenitor growth, and smaller erythrocytes, though bone marrow cellularity was preserved and hemoglobin levels were slightly higher. Seven days after severe trauma and chronic stress, aged animals had persistently higher urine norepinephrine, had proportionally greater losses in bone marrow cellularity, continued to have lower progenitor growth, and developed an iron-restricted anemia phenotype. Mobilization of hematopoietic progenitors from the bone marrow to peripheral blood affected young animals to a greater degree than aged animals. Therefore, although both groups had significantly lower hemoglobin levels seven days after severe trauma and chronic stress, the mechanisms contributing to persistent-injury associated anemia were different between aged and young animals.
The finding that aged animals had higher naïve basal and peak levels of urine norepinephrine than young counterparts is consistent with previous reports. It has been proposed that this phenomenon may be attributable to increased activity of dopamine-β-hydroxylase, the enzyme that synthesizes norepinephrine (31). In addition, norepinephrine end-organ metabolism may be subject to age-related changes. Uptake of norepinephrine into the heart is greater among aged rats than younger counterparts (32), though the inotropic response to norepinephrine is attenuated in aged animals (33). To our knowledge, the impact of aging on catecholamine levels following traumatic injury has not been previously reported, however, similar results have been reported in non-trauma stress. In a study comparing aged and young Fischer rats, McCarty et al. (10) found that one hour after cold water immersion, norepinephrine levels were significantly higher in the aged rats compared to young rats. In a study comparing elderly and young human subjects, Barnes et al. (11) found that psychologic stress-induced norepinephrine levels were significantly higher among the elderly subjects.
Total bone marrow cellularity among aged animals was preserved, and slightly increased, compared to young animals. This observation was consistent with that of Stelzer et al. (14), who found that the frequency of myeloid progenitor cells in the bone marrow was not significantly different between aged and young Sprague-Dawley rats. Notably, this study also found that aged rats had significantly reduced myeloid progenitor proliferative capacity, similar to our findings. These observations have also been made in human subjects. Human bone marrow cellularity and hematopoietic progenitor counts remain stable or slightly increased with advanced age (34-36). However, functional inhibition is suggested by the finding that both anemic and non-anemic elderly humans have reduced proliferation of CFU-E colonies (36, 37).
In this study, mobilization of hematopoietic progenitors from the bone marrow to peripheral blood was greater in young rats than aged rats. Unfortunately, there is a paucity of previously reported data regarding the effects of aging on hematopoietic progenitor mobilization. In a study of rhesus monkeys, Lee et al. (38) found that there was a greater proportion of circulating erythroid and myeloid progenitors among young animals compared to adult and elderly animals, consistent with our results. In humans, first trimester fetal blood contains high quantities of hematopoietic progenitors, and the quantity appears to decrease with increasing age (39, 40). Therefore, our observation that hematopoietic progenitor mobilization seven days following LCSH/CS was greater in young animals may be attributable to general aging pathophysiology rather than the unique pathophysiology of severe trauma and chronic stress.
Aged animals had significantly greater hemoglobin levels than young animals at naïve baseline. F44-BN animals have been previously shown to have increased hemoglobin levels later in their lifespan, with average hemoglobin 15.3 g/dL at 30 months of age, similar to our observation that 25 month old animals had hemoglobin 15.2 g/dL (22). However, this phenomenon does not occur in humans. The prevalence of anemia among human subjects increases with increasing age, whether considering community-dwelling subjects (6) or critically ill medical and surgical patients (41). It is likely that these trends are observed in trauma patients as well. In a propensity matched analysis comparing trauma patients age 65 or greater to trauma patients age 18-64, we have previously observed that admission hemoglobin levels are significantly lower among the elderly (10.2 ±1.9 vs. 11.3 ±2.0 g/dL, p =0.012, unpublished data). In the present study, the significantly higher baseline hemoglobin level in aged animals was attenuated following LCHS and LCHS/CS, and both groups were significantly anemic seven days after LCHS/CS. The authors believe that injured animals exhibited a mild-moderate anemia rather than severe anemia because compensatory mechanisms to upregulate erythropoiesis were partially effective in mitigating post-injury anemia. In the clinical setting, bone marrow failure alone may not manifest as severe anemia requiring red cell transfusion, but it may contribute to severe anemia when considered in the context of acute and chronic blood loss among critically ill trauma patients.
Notably, aged animals had significantly lower mean corpuscular volume and slightly greater red cell distribution width, and these differences were exaggerated by severe trauma and chronic stress. These findings suggest the presence of iron-restricted erythropoiesis the aged animals (42, 43). To our knowledge, the effects of trauma on erythrocyte phenotype in rats have not been previously reported. In humans, the erythrocyte phenotype of anemic and non-anemic subjects varies according to the underlying disease process. In our study, F344-BN rats were used for the aged group because they are less affected by age-related degenerative disease (19-21). However, it is possible that they were affected by anemia of chronic disease, causing iron sequestration and an iron-restricted erythrocyte phenotype. Unfortunately, the use of heparin as an anticoagulant in our plasma samples limits our ability to investigate the iron-sequestration pathway in greater detail.
The major limitations of this study are the small number of animals (n=8-10 per group), the use of different rat strains to represent aged and young groups, and lack of mechanistic detail regarding iron-restricted anemia. Although the sample sizes were small, they were adequate to detect statistically significant differences between groups that were consistent with previously reported findings under different experimental conditions, suggesting that this study was adequately powered. Ideally, aged F344-BN rats would be compared to young F344-BN rats to perform these analyses. Unfortunately, young F344-BN rats were not available when these experiments were being performed. In addition, the young Sprague-Dawley trauma model has been well validated in previous studies (15, 44, 45), establishing a reliable foundation for comparison to aged animals. Future studies should investigate the impact of hematopoietic progenitor mobilization on wound healing and tissue repair and elaborate on the impact of aging on iron-restricted post-injury anemia by incorporating plasma and serum iron studies and analysis of bone marrow iron stores by histology. Because hematopoietic progenitor mobilization appears to be necessary for wound healing and tissue repair (17, 46), and aged animals had less mobilization than young animals in the present study, this phenomenon may have clinically important implications for severely injured elderly trauma patients. In particular, decreasing progenitor mobilization abrogating the neuroendocrine stress response with non-selective beta adrenergic receptor blockers may be ineffective among elderly trauma patients. Further investigation of the impact of aging on post-injury iron dysregulation may elucidate a role for modulating hepcidin to improve iron bioavailability.
Conclusions
Compared to young rats, aged rats were disproportionately affected by post-injury hypercatecholaminemia, impaired hematopoietic progenitor growth, and an iron-restricted red blood cell phenotype at baseline which persisted seven days after injury. Although both groups developed significantly lower hemoglobin levels seven days after severe trauma and chronic stress compared to age-matched naïve animals, the mechanisms contributing to persistent-injury associated anemia appear to be different between aged and young animals. Further research is needed to determine if the clinical approach to persistent injury-associated anemia should differ between elderly and young trauma patients.
Acknowledgments
This work was supported in part by grants R01 GM113945-01 (PAE), R01 GM105893-01A1 (AMM), and P50 GM111152–01 (SCB, CLL, PAE, AMM) awarded by the National Institute of General Medical Sciences (NIGMS). TJL and JCM were supported by a post-graduate training grant (T32 GM-008721) in burns, trauma and perioperative injury by NIGMS.
Footnotes
This work was presented as a poster at the 76th Annual Meeting of AAST in Baltimore, MD on September 13, 2017.
This manuscript has not been submitted elsewhere and the authors have nothing to disclose.
The authors have no relevant conflicts of interest.
Author contributions
TJL, KBK, CSC, SCB, CL, PAE, and AMM contributed to study design. TJL, KBK, CSC, JMP, and JCM contributed to data collection and analysis. All authors contributed to data interpretation and provided critical revisions to the manuscript.
References
- 1.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238(5):748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–60. doi: 10.1097/TA.0000000000000264. discussion 59-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirie K, Myles P, Wood E. Anemia and iron-restricted erythropoiesis in traumatic critical illness. J Trauma Acute Care Surg. 2016;80(3):538–45. doi: 10.1097/TA.0000000000000939. [DOI] [PubMed] [Google Scholar]
- 4.Robinson Y, Hostmann A, Matenov A, Ertel W, Oberholzer A. Erythropoiesis in multiply injured patients. J Trauma. 2006;61(5):1285–91. doi: 10.1097/01.ta.0000240969.13891.9b. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care. 2001;16(1):36–41. doi: 10.1053/jcrc.2001.21795. [DOI] [PubMed] [Google Scholar]
- 6.Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45(4):210–7. doi: 10.1053/j.seminhematol.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281(18):1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261(5558):333–5. doi: 10.1038/261333a0. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JW, Troen BR. Sympathetic nervous system and aging in man. Endocr Rev. 1980;1(2):167–79. doi: 10.1210/edrv-1-2-167. [DOI] [PubMed] [Google Scholar]
- 10.McCarty R. Age-related alterations in sympathetic-adrenal medullary responses to stress. Gerontology. 1986;32(3):172–83. doi: 10.1159/000212785. [DOI] [PubMed] [Google Scholar]
- 11.Barnes RF, Raskind M, Gumbrecht G, Halter JB. The effects of age on the plasma catecholamine response to mental stress in man. J Clin Endocrinol Metab. 1982;54(1):64–9. doi: 10.1210/jcem-54-1-64. [DOI] [PubMed] [Google Scholar]
- 12.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109(4):1736–42. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42(5):385–90. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelzer I, Fuchs R, Schraml E, Quan P, Hansalik M, Pietschmann P, Quehenberger F, Skalicky M, Viidik A, Schauenstein K. Decline of bone marrow-derived hematopoietic progenitor cell quality during aging in the rat. Exp Aging Res. 2010;36(3):359–70. doi: 10.1080/0361073X.2010.484785. [DOI] [PubMed] [Google Scholar]
- 15.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg. 2015;79(1):91–6. doi: 10.1097/TA.0000000000000686. discussion 6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69(2):338–43. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannoush EJ, Sifri ZC, Elhassan IO, Mohr AM, Alzate WD, Offin M, Livingston DH. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma. 2011;71(2):283–9. doi: 10.1097/TA.0b013e318222f380. discussion 9-91. [DOI] [PubMed] [Google Scholar]
- 18.Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of wistar furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol. 2003;14(10):2526–33. doi: 10.1097/01.asn.0000086476.48686.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mech Ageing Dev. 2011;132(1–2):1–7. doi: 10.1016/j.mad.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51(1):B54–9. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker EM, Jr, Nillas MS, Mangiarua EI, Cansino S, Morrison RG, Perdue RR, Triest WE, Wright GL, Studeny M, Wehner P, et al. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36(4):427–38. [PubMed] [Google Scholar]
- 22.Smith DBR. Clinical Chemistry and Hematology Profiles of the Aging Rat. Lab Animal. 1992;21(9):32–45. [Google Scholar]
- 23.Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med. 2013;4(6):624–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015;158(3):595–601. doi: 10.1016/j.surg.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore AV, Bible LE, Livingston DH, Mohr AM, Sifri ZC. Can mesenchymal stem cells reverse chronic stress-induced impairment of lung healing following traumatic injury? J Trauma Acute Care Surg. 2015;78(4):767–72. doi: 10.1097/TA.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67(2):315–21. doi: 10.1097/TA.0b013e3181a5c9c7. discussion 21-2. [DOI] [PubMed] [Google Scholar]
- 27.Linnoila M, Guthrie S, Lane EA, Karoum F, Rudorfer M, Potter WZ. Clinical studies on norepinephrine metabolism: how to interpret the numbers. Psychiatry Res. 1986;17(3):229–39. doi: 10.1016/0165-1781(86)90051-x. [DOI] [PubMed] [Google Scholar]
- 28.Potter WZ, Calil HM, Extein I, Gold PW, Wehr TA, Goodwin FK. Specific norepinephrine and serotonin uptake inhibitors in man: a crossover study with pharmacokinetic, biochemical, neuroendocrine and behavioral parameters. Acta Psychiatr Scand Suppl. 1981;290:152–65. doi: 10.1111/j.1600-0447.1981.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004;5(4):385–93. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 30.Mohr AM, ElHassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, Rameshwar P, Livingston DH. Does beta blockade postinjury prevent bone marrow suppression? J Trauma. 2011;70(5):1043–9. doi: 10.1097/TA.0b013e3182169326. discussion 9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman LS, Ohuchi T, Goldstein M, Axelrod F, Fish I, Dancis J. Changes in human serum dopamine- -hydroxylase activity with age. Nature. 1972;236(5345):310–1. doi: 10.1038/236310a0. [DOI] [PubMed] [Google Scholar]
- 32.Gey KF, Burkard WP, Pletscher A. Variation of the norepinephrine metabolism of the rat heart with age. Gerontologia. 1965;11(1):1–11. doi: 10.1159/000211461. [DOI] [PubMed] [Google Scholar]
- 33.Lakatta EG, Gerstenblith G, Angell CS, Shock NW, Weisfeldt ML. Diminished inotropic response of aged myocardium to catecholamines. Circ Res. 1975;36(2):262–9. doi: 10.1161/01.res.36.2.262. [DOI] [PubMed] [Google Scholar]
- 34.Frisch B, Bartl R. Bone marrow histology in myelodysplastic syndromes. Scand J Haematol Suppl. 1986;45:21–37. doi: 10.1111/j.1600-0609.1986.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 35.Tricot G, De Wolf-Peeters C, Hendrickx B, Verwilghen RL. Bone marrow histology in myelodysplastic syndromes. I. Histological findings in myelodysplastic syndromes and comparison with bone marrow smears. Br J Haematol. 1984;57(3):423–30. doi: 10.1111/j.1365-2141.1984.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 36.Lipschitz DA, Udupa KB, Milton KY, Thompson CO. Effect of age on hematopoiesis in man. Blood. 1984;63(3):502–9. [PubMed] [Google Scholar]
- 37.Hirota Y, Okamura S, Kimura N, Shibuya T, Niho Y. Haematopoiesis in the aged as studied by in vitro colony assay. Eur J Haematol. 1988;40(1):83–90. doi: 10.1111/j.1600-0609.1988.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee CC, Fletcher MD, Tarantal AF. Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatr Res. 2005;58(2):315–22. doi: 10.1203/01.PDR.0000169975.30339.32. [DOI] [PubMed] [Google Scholar]
- 39.Campagnoli C, Fisk N, Overton T, Bennett P, Watts T, Roberts I. Circulating hematopoietic progenitor cells in first trimester fetal blood. Blood. 2000;95(6):1967–72. [PubMed] [Google Scholar]
- 40.Huang S, Law P, Young D, Ho AD. Candidate hematopoietic stem cells from fetal tissues, umbilical cord blood vs. adult bone marrow and mobilized peripheral blood. Exp Hematol. 1998;26(12):1162–71. [PubMed] [Google Scholar]
- 41.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, Investigators ABC Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 42.Keel SB, Abkowitz JL. The microcytic red cell and the anemia of inflammation. N Engl J Med. 2009;361(19):1904–6. doi: 10.1056/NEJMcibr0906391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185(10):1049–57. doi: 10.1164/rccm.201110-1915CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alamo IG, Kannan KB, Ramos H, Loftus TJ, Efron PA, Mohr AM. Clonidine reduces norepinephrine and improves bone marrow function in a rodent model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2016 doi: 10.1016/j.surg.2016.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alamo IG, Kannan KB, Bible LE, Loftus TJ, Ramos H, Efron PA, Mohr AM. Daily propranolol administration reduces persistent injury-associated anemia after severe trauma and chronic stress. J Trauma Acute Care Surg. 2017;82(4):714–21. doi: 10.1097/TA.0000000000001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannoush EJ, Elhassan I, Sifri ZC, Mohr AA, Alzate WD, Livingston DH. Role of bone marrow and mesenchymal stem cells in healing after traumatic injury. Surgery. 2013;153(1):44–51. doi: 10.1016/j.surg.2012.06.020. [DOI] [PubMed] [Google Scholar]


