Abstract
The rare, chronic, autosomal-recessive lysosomal storage disease Niemann-Pick disease type C1 (NPC1) is characterized by progressively debilitating and ultimately fatal neurological manifestations. There is an urgent need for disease-modifying therapies that address NPC1 neurological pathophysiology, and passage through the blood-brain barrier represents an important consideration for novel NPC1 drugs. Animal investigations of 2-hydroxypropyl-β-cyclodextrins (HPβCD) in NPC1 in mice demonstrated that HPβCD does not cross the blood-brain barrier in significant amounts but suggested a potential for these complex oligosaccharides to moderately impact CNS manifestations when administered subcutaneously or intraperitoneally at very high doses; however, safety concerns regarding pulmonary toxicity were raised. Subsequent NPC1 investigations in cats demonstrated far greater HPβCD efficacy at much lower doses when the drug was administered directly to the CNS. Based on this, a phase 1/2a clinical trial was initiated with intrathecal administration of a specific, well-characterized mixture of HPβCD, with a tightly controlled molar substitution specification and a defined molecular “fingerprint” of the different species. The findings were very encouraging and a phase 2b/3 clinical trial has completed enrollment and is underway. In addition, phase 1 clinical studies utilizing high-dose intravenous administration of a different HPβCD are currently recruiting. Independent studies are needed for each product to satisfactorily address questions of safety, efficacy, dosing, and route of administration. The outcomes cannot be assumed to be translatable between HPβCD products and/or routes of administration.
Keywords: 2-hydroxypropyl-β-cyclodextrins, blood-brain barrier, cyclodextrins, intraperitoneal, intrathecal, lysosomal storage disease, neurodegenerative, Niemann-Pick disease type C, subcutaneous
1. Introduction
Niemann-Pick disease type C1 (NPC1) is an autosomal-recessive, rare lysosomal storage disease caused by mutations in the NPC1 gene that lead to endo-lysosomal accumulation of cholesterol and other lipids [1, 2]. It is a neurovisceral disease with clinical manifestations dominated by progressive and ultimately fatal neurodegenerative involvement [1, 2]. There are no approved pharmacologic drugs for the treatment of NPC1 in the United States [3]. Miglustat, a partial inhibitor of the synthesis of some glycosphingolipids, has been approved for NPC1 in several countries outside the United States and is used off-label in the United States [4]. Miglustat reduces the substrates that eventually lead to endosomal accumulation but has no effect on the cholesterol deposits, and has limited efficacy in slowing the worsening of neurological manifestations [3]. Currently, patient management is primarily focused on supportive therapy and relief of symptoms [4-6]. As neurodegenerative features are the predominant, most debilitating, and life-threatening features of NPC1 [2], there is a need for therapies that reach the central nervous system (CNS) to address neurological pathophysiology [3, 7, 8].
The blood-brain barrier (BBB) is a complex biological system that selectively permits movement of substances from the blood to the CNS. Most pharmacologic agents are restricted from crossing the BBB in substantial amounts; thus the BBB represents a key challenge in developing drugs that can access the brain and CNS to treat neurological pathophysiology such as that found in NPC1 [9-11]. Drugs that are not administered directly to the CNS must contend with nonspecific tissue uptake, elimination, and degradation prior to accessing one of the specific transport mechanisms for crossing the BBB to enter the brain [9]. Prodrug or “Trojan horse” strategies, which employ drug carriers, have been of limited use in delivering therapeutically relevant drug levels to the CNS in many disease settings. For many neurological diseases, including NPC1, direct administration to the CNS, bypassing the BBB, represents the most effective way to deliver therapeutics that act upon neural tissues. Routes of administration include intraperitoneal and subcutaneous injection, where drug is delivered to the abdominal cavity or beneath the dermis of the skin, respectively, and subsequently absorbed into the systemic circulation prior to crossing the BBB. Intravenous administration directly accesses the systemic circulation, but the drug must still cross the BBB. In contrast, intrathecal and intracerebroventricular administration delivers drug directly to the cerebrospinal fluid that bathes the brain and CNS.
Cyclodextrins are composed of glucose units in a ring configuration. The oligosaccharides have the shared features of lipophilic central cavities and hydrophilic outer surfaces that help molecules transport lipids in aqueous environments [10, 12]. For many years, cyclodextrins have been used as pharmaceutical excipients in the formulation of hydrophobic drugs [3, 12]. There are different types of cyclodextrins with different structures and derivatives and as such, different cyclodextrins have different physicochemical properties [12]. Furthermore, cyclodextrins are not produced as single chemical entities, but rather, they are complex mixtures of different chemical species, and variations in production lead to differences in composition of these mixtures [13]. Consequently, different cyclodextrin compositions should not be expected to have the same biological activity and/or clinical safety/efficacy profile [10, 14]. One particular type of cyclodextrin, 2-hydroxypropyl-β-cyclodextrin (HPβCD; Fig. 1), has gained attention as a potential therapeutic intervention for NPC1 [3]. In the United States and European Union, two different HPβCD products, VTS-270 (Vtesse, Inc., Gaithersburg, MD) and Trappsol® Cyclo™ (CTD Holdings, Inc., Alachua, FL) have received orphan drug designations for the treatment of NPC1. VTS-270 is a specific and well-characterized mixture of HPβCD, with a tightly controlled molar substitution specification and a defined molecular “fingerprint” of the different chemical species present in the mixture based on Kleptose® HPB (Roquette Pharma, France). Highly encouraging results were seen in a phase 1/2a clinical trial of VTS-270 [15] and VTS-270 is currently being studied in a global pivotal phase 2b/3 clinical trial (NCT02534844) as a treatment for the neurological manifestations of NPC1. The trial is now fully enrolled. Additionally, recruitment has recently been announced for investigation of Trappsol Cyclo in a phase 1 and a phase 1/2 study. Due to their relatively large size and the physicochemical properties, HPβCD products are not expected to passively cross the BBB in substantial amounts [16] and thus, questions surrounding the ability of HPβCD to cross the BBB are central to the understanding of potential efficacy in the NPC setting. The purpose of this review is to examine the key issues surrounding HPβCD as related to the BBB and route of administration in the treatment of NPC1.
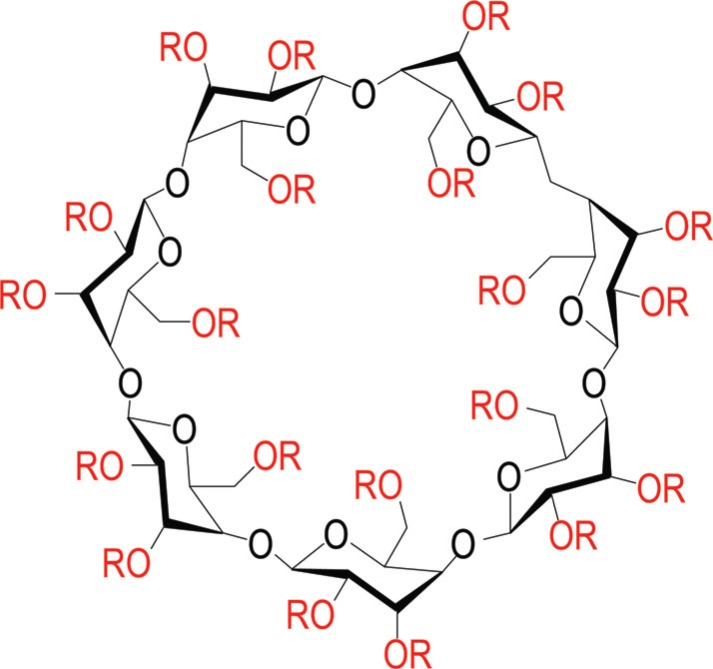
Fig. (1).
Structure of 2-hydroxypropyl-β-cyclodextrin (HPβCD). There are 21 sites (red) on the large β cyclodextrin ring that are potential substitution sites for condensation with propylene oxide to yield various species of HPβCD with different degrees of substitution, depending upon how many sites are substituted. HPβCD products exist as complex mixtures of different chemical species with varying degrees of substitution, not as a single chemical structure with set numbers and positions of substitutions. Variations in proprietary production processes lead to differences in composition of these mixtures. Consequently, different cyclodextrin products should not be expected to have the same formulation, biological activity, and/or clinical safety/efficacy profile. R = H or CH2-CHOH-CH3. (The color version of the figure is available in the electronic copy of the article).
2. Animal Investigations of HPβCD and the BBB
Initial observations and suggestions that HPβCD could potentially have an impact on NPC1 disease came from investigations in mice and cats (Table 1) [7, 8, 16-20].
Table 1.
Animal studies of different HPβCD products in the NPC1 setting.
| Citation |
Route of
Administration |
HPβCD Dose, Frequency, and Duration |
Key BBB
Penetration Findings |
Key NPC1 Safety/Efficacy Findings |
|---|---|---|---|---|
| NPC1-/- Mouse Model | ||||
| Camargo, 2001a | Intraperitoneal | 100 or 500 mg/kg, three times weekly | No significant penetration |
Small delay of onset of neurological symptoms (~20%); lowered unesterified cholesterol |
| Davidson, 2009b | Subcutaneous, intraperitoneal | 4000 mg/kg every other day Short term: 2 weeks Chronic: to end-stage disease |
Not assessed | Short term: Little to no accumulation of cholesterol or gangliosides; autophagosome marker expression similar to wild type Chronic: Delayed onset of ataxic gait and tremor; increased lifespan; reduced accumulation of cholesterol and glycosphingolipid; reduced markers of neurodegeneration; autophagosome marker expression similar to wild type Safety: Concerns raised about possible pulmonary complications with long-term high-dose subcutaneous treatment |
| Liu, 2009c | Subcutaneous | 4000 mg/kg single injection | Not assessed | Reduced total body cholesterol burden and macrophage activation; improved liver function, Purkinje cell survival; increased lifespan |
| Aqul, 2011d | Subcutaneous; intracerebroventricular infusion | Subcutaneous: 4000 mg/kg every 7 days from day 7 until day 49 Intracerebro-ventricular: 23 mg/kg per day from day 21 until day 49 (along with subcutaneous administration) |
Not assessed | Subcutaneous: Some slowing of neuro-degeneration; increased lifespan Intracerebroventricular: Histology indistinguishable from control mice; all animals remained clinically well HPβCD far more effective when administered directly to the brain |
| Ramirez, 2011e | Subcutaneous | 4000 mg/kg (single dose) | Not assessed directly | HPβCD dose achieving 50% inhibition of unesterified cholesterol synthesis was 2-fold higher in the brain vs the liver |
| Pontikis, 2013f | Intraperitoneal | 10 μCi (single dose) | No significant penetration | Not assessed |
| Lopez, 2014g | Subcutaneous | 4000 mg/kg weekly | Not assessed directly | In adult (49 days old) mice, HPβCD reduced cholesterol in liver and spleen but not brain by 77 days, with only marginal increase in lifespan |
| NPC1 Cat Model | ||||
| Vite, 2015h | Subcutaneous; intrathecal | Subcutaneous: 1000, 4000, or 8000 mg/kg every 7 days Intrathecal: 3.8, 7.5, 15, 30, 60, or 120 mg every 14 days |
No significant penetration |
Subcutaneous: Improved hepatic disease; increased body weight; decreased hepatic cholesterol, sphingomyelin, neutral glycolipids, free sphingosine, and ganglioside storage; improved Purkinje cell survival and increased mean survival time from 21 weeks in untreated cats to 35 weeks at 8000 mg/kg Intrathecal: Delayed cerebellar dysfunction; reduced Purkinje cell loss; normalized cholesterol and sphingolipid levels in the brain; reduced storage of gangliosides; slowed disease progression at 24 weeks of age; at doses ≥30 mg, all cats were still living at the end of the 76-week study period and exhibited only mild to moderate ataxia Safety: Increase in hearing threshold/ototoxicity; subcutaneous doses high enough to reduce neurological disease resulted in pulmonary toxicity |
2.1. Mouse Studies
Camargo et al. found a lack of brain penetration of radiolabeled HPβCD when administered at a dose of 500 mg/kg intraperitoneally 3 times per week that was comparable to that of sucrose (known to not cross the BBB in mice) in NPC1-/- mice. Similarly, sucrose and HPβCD were not detected in the brain of MDR1a-/- mice, which are known for increased BBB permeability for a wide variety of pharmacologic agents [16]. Penetration by sucrose and HPβCD was only achieved in the presence of RMP-7, a bradykinin agonist that relaxes the tight junctions of the BBB. In that study, the authors concluded that HPβCD was not significantly penetrating the BBB [16]. However, HPβCD did lower the levels of cholesterol in the liver and delayed the onset of neurological signs (as measured by limb tremor) by 20% [16]. This suggested a potential for some, albeit modest, degree of neurological efficacy in this mouse model without substantial penetration of the BBB. This raised the possibility that direct administration to the CNS could improve neurological efficacy, although the reported initial attempts to investigate CNS delivery were confounded by technical difficulties [16].
In a later study in 2009 by Davidson et al., NPC1-/- mice were given subcutaneous or intraperitoneal injections of HPβCD at a dose of 4000 mg/kg on postnatal day 7 or shortly after weaning. They were dosed every other day and followed for signs of end-stage disease [17]. This chronic treatment with HPβCD led to a moderate delay in the onset of clinical signs, an increase in lifespan, a reduction in neuronal cholesterol and ganglioside accumulation, reduced neurodegeneration, and normalization of markers of lysosomal inflammation [17]. Based on lung histopathology, concerns were raised about the possibility of pulmonary complications with long-term, high-dose, subcutaneous administration of HPβCD [17]. Although the treatment had moderate beneficial effects on CNS neurons, the authors acknowledged that the mechanisms by which the effects occurred were unknown because HPβCD was not administered directly to the CNS and would not be expected to cross the BBB [17]. Also in 2009, Liu et al. found that a single subcutaneous injection of HPβCD at a dose of 4000 mg/kg in 7-day-old NPC1-/- mice suggested efficacy via reduced total body burden of cholesterol, reduced neurodegeneration, and increased lifespan [18].
Subsequently, in 2011, Aqul et al. found that when NPC1-/- mice were treated with 4000 mg/kg HPβCD via weekly subcutaneous injection from 7 to 49 days of age, neurodegeneration was slowed. When mice were treated via the same subcutaneous injection from 7 to 49 days of age together with continuous intracerebroventricular injections from 21 to 49 days of age into the left ventricle of the brain at a dose of 23 mg/kg of body weight, the histology in all regions of the CNS was indistinguishable from that of control mice [19]. This suggested that HPβCD is far more effective in reversing the lysosomal transport defect in the NPC1-/- mice when delivered directly to the brain, presumably due to the inability of HPβCD to traverse the BBB. This was supported by another study in 2011 by Ramirez et al., who also investigated subcutaneous injection of HPβCD at a single dose of 4000 mg/kg in NPC1-/- mice. Here, the investigators found that the dose of HPβCD that resulted in 50% inhibition of unesterified cholesterol synthesis in the brain was 2 log units higher than the dose that resulted in a 50% inhibition of cholesterol synthesis in the liver [8]. This underscored the very low rate of BBB penetration of HPβCD in this animal model of NPC1. Furthermore, it was realized that direct CNS administration to circumvent the BBB could have significantly greater impact on CNS manifestations of NPC1.
To better understand these early findings of efficacy, Pontikis et al. examined the ability of HPβCD to cross the BBB in NPC1-/- mice using a single-dose in situ brain perfusion and a multi–time-point regression analysis after intraperitoneal injection [7]. These techniques are well-established for determining brain uptake of slowly or rapidly penetrating tracers [7]. No significant penetration of labeled HPβCD into any of the examined brain regions was found [7]. However, analysis of the volume of distribution of HPβCD in wild-type mice suggested significant binding of HPβCD to the brain vasculature [7]. One suggestion for the observed moderate neurological efficacy findings of HPβCD administered by intraperitoneal or subcutaneous injection in neonatal mice was that the BBB might be more permeable earlier in life. However, the study by Pontikis et al. found that the BBB was just as robust in 7-day-old NPC1-/- mice as in adult NPC1-/- mice, and the authors noted that other studies using a range of other tracers have refuted non-specific permeability of the neonatal BBB in general [7].
In 2014, Lopez et al. investigated the effects of subcutaneous administration of 4000 mg/kg HPβCD weekly in adult (49 days old) NPC1-/- mice in which large amounts of cholesterol had accumulated in various tissues and organs [21]. They found cholesterol reductions in the liver and spleen and improved liver function. However, there was no reduction of cholesterol accumulation in the brain or lungs through 77 days and only a marginal effect on lifespan (increase of 5 days) compared with saline-treated NPC1-/- control mice [21]. Together, these data on HPβCD in the NPC1-/- mouse model suggest limited penetration of the BBB, with a modest degree of neurological efficacy when administered subcutaneously or intraperitoneally in very young mice but not in older mice. Conversely, there is much greater efficacy when the drug is administered directly to the CNS.
2.2. Cat Studies
Later investigations of HPβCD for the potential treatment of NPC1 involved a model in cats bearing a naturally occurring missense mutation in NPC1 with clinical, neuropathologic, and biochemical abnormalities similar to those observed in humans with NPC1 disease [20, 22]. Progression of the disease is similar to that in humans and most closely parallels the disease in children [23].
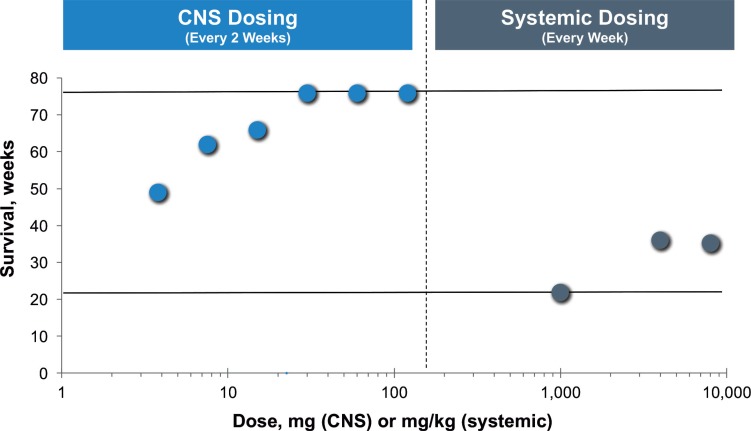
In 2015, Vite et al. investigated the effects of HPβCD in this cat model in an approach that compared NPC1 disease amelioration achieved with subcutaneous injection at weekly doses of 1000, 4000, and 8000 mg/kg and intrathecal administration at the cerebromedullary cistern at doses ranging from 3.8 to 120 mg every other week [20]. Subcutaneous administration was found to improve hepatic disease, but pulmonary toxicity was observed with the very high doses needed to elicit CNS effects [20]. At the two highest subcutaneous doses, only very low levels of HPβCD (approximately 20 μg/mL) were detected in the brain when measured 60 minutes after administration [20]. Subcutaneous administration resulted in a low brain/plasma ratio of 0.7% and low serum/cerebrospinal fluid ratio of 0.3% for HPβCD. This was similar to what has been observed for HPβCD in mice, and the low serum/cerebrospinal fluid ratio of HPβCD was similar to that of human albumin, which does not cross the BBB in substantial amounts [20, 24, 25]. These findings indicate poor penetration of HPβCD across the BBB. Upon direct administration to the CNS via intrathecal injection, HPβCD was found to reach not just the bathing surfaces of the brain, but also to penetrate deeply into the cerebellum and cerebrum at high concentrations [20]. HPβCD that was administered intrathecally prevented the onset of cerebellar dysfunction in presymptomatic cats for over a year, reduced Purkinje cell loss, and normalized cholesterol and sphingolipid levels in the brain [20]. In untreated NPC1 cats (n=39) in this study, the mean survival time was 21 weeks, whereas in the group of NPC1 cats that were treated with the maximal subcutaneous dose of 8000 m/kg (n=5), the mean survival increased marginally to 35 weeks (Fig. 2) [20]. The survival time observed for cats treated subcutaneously with HPβCD was similar to that of NPC1 cats treated with miglustat [26]. In marked contrast, all the NPC1 cats that were treated intrathecally with ≥30 mg HPβCD (n=26) were still alive at the end of the 76-week study period (Fig. 2) [20]. Male NPC1 cats from the 120-mg group were still alive at 2 years of age and were breeding successfully [20]. Other HPβCD-treated NPC1 cats have survived for over 4 years (Vite, personal communication). Even after late onset of the disease, intrathecally administered HPβCD slowed the progression of symptoms and significantly increased survival time [20].
Fig. (2).
Effects of CNS and systemic HPβCD dosing on survival time in NPC1 cats. CNS dosing was intrathecal (n=27); systemic dosing was subcutaneous (n=13). All NPC1 cats that were treated intrathecally with ≥30 mg HPβCD were still alive at the end of the 76-week study period. Upper line indicates maximum follow-up time in study (76 weeks). Lower line indicates the average lifespan of untreated NPC1 cats in the study (21 weeks). The 1000 mg/kg systemic dose was given with 25 mg/kg allopregnanolone.
The data from the Vite cat studies support the results observed in the mouse studies wherein much higher doses were needed to affect CNS disease manifestations compared with peripheral disease manifestations when HPβCD was administered subcutaneously. This is most likely due to the inability of therapeutically relevant amounts of HPβCD to traverse the BBB. Data from the cat model also underscore the increased safety/toxicity concerns that arise when very high subcutaneous doses are used to elicit only moderate neurological responses. These data from the cat model support the results from the mouse model that while there may be some very modest neurological efficacy of HPβCD when administered subcutaneously, it is relatively very minimal compared with the neurological efficacy observed when administered directly to the CNS. It is not known if the efficacy observed when HPβCD is administered via subcutaneous or intraperitoneal injection is due to a direct effect arising from the small amount of drug that does permeate the BBB or to some other indirect mechanism. Overall, the evidence strongly supports that direct administration to the CNS ameliorates the neurological manifestations of NPC1 disease, even after disease onset, and also avoids the need for the high serum concentrations that were associated with pulmonary toxicity when administered systemically.
3. Clinical Investigations and Case Reports of HPβCD in NPC1
Given the promising findings of a potential for HPβCD efficacy in the animal studies of NPC1, attention began to turn to treating humans [3]. Based on United States Food and Drug Administration (FDA) individual compassionate use investigational new drug (IND) programs, twin 3-year-old girls were initially treated with intravenous HPβCD in 2009 [3, 27]. Escalating doses were administered twice weekly over an 8-hour infusion with a maximum dose of 2900 mg/kg for more than a year and were well tolerated. However, due to continued disease progression while receiving HPβCD intravenously, the patients later began receiving intrathecal HPβCD in 2010 [3, 27]. In 2011, a collaborative approach was established among multiple departments of the NIH, academic scientists, non-profit organizations, and industry to evaluate and accelerate the development of HPβCD as a candidate treatment for NPC1 [3]. Selection of the route of administration for clinical trials in humans was a key first step in the development program of HPβCD for NPC [3]. An overview of these and other clinical studies of NPC1 in humans is shown in Table 2. Given the results of the nonclinical animal investigations, a multidisciplinary team of experts determined that administration directly into the CNS represented the safest and most effective approach to ameliorating the neurological symptoms of NPC1 by overcoming the challenges of BBB penetration [3]. This led to the initiation of a phase 1/2a clinical trial in the United States to investigate the effects of intrathecally administered HPβCD.
Table 2.
Clinical studies of different HPβCD products in humans with NPC1.
| ClinicalTrials. gov Identifier | Drug and Dose |
Route of
Administration |
Na |
Phase and
Objectives |
Duration | Location | Sponsor | Status |
|---|---|---|---|---|---|---|---|---|
| NCT01747135 | VTS-270 50–1200 mg |
Lumbar IT infusion monthly | 14 | Phase 1/2a Safety, tolerability, PK, and dose-finding |
12–18 months | US | Vtesse, Inc. Collaborators: Eunice Kennedy Shriver National Institute of Child Health and Human Development |
Complete: results indicate acceptable safety profile and evidence for neurological efficacyb |
| NCT02534844 | VTS-270 900, 1200, and 1800 mg |
Lumbar IT infusion EOW | 51 | Phase 2b/3 Pivotal safety and efficacy |
Parts A & B: 52 weeks; with part C open-label extension | Australia, France, Germany, Spain, Turkey, UK, and US | Vtesse, Inc. | Part A complete; 900-mg dose selected for parts B&C; Part B fully/over enrolled |
| NCT02939547 | Trappsol Cyclo 1500 or 2500 mg/kg |
IV infusion EOW | 12 | Phase 1 Safety and PK |
20 weeks | US | CTD Holdings | Recruiting |
| NCT02912793 | Trappsol Cyclo 1500, 2000, or 2500 mg/kg |
IV infusion EOW | 12 | Phase 1/2 Safety, PK, preliminary efficacy |
56 weeks | UK, Italy, and Sweden | CTD Holdings | Recruiting |
EOW, every other week; IT, intrathecal; IV, intravenous; NPC1, Niemann-Pick disease type C1; PK, pharmacokinetics.
aEstimated/current enrollment.
b[15].
3.1. Clinical Studies
The phase 1/2a clinical trial (NCT01747135) enrolled 14 NPC1 patients aged 4 to 24 years who had neurological manifestations of the disease [15]. The formulation of HPβCD used was VTS-270, and it was administered monthly directly to the CNS at doses ranging from 50 to 1200 mg. The study demonstrated an acceptable safety profile for VTS-270 and showed evidence for restoration of neuronal cholesterol homeostasis and slowing of neurological disease progression [15]. To date, these 14 patients have been treated monthly for 20 to 40 months, and 3 additional patients have been treated every 2 weeks for 32 to 39 months in a parallel study at Rush University Medical Center.
The promising results of the VTS-270 phase 1/2a study supported the further development of the drug in a late-stage pivotal phase 2b/3 study of clinical safety and efficacy (NCT02534844). The study is a randomized, double-blind, sham-controlled, 3-part, multi-dose trial of patients with neurological manifestations of NPC1. Patients aged 4 to 21 years with NPC1 and onset of neurological symptoms prior to 15 years of age have been recruited, screened, enrolled, and treated with VTS-270 in the United States and multiple other countries; the trial is now fully enrolled. In part A of the study (dose-selection phase), VTS-270 was dosed at 900, 1200, or 1800 mg by intrathecal lumbar administration every other week for 8 weeks in 9 patients, and 3 patients received sham treatment. Part A of the study has completed, and 900 mg has been established as the dose for part B (double-blind phase) and part C (open-label extension). Part B is fully enrolled and includes over 51 patients; all patients completing part B will be eligible to enroll in part C along with the patients who were part of the phase 1/2a trial.
Two additional early development (phase 1/2) clinical trials of a different HPβCD, Trappsol Cyclo, are planned (NCT02939547; NCT02912793); recruitment has begun in the United States and United Kingdom. The stated route of administration is intravenous infusion of doses of 1500 and 2500 mg/kg in one trial and doses of 1500, 2000, and 2500 mg/kg in the other trial. The doses are stated to be below the maximum dose for which there are long-term clinical data in 2 NPC1 patients who were treated with 2800 mg/kg for 3 to 5 years.
3.2. Case Reports
A 2013 report by Matsuo et al. described 2 patients from Japan aged 4 and 14 years who were treated with intravenous HPβCD up to 2500 mg/kg twice per week (first patient) and 2000 mg/kg three times per week (second patient) [28]. The HPβCD used was Kleptose HPB, the same HPβCD that VTS-270 is based upon [14]. Prior to treatment, the first patient began to exhibit rapid neurological deterioration at age 3 years including progressive ataxia, cataplexy, dysarthria, dysphagia, and convulsions; hepatosplenomegaly was also present [28]. The second patient had hepatosplenomegaly at 7 months of age and began to exhibit neurological signs including ataxic gait and frequent falls at 4 years of age, which progressed to include psychomotor deterioration, seizures, and deterioration of mental and motor function through 7 years of age; the patient was bedridden by 13 years of age and had poor spontaneous movements, facial myoclonus, and poor/no blink reflex [28]. Both patients underwent clinical assessments prior to treatment initiation and after 3, 6, and 12 months of treatment [28]. While hepatosplenomegaly was partially ameliorated, effects on neurological function, if any, were partial, transient, and only apparent in the first few months of treatment [28]. The authors concluded that the results were consistent with HPβCD remaining primarily in the systemic circulation, and that delivery of HPβCD directly to the CNS was needed to address the neurological manifestation of the disease [28]. Given the pulmonary toxicity noted previously with high-dose subcutaneous administration in the cat model and in NPC1-/- mice, it may be notable that at 23 months of intravenous HPβCD administration, the younger patient presented with severe aspiration pneumonia followed by fever and pulmonary cloudiness; compromised CNS function may have been a factor in these sequelae in addition to any potential toxicity from the drug [28].
In 2014, Matsuo et al. described HPβCD administration in another Japanese patient, a female child who was diagnosed with NPC1 at 2 months. Prior to treatment, rapid neurological deterioration was exhibited by 3 years of age including ataxia, cataplexy, dysarthria, dysphagia, and convulsions [29]. At 4 years of age, the patient began treatment with intravenous HPβCD (dose and source of HPβCD not specified) but experienced only slight improvement in hepatosplenomegaly with continued worsening of neurological symptoms; she became bedridden and lost the ability to speak [29]. Intrathecal administration of HPβCD at a weekly dose of 450 mg (22.5 mg/kg) was added at the age of 6 years, and intravenous administration was stopped a year later [29]. Although the patient’s NPC1 disease had already progressed substantially and was continuing to do so at a rapid rate by the time intrathecal administration was initiated, the patient maintained neurological function for an additional 2 years, demonstrating disease stabilization in the neuroglial domain with no adverse effects [29].
In 2015, Maarup et al. described intrathecal administration of 200 mg of Kleptose HPB every other week via lumbar puncture in a 12-year-old male with mild NPC in the United States. After 1.5 years, neurological disease manifestations were stabilized, with some improvement in some neurological domains including supranuclear gaze palsy and NPC1 severity score; high-frequency hearing loss was an expected observation attributable to the drug based on animal studies [30].
More recently, in 2016, Garcia-Robles reported on 2 adult female patients in Spain who received CNS administration of Trappsol [31]. The first patient was diagnosed with NPC1 at 49 years of age and presented with progressive symptoms of ataxia, dysarthria, abnormal behavior, supranuclear gaze palsy, and hepatosplenomegaly [31]. She received Trappsol every 2 weeks by lumbar puncture at 4 doses escalating from 175 to 700 mg [31]. Treatment was well tolerated, but after 2 months of treatment no significant neurological changes or changes in the NPC severity scale were observed [31]. The second patient displayed symptoms of NPC1 as early as 14 years of age and was diagnosed at 30 years of age. Symptoms included progressive deterioration of executive function, loss of vocabulary, inability to count or calculate, impaired balance, clumsiness, hand tremors, dysphagia, and splenomegaly [31]. The patient received Trappsol every other week for a total of 8 doses ranging from 175 to 875 mg (an Ommaya reservoir was implanted after the first 2 doses due to chemical meningitis after the second dose) [31]. The patient experienced a range of adverse events including 2 incidences of toxic meningitis, aspiration pneumonia, febrile syndrome, and candidiasis [31]. While the authors noted that many of these adverse reactions could have been due to the method of administration and not to Trappsol, upon analysis using the Naranjo algorithm, the authors concluded that the meningitis was likely to have been caused by Trappsol [31]. No substantial improvement or slowing of disease progression was observed, and the patient died 4.5 months after discontinuing Trappsol [31]. The authors noted that the lack of efficacy observed in both patients could have been related to the late onset and severity of NPC disease when Trappsol was initiated and/or to the short duration of treatment [31].
4. Considerations and Future Perspectives
Findings from the animal models of NPC1, early clinical studies, and case reports of patients with NPC1 all suggest that HPβCD does not readily cross the BBB in therapeutically relevant amounts. Therefore, direct administration to the CNS is likely to be the only treatment modality expected to have a marked effect on NPC1 neurological manifestations. There are, however, considerations and understandable concerns regarding administration of HPβCD directly to the CNS, especially in pediatric patients, due to the invasiveness of such procedures, the perceived patient discomfort, and the potential safety concerns, such as CNS infection. When administering drugs directly to the CNS, it is critical that the agent being administered is highly purified and well characterized in order to avoid introducing any contaminants and/or unknown agents that could adversely affect neurological development and/or function. While intravenous administration is less invasive, very high doses of HPβCD are expected to be required to achieve even a small impact on neurological NPC1 manifestations, and it has not been demonstrated if a meaningful impact on disease can be achieved via this route of administration. Furthermore, the high doses of HPβCD needed for IV treatment may be associated with safety concerns, such as pulmonary complications noted in the mouse and cat studies and in the case reports [17, 20, 28]. While intravenous infusions may be less invasive than direct CNS administration, infusion times can be protracted (lasting 8 hours in many cases), which can be challenging, particularly in a young patient population. Audiological pathology is a known component of NPC1 disease [32], and in the animal models and the phase 1/2a human clinical trial, ototoxicity (high-frequency tone hearing loss), has been reported following administration of HPβCD. In the cat model, ototoxicity occurred with both intrathecal administration and subcutaneous administration [20, 22]. The high-frequency hearing loss experienced by some patients in the phase 1/2a study is being managed with hearing aids [15]. Clinical studies are currently underway investigating the safety and potential toxicity (generalized and local) of intrathecal and intravenous administration of HPβCD and their potential relationship with dose so as to optimize the safest, most efficacious dose for patients with NPC1. The completed phase 1/2a study of intrathecally administered HPβCD was a dose-escalation study wherein dose was advanced based on safety and tolerance data from higher-dose cohorts and helped to inform doses to be explored in the ongoing phase 2b/3 study [15]. Ongoing phase 1 and phase 1/2 studies of intravenous HPβCD will also explore several doses (Table 2).
As noted earlier, cyclodextrins are complex mixtures of different chemical species and different cyclodextrin products are therefore not the same as one another. This holds true for HPβCD, as differences in production by different manufacturers result in differences in the complex mixtures of similar, but not identical, chemical species. In the animal studies, a range of HPβCD products/suppliers was employed within and among the studies. Thus, direct and/or quantitative comparisons of results should be approached with caution. VTS-270 is currently the only HPβCD with an extensive preclinical safety and toxicology dataset specifically for this particular form of HPβCD. Use of VTS-270 and Trappsol Cyclo has been described in case reports as noted earlier. A recent mass spectrometry study demonstrated significant compositional differences between VTS-270 and another HPβCD product: although both are HPβCDs, they have unique compositional fingerprints and differences in the amounts and types of various ions, monomers, and dimers, and thus should not be considered to be identical [33]. They may also have different biological and clinical activities and different impurity profiles. Importantly, anecdotal reports, case reports, and the results of the completed clinical trials for one product should not be assumed to be translatable to other products, as seen in the Garcia-Robles 2016 report [31]. In other examples of pharmaceutical agents containing different mixtures of similar but non-identical chemical species, separate pivotal studies were required for each for FDA approval [34]. In looking forward to ongoing and planned clinical studies, it is important to understand that any demonstration of safety and/or efficacy by one product for any given route of administration should not be assumed to be translatable to another product or another route of administration. Studies are needed for each product to satisfactorily address safety, efficacy, dosing, and route of administration questions.
Conclusion
There is an unmet medical need for disease-modifying therapies that address NPC1 neurological manifestations. Consequently, the BBB represents an important consideration for novel NPC1 drugs. Based upon physicochemical properties, findings in animal models, early clinical studies, and patient case reports, the evidence to date suggests that HPβCD does not cross the BBB in therapeutically relevant amounts in the NPC1 setting. Administration directly to the CNS is expected to provide the greatest NPC1 neurological efficacy; this concept was supported by a phase 1/2a clinical study. Conversely, intravenous administration is not expected to have a clinically meaningful benefit on the CNS manifestations of NPC1 and has not yet been prospectively studied in NPC1 patients. Clinical trials of different HPβCD agents are currently underway and the route of administration is an important point of consideration for the anticipated results of these trials with regard to safety, tolerability, and efficacy in the NPC1 population.
Consent for Publication
Not applicable.
Acknowledgements
Medical writing assistance was provided by Peloton Advantage, Parsippany, NJ, and was funded by Vtesse, Inc., Gaithersburg, MD.
CONFLICT OF INTEREST
Pericles Calias was a consultant to, and a member of, the preclinical scientific advisory board for Vtesse Inc. at the time of article development and is currently an employee of Sucampo Pharmaceuticals, Inc., which acquired Vtesse in April 2017.
references
- 1.Vanier M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanier M.T. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2015;38(1):187–199. doi: 10.1007/s10545-014-9794-4. [DOI] [PubMed] [Google Scholar]
- 3.Ottinger E.A., Kao M.L., Carrillo-Carrasco N., et al. Collaborative development of 2-hydroxypropyl-beta-cyclodextrin for the treatment of Niemann-Pick type C1 disease. Curr. Top. Med. Chem. 2014;14(3):330–339. doi: 10.2174/1568026613666131127160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papandreou A., Gissen P. Diagnostic workup and management of patients with suspected Niemann-Pick type C disease. Ther. Adv. Neurol. Disorder. 2016;9(3):216–229. doi: 10.1177/1756285616635964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alobaidy H. Recent advances in the diagnosis and treatment of niemann-pick disease type C in children: a guide to early diagnosis for the general pediatrician. Int. J. Pediatr. 2015;2015:816593. doi: 10.1155/2015/816593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson M.C., Hendriksz C.J., Walterfang M., Sedel F., Vanier M.T., Wijburg F. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol. Genet. Metab. Rep. 2012;106(3):330–344. doi: 10.1016/j.ymgme.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Pontikis C.C., Davidson C.D., Walkley S.U., Platt F.M., Begley D.J. Cyclodextrin alleviates neuronal storage of cholesterol in Niemann-Pick C disease without evidence of detectable blood-brain barrier permeability. J. Inherit. Metab. Dis. 2013;36(3):491–498. doi: 10.1007/s10545-012-9583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez C.M., Liu B., Aqul A., et al. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 2011;52:688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vecsernyes M., Fenyvesi F., Bacskay I., Deli M.A., Szente L., Fenyvesi E. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014;45(8):711–729. doi: 10.1016/j.arcmed.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Calias P., Banks W.A., Begley D., Scarpa M., Dickson P. Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacol. Ther. 2014;144(2):114–122. doi: 10.1016/j.pharmthera.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Loftsson T., Jarho P., Masson M., Jarvinen T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005;2(2):335–351. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- 13.Pitha J., Milecki J., Fales H., Pannell L., Uekama K. Hydroxypropyl-B-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 1986;29:73–82. [Google Scholar]
- 14.Davidson C.D., Fishman Y.I., Puskas I., et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016;3(5):366–380. doi: 10.1002/acn3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ory D.S., Ottinger E.A., Farhat N.Y., et al. Intrathecal 2-hydroxypropyl-B-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial. Lancet. 2017;390(10104):1758–1768. doi: 10.1016/S0140-6736(17)31465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo F., Erickson R.P., Garver W.S., et al. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70(2):131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 17.Davidson C.D., Ali N.F., Micsenyi M.C., et al. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4(9):e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Turley S.D., Burns D.K., Miller A.M., Repa J.J., Dietschy J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc. Natl. Acad. Sci. USA. 2009;106(7):2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aqul A., Liu B., Ramirez C.M., et al. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011;31(25):9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vite C.H., Bagel J.H., Swain G.P., et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci. Transl. Med. 2015;7(276):276ra26. doi: 10.1126/scitranslmed.3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez A.M., Terpack S.J., Posey K.S., Liu B., Ramirez C.M., Turley S.D. Systemic administration of 2-hydroxypropyl-beta-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function. Clin. Exp. Pharmacol. Physiol. 2014;41(10):780–787. doi: 10.1111/1440-1681.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward S., O’Donnell P., Fernandez S., Vite C.H. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 2010;68(1):52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vite C.H., Ding W., Bryan C., et al. Clinical, electrophysiological, and serum biochemical measures of progressive neurological and hepatic dysfunction in feline Niemann-Pick type C disease. Pediatr. Res. 2008;64(5):544–549. doi: 10.1203/PDR.0b013e318184d2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tortelli B., Fujiwara H., Bagel J.H., et al. Cholesterol homeostatic responses provide biomarkers for monitoring treatment for the neurodegenerative disease Niemann-Pick C1 (NPC1). Hum. Mol. Genet. 2014;23(22):6022–6033. doi: 10.1093/hmg/ddu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks W.A. Developing drugs that can cross the blood-brain barrier: Applications to Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl. 3):S2. doi: 10.1186/1471-2202-9-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein V.M., Crooks A., Ding W., et al. Miglustat improves purkinje cell survival and alters microglial phenotype in feline Niemann-Pick disease type C. J. Neuropathol. Exp. Neurol. 2012;71(5):434–448. doi: 10.1097/NEN.0b013e31825414a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastings C. Request for intrathecal delivery of HPBCD for Niemann Pick Type C patients, Caroline Hastings, M.D. Principal Investigator Department of Pediatric Hematology Oncology Children’s Hospital & Research Center Oakland Submission Date to FDA: August 13, 2010 Oakland. CA: Children's Hospital & Research Center Oakland; 2010. http: //addiandcassi.com/wordpress/wp-content/uploads/Hempel-Cyclodextrin-Intrathecal-FDA-Filing-2010-Aug.pdf [Google Scholar]
- 28.Matsuo M., Togawa M., Hirabaru K., et al. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol. Genet. Metab. Rep. 2013;108(1):76–81. doi: 10.1016/j.ymgme.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo M., Shraishi K., Wada K., et al. Effects of intracerebroventricular administration of 2-hydroxypropyl-beta-cyclodextrin in a patient with Niemann-Pick Type C disease. Mol. Genet. Metab. Rep. 2014;1:391–400. doi: 10.1016/j.ymgmr.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maarup T.J., Chen A.H., Porter F.D., et al. Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol. Genet. Metab. 2015;116(1-2):75–79. doi: 10.1016/j.ymgme.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Robles A.A., Company-Albir M.J., Megias-Vericat J.E., et al. Use of 2 hydroxypropyl-beta-cyclodextrin therapy in two adult Niemann Pick Type C patients. J. Neurol. Sci. 2016;366:65–67. doi: 10.1016/j.jns.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 32.King K.A., Gordon-Salant S., Yanjanin N., et al. Auditory phenotype of Niemann-Pick disease, type C1. Ear Hear. 2014;35(1):110–117. doi: 10.1097/AUD.0b013e3182a362b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yergey A.L., Blank P.S., Cologna S.M., Backlund P.S., Porter F.D., Darling A.J. Characterization of hydroxypropyl-beta-cyclodextrins used in the treatment of Niemann-Pick Disease Type C1. PLoS One. 2017;12(4):e0175478. doi: 10.1371/journal.pone.0175478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M.K. A comparative overview of prescription omega-3 fatty acid products. P&T. 2015;40(12):826–857. [PMC free article] [PubMed] [Google Scholar]