Abstract
Significance: Sirtuins are an evolutionarily conserved family of NAD+-dependent lysine deacylases and ADP ribosylases. Their requirement for NAD+ as a cosubstrate allows them to act as metabolic sensors that couple changes in the energy status of the cell to changes in cellular physiological processes. NAD+ levels are affected by several NAD+-producing and NAD+-consuming pathways as well as by cellular respiration. Thus their intracellular levels are highly dynamic and are misregulated in a spectrum of metabolic disorders including cerebral ischemia. This, in turn, compromises several NAD+-dependent processes that may ultimately lead to cell death.
Recent Advances: A number of efforts have been made to replenish NAD+ in cerebral ischemic injuries as well as to understand the functions of one its important mediators, the sirtuin family of proteins through the use of pharmacological modulators or genetic manipulation approaches either before or after the insult.
Critical Issues and Future Directions: The results of these studies have regarded the sirtuins as promising therapeutic targets for cerebral ischemia. Yet, additional efforts are needed to understand the role of some of the less characterized members and to address the sex-specific effects observed with some members. Sirtuins also exhibit cell-type-specific expression in the brain as well as distinct subcellular and regional localizations. As such, they are involved in diverse and sometimes opposing cellular processes that can either promote neuroprotection or further contribute to the injury; which also stresses the need for the development and use of sirtuin-specific pharmacological modulators. Antioxid. Redox Signal. 28, 691–710.
Keywords: : sirtuins, SIRT, NAD+, cerebral ischemia, preconditioning, cerebral ischemic tolerance
Sirtuins: Function and Background
Sirtuins first sparked interest in 1999, when a member of the sirtuin family known as silent information regulator 2 (Sir2) was shown to modulate lifespan in Saccharomyces cerevisiae (71). Cells carrying a mutant form of Sir2 had a significantly reduced lifespan compared to wild types, whereas strains overexpressing Sir2 exhibited a noticeably increased lifespan (71). It was later shown that the lifespan extension induced by caloric restriction is abolished in strains carrying a mutant Sir2 (100). Since then, there has been an explosion in the study of sirtuins in different model organisms that ultimately linked them to processes beyond aging, including metabolic diseases such as diabetes, cancer, and neurological disorders. For a thorough discussion of these studies, the readers are guided to the following reviews among others that further elaborate on the functions of sirtuins in the context of these metabolic disorders (20, 130, 136, 137, 141, 153, 163).
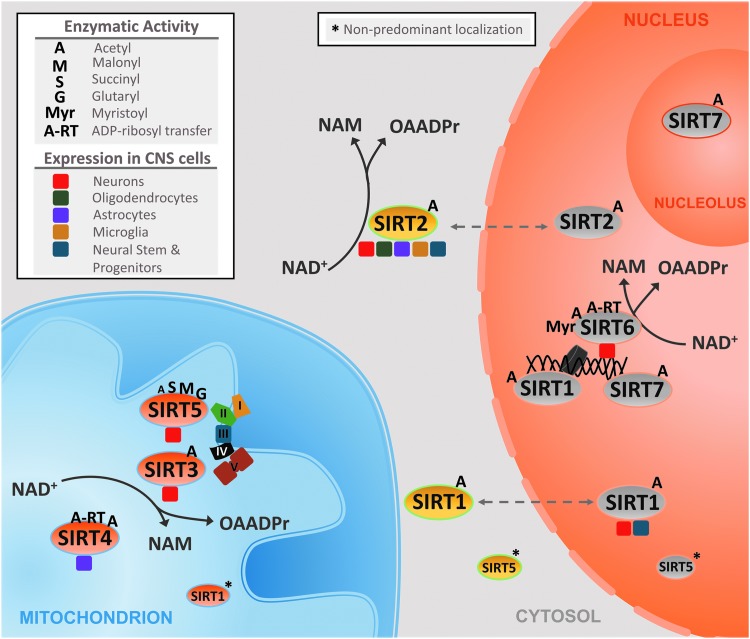
In mammals, the sirtuin family of proteins consists of seven members (SIRT1–7), which share a conserved catalytic domain of 275 amino acids (16). They were originally classified as class III histone deacetylases (HDACs) owing to their ability to remove acetyl groups from lysine residues on histones proteins, yet they are currently known to act on nonhistone proteins as well as to remove additional acyl groups from lysine residues (151). Among the four classes of HDACs, the sirtuins are the only class that requires nicotinamide adenine dinucleotide (NAD+) as a co-substrate to hydrolyze acylated amino acid residues (151). Sirtuins couple the deacylation of amino acid residues to the consumption of NAD+ and as such generate two byproducts, nicotinamide (NAM) and O-acetyl-ADP ribose (OAADPr) (158) (Fig. 1). The removal of acyl groups from the lysine residues of target proteins can affect their enzymatic function, localization, or stability.
FIG. 1.
The enzymatic activities, subcellular localizations, and cell-type specific expression of sirtuins. The figure depicts the predominant, but not exclusive, subcellular localizations of the seven sirtuin members (SIRT1–7) along with their predominant enzymatic activities. The presented subcellular localizations and enzymatic activities reported are not brain specific and represent general known activities for sirtuins studied in different cell types as well as in vitro. The enzymatic activities are depicted by a letter that represents the acyl group removed by the respective sirtuin. Colored boxes reflect the cell-type specific expression of sirtuins reported in the central nervous system. The enzymatic activities of sirtuins involve the coupled deacylation of amino acid residues to the consumption of NAD+ and as such generate two byproducts, NAM and OAADPr or O-acyl-ADP ribose. Asterisks* represents some of the nonpredominant subcellular localizations of sirtuins. NAM, nicotinamide; OAADPr, O-acetyl-ADP ribose.
In addition, not all sirtuin members are primarily or strictly lysine deacetylates (Fig. 1). SIRT1, SIRT2, and SIRT3 show the highest deacetylase activity (4). Yet, side chains of lysine residues could be modified by additional acyl groups that contain varying number of carbon residues, have different structures, or even carry different electrical charges (29). Such acyl groups include formyl, propionyl, butyryl, crotonyl, and succinyl, among others (29). Thus, the sirtuins have a diverse array of enzymatic activities with each member having preferences for specific acyl groups (Fig. 1) (4). In addition to their diverse enzymatic functions, sirtuins also exhibit distinct subcellular localizations (Fig. 1) (4, 113). As such, sirtuins act on a variety of substrates and regulate various cellular processes through their distinct enzymatic functions and subcellular localizations.
Dynamic Regulation of Intracellular NAD+
A feature common to all sirtuin members is their requirement for NAD+ as a cosubstrate (88, 151). NAD+ consists of two nucleotides joined by a phosphate group with one carrying an adenine and the other carrying nicotinamide (17). It exists in two forms, an oxidized form (NAD+) and a reduced form (NADH), with NAD+ being the predominant form under basal conditions (17, 163).
Role of sirtuins as metabolic sensors: reliance on NAD+ as a coenzyme
A crucial function for NAD+ is its role as a coenzyme in cellular redox reactions (Fig. 2) (163). In the cytosol, NAD+ is reduced to NADH during glycolysis, whereas in the mitochondria tricarboxylic acid the TCA cycle enzymes reduce additional NAD+ molecules (163, 181). In the presence of oxygen, reduced NADH molecules get oxidized by the electron transport chain (ETC) to produce the proton gradient necessary for adenosine triphosphate (ATP) synthesis (163, 181). Alternatively, in the absence of oxygen, NADH gets oxidized in the cytoplasm through the reduction of pyruvate to lactate. As such, NAD+ plays a crucial role as a coenzyme necessary for ATP production using both aerobic and anaerobic respiration (163, 181).
FIG. 2.
The dynamic regulation of intracellular NAD+ levels. Intracellular NAD+ levels are highly dynamic and are regulated by NAD+-producing and NAD+-consuming pathways as well as by cellular respiration. Three NAD+-producing pathways have been identified, which are the (i) de novo synthesis pathway (ii) the Preiss–Handler, and (iii) the NAD+-salvage pathway. Each pathway uses different precursor molecules and enzymes for the synthesis of NAD+. The precursor molecules surrounded by boxes are obtained from the diet. Similarly, three NAD+-consuming pathways have been identified and they include the (i) sirtuins (SIRTs) (ii) the PARPs, and (iii) the cADPR synthases known as CD38 and CD157, which are reported to exist both intra- and extracellularly (only depicted as extracellularly in the figure). NAD+ levels are further regulated by cellular redox reactions involved in cellular respiration, where NAD+ plays a role as a coenzyme. Enzymes of glycolysis reduce NAD+ in the cytoplasm, whereas the TCA cycle enzymes reduce additional NAD+ molecules in the mitochondria. Reducing equivalents of cytoplasmic NAD+ are then transferred to the mitochondrion through the G3P and MAS enzymes, ultimately reaching the electron transport chain complexes, where they get oxidized to generate the proton gradient necessary for ATP production. Another important factor regulating NAD+ not depicted in this figure is the circdian regulation of NAD+ by the circadian genes. cADPR, cyclic ADP ribose; G3P, glycerol-3-phoshate; CD38/157, ADP ribosyl cyclase/cyclic ADP ribose hydrolase 38/157; ETC, electron transport chain; MAS, malate–aspartate shuttle; NA, nicotinic acid; NAAD, nicotinic acid adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide (oxidized form); NADH, nicotinamide adenine dinucleotide (reduced form); NADSYN, NAD synthetase; NAM, nicotinamide; NAMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinate phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide mononucleotide adenylyl transferase; NR, nicotinamide riboside; NRK, nicotinamide riboside kinase; PARPs, poly-ADP ribose transferases; QA, quinolinic acid; QPRT, quinolinate phosphoribosyl transferase; TCA cycle, tricarboxylic acid cycle; Trp, tryptophan.
As a result of its involvement in the redox reactions of glycolysis and oxidative phosphorylation, the ratio of oxidized NAD+ to reduced NADH fluctuates with nutrient availability (Fig. 2). NAD+ levels are increased in response to conditions of energetic stress, because of limited energy substrates, such as in the cases of calorie restriction, fasting, and exercise (14, 22, 81, 179). In contrast, NAD+ levels decrease in response to an excess of energy substrates such as in the cases of high-fat diets and type-2 diabetes (77, 186). Since NAD+ acts as a cosubstrate for the sirtuins and its relative concentration fluctuates with energy status, this allows the sirtuins to act as metabolic sensors (31). By sensing the levels of NAD+, sirtuins couple changes in the energy status of the cell to adaptive responses, ultimately regulating metabolic efficiency (31). In addition to being regulated by NAD+, sirtuins have been reported to be negatively regulated NAM, the byproduct of their enzymatic reaction (5, 44). NADH is thought to be an ineffective inhibitor because the concentration required should be in the millimolar range, significantly higher than its physiological levels (101, 145). Thus, the ratio of NAD+/NAM better regulates the sirtuins activity than NAD+/NADH (16).
NAD+-producing and NAD+-consuming pathways
Intracellular NAD+ levels are affected not only by the cell's redox status but also by the rate of NAD+ production and consumption (129). Three pathways have been characterized that synthesize NAD+ (Fig. 2) (16, 163). They are known as (i) de novo synthesis (ii) the Preiss–Handler pathway, and (iii) the NAD+-salvage pathway (16, 163). In the de novo pathway, tryptophan obtained from the diet is converted into quinolinic acid, which feeds into the Preiss–Handler pathway through its conversion into nicotinic acid mononucleotide (NAMN) (89). In the Preiss–Handler pathway, NAMN is converted into nicotinic acid adenine dinucleotide, which is then directly converted to NAD+. The Preiss–Handler pathway can also utilize nicotinic acid (NA; niacin) from the diet as a precursor (16, 163). The third pathway, which is of interest in many metabolic disorders, is the NAD+-salvage pathway (165). It recycles NAM produced by NAD+ breakdown into nicotinamide mononucleotide (NMN) through the action of nicotinamide phosphoribosyltransferase (NAMPT). NMN is then directly converted into NAD+ by NMNAT. Dietary NAM (vitamin B3) and nicotinamide riboside (NR) can feed into the NAD+-salvage pathway (12, 165). Although dietary tryptophan, NR, NA, and NAM are all important precursors, their plasma levels are too low to maintain sufficient NAD+ production, thus it is thought that mammals rely mostly on the NAD+-salvage pathway to preserve intracellular NAD+ (15, 51, 56, 65). To add to its dynamic regulation, intracellular levels of NAD+ oscillate in a circadian manner because of the cyclical expression of the NAMPT enzyme, which is regulated by SIRT1 along with the circadian core genes (123).
Within the cell, pools of NAD+ are found in specific subcellular compartments and regulate compartment-specific pathways (Fig. 2) (46). NAD+ can freely diffuse between the cytosol and nucleus and can cross the outer mitochondrial membrane. However, a transport mechanism is required for NAD+ export and NADH import across the inner mitochondrial membrane (16, 163). Reducing equivalents of NADH entering the mitochondrial matrix are exchanged for NAD+ leaving the matrix through the glycerol-3-phoshate and malate–aspartate shuttles (16, 163). NAM produced in each compartment is recycled back into NAD+ by compartment-specific isoforms of NMNAT and possibly NAMPT enzymes (16, 46, 163).
In addition to its role as a cosubstrate for sirtuins, NAD+ acts as a substrate for poly-ADP ribose transferases (PARPs) as well as cyclic ADP ribose (cADPR) synthases (Fig. 2) (163). The PARP family consists of 17 members in humans. Among them, PARP1 and PARP2 consume the largest amount of NAD+ as they catalyze the transfer of multiple ADP ribose units to target molecules. The remaining members are catalytically inactive or only transfer a single ADP ribose (84). PARP1 and PARP2 play a crucial role in the DNA damage repair (115). Upon DNA damage, these enzymes transfer linear or branched ADP ribose polymers to histones and proteins, which then recruit the DNA repair machinery (84). Depending on the severity of DNA damage, PARPs can either lead to DNA damage repair or to cell death caused by an energy failure because of NAD+ and subsequent ATP depletion (115). In addition to DNA repair, PARPs are involved in several other cellular processes (84, 115). The third family of NAD+-consuming enzymes is the cADPR synthases, or ADP ribosyl cyclases that consist of two proteins CD38 and CD157 that exists both on cell membranes and intracellularly (16, 106, 163). Besides their role in signaling, these enzymes have an ADP ribosyl cyclase activity that allows them to produce cADPR from NAD+ (16, 106, 163). Molecules of cADPR then act as a second messenger to trigger the release of calcium stores from the endoplasmic reticulum (ER) (16, 106).
Thus, NAD+ levels within the cell are highly dynamic and are determined not only by the energy status of the cell that regulates its aerobic and anaerobic respiration rates but also by the balance of the three NAD+-producing and three NAD+-consuming pathways (Fig. 2) (16, 163).
NAD+ in Cerebral Ischemia and Preconditioning
The brain only constitutes around 2% of total body weight yet it consumes around 20% of total body energy (30). This high energy requirement makes the brain highly susceptible to ischemic injuries, which usually results from either an ischemic or hemorrhagic stroke or from cardiac arrests (30, 154, 174). Despite decades of research, cerebral ischemia remains one of the leading causes of adult disability and death in the United States and worldwide (174). Currently, recombinant tissue plasminogen activator (rtPA), a thrombolytic agent, is the sole FDA-approved treatment for ischemic stroke, yet it is administered to <5% of stroke patients (91). Numerous neuroprotective drugs have been tested in the clinic, yet nearly all have failed to promote significant recovery (76, 114). New avenues of research now aim to take advantage of other therapeutic windows as well as novel drug targets to combat cerebral ischemia (47, 75, 157).
During cerebral ischemia, the interruption of blood flow deprives cells of oxygen and glucose, which prevents them from generating sufficient ATP (154). This affects all the energy-dependent processes within the brain, specifically the Na+/K+ ATPase, which is required to maintain the plasma membrane potential (154). The failure of the Na+/K+ ATPase causes cells to depolarize and release excessive amounts of glutamate that activate postsynaptic AMPA and NMDA receptors, causing a massive influx of sodium and calcium (154). The massive increase in calcium initiates a complex cascade of events leading to increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) that interact with and damage cellular components, including nucleic acids (104). DNA damage is then detected by the PARP enzymes, which respond by synthesizing linear and branched forms of poly-ADP ribose units from NAD+ and attach them to histones and nuclear proteins to recruit the DNA repair machinery (7, 104). When modestly activated this leads to DNA repair, but when PARPs are over-activated such as in regions experiencing severe oxidative and nitrosative stresses, they consume as much as 80% of intracellular NAD+ (7, 104). The depletion of NAD+ then impairs the generation of ATP from glycolysis and oxidative phosphorylation, causing an energy failure that could lead to cell death (78, 79, 104, 184). In addition, the reduction in NAD+ limits its availability to other NAD+-consuming enzymes, the sirtuins and the cADPR synthases, both of which play crucial cellular functions that further contributes to the injury (Fig. 3) (16, 163).
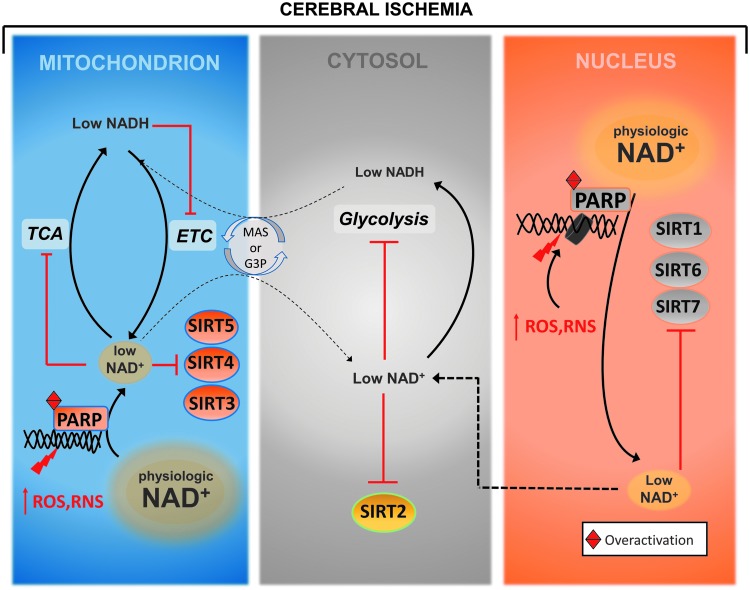
FIG. 3.
NAD+ depletion by PARP enzymes during cerebral ischemia. During cerebral ischemia, levels of ROS, RNS increase in the brain and, in turn, these highly reactive molecules interact with and damage different cellular components including nucleic acids. The DNA damage is then detected by the PARP enzymes that respond by consuming large amounts of NAD+ to synthesize linear and branched forms of poly-ADP ribose units and attach them to histones and nuclear proteins, a signal needed to recruit the DNA repair machinery. When modestly activated, this leads to DNA repair, but when PARPs are over activated such as in regions experiencing severe oxidative and nitrosative stresses, they consume as much as 80% of intracellular NAD+. The depletion of NAD+ then impairs the generation of ATP from glycolysis and oxidative phosphorylation, causing an energy failure that could lead to cell death. In addition, the reduction in NAD+ levels also limits its availability for other NAD+-consuming enzymes, the sirtuins and cADPR synthases, which play crucial cellular functions, thus further contributing to the injury. RNS, reactive nitrogen species; ROS, reactive oxygen species.
Taking into account the importance of maintaining intracellular NAD+ levels during cerebral ischemia, many efforts have been directed toward this approach as a potential therapeutic intervention. Several studies have used PARP inhibitors or transgenic animals lacking PARP to maintain NAD+ levels to protect against the ischemia-induced injuries (7, 10, 156). The successful results of these studies allowed minocycline, an antibiotic with PARP-inhibiting activities, to reach clinical trials for the treatment of stroke (3, 80). Other approaches to maintaining NAD+ levels were made by supplementing NAD+ itself or its precursors (NAM and NMN) before or after the injury (6, 79, 165, 166, 170, 184, 185). Similarly, administering NAMPT activators has also shown successful outcomes in cerebral ischemic models (26, 166).
Preconditioning also constitutes a promising alternative approach that can modulate NAD+ levels. Ischemic preconditioning (IPC) refers to the ability of a mild, noninjurious ischemic insult to protect against a subsequent injurious one (75). Although IPC may not be directly translatable because of the concerns and risks associated with it, IPC in the form remote limb preconditioning constitutes an alternative approach for inducing ischemic tolerance in multiple organs of the body, including the brain (109). Remote IPC is now being extensively studied in clinical trials in the context of several disorders, including cardiovascular and cerebrovascular diseases (60, 90, 96). Similarly, the administration of pharmacological agents has been shown to mimic IPC in a phenomena referred to as pharmacological preconditioning that is of immense therapeutic value as well because of its potential of being translated into the clinic (75). The endogenous mechanisms activated by preconditioning have been the focus of research by our laboratory and others with the aim of identifying novel targets and adaptations that mediate ischemic tolerance (75, 157). Preconditioning is known to increase antioxidant levels that reduce oxidative and nitrosative stresses thus, in turn, reducing DNA damage (95, 124). This approach could be indirectly used to reduce PARP activity and thus preserve intracellular NAD+ pools (156). Furthermore, our laboratory has previously shown that preconditioning stimulates NAMPT expression and increases NAD+ levels in mitochondrial fractions and as such protects against cerebral ischemic injuries (116).
Thus, several studies have shown promising outcomes of NAD+ supplementation in the context of cerebral ischemic injuries, yet because NAD+ plays diverse roles as a coenzyme in redox reactions as well as cosubstrate for three protein families, its supplementation may affect numerous downstream pathways (16, 163). In this review, we focus on the importance of one of its downstream mediators, the sirtuin family of NAD-consuming enzymes, and summarize the studies up to date that has explored their roles in cerebral ischemic injuries and preconditioning.
Sirtuins in Cerebral Ischemia and Preconditioning
All members of the sirtuin family are known to be expressed in the brain, and they display distinct regional, cellular, and subcellular localizations (Figs. 1 and 4). Cerebral ischemia and preconditioning differentially regulate the expression and activity of different sirtuins and, in turn, they play significant roles in determining the outcome of these insults (Fig. 5 and Table 1).
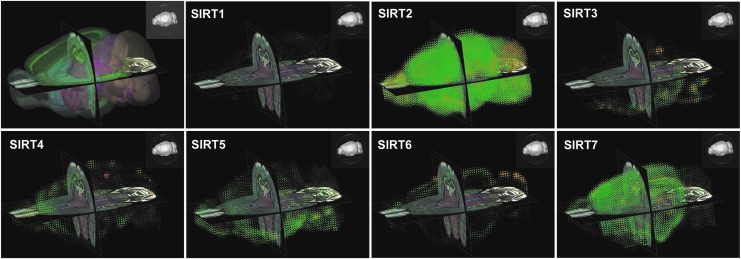
FIG. 4.
The mRNA distribution of sirtuins in a mouse brain reveals region-specific gene expression. Images obtained from the Allen Mouse Brain Atlas-Brain Explorer® 2 based on an ISH experiment targeting sirtuins. Gene-specific antisense probes were used to detect the mRNA distribution of the seven sirtuin members (SIRT1–7) in the brain of a 56-day-old male mouse using sagittal brain sections. The top left image represents the three-dimensional anatomical structures of the mouse brain. Original ISH sagittal images can be viewed on the Allen Brain Atlas website along with the ISH probe sequences used for each experiment. ISH, in situ hybridization.
FIG. 5.
Pathways of cerebral ischemic protection and injury regulated by sirtuins. The figure represents the main pathways regulated by the seven sirtuin members (SIRT1–7) in the context of cerebral ischemic injuries. These pathways were shown to be modulated upon the pharmacological or genetic manipulation of sirtuins using in vitro and in vivo models of cerebral ischemia and, in turn, either promote ischemic protection against the insult or further contribute to it.
Table 1.
Summary of In Vivo Studies Involving the Genetic or Pharmacological Manipulation of Sirtuins in the Context of Cerebral Ischemia
| Sirtuin modulating compound | Treatment | Injury | Method of sirtuin inhibition | Evaluation | Outcome | Targets | Ref |
|---|---|---|---|---|---|---|---|
| Multisirtuin modulation | |||||||
| Resveratrol (10 mg/kg), IPC (2 min BCAo w/HT) | Once 2 days prior, once 2 days prior | ACA | Sirtinol (10 μl of 1 mM) ICV | 7 days post | Reduced hippocampal cell death, improved neurological score | UCP2, mitochondrial function | (36) |
| Curcumin (50 mg/kg) | Once/day for 5 days prior | 2 h tMCAo | Sirtinol (15 mg/kg) IP | 24 h post | Reduced infarct, brain edema, improved neurological score | TNFα, IL-6, mitochondrial function, Bcl-2, p53, Bax | (110) |
| Sirt1 | |||||||
| Hyperbaric oxygen preconditioning (1 h 100% oxygen) | Once/day for 5 days prior | 2 h tMCAo | siRNA (10 μl of 50 nM), ICV | 7 days post | Reduced infarct volume ratio, improved neurobehavioral deficit | Nrf2, HO-1, SOD1 | (177) |
| Melatonin (10 mg/kg) | Once at ischemia onset, once at reperfusion onset | 30 min tMCAo | EX-527 (10 μg) 3x/day for 2 days prior, ICV | 24 h post | Reduced infarct, brain edema, improved neurological score | Bcl2, Bax, mitochondrial function | (180) |
| NAD+–lentiviral NAMPT overexpression (0.5 μl per site of 1 × 109 TU/ml) | 4 sites (cortex and hippocampus) 3 weeks prior | 2 h tMCAo | Sirt1+/− mice | 24 h post | Reduced infarct volume, improved neurological deficit | AMPK, LKB1 | (166) |
| Activator 3 (10 mg/kg) | 10 min, 24 h, 40 h after | pMCAo | Sirt1−/−, Sirtinol (10 mg/kg) 10 min, 24 h, 40 h after IP | 48 h post | Reduced infarct volume | p53, NF-κB | (58) |
| Sirt1 overexpression | N/A | BCAS | N/A | 28 days post | Improved histopathology, spatial working memory | eNOS, cerebral blood flow | (53) |
| Sirt1 overexpression | N/A | 10 min BCAo | Sirtinol (1 mg/kg), IV | 7 days post | less hippocampal damage | Cerebral blood flow | (54) |
| Sirt2 | |||||||
| Sirt2 knockout | immediately after | 45 or 1 h tMCAo | Sirt2−/−, AGK2 (0.764 mg/kg), superficial cervical vein | 24 h post | Reduced infarct, improved neurological deficit | N/A | (176) |
| N/A | 30 min after | 8 min CA/CPR | AGK2, 30 min after (10 μg/kg), IV | 3 days, 7 days or 30 days after | Reduced CA1 injury in males | TRPM2, long-term potentiation | (148) |
| Sirt2 knockout | N/A | 45 min or 15 min of tMCAo | Sirt2−/− | 48 h, 7 days | Improved neurological deficit, no change in stroke volume | N/A | (85) |
| Sirt3 | |||||||
| Sirt3 knockout | N/A | 1 h tMCAo | Sirt3−/− | 24 h | Reduced infarct | Ceramide synthases, mitochondrial function, ROS | (128) |
| Sirt4 | |||||||
| None | |||||||
| Sirt5 | |||||||
| Sirt5 knockout | ΨɛRACK (PKCɛ activator, 0.75 mg/kg) | 85 min tMCAo | Sirt5−/− | 24 h | Reduced infarct | NAMPT, AMPK, mitochondrial function | (117) |
| Sirt6 | |||||||
| None | |||||||
| Sirt7 | |||||||
| None | |||||||
ACA, asphyxial cardiac arrest; BCAS, bilateral common carotid artery stenosis; BCAo, bilateral common carotid artery occlusion; eNOS, endothelial nitric oxide synthase; ICV, intracerebroventricular; N/A, not available; PKCɛ, protein kinase C epsilon; ROS, reactive oxygen species; tMCAo, transient middle cerebral artery occlusion; TU, transduction units.
Nuclear sirtuins
SIRT1
SIRT1 is the most conserved sirtuin across species and so far the best studied (144). Its predominant enzymatic activity is deacetylation although it hydrolyzes other acyl groups as well (4, 8, 120). SIRT1 plays a role in transcriptional repression by deacetylating histone residues such as H3K9, H3K56, and H4K16. SIRT1 also acts on nonhistone proteins to regulate DNA repair, glycolysis, mitochondrial biogenesis, inflammation, oxidative stress, and apoptosis (21, 34, 118, 162, 169). The systemic overexpression of SIRT1 mimics the effects of caloric restriction and increases the lifespan of several model organisms, yet in mice, although its systemic overexpression does not increase lifespan, it can still promote several health benefits (13, 59). In addition, numerous studies have suggested that the health benefits promoted by calorie restriction in different organs of the body including the brain using various models of ischemia and neurodegeneration are mediated at least, in part, through the activity of SIRT1 (66, 87, 138, 139, 150).
In the brain, SIRT1 is predominately localized in neuronal nuclei, yet has been detected in neural stem cells, astrocytes, and microglia in vitro, and in glial cells of postmortem human brains (83). It regulates important processes in the brain including genomic stability, neurogenesis, neurite outgrowth, synaptic plasticity, and cognition (126). Interestingly, SIRT1 in the hypothalamus can regulate systemic metabolic activities, and when overexpressed in the hypothalamus, it increases the lifespan of mice (143).
In the context of cerebral ischemia, most but not all studies support a neuroprotective role for SIRT1 (83). These studies support a role for SIRT1 in preserving mitochondrial function, mediating the neuroprotective effects of NAD+, reducing oxidative stress, preserving blood flow, and reducing inflammation among other pathways (36, 54, 83, 166, 171). For a detailed discussion of these and other studies, the readers are directed to a recent review that focuses solely on the role of SIRT1 in cerebral ischemia (83). In this review, we discuss the most recent and compelling studies.
SIRT1 levels are regulated by cerebral ischemic injuries, as levels fall in the ischemic brain and continue to decline with tissue reperfusion (72). Interestingly, SIRT1 increases in peri-infarct areas of the cortex up to 7 days after permanent middle cerebral artery occlusion (pMCAo) (58). In turn, SIRT1 activity modulates the outcome of cerebral ischemic injuries. Sirt1−/− mice display larger infarct volumes than wild-type mice (58), whereas mice overexpressing SIRT1 are more resistant to the injury (53, 54).
Both preconditioning and postconditioning approaches can tap into the neuroprotective properties of SIRT1. IPC reduced hippocampal cell death and improved outcome after asphyxial cardiac arrest (ACA), a rodent model of global cerebral ischemia (36). Intracerebroventricular (ICV) administration of the sirtuin inhibitor sirtinol blocked these protective effects. It should be noted that sirtinol is a multisirtuin inhibitor and although these studies may stress the importance of SIRT1, other sirtuins may be contributing to the observed results. Another preconditioning intervention, hyperbaric oxygen exposure, also reduced infarct and improved the neurobehavioral score from transient middle cerebral artery occlusion (tMCAo), effects that were abolished with ICV administration of Sirt1-targeted siRNA (177). Pharmacological preconditioning mimetics are of potential therapeutic value and have shown efficacy in several studies. For example, introducing resveratrol, a SIRT1 activator, 2 days before ACA mimics the effects of IPC and protects rats against ACA in a sirtuin-dependent manner (36). Curcumin, another naturally occurring compound with SIRT1-activating properties, when administered for 5 days before the injury, protects against a tMCAo but not in the presence of sirtinol (110). Targeting SIRT1 postinjury is also efficacious. Melatonin, the indoleamine produced in the body and found in foods, is another SIRT1-modulating compound. Administration of melatonin at ischemia and reperfusion onset reduced infarct, brain edema, and improved neurological score against tMCAo in an SIRT1-dependent manner (180). Many studies highlight the ability of resveratrol to induce ischemic tolerance in rodent models when administered hours or even days after injury (41, 57, 155, 168). Nevertheless, in addition to SIRT1 activation, resveratrol was shown to regulate SIRT3 and SIRT5 as well as nonsirtuin proteins, thus its protective effects should be carefully interpreted in a context-dependent manner as it may not be solely dependent on SIRT1 (48). In addition, it should be noted that resveratrol has been tested in clinical trials in the context of several disorders, including stroke wherein it was administered along with tPA and have shown promising outcomes (23). Resveratrol has also been tested in other cardiovascular disorders (1, 105, 188) as well as neurodegenerative disorders such as Alzheimer's disease (160). These studies could in the future facilitate its translation into clinical trials to be tested against cerebral ischemic insults in the form of preconditioning as well as postconditioning.
Intriguingly, both brain-derived and peripherally derived mechanisms of SIRT1-mediated ischemic tolerance are evident. In favor of brain-derived protection, preconditioning and pharmacological agents are effective at protecting primary neuronal and organotypic cultures in vitro (45, 140). In addition, in vivo studies have shown that ICV administration of SIRT1 or sirtuin-inhibiting compounds or direct injections of siRNA abolishes the protection despite the fact that peripheral SIRT1 remains active (36, 177).
Furthermore, several studies support SIRT1-mediated mechanisms that arise in the periphery or in non-neuronal cell types. Bilateral common carotid artery occlusion, a model of global ischemia, reduced cerebral blood flow to 20–25% of baseline in wild-type mice but only to 45–50% in SIRT1 overexpressing mice, suggesting that SIRT1 can preserve cerebral flow and in turn reduce the severity of ischemia (54). Also, SIRT1 overexpression maintained cerebral blood flow to near baseline levels hours and days after bilateral common carotid artery stenosis by preventing endothelial nitric oxide synthase acetylation, promoting its activation, and preserving of cerebral flow (53). These effects were abolished by sirtinol administration and were not attributed to the generation of collateral vasculature that may circumvent anterior and posterior circulation of the brain (53). Angiogenesis is another process by which enhanced cerebral blood flow can improve outcome after ischemic injury. SIRT1 is expressed in endothelial cells in the brain and is known to mediate their migration, sprouting, and production of erythropoietin (135). SIRT1 deacetylates hypoxia-inducible factor 2 alpha (HIF-2α), increasing its transcription and ultimately the erythropoietin-derived adaptation of red blood cells to low oxygen (37). This effect is seen with IPC and sufficient to induce neuroprotection in hippocampal neurons (189). Inflammation is another SIRT1-regulated pathway that could culminate in neuroprotection. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a proinflammatory mediator that contributes to ischemic injury during the reperfusion phase (133). SIRT1 deacetylates NF-κB, thus decreasing its activity (182). Resveratrol inhibits NF-κB by deacetylation, which is associated with increased SIRT1 activity (61). Together these studies demonstrate several non-neuronal-derived mechanisms of SIRT1-mediated ischemic tolerance.
Although most studies support a neuroprotective role for SIRT1, a few lines of evidence suggest that SIRT1 does not protect against ischemia-like insults or may even be detrimental. One interesting study found that overexpressing SIRT1 protected against low potassium-induced cell death in cerebellar granule neurons (CGNs); however, this protection was not abolished by the sirtuin inhibitor NAM or sirtinol, suggesting deacetylase-independent mechanisms of neuroprotection (132). Overexpressing a mutant SIRT1 that lacks deacetylase activity was reported to afford protection, leading to the hypothesis that SIRT1 may have chaperone-like activity that mediates neuroprotection (132). Moreover, some studies have postulated that SIRT1 may contribute to the rapid decline of NAD+ seen after ischemia (2, 115). NAM, which inhibits sirtuins, protected against excitotoxic cell death by preserving NAD+ levels (102). More studies are needed to tease apart these intricate aspects of SIRT1 function in ischemia and to refine the involvement of SIRT1 in preconditioning and postischemia neuroprotection.
SIRT6
SIRT6 possesses a HDAC, ADP ribosylase, and a demyristoylase activity (69, 107, 111). It acts on acetylated lysine residues such as H3K9ac and H3K56ac to repress gene expression and promote genome stability, particularly important in telomere maintenance (111, 112). SIRT6 also plays a role in DNA damage repair through the ADP ribosylation and thus activation of PARP1 (107). Furthermore, SIRT6 has been reported to possess anti-inflammatory functions and to play a role in maintaining metabolic homeostasis and preventing aging and senescence (19, 175, 191). Mice lacking Sirt6 gene have a shortened lifespan, exhibit aging-like degenerative process, are susceptible to DNA damage, and show a severe hypoglycemic phenotype because of increased glucose uptake and glycolysis (119, 191). Yet mice overexpressing Sirt6 are resistant to the metabolic damages caused by high-fat diets and show extended lifespan in male but not female mice (73, 74).
In the context of cerebral ischemic injuries, few studies have explored the functions of SIRT6. During ischemic/reperfusion (I/R) injuries, levels of hydrogen peroxide (H2O2) are elevated and contribute to the oxidative stress-induced injury (32). Shao et al. showed that in response to H2O2, SIRT6 is reduced in SH-SY5Y neuronal cells (146). The overexpression of SIRT6 further exacerbated the necrotic cell death and ROS production. In these cells, SIRT6 induced autophagy by inhibiting the AKT signaling pathway, whereas the inhibition of either SIRT6 or autophagy was able to rescue them from H2O2-induced cell death (146). Cardinale et al. showed that SIRT6 is highly expressed in the cortex and hippocampus of mice and is specifically abundant in the nucleus and synaptosomal fractions (18). When cultured in vitro, cortical and hippocampal neurons showed decreased SIRT6 expression with increased maturation. The overexpression of SIRT6 decreased the viability of these cells in response to H2O2 treatment (18).
Yet, other reports support a protective role for SIRT6. A study by Hu et al. showed that pretreating brain endothelial cell line (bEnd.3) with Na2S (an exogenous donor of H2S) protected the cells from an oxygen and glucose deprivation (OGD)-induced cell death (63). This correlated with decreased ROS production and increased activities of antioxidant genes superoxide dismutase (SOD) and CAT (catalase). Treatment with Na2S also increased the expression and activity of SIRT6 which was reduced by OGD. Knocking down SIRT6 reduced the activities of SOD and CAT and abolished the Na2S-mediated protection against an OGD in endothelial cells (63). As mentioned above, a study by Zhao et al. showed that the overexpression of NAMPT increased the survival of mice and their functional recovery when subjected to an MCAo which correlated with increased neurogenesis (190). The knockdown of SIRT1, 2, or 6 prevented the differentiation of neural stem cells in vitro in response to NAD+ or NMN supplementation. Although the study supports a positive role for SIRT6 in promoting the differentiation of neural stem cells (NSC), its importance in cerebral ischemia was not experimentally validated (190). Another study which potentially hints toward a protective role for SIRT6 in the brain was performed by Lee et al. (93). The authors report SIRT6 to be predominately found in neuronal cells. In response to an in vitro and in vivo models of cerebral ischemia, SIRT6 is reduced and this correlated with the translocation of high mobility group box-1 protein (HMGB1) from the nucleus to the cytoplasm and its extracellular release, a process known to promote inflammation in the brain. The knockdown of SIRT6 in SH-SY5Y cells did not affect OGD-induced cell death but increased the nuclear translocation of HMGB1 and its extracellular secretion. The study supports a role for SIRT6 in preventing the HMGB1 release which might potentially reduce inflammation in the brain although not directly shown (93).
The study by Pfister et al. showed that in CGNs, the overexpression of SIRT6 induced apoptosis in healthy neurons. Yet in a neuroblastoma cell line (HT22), SIRT6 overexpression was protective against homocysteic acid-induced apoptosis (132).
Dong et al. while assessing the association between genetic variants in the sirtuin members and carotid plaque presence and abundance found an association between a single nucleotide polymorphism (SNP) (rs107251) and two haplotypes in SIRT6 gene to correlate with an increased risk for carotid plaque presence as well as plaque abundance (39). In a follow-up study, the authors showed that the SNP rs107251 also significantly correlated with total plaque area. Since the accumulation of carotid plaque increases the risk of clinical atherosclerosis, an underlying cause of ischemic strokes, this study suggests a potential role for SIRT6 in increasing the risk ischemic strokes (38).
Furthermore, SIRT6 was reported to be post-translationally modified by nitrogen species under conditions of nitrosative stresses (62). Using primary human retinal microvascular endothelial cells, Hu et al. showed that the treatment with a peroxynitrite donor induces the nitration of SIRT6 which reduces its deacetylase activity. Similarly, using an in vivo model of LPS-induced retinal inflammation, SIRT6 was modified by nitration and showed reduced activity. This study shows the importance of nitrosative stresses in regulating SIRT6 activity (62).
Thus the limited studies assessing the role of SIRT6 in cerebral ischemic injuries are somehow contradictory with evidence supporting both a detrimental and a protective role for SIRT6.
SIRT7
SIRT7 is mainly found in the nucleolus where it plays a positive role in the regulation of ribosomal RNA transcription through the deacetylation of RNA polymerase I subunit (24, 43, 159). SIRT7 also regulates tRNA transcription through the indirect activation of RNA pol III (159). Both events are required for protein translation. Moreover, SIRT7 acts as a HDAC acting on the H3K18ac mark (9). The increased activity of SIRT7 associated with hypoacetylation of H3K18 has been linked to tumor progression, the reason why SIRT7 is considered a drug target for cancer therapy (9, 144). Furthermore, SIRT7 plays a positive role in the regulation of nuclear-encoded mitochondrial genes through its deacetylation of GABPβ1 (142). Additionally, SIRT7 exhibits a histone desuccinylase activity (97). In response to DNA damage SIRT7 desuccinylates H3K122 and promotes chromatin condensation and DNA damage repair (97). Mice lacking Sirt7 have reduced lifespan, exhibit inflammatory cardiomyopathies, and fatty liver diseases among others (4, 149, 161).
The role of SIRT7 in cerebral ischemic injuries has not been studied. A single study that aimed at identifying blood biomarkers for cardioembolic stroke has revealed SIRT7 to be among the stroke-associated genes (173). Following a systems biology approach, the authors combined publically available microarray data from human blood samples taken at three different timepoints after a cardioembolic stroke with protein-protein interaction information obtained from a publically available database, to identify protein biomarkers with significant stroke relevance. The authors revealed six genes that show high association with time after a stroke, among them is SIRT7. Based on pathway analysis and previously known functions, the authors predicted SIRT7 to play a protective role in cardioembolic stroke, yet this was not validated experimentally (173).
Cytoplasmic sirtuins
SIRT2
SIRT2 is predominately localized in the cytoplasm of cells but is also known to translocate to the nucleus (4). Among the known functions of SIRT2, is the deacetylation of the microtubule protein α-tubulin in the cytoplasm and the deacetylation histone 4 lysine 16 in the nucleus, which is necessary for chromosome condensation (52). SIRT2 regulates both processes to control the entry and exit of the cells from mitosis (52). SIRT2 is highly abundant in the brain (Fig. 4) and was thought to be predominately expressed in oligodendrocytes; yet several studies have reported SIRT2 expression in microglia, astrocytes, neurons and neural stem cells (98, 99, 172, 176, 190). In the brain, SIRT2 controls different processes including the inhibition of oligodendrocyte differentiation by microtubule deacetylation, pro- and anti-inflammatory functions in microglia, induction of cell death in neurons, and inhibition of neurite outgrowth (52, 98, 99, 127, 187).
Contrary to the overgeneralization that activating sirtuins promotes beneficial outcomes in the brain, SIRT2 seems to play an unconventional detrimental role. In the context of Parkinson's and Huntington's disease as well as in cerebral ischemia, the pharmacological inhibition of SIRT2 or its genetic ablation promotes beneficial outcomes (25, 28, 131, 148, 176). A recent study by Xie et al. showed that downregulating SIRT2 in the brain was neuroprotective against cerebral ischemic injuries (176). In the study, SIRT2 was reported to be highly expressed in the cytoplasm of neurons and absent in microglia and astrocytes. In response to in vitro and in vivo models of cerebral ischemia, SIRT2 translocated to the nucleus of neurons and increased in abundance. The authors reported the same observation in the brains of postmortems ischemic stroke patients. The administration of AGK2, a SIRT2 inhibitor, immediately after the injury or ablating Sirt2 genetically significantly reduced infarct volume of mice subjected to tMCAo and improved their neurological impairments (176). Yet the exact nuclear functions of SIRT2 were not determined by the study. Although the authors show an increase in the H4K16 acetylation mark in Sirt2−/− mice compared to wild types in response to the tMCAo, the genomic targets of this modification were not identified. Other potential nuclear targets suggested by the authors but not experimentally addressed are the FOXO transcription factors. Under cellular stresses, SIRT2 is known to deacetylate FOXO1 and FOXO3a, which in turn, induces an increase in autophagy and mitochondrial superoxide dismutase (MnSOD) expression, respectively (176). Another study by Shimizu et al. showed that SIRT2 mediates a male-specific form of injury in the brain of mice subjected to a global cerebral ischemia (148). In response to cerebral ischemia, SIRT2 gets activated and, in turn, produces the OAADPr as a byproduct of its enzymatic activity. The OAADPr then activates the calcium-permeable transient receptor potential channel M2 (TRPM2), which is known to induce cell death when activated for a prolonged time (49, 68, 148). Although all sirtuins can produce OAADPr as a byproduct of their enzymatic activities, this study may suggest that the subcellular localization of OAADPr can regulate different subcellular signaling pathways. Introducing AGK-2 was able to reduce the cerebral injury and alleviate the functional deficits in wild-type mice but not in TRPM2 knockout mice (148). Interestingly, AGK-2 was not able to protect female mice from the same ischemic injury (68, 148). The authors partly attribute this sex-specific difference to the dynamic activation of SIRT2. In females, SIRT2 activity quickly returns to baseline after the injury, which the authors believe was not sufficient to activate the TRPM2 receptor, whereas in males it remains elevated for 24 h after. The possible mechanism behind this sex-specific differential activation of SIRT2 was not addressed in the study, yet the authors believe their results support the oxidative stress-mediated male-specific mechanisms of cell death because of the involvement of the TRPM2 receptor, which is known to be predominately activated in males by oxidative stress (148). Krey et al. also showed that the knockout of SIRT2 was able to prevent neurological deficits in male mice subjected to two models of MCAo although no reductions in infarct volume or inflammatory cell count were observed (85). In ischemic brains, SIRT2 was reported to be upregulated and enriched in myelin-rich regions, whereas its expression was not detected in astrocytes, microglia, or neurons. Owing to the role of SIRT2 in regulating myelination and to the absence of inflammatory changes or infarct volume reductions, the authors concluded that SIRT2 induces a myelin-dependent form of axonal dysfunction in neurons, which was alleviated by its inhibition (85). In addition, an in vitro study by Nie et al. showed that in response to oxidative stress, SIRT2 was upregulated and promoted apoptosis in a differentiated PC12 cell line, a neuronal-like cellular model (127). The inhibition of SIRT2 with AGK2 or its knockdown reduced the cell death induced by hydrogen peroxide by reducing ROS production, the results of which support a role for SIRT2 in mediating oxidative stress-induced apoptosis (127). Furthermore, a study by Pfister et al. showed that the mere overexpression of SIRT2 in healthy CGNs and in HT22 cells was able to induce apoptosis (132).
In contrast, few studies have demonstrated a neutral or potentially neuroprotective role for SIRT2. A study by Chen et al. showed that the inhibition of SIRT2 by a different inhibitor AK7 was not able to reduce the infarct volumes or prevent neurological deficits of mice (25). Yet in this study, the authors pooled animals exposed to different treatments in their analysis that may have masked any protective effect of the drug (25). In 2015, Zhao et al. showed that transgenic mice overexpressing NAMPT had increased the number of neural stem cells, which correlated with enhanced functional recovery and survival after MCAo (190). The authors then knocked down all the sirtuin members in NSC cultured in vitro to identify downstream mediators of NAD+ and showed that the knockdown of SIRT2 limited the cell's proliferation and prevented their differentiation. This study supports a positive role for SIRT2 in promoting NSC proliferation and differentiation, yet its direct role in cerebral ischemic injury was not shown experimentally (190).
Thus so far the majority of studies support a detrimental role for SIRT2 in cerebral ischemic injuries, although through different proposed mechanisms (85, 148, 176). The study by Shimizu et al. raises the important point that these effects are sex specific, a concept that warrants further scrutiny when targeting sirtuins in cerebral ischemia (148).
Mitochondrial sirtuins
SIRT3
SIRT3 is known as the main mitochondrial lysine deacetylase (130). It plays a crucial role in maintaining mitochondrial homeostasis by acting as a stress response protein. SIRT3 regulates mitochondrial energy metabolism and ROS levels in response to reduced nutrient availability and increased oxidative stress (48, 130). It deacetylates and activates several protein targets that play a role in the TCA cycle, oxidative phosphorylation, fatty acid oxidation, amino acid metabolism, urea cycle, and ROS detoxification (48, 130). Interestingly, the presence of an active enhancer in the SIRT3 gene has been linked to increased longevity in humans (11).
In the context of cerebral ischemic injuries, several studies have explored the potential functions of SIRT3. Using an in vitro model of neuronal cultures subjected to excitotoxic stress, Kim et al. showed that SIRT3 promotes neuroprotection against NMDA-mediated toxicity (78). Treating neuronal cultures with NMDA depleted cytosolic but not mitochondrial NAD+ levels through PARP activation and only activated SIRT3 among the mitochondrial sirtuins. Inhibiting PARP-1 reduced SIRT3 activation, and transfecting cells with an enzyme that consumes cytosolic but not mitochondrial NAD+ was sufficient to activate SIRT3. Furthermore, the overexpression of SIRT3 reduced cell death and lowered ROS levels in response to NMDA, whereas its knockdown exacerbated it. Thus, the study revealed a neuroprotective role of SIRT3 against excitotoxic injuries (78). Using a similar model, a study by Cheng et al. showed that SIRT3 mediates adaptive responses in neurons to excitotoxic and bioenergetics stresses (27). The knockout of SIRT3 increased the susceptibility of neurons to oxidative and mitochondrial stresses, while its rescue restored their resistance. This protection was proposed to be through the suppression of mitochondrial ROS levels. Notably, the stimulation of SIRT3 seen in hippocampal neurons by running on a wheel was required to mediate the neuroprotective effects of running (27).
Using in vitro models of primary neuronal cultures or neuronal cell lines subject to oxidative stress by hydrogen peroxide (H2O2), SIRT3 was also reported to be neuroprotective. In 2013, Wang et al. showed that H2O2 treatment reduced SIRT3 levels in motor neuron-like cells NSC34 (164). Pretreatment with valproic acid or lithium alleviated this toxicity. The induced protection correlated with increased SIRT3 levels, yet the authors did not experimentally show whether the protection is SIRT3 dependent (164). Dai et al. showed that treating mouse hippocampal cells HT22 with H2O2 increased SIRT3 levels in a time- and dose-dependent manner. Similarly, the knockdown of SIRT3 worsened the injury, whereas its overexpression provided protection that correlated with reduced ROS and lipid peroxidation, yet with no changes in antioxidant enzyme activities (32). SIRT3 also reduced apoptosis and reversed the H2O2-mediated damage to the respiratory chain complexes, calcium buffering capacity, ATP production, and mitochondrial swelling (32). The same authors reported the same observation in rat neuronal cultures wherein they additionally observed increased antioxidant enzyme activity in response to SIRT3 overexpression, which correlated with reduced ROS (33).
Using an in vitro model of ischemia that is the OGD, SIRT3 was also reported to be neuroprotective. Shulyakova et al. showed that overexpressing SIRT3 in neuronally differentiated PC12 cells was protective against an OGD and reversed the OGD-induced increase in ROS (152). A similar study by Wang et al. also showed that the knockdown of SIRT3 in PC12 cells worsened the OGD-induced injury, whereas a recombinant form of SIRT3 rescued the cells and activated the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and MnSOD, both of which reduce oxidative stress. The knockdown of both genes abolished the SIRT3-mediated neuroprotection (167).
The role of SIRT3 in cerebral ischemia has also been explored using in vivo animal models. Yin et al. showed that ketones reduced the infarct volume and improved the neurological deficits of mice when introduced directly after a tMCAo (183). The protection correlated with increased NAD+/NADH, SIRT3 levels, and SIRT3 downstream antioxidant genes forkhead box O3a (FoxO3a) and superoxide dismutase 2 (SOD2). A reduction in protein oxidation and preservation of mitochondrial function were also reported. Overexpressing SIRT3 in primary neurons increased their resistance to rotenone, an inhibitor of mitochondrial complex I, whereas its knockdown abolished the protection and decreased the levels of FoxO3a and SOD2. Thus, the study supports an SIRT-3-dependent neuroprotective role of ketones through the activation antioxidant genes, which, in turn, preserve mitochondrial function (183). In another study, Huang et al. showed that in response to a subarachnoid hemorrhage (SAH), SIRT3 levels are reduced (64). SIRT3 was detected in both neurons and endothelial cells and by measuring SOD2 levels, an SIRT3 downstream target; the authors reported a time-dependent increase in its expression after the SAH, which they interpreted as an increase in ROS production. Because of the downregulation in SIRT3 that correlated with increased cell death, the authors concluded that SIRT3 is required for the neuroprotection, yet this was not shown experimentally (64). Another study conducted in vivo by Yang et al. assessed the role of SIRT3 in endothelial cells against hypoxic injuries (178). Using in vitro and in vivo models, minocycline protected the blood brain barrier (BBB) against hypoxic injuries by inhibiting the expression of HIF-1α (hypoxia-inducible factor 1 alpha) and its nuclear translocation. This correlated with reduced expression of its downstream targets that increase BBB permeability the matrix metallopeptidase −2 and −9 (MMP-2, MMP-9), and an increased expression of tight junction genes. In response to hypoxia, prolyl hydroxylase domain-containing protein 2, which targets HIF-1α for degradation, was reduced concurrently with SIRT3, yet minocycline was able to upregulate both genes under hypoxic conditions. Interestingly, the knockdown of SIRT-3 in vivo abolished the protective effects of minocycline on BBB integrity and increased HIF-1α expression, along with its downstream targets (178).
Yet contrary to previous studies, some studies have reported detrimental roles for SIRT3. A recent study by Novgorodov et al. showed that SIRT3 contributes to the cerebral ischemic injury by increasing ceramide production (128). Ceramide is found in the plasma, ER, and mitochondrial membranes where it plays important physiological functions, yet during cerebral ischemic injuries, its levels increase, which induces defects in the mitochondrial respiratory complexes and increases ROS production (128, 192). The study showed that I/R injury activates SIRT3, which, in turn, deacetylates the mitochondrial ceramide synthase, leading to its increased enzymatic activity and ceramide abundance. SIRT3 KO mice had reduced ceramide synthases activity in response to I/R that correlated with reduced mitochondrial ceramide, and as such had preserved mitochondrial respiration, reduced oxidative damage, and reduced infarct volume in response to a tMCAo (128). Furthermore, a study by Pfister et al. showed that in CGNs and in HT22 cells, the mere overexpression of SIRT3 was able to induce apoptosis (132).
In the context of preconditioning, a recent work from our laboratory by Morris-Blanco et al. showed that the ischemic tolerance mediated by protein kinase C epsilon (PKCɛ) preconditioning is independent of SIRT3 activity but relies on the activity of the mitochondrial SIRT5 (117). Our laboratory had previously shown that PKCɛ increases the mitochondrial NAD+/NADH ratio. In a follow-up study, the increase in mitochondrial NAD+/NADH ratio was shown to only affect the activity of mitochondrial SIRT5 with no effects on that of SIRT3, suggesting that the ischemic tolerance mediated by PKCɛ preconditioning is independent of SIRT3 activity (116, 117).
Although several studies support a neuroprotective role for SIRT3 in cerebral ischemic injuries, additional studies would be required to better resolve its role because some studies have reported detrimental functions for SIRT3.
SIRT4
SIRT4 has a low deacetylase activity, yet possesses an ADP ribosyl transferase activity as well as a lipoamidase activity (130). In nutrient-replete conditions, SIRT4 inhibits fatty acid oxidation in skeletal muscles and promotes lipogenesis in white adipose tissues through the deacetylation and repression of the malonyl CoA decarboxylase. Thus Sirt4 KO mice are resistant to diet-induced obesity (92). During nutrient-sufficient conditions, SIRT4 in the pancreas reduces the activity of the glutamate dehyrdrogenase (GDH) by ADP ribosylation, and thus reduces insulin secretion by β cells. While during calorie restriction, SIRT4 activity is downregulated in the pancreas and allows β cells to release insulin in response to amino acids (50). In liver and muscle cells, the knockdown of SIRT4 has been shown to increase fatty acid oxidation (125). Based on these studies, the pharmacological inhibition of SIRT4 is considered a potential therapeutic strategy against metabolic diseases (48, 125).
In the brain, very little is known about the functions of SIRT4 or its enzymatic targets. A study in 2013 reported SIRT4 to be highly expressed in astrocytes and radial glial cells in the postnatal brain specifically in the mitochondria and to be reduced in expression during development (82). Using a radial glial cell line, CTX8, the overexpression of SIRT4 reduced its astrocytic development in vitro possibly by inhibiting the SIRT4 downstream target GDH1, thus supporting a role for SIRT4 in regulating gliogenesis (82).
In the context of cerebral ischemia, the role of SIRT4 has been largely unexplored, with only one study assessing its role in excitotoxic injuries (147). In this study, SIRT4 levels were reported to be upregulated in the hippocampus of mice after kainic acid (KA) treatment. SIRT4 knockout mice showed increased cell death and increased seizure phenotypes in response to KA exposure. In addition, using in vitro cultures of either astrocytes or mixed neuronal-astrocytic cultures, SIRT4 was reported to be predominately expressed in astrocytes and present at lower levels in neurons. Mice lacking SIRT4 showed significantly reduced rates of GLT-1-dependent glutamate uptake compared with wild types under baseline as well as in response to KA. Supporting this observation, hippocampal lysates lacking SIRT4 showed significantly reduced levels of GLT-1 at the cell surface yet with no differences observed in total cell lysates. This study supports a protective role for SIRT4 against excitotoxic injuries in astrocytes through the increased surface expression of GLT-1. In the same study, the authors showed that neuronal cultures lacking SIRT4 had lower baseline ATP levels and a dose-dependent decrease in ATP levels in response to KA. These data support a role for SIRT4 in energy production in neurons, which may be important in their protection against excitotoxic injuries (147). Thus the study supports a neuroprotective role for SIRT4 in the brain by reducing excitotoxicity, yet the overall functions of SIRT4 in the brain are still largely unexplored specifically in the context of ischemic injuries.
SIRT5
SIRT5 possesses several enzymatic activities including demalonylation, deglutarylation, desuccinylation, and a low deacetylation activity (86). SIRT5 regulates several metabolic processes, including glycolysis, respiration, fatty acid oxidation, apoptosis, and ROS detoxification (130, 144). One of the well-studied functions of SIRT5 is its regulation of the urea cycle in the liver. The urea cycle detoxifies ammonia derived from the deamination of amino acids through its conversion into urea, a less toxic molecule (121, 122). In the liver, SIRT5 within the mitochondrial matrix deacetylates and activates the rate-limiting enzyme of the urea cycle called carbamoyl phosphate synthetase 1 (CPS1) (121, 122). During fasting and calorie restriction, the regulation of CPS1 by SIRT5 is increased to detoxify the excess ammonia released from amino acids metabolism (121, 122). In nonliver cells, SIRT5 can also regulate the urea cycle through the desuccinylation of glutaminase (134).
In the brain, SIRT5 has not been extensively studied. A study by Liu et al. supports a neuroprotective role for SIRT5 in Parkinson's disease. (103). In addition, Li et al. showed that the absence of SIRT5 in mice exacerbates the KA-induced seizures and increases the neuronal degeneration in the hippocampus (94). KA increased SIRT5 levels in the surviving mitochondria of the hippocampus, which the authors interpreted as a reflection of its neuroprotective role.
A study by Pfister et al. assessed the role of all the sirtuin genes on the survival of neuronal cells in vitro. In CGNs induced to undergo apoptosis, SIRT5 is protective when localized in the nucleus and cytoplasm, but when localized in the mitochondria it induces apoptosis. While using HT22 cells, SIRT5 overexpression, which localized to the mitochondria, induced apoptosis. Thus it is possible that the subcellular localization of SIRT5 determines its outcome on the survival of neurons (132).
In the context of cerebral ischemic injuries, a study by Morris-Blanco et al. showed that SIRT5 is required for the neuroprotection afforded by PKCɛ and IPC against cerebral ischemic insults (117). Using mixed neuronal-astrocytic cultures, PKCɛ and IPC increased both the activity and expression of mitochondrial SIRT5 but not SIRT3, which correlated with a reduction in mitochondrial succinylation but not acetylation. Consistently, SIRT5 knockout mice showed increased levels of mitochondrial lysine succinylation in the cortex, which correlated with a reduction in the oxygen consumption rate of mitochondrial respiratory complexes. Also, PKCɛ preconditioning enhanced mitochondrial respiration in wild-type mice but not in SIRT5 knockouts. Furthermore, SIRT5 knockout mice lose the neuroprotection mediated by PKCɛ preconditioning against cerebral ischemic insults as revealed by increased necrotic cell death and larger infarct volumes. Thus, this study supports a role for SIRT5 as a mediator of cerebral ischemic tolerance afforded by preconditioning possibly through the regulation of mitochondrial respiration (117).
Dong et al. in 2011 evaluated the association between genetic variants in the sirtuin genes and presence or number of carotid plaques (39). The study revealed two SNPs and a 4-SNP haplotype in the SIRT5 gene to be associated with increased number of carotid plaques. The study also revealed an association between smoking, hypertension, and diabetes and SIRT5 SNPs with number and abundance of carotid plaques. Since the accumulation of carotid plaques increases the risk of clinical atherosclerosis, an underlying cause of ischemic strokes, this study suggests a potential role for SIRT5 in increasing the risk of ischemic strokes. (39).
Thus although some reports support a neuroprotective role of SIRT5 in cerebral ischemic injuries, its functions are still largely unexplored and await further studies.
Conclusion
In conclusion, numerous studies have supported the importance of targeting the sirtuin family members either before or after a cerebral ischemic injury (Fig. 5 and Table 1). Both approaches would be of significant interest in clinical settings for the therapeutic targeting of cerebral ischemic injuries. Preconditioning offers an appealing approach to target the sirtuin family because it induces a coordinated response of multiple rather than single sirtuin members, which, in turn, integrates many cellular physiological processes to create the ischemic tolerant phenotype. The processes regulated by sirtuins in the context of cerebral ischemic injuries as reviewed above involve DNA repair, mitochondrial respiration, oxidative stress regulation, calcium buffering, and glutamate uptake among several others (Fig. 5). Yet there is still a need to better understand the functions of the less characterized sirtuin members, especially that many of them are highly abundant in the brain and display distinct regional and cellular localization (Figs. 1 and 4). Also, there is a need for the use of sirtuin-specific pharmacological activators or inhibitors as opposed to multisirtuin modulators, which were used in the initial studies mainly because of the opposing functions reported by different sirtuin members.
Most studies discussed above have used male model organism in their studies, whereas female model organisms have been underrepresented. Taking into account the sex-specific difference seen with some members of the sirtuin family as well as with the use of PARP modulators, this stresses the need to better understand the sex-specific mechanisms of sirtuins in a preclinical setting before translating some of these findings into the clinic. Moreover, since sirtuins rely on NAD+ to conduct their enzymatic functions, future studies can potentially address combinatorial approaches that regulate both NAD+ levels and sirtuins' activity to combat cerebral ischemic injuries and promote ischemic tolerance or recovery.
Moreover, interesting findings from a recent study have revealed that chronic exposure to hypoxia can actually compensate for defects in mitochondrial complex respiration through the activation of the endogenous hypoxia response (67). Findings from this study can have promising applications in the context of cerebral ischemia, which is known to induce defects in the mitochondrial complexes. Future studies can also determine whether members of the sirtuin might be playing a role in this hypoxic response as has been suggested recently by some studies (40, 70).
In addition, taking into account the recent findings that mitochondria can be transferred between cells of the central nervous system which can potentially mediate protective effects and that many of the cellular physiological processes that are regulated by the sirtuins converge into mitochondrial regulated processes, future studies should address this avenue to determine whether sirtuins themselves are part of the exchange or whether they play a role in a cell nonautonomous manner through mitochondrial exchange (35, 55). Studies of mitochondrial transfer are now being assessed in clinical trials using cardiac tissues, which could potentially open the door for future trials that could introduce them into the brain (42, 108).
Abbreviations Used
- ACA
asphyxial cardiac arrest
- BBB
blood brain barrier
- BCAo
bilateral common carotid artery occlusion
- BCAS
bilateral common carotid artery stenosis
- bEnd.3
brain endothelial cell line
- cADPR
cyclic ADP ribose
- CAT
catalase
- CGNs
cerebellar granule neurons
- CPS1
carbamoyl phosphate synthetase 1
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FoxO3a
forkhead box O3a
- GDH
glutamate dehyrdrogenase
- H2O2
hydrogen peroxide
- HDACs
histone deacetylases
- HIF-1α
hypoxia-inducible factor 1 alpha
- HIF-2α
hypoxia-inducible factor 2 alpha
- HMGB1
high mobility group box-1 protein
- HT22
immortalized mouse hippocampal neuronal cell line
- I/R
ischemic/reperfusion
- ICV
intracerebroventricular
- IPC
ischemic preconditioning
- KA
kainic acid
- MMP-2
matrix metallopeptidase 2
- MMP-9
matrix metallopeptidase 9
- MnSOD
mitochondrial superoxide dismutase
- NA
nicotinic acid
- Na2S
sodium sulfide
- NAAD
nicotinic acid adenine dinucleotide
- NAADP
nicotinic acid adenine dinucleotide phosphate
- NAD+
nicotinamide adenine dinucleotide
- NAM
nicotinamide
- NAMN
nicotinic acid mononucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMN
nicotinamide mononucleotide
- NMNATs
nicotinamide mononucleotide adenylyl transferases
- NR
nicotinamide riboside
- NRK
nicotinamide riboside kinase
- OAADPr
O-acetyl-ADP ribose
- OGD
oxygen and glucose deprivation
- PARPs
poly-ADP ribose transferases
- PGC-1α
PPAR-γ coactivator 1-α
- PKCɛ
protein kinase C epsilon
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- rtPA
recombinant tissue plasminogen activator
- SAH
subarachnoid hemorrhage
- Sir2
silent information regulator 2
- SIRT
sirtuin
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- SOD2
superoxide dismutase 2
- TCA cycle
tricarboxylic acid cycle
- tMCAo
transient middle cerebral artery occlusion
- TRPM2
calcium permeable transient receptor potential channel M2
Acknowledgments
This work was supported by grants from NIH/NINDS NS45676, NS054147, and NS34773 (to M.A.P.P), by the American Heart Association (AHA) predoctoral award 16PRE29170004 (to N.K.), and by the NIH F31 predoctoral award NS089356-01A1 (to K.B.K.).
References
- 1.Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, Baur JA, and Smoliga JM. Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol 166: 246–248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, and Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci 30: 2967–2978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, and Rezaei Y. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand 131: 45–50, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Anderson KA, Green MF, Huynh FK, Wagner GR, and Hirschey MD. SnapShot: mammalian sirtuins. Cell 159: 956–956 e951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, and Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423: 181–185, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoub IA, Lee EJ, Ogilvy CS, Beal MF, and Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal cerebral ischemia in Wistar rats. Neurosci Lett 259: 21–24, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Banasik M, Stedeford T, and Strosznajder RP. Natural inhibitors of poly(ADP-ribose) polymerase-1. Mol Neurobiol 46: 55–63, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T, Wong CF, Zhang J, Hao Q, and Li XD. Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. Elife 3, 2014, DOI: 10.7554/eLife.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 487: 114–118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter P, Chen Y, Xu Y, and Swanson RA. Mitochondrial dysfunction induced by nuclear poly(ADP-ribose) polymerase-1: a treatable cause of cell death in stroke. Transl Stroke Res 5: 136–144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85: 258–263, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bieganowski P. and Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117: 495–502, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6: 759–767, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, and Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto C, Menzies KJ, and Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab 22: 31–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canto C, Sauve AA, and Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med 34: 1168–1201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale A, de Stefano MC, Mollinari C, Racaniello M, Garaci E, and Merlo D. Biochemical characterization of sirtuin 6 in the brain and its involvement in oxidative stress response. Neurochem Res 40: 59–69, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Cardus A, Uryga AK, Walters G, and Erusalimsky JD. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res 97: 571–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalkiadaki A. and Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer 15: 608–624, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Chang HC. and Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25: 138–145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, and Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22: 1753–1757, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Bai Q, Zhao Z, Sui H, and Xie X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol Scand 134: 54–60, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Seiler J, Santiago-Reichelt M, Felbel K, Grummt I, and Voit R. Repression of RNA polymerase I upon stress is caused by inhibition of RNA-dependent deacetylation of PAF53 by SIRT7. Mol Cell 52: 303–313, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H, Silverman RB, Ayata C, et al. The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson's disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS One 10: e0116919, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Zhao S, Song Y, Shi Y, Leak RK, and Cao G. The Role of Nicotinamide Phosphoribosyltransferase in Cerebral Ischemia. Curr Top Med Chem 15: 2211–2221, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab 23: 128–142, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington's disease mouse models. Cell Rep 2: 1492–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 15: 536–550, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Clarke DD. and Sokoloff L. Circulation and Energy Metabolism of the Brain. In: Basic Neurochemistry, 6th edition Molecular, Cellular and Medical Aspects. edn. Edited by Siegel GJ, Editor-in-Chief, Agranoff BW, Albers RW, Fisher SK, Uhler MD. Philadelphia: Lippincott-Raven, 1999, pp. 637–669 [Google Scholar]
- 31.Covington JD. and Bajpeyi S. The sirtuins: markers of metabolic health. Mol Nutr Food Res 60: 79–91, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, and Jiang XF. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med 34: 1159–1168, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, and Jiang XF. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int J Mol Sci 15: 14591–14609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das C, Lucia MS, Hansen KC, and Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459: 113–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A 111: 9633–9638, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, and Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159: 993–1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, and Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Dong C, Della-Morte D, Cabral D, Wang L, Blanton SH, Seemant C, Sacco RL, and Rundek T. Sirtuin/uncoupling protein gene variants and carotid plaque area and morphology. Int J Stroke 10: 1247–1252, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong C, Della-Morte D, Wang L, Cabral D, Beecham A, McClendon MS, Luca CC, Blanton SH, Sacco RL, and Rundek T. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS One 6: e27157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong SY, Guo YJ, Feng Y, Cui XX, Kuo SH, Liu T, and Wu YC. The epigenetic regulation of HIF-1alpha by SIRT1 in MPP(+) treated SH-SY5Y cells. Biochem Biophys Res Commun 470: 453–459, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong W, Li N, Gao D, Zhen H, Zhang X, and Li F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg 48: 709–714, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, and McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 154: 286–289, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Ford E, Voit R, Liszt G, Magin C, Grummt I, and Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev 20: 1075–1080, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallo CM, Smith DL, Jr., and Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol 24: 1301–1312, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao D, Huang T, Jiang X, Hu S, Zhang L, and Fei Z. Resveratrol protects primary cortical neuron cultures from transient oxygen-glucose deprivation by inhibiting MMP-9. Mol Med Rep 9: 2197–2204, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, and Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11: 535–546, 2015 [DOI] [PubMed] [Google Scholar]
- 47.George PM. and Steinberg GK. Novel Stroke Therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87: 297–309, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gertz M. and Steegborn C. Using mitochondrial sirtuins as drug targets: disease implications and available compounds. Cell Mol Life Sci 73: 2871–2896, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL, Scharenberg AM, and Denu JM. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem 281: 14057–14065, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Hara N, Yamada K, Shibata T, Osago H, and Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS One 6: e22781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harting K. and Knoll B. SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol 89: 262–269, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Hattori Y, Okamoto Y, Maki T, Yamamoto Y, Oishi N, Yamahara K, Nagatsuka K, Takahashi R, Kalaria RN, Fukuyama H, et al. Silent information regulator 2 homolog 1 counters cerebral hypoperfusion injury by deacetylating endothelial nitric oxide synthase. Stroke 45: 3403–3411, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Hattori Y, Okamoto Y, Nagatsuka K, Takahashi R, Kalaria RN, Kinoshita M, and Ihara M. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. Neuroreport 26: 113–117, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, and Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535: 551–555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henderson LM. Tryptophan's role as a vitamin precursor (Krehl et al., 1945). J Nutr 127(5 Suppl): 1043S–1045S, 1997 [PubMed] [Google Scholar]
- 57.Hermann DM, Zechariah A, Kaltwasser B, Bosche B, Caglayan AB, Kilic E, and Doeppner TR. Sustained neurological recovery induced by resveratrol is associated with angioneurogenesis rather than neuroprotection after focal cerebral ischemia. Neurobiol Dis 83: 16–25, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Hernandez-Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, and Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44: 2333–2337, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, and Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun 1: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 45: 159–167, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Hu S, Liu H, Ha Y, Luo X, Motamedi M, Gupta MP, Ma JX, Tilton RG, and Zhang W. Posttranslational modification of Sirt6 activity by peroxynitrite. Free Radic Biol Med 79: 176–185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Y, Li R, Yang H, Luo H, and Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. J Stroke Cerebrovasc Dis 24: 601–609, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Huang W, Huang Y, Huang RQ, Huang CG, Wang WH, Gu JM, and Dong Y. SIRT3 Expression Decreases with Reactive Oxygen Species Generation in Rat Cortical Neurons during Early Brain Injury Induced by Experimental Subarachnoid Hemorrhage. Biomed Res Int 2016: 8263926, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobson EL, Dame AJ, Pyrek JS, and Jacobson MK. Evaluating the role of niacin in human carcinogenesis. Biochimie 77: 394–398, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Jadiya P, Chatterjee M, Sammi SR, Kaur S, Palit G, and Nazir A. Sir-2.1 modulates ‘calorie-restriction-mediated’ prevention of neurodegeneration in Caenorhabditis elegans: implications for Parkinson's disease. Biochem Biophys Res Commun 413: 306–310, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, et al. Hypoxia as a therapy for mitochondrial disease. Science 352: 54–61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, and Herson PS. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab 31: 2160–2168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496: 110–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joo HY, Yun M, Jeong J, Park ER, Shin HJ, Woo SR, Jung JK, Kim YM, Park JJ, Kim J, et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1alpha (HIF-1alpha) via direct interactions during hypoxia. Biochem Biophys Res Commun 462: 294–300, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Kaeberlein M, McVey M, and Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13: 2570–2580, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalaivani P, Ganesh M, Sathiya S, Ranju V, Gayathiri V, and Saravana Babu C. Alteration in bioenergetic regulators, SirT1 and Parp1 expression precedes oxidative stress in rats subjected to transient cerebral focal ischemia: molecular and histopathologic evidences. J Stroke Cerebrovasc Dis 23: 2753–2766, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, and Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483: 218–221, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, Kronfeld-Schor N, and Cohen HY. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 9: 162–173, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Khoury N, Koronowski KB, and Perez-Pinzon MA. Long-term window of ischemic tolerance: An evolutionarily conserved form of metabolic plasticity regulated by epigenetic modifications? J Neurol Neuromed 1: 6–12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kikuchi K, Tanaka E, Murai Y, and Tancharoen S. Clinical trials in acute ischemic stroke. CNS Drugs 28: 929–938, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, Park JH, Yang HJ, Kim MS, Kwon DY, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res 10: 722–731, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Kim SH, Lu HF, and Alano CC. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One 6: e14731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klaidman L, Morales M, Kem S, Yang J, Chang ML, and Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 69: 150–157, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Kohler E, Prentice DA, Bates TR, Hankey GJ, Claxton A, van Heerden J, and Blacker D. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke 44: 2493–2499, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, and Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 131: 21–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komlos D, Mann KD, Zhuo Y, Ricupero CL, Hart RP, Liu AY, and Firestein BL. Glutamate dehydrogenase 1 and SIRT4 regulate glial development. Glia 61: 394–408, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koronowski KB. and Perez-Pinzon MA. Sirt1 in cerebral ischemia. Brain Circ 1: 69–78, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kraus WL. PARPs and ADP-Ribosylation: 50 Years … and Counting. Mol Cell 58: 902–910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krey L, Luhder F, Kusch K, Czech-Zechmeister B, Konnecke B, Fleming Outeiro T, and Trendelenburg G. Knockout of silent information regulator 2 (SIRT2) preserves neurological function after experimental stroke in mice. J Cereb Blood Flow Metab 35: 2080–2088, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar S. and Lombard DB. Mitochondrial sirtuins and their relationships with metabolic disease and cancer. Antioxid Redox Signal 22: 1060–1077, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, and Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kupis W, Palyga J, Tomal E, and Niewiadomska E. The role of sirtuins in cellular homeostasis. J Physiol Biochem 72: 371–380, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurnasov O, Goral V, Colabroy K, Gerdes S, Anantha S, Osterman A, and Begley TP. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem Biol 10: 1195–1204, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Laiwalla AN, Ooi YC, Liou R, and Gonzalez NR. Matched Cohort Analysis of the Effects of Limb Remote Ischemic Conditioning in Patients with Aneurysmal Subarachnoid Hemorrhage. Transl Stroke Res 7: 42–48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lansberg MG, Bluhmki E, and Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke 40: 2438–2441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell 50: 686–698, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee OH, Kim J, Kim JM, Lee H, Kim EH, Bae SK, Choi Y, Nam HS, and Heo JH. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun 438: 388–394, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Li F. and Liu L. SIRT5 Deficiency Enhances susceptibility to kainate-induced seizures and exacerbates hippocampal neurodegeneration not through mitochondrial antioxidant enzyme SOD2. Front Cell Neurosci 10: 171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, and Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res 1210: 223–229, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Li L, Luo W, Huang L, Zhang W, Gao Y, Jiang H, Zhang C, Long L, and Chen S. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res 164: e21–e26, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun 7: 12235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, and Liang F. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci 27: 2606–2616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Nie H, Wu D, Zhang J, Wei X, and Ying W. Poly(ADP-ribose) polymerase mediates both cell death and ATP decreases in SIRT2 inhibitor AGK2-treated microglial BV2 cells. Neurosci Lett 544: 36–40, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Lin SJ, Defossez PA, and Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Lin SJ, Ford E, Haigis M, Liszt G, and Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev 18: 12–16, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu D, Gharavi R, Pitta M, Gleichmann M, and Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromol Med 11: 28–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L, Peritore C, Ginsberg J, Shih J, Arun S, and Donmez G. Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson's disease. Behav Brain Res 281: 215–221, 2015 [DOI] [PubMed] [Google Scholar]
- 104.Love S. Oxidative stress in brain ischemia. Brain Pathol 9: 119–131, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, and Szabados E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc 50: 179–187, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, and Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88: 841–886, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, and Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332: 1443–1446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCully JD, Cowan DB, Emani SM, and Del Nido PJ. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion 34: 127–134, 2017 [DOI] [PubMed] [Google Scholar]
- 109.Meller R. and Simon RP. A critical review of mechanisms regulating remote preconditioning-induced brain protection. J Appl Physiol (1985) 119: 1135–1142, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, and Luo T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull 121: 9–15, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452: 492–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, and Chua KF. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle 8: 2664–2666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michishita E, Park JY, Burneskis JM, Barrett JC, and Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16: 4623–4635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minnerup J, Sutherland BA, Buchan AM, and Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13: 11753–11772, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moroni F. Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol 8: 96–103, 2008 [DOI] [PubMed] [Google Scholar]
- 116.Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, and Perez-Pinzon MA. Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab 34: 1024–1032, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, and Perez-Pinzon MA. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci Rep 6: 29790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morris KC, Lin HW, Thompson JW, and Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab 31: 1003–1019, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Nakagawa T. and Guarente L. SnapShot: sirtuins, NAD, and aging. Cell Metab 20: 192–192 e191, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Nakagawa T. and Guarente L. Urea cycle regulation by mitochondrial sirtuin, SIRT5. Aging (Albany NY) 1: 578–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakagawa T, Lomb DJ, Haigis MC, and Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137: 560–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakahata Y, Sahar S, Astarita G, Kaluzova M, and Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Narayanan SV, Dave KR, Saul I, and Perez-Pinzon MA. Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 46: 1626–1632, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, Ren X, Wu Z, Streeper RS, and Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem 285: 31995–32002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ng F, Wijaya L, and Tang BL. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci 9: 64, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nie H, Hong Y, Lu X, Zhang J, Chen H, Li Y, Ma Y, and Ying W. SIRT2 mediates oxidative stress-induced apoptosis of differentiated PC12 cells. NeuroReport 25:838–842, 2014 [DOI] [PubMed] [Google Scholar]
- 128.Novgorodov SA, Riley CL, Keffler JA, Yu J, Kindy MS, Macklin WB, Lombard DB, and Gudz TI. SIRT3 Deacetylates Ceramide Synthases: IMPLICATIONS FOR MITOCHONDRIAL DYSFUNCTION AND BRAIN INJURY. J Biol Chem 291: 1957–1973, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Opitz CA. and Heiland I. Dynamics of NAD-metabolism: everything but constant. Biochem Soc Trans 43: 1127–1132, 2015 [DOI] [PubMed] [Google Scholar]
- 130.Osborne B, Bentley NL, Montgomery MK, and Turner N. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med 100: 164–174, 2016 [DOI] [PubMed] [Google Scholar]
- 131.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317: 516–519, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Pfister JA, Ma C, Morrison BE, and D'Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One 3: e4090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pizzi M, Sarnico I, Lanzillotta A, Battistin L, and Spano P. Post-ischemic brain damage: NF-kappaB dimer heterogeneity as a molecular determinant of neuron vulnerability. FEBS J 276: 27–35, 2009 [DOI] [PubMed] [Google Scholar]
- 134.Polletta L, Vernucci E, Carnevale I, Arcangeli T, Rotili D, Palmerio S, Steegborn C, Nowak T, Schutkowski M, Pellegrini L, et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 11: 253–270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Poulose N. and Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta 1852: 2442–2455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pulla VK, Battu MB, Alvala M, Sriram D, and Yogeeswari P. Can targeting SIRT-1 to treat type 2 diabetes be a good strategy? A review. Expert Opin Ther Targets 16: 819–832, 2012 [DOI] [PubMed] [Google Scholar]
- 138.Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281: 21745–21754, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Ran M, Li Z, Yang L, Tong L, Zhang L, and Dong H. Calorie restriction attenuates cerebral ischemic injury via increasing SIRT1 synthesis in the rat. Brain Res 1610: 61–68, 2015 [DOI] [PubMed] [Google Scholar]
- 140.Raval AP, Dave KR, and Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab 26: 1141–1147, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Roth M. and Chen WY. Sorting out functions of sirtuins in cancer. Oncogene 33: 1609–1620, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ryu D, Jo YS, Lo Sasso G, Stein S, Zhang H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, et al. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab 20: 856–869, 2014 [DOI] [PubMed] [Google Scholar]
- 143.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, and Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18: 416–430, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schiedel M, Robaa D, Rumpf T, Sippl W, and Jung M. The current state of NAD+ -dependent histone deacetylases (sirtuins) as novel therapeutic targets. Med Res Rev 0: 1–54, 2017 [DOI] [PubMed] [Google Scholar]
- 145.Schmidt MT, Smith BC, Jackson MD, and Denu JM. Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem 279: 40122–40129, 2004 [DOI] [PubMed] [Google Scholar]
- 146.Shao J, Yang X, Liu T, Zhang T, Xie QR, and Xia W. Autophagy induction by SIRT6 is involved in oxidative stress-induced neuronal damage. Protein Cell 7: 281–290, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shih J, Liu L, Mason A, Higashimori H, and Donmez G. Loss of SIRT4 decreases GLT-1-dependent glutamate uptake and increases sensitivity to kainic acid. J Neurochem 131: 573–581, 2014 [DOI] [PubMed] [Google Scholar]
- 148.Shimizu K, Quillinan N, Orfila JE, and Herson PS. Sirtuin-2 mediates male specific neuronal injury following experimental cardiac arrest through activation of TRPM2 ion channels. Exp Neurol 275(Pt 1): 78–83, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K, et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep 5: 654–665, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shinmura K, Tamaki K, and Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 295: H2348–H2355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shirakawa K, Chavez L, Hakre S, Calvanese V, and Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol 21: 277–285, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shulyakova N, Sidorova-Darmos E, Fong J, Zhang G, Mills LR, and Eubanks JH. Over-expression of the Sirt3 sirtuin Protects neuronally differentiated PC12 Cells from degeneration induced by oxidative stress and trophic withdrawal. Brain Res 1587: 40–53, 2014 [DOI] [PubMed] [Google Scholar]
- 153.Silva JP. and Wahlestedt C. Role of Sirtuin 1 in metabolic regulation. Drug Discov Today 15: 781–791, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Sims NR. and Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802: 80–91, 2010 [DOI] [PubMed] [Google Scholar]
- 155.Singh N, Agrawal M, and Dore S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem Neurosci 4: 1151–1162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sriram CS, Jangra A, Bezbaruah BK, V AK, and Sykam S. Alternative mechanisms of inhibiting activity of poly (ADP-ribose) polymerase-1. Front Biosci (Schol Ed) 8: 123–128, 2016 [DOI] [PubMed] [Google Scholar]
- 157.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, and Gao Y. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 114: 58–83, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tanny JC. and Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci U S A 98: 415–420, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsai YC, Greco TM, and Cristea IM. Sirtuin 7 plays a role in ribosome biogenesis and protein synthesis. Mol Cell Proteomics 13: 73–83, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85: 1383–1391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, and Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102: 703–710, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, and Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16: 93–105, 2004 [DOI] [PubMed] [Google Scholar]
- 163.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science 350: 1208–1213, 2015 [DOI] [PubMed] [Google Scholar]
- 164.Wang J, Feng H, Zhang J, and Jiang H. Lithium and valproate acid protect NSC34 cells from H2O2-induced oxidative stress and upregulate expressions of SIRT3 and CARM1. Neuro Endocrinol Lett 34: 648–654, 2013 [PubMed] [Google Scholar]
- 165.Wang P. and Miao CY. NAMPT as a Therapeutic Target against Stroke. Trends Pharmacol Sci 36: 891–905, 2015 [DOI] [PubMed] [Google Scholar]
- 166.Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, Zhai QW, Su DF, and Miao CY. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol 69: 360–374, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Wang Q, Li L, Li CY, Pei Z, Zhou M, and Li N. SIRT3 protects cells from hypoxia via PGC-1alpha- and MnSOD-dependent pathways. Neuroscience 286: 109–121, 2015 [DOI] [PubMed] [Google Scholar]
- 168.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, and Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 958: 439–447, 2002 [DOI] [PubMed] [Google Scholar]
- 169.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14: 312–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, Signore AP, Stetler RA, Gao Y, and Chen J. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke 39: 2587–2595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, and Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med 47: 229–240, 2009 [DOI] [PubMed] [Google Scholar]
- 172.Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, Orfaniotou F, Dhaunchak A, Brinkmann BG, Mobius W, et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci 27: 7717–7730, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wong YH, Wu CC, Lai HY, Jheng BR, Weng HY, Chang TH, and Chen BS. Identification of network-based biomarkers of cardioembolic stroke using a systems biology approach with time series data. BMC Syst Biol 9(Suppl 6): S4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133: 447–454, 2016 [DOI] [PubMed] [Google Scholar]
- 175.Wu Y, Chen L, Wang Y, Li W, Lin Y, Yu D, Zhang L, Li F, and Pan Z. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-kappaB mediated inflammatory responses in osteoarthritis development. Sci Rep 5: 17602, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie XQ, Zhang P, Tian B, and Chen XQ. Downregulation of NAD-Dependent Deacetylase SIRT2 Protects Mouse Brain Against Ischemic Stroke. Mol Neurobiol 2016, DOI: 10.1007/s12035-016-0173-z [DOI] [PubMed] [Google Scholar]
- 177.Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, and Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J Cereb Blood Flow Metab 33: 396–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang F, Zhou L, Wang D, Wang Z, and Huang QY. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1alpha through SIRT-3/PHD-2 degradation pathway. Neuroscience 304: 250–259, 2015 [DOI] [PubMed] [Google Scholar]
- 179.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130: 1095–1107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, Li J, Di S, Yue L, Liang G, et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 58: 61–70, 2015 [DOI] [PubMed] [Google Scholar]
- 181.Yang Y. and Sauve AA. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta 1864: 1787–1800, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, and Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yin J, Han P, Tang Z, Liu Q, and Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35: 1783–1789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ying W, Garnier P, and Swanson RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun 308: 809–813, 2003 [DOI] [PubMed] [Google Scholar]
- 185.Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, Zhang P, and Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci 12: 2728–2734, 2007 [DOI] [PubMed] [Google Scholar]
- 186.Yoshino J, Mills KF, Yoon MJ, and Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying WH, and Tian HL. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. J Neurochem 136: 581–593, 2016 [DOI] [PubMed] [Google Scholar]
- 188.Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventos RM, Martinez-Gonzalez MA, Salas-Salvado J, Aros F, Fito M, Lapetra J, Estruch R, Andres-Lacueva C, et al. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol Res 65: 615–620, 2012 [DOI] [PubMed] [Google Scholar]
- 189.Zhang F, Signore AP, Zhou Z, Wang S, Cao G, and Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res 83: 1241–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 190.Zhao Y, Guan YF, Zhou XM, Li GQ, Li ZY, Zhou CC, Wang P, and Miao CY. Regenerative neurogenesis after ischemic stroke promoted by nicotinamide phosphoribosyltransferase-nicotinamide adenine dinucleotide cascade. Stroke 46: 1966–1974, 2015 [DOI] [PubMed] [Google Scholar]
- 191.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou K. and Blom T. Trafficking and functions of bioactive sphingolipids: lessons from cells and model membranes. Lipid Insights 8(Suppl 1): 11–20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]