Abstract
Tumor metastasis to the oral cavity is rare and is usually an indication of late-stage disease and poor prognosis. While, there are reports of renal cell carcinoma (RCC) metastatic to oral cavity, vast majority of them are to the jaw. Herein, we present a case of a 78-year-old woman with RCC metastasis limited to the oral soft tissue without any bone involvement. As the lesion solely involved maxillary gingiva, it clinically mimicked that of a pyogenic granuloma, which is a reactive, nonneoplastic condition. This case was further complicated as the patient was unaware of primary cancer and appeared to be in good physical health. Her oral metastasis marked the initial manifestation of an otherwise silent primary renal cancer.
Keywords: Maxillary gingiva, metastatic cancer, pyogenic granuloma, renal cell carcinoma
INTRODUCTION
Tumor metastasis to the oral cavity is a rare phenomenon, accounting for only 1% of all oral malignancies.[1] When metastases occur, the jawbones are twice as likely to be involved than the oral mucosa. Most often, oral metastases are a late-stage manifestation found in the presence of widespread disease and are associated with poor long-term prognosis. However, oral metastases may occasionally be the first presentation of an otherwise nonmanifesting malignancy at a distant site.[1]
Renal cell carcinoma (RCC) is the third most common neoplasm to metastasize to the oral cavity, after lung and breast.[1] RCC originates in the lining of the proximal convoluted tubule and accounts for roughly 3% of adult malignancies.[2] Common sites of metastasis include lung, bone, lymph nodes and liver, with less frequent involvement of the head and neck region. The risk of metastasis to the latter is 15%, and most often affects facial skin.[3,4,5] Within the oral cavity, RCC is primarily metastatic to the tongue.[5]
Herein, we present a case of a 78-year-old woman with RCC metastatic to the maxillary anterior gingiva. This case is unique in that it not only represents an unusual location for metastasis but it also was the first presentation of an otherwise unknown primary malignancy. A literature review of the past 10 years (2007–2017) revealed only 25 cases of metastatic RCC to oral soft tissues, of which 12 were initial manifestations of a primary occult tumor. Our case adds to the small though growing collection of literature on this entity.
CASE REPORT
A 78-year-old woman presented to her general dentist with a chief complaint of an enlarging soft tissue mass of several months duration. The patient stated to be otherwise healthy, with no history of malignancy. She was not in acute distress on presentation.
Intraoral examination revealed a fluctuant, exophytic lesion of the maxillary anterior gingiva extending from the right lateral incisor to the left central incisor (teeth #12, 11, 21, F. D. I. System). The lesion measured 3.0 cm × 1.5 cm in greatest dimension and appeared dark-red color with secondary tan-gray ulceration [Figure 1a]. The dentist described the involved tissue as edematous and hyperemic and stated that on incisional biopsy the tissue partially collapsed under pressure from the forceps. A smaller, similar appearing lesion was identified in the right maxillary vestibule adjacent to the labial frenum [Figure 1b]. A periapical radiograph of the area showed no changes in the quality or quantity of bone and no evidence of tooth-related infections [Figure 2]. Based on the appearance of the lesion, a clinical diagnosis of pyogenic granuloma was made before the biopsy.
Figure 1.
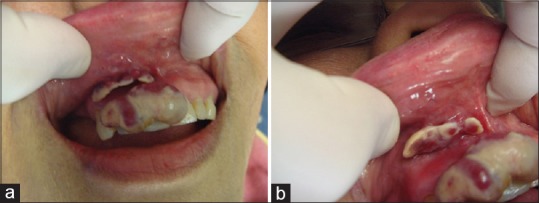
(a) Clinical image showing a tan-red exophytic, lobulated mass of the maxillary anterior facial gingiva. (b) A separate, similar appearing smaller lesion was identified in the right maxillary vestibule
Figure 2.
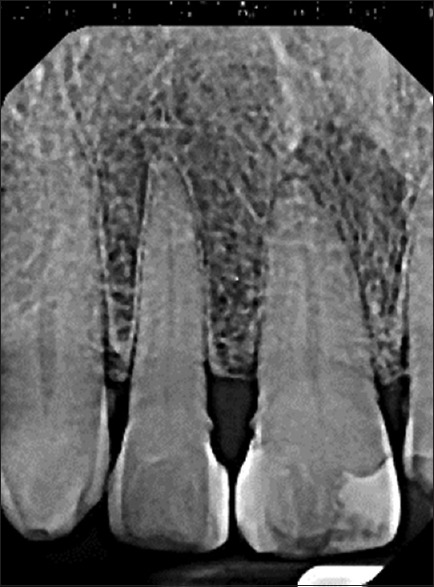
Periapical radiograph showing no changes in the quality or quantity of bone and no evidence of odontogenic infections
Histologic examination revealed soft tissue covered by stratified squamous epithelium. The epithelium appeared focally ulcerated but was otherwise unremarkable. Beneath the epithelium, tumor cells were found to completely efface the lamina propria [Figure 3a]. These cells were predominately arranged in lobular aggregates separated by thin fibrous septae [Figure 3b]. Some of the aggregates had a perivascular pattern, and the lesion itself had a rich vascular network. On high-power magnification, the cells displayed pink to vacuolated cytoplasm with vesicular nuclei and prominent nucleoli. Significant nuclear pleomorphism was present and the lesion demonstrated brisk mitotic activity [Figure 3c]. Based on these findings, the lesion was initially diagnosed as a carcinoma of unknown primary origin.
Figure 3.
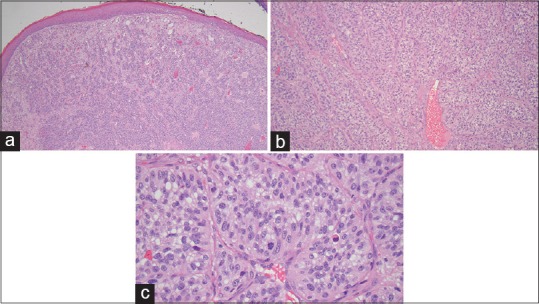
(a) Histopathologic image showing tumor cell nests completely effacing the lamina propria, (H&E, ×40). (b) These tumor nests were arranged in lobular aggregates separated by thin fibrous septae, (H&E, ×100). (c) On high power magnification, the cells displayed pink to vacuolated cytoplasm with vesicular nuclei and prominent nucleoli. Significant nuclear pleomorphism was present and the lesion demonstrated brisk mitotic activity, (H&E, ×400)
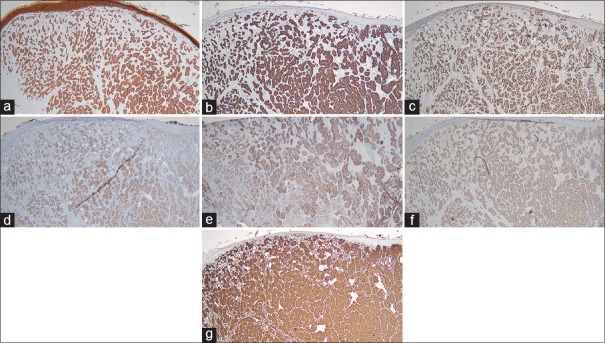
A wide panel of immunohistochemical markers was subsequently used to further classify the cells of origin. The tumor cells were strongly positive for pancytokeratin, CK8/18, Pax-8, CD10, CA9, CK19 and vimentin [Figure 4a–g] and were focally positive for EMA. The cells were negative for CK20, CK7, p63, p40, CK5, synaptophysin, c-kit, GATA3, TTF-1, S100, CDX-2, calponin, calcitonin, EBER, HMB45, PR, ER and CD31. These findings were consistent with a primary malignancy of renal origin.[6]
Figure 4.
Histopathologic image showing strong positivity for (a) pan-cytokeratin, ×40, (b) CK8/18, ×40, (c) Pax-8, ×40, (d) CD10, ×40, (e) CA9, ×40, (f) CK19, ×40 and (g) vimentin, ×40, in the tumor cell population
The patient was subsequently referred to her primary care physician and an oncologist for full body imaging. A whole body bone scan was performed which showed uptake in the right kidney consistent with neoplasm. In addition, focal increased uptake consistent with possible metastatic deposits was seen in the right femoral head and greater trochanter.
DISCUSSION
More than 90% of all malignancies of the oral cavity are squamous cell carcinomas. The second most common are of minor salivary gland origin.[7] Metastatic dissemination to the oral cavity from distant primary sites is a rare phenomenon and is usually an indication of a late-stage widespread disease and is associated with poor long-term prognosis. However, in a recent literature review, about 23% of oral metastases have been found to be the first sign of an undiscovered malignancy at a distant site.[1]
The most common primary sites for oral metastases in men are the lung, followed with much lower incidence by the kidney and liver; in women, breast, female genital organs and kidney represent the most common sites of primary cancer. The jawbones, particularly the mandible, are twice as likely to be affected by metastatic spread than the oral soft tissues.[1] In the oral soft tissues, the attached gingiva is most commonly involved. Depending on the origin, metastatic cancer favors different oral sites: in men, cancer of the lung preferentially metastasizes to both the jawbones and the oral mucosa and the kidney to oral soft tissues. In women, cancer of the breast most commonly metastasizes to both the jawbones and soft tissues, whereas dissemination from the female genital organs primarily affects oral soft tissues.[1]
RCC is the third most frequent neoplasm to metastasize to the orofacial region, preceded by lung and breast cancer.[1] RCC originates in the lining of the proximal convoluted tubule and accounts for roughly 3%of adult malignancies.[2] About one in three patients with RCC eventually develops distant metastatic disease, at which case the disease is considered incurable. Common sites of metastasis are lung (45.2%), bone (29.5%), lymph nodes (21.8%) and liver (20.3%). Although less frequent, the head and neck region is under 15% risk of being affected by metastatic dissemination of RCC.[3]
A recent literature review by Suojanen et al. reports a total of 75 cases of RCC metastatic to the head and neck region (1976–2014). Of those cases, the most commonly involved sites are facial skin, the parotid gland, and paranasal sinuses, followed by the oral cavity.[3,4,5] Curiously, oral metastatic RCCs do not predominantly affect the gingiva, which most oral soft tissue metastases do, but rather occur in minor salivary gland-containing areas and the tongue.[5,8]
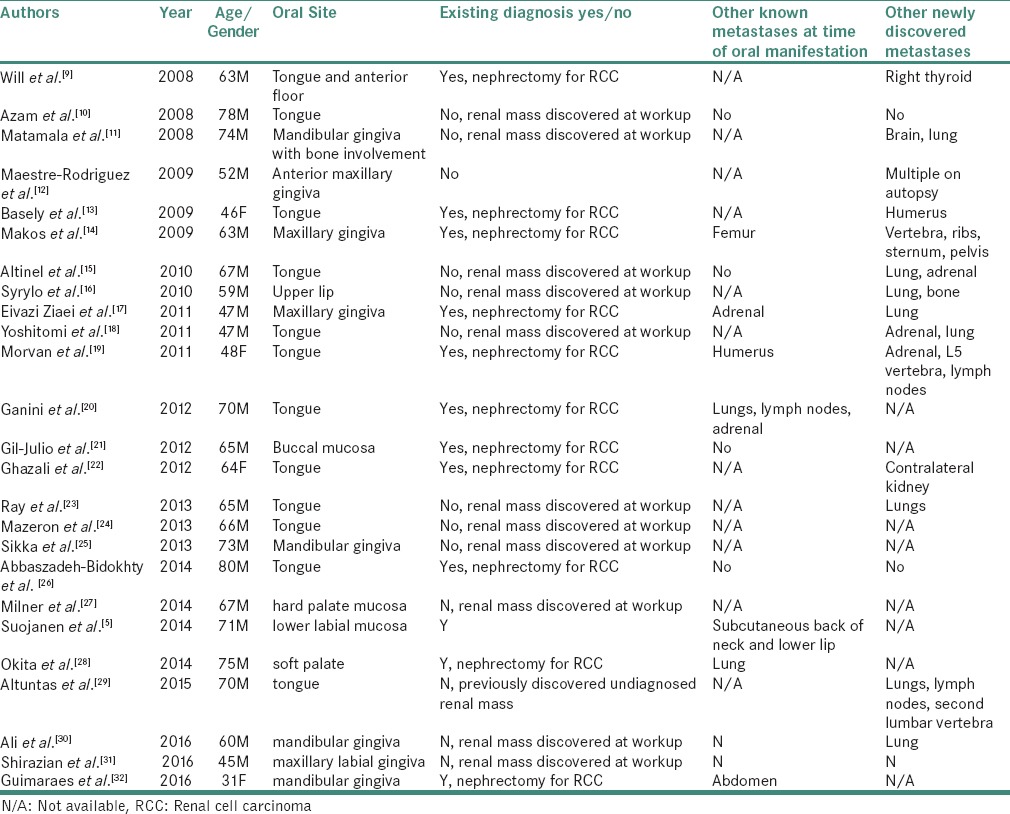
We conducted a literature review of the last 10 years (2007–2017), which revealed 25 cases of RCC metastatic to the oral soft tissues (excluding the current case). The choice of the time frame was dictated by the fact that the last comprehensive literature review of metastatic tumors to the oral cavity, including metastatic renal carcinoma, covered cases reported between 1916 and 2006.[1] Furthermore, more recent reviews which deal specifically with RCC metastases, such as that of Suojanen et al. are not limited to the oral cavity proper.[5] In 12 of the cases reported between 2007 and 2017, oral metastasis was the first presentation of an otherwise occult renal tumor discovered at subsequent workup. The full listing of cases can be found in Table 1.[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]
Table 1.
Reported cases of RCC metastatic to the oral soft tissues in 2007-2017
Metastases to oral soft tissues often present as focally ulcerated, nodular masses resembling a hyperplastic or reactive growth. For this reason, they may be initially misdiagnosed as peripheral giant cell granulomas or pyogenic granuloma;[33] this was the case with our patient. Personal history of malignancy may be useful in establishing a diagnosis, however, as was previously stated, approximately 23% of oral metastases present as the first sign of a malignant disease.[1] Histologic analysis can readily distinguish between oral metastases and primary reactive processes; the presence of nuclear enlargement and pleomorphism, brisk mitotic activity and cellular atypia are features of malignancy not seen in benign processes such as pyogenic granulomas. Furthermore, the microscopic appearance of the metastatic neoplasm should resemble the tumor of origin; however, this may not hold true in late-stage lesions where the tumor cells have become dedifferentiated.[33] In our case, the cells displayed pink to vacuolated cytoplasm with vesicular nuclei and prominent nucleoli. The differential diagnosis based on this cellular morphology included metastasis from a breast, thyroid, ovarian, colon or renal primary, malignant melanoma, epithelioid sarcoma, angiosarcoma and carcinosarcoma. A metastatic lesion was favored despite the lack of a known primary lesion. Specifically, metastatic RCC was considered likely due to the high degree of vascularity of the lesion.[7] Angiosarcoma was also included in the differential diagnosis for this reason. Melanoma was considered because it is known to have diverse morphologic presentations and rare cases with perivascular pseudorosettes have been reported.[34] In many cases, as with the current case, immunohistochemical analysis is necessary to confirm the tumor lineage.
Prognosis for patients with metastatic tumors is usually poor because multiple other metastatic sites are usually present.[33] Of the 25 cases of metastatic RCC included in Table 1, seven had other known metastases at the time of oral manifestation and 14 had other newly discovered metastases following detection of the oral lesion (one at autopsy). Therefore, management of the oral lesion is usually palliative and coordinated with the patient's overall treatment. In cases where the oral lesion represents the initial presentation of an occult primary malignancy, additional workup including full body imaging is recommended to determine the extent of the malignant process.
Metastatic lesions to the oral cavity are an uncommon process associated with poor long-term prognosis. In most cases, the patient has a known history of malignancy and the oral lesion represents one of several metastases. Infrequently, oral metastases represent the initial presenting sign of an otherwise silent primary tumor. Our case represents only the 26th documented report of RCC metastatic to oral soft tissues over the past 10 years. Furthermore, it is only the 13th documented case in which the oral lesion was the first sign of an occult renal primary. Awareness of such a presentation is essential to the clinician when establishing a working differential for nodular or hyperplastic masses of unknown cause.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity-pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44:743–52. doi: 10.1016/j.oraloncology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Brufau BP, Cerqueda CS, Villalba LB, Izquierdo RS, González BM, Molina CN, et al. Metastatic renal cell carcinoma: Radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. Radiographics. 2013;33:1691–716. doi: 10.1148/rg.336125110. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol. 2012;23:973–80. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 4.Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: A review of case reports. J Med Case Rep. 2011;5:429. doi: 10.1186/1752-1947-5-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suojanen J, Färkkilä E, Helkamaa T, Loimu V, Törnwall J, Lindqvist C, et al. Rapidly growing and ulcerating metastatic renal cell carcinoma of the lower lip: A case report and review of the literature. Oncol Lett. 2014;8:2175–8. doi: 10.3892/ol.2014.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 8.Pires FR, Azevedo RS, Ficarra G, Cardoso AS, Carlos R, Kowalski LP, et al. Metastatic renal cell carcinoma to the oral cavity and clear cell mucoepidermoid carcinoma: Comparative clinicopathologic and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e22–7. doi: 10.1016/j.tripleo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Will TA, Agarwal N, Petruzzelli GJ. Oral cavity metastasis of renal cell carcinoma: A case report. J Med Case Rep. 2008;2:313. doi: 10.1186/1752-1947-2-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azam F, Abubakerr M, Gollins S. Tongue metastasis as an initial presentation of renal cell carcinoma: A case report and literature review. J Med Case Rep. 2008;2:249. doi: 10.1186/1752-1947-2-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narea-Matamala G, Fernández-Toro Mde L, Villalabeitía-Ugarte E, Landaeta-Mendoza M, Rojas-Alcayaga G. Oral metastasis of renal cell carcinoma, presentation of a case. Med Oral Patol Oral Cir Bucal. 2008;13:E742–4. [PubMed] [Google Scholar]
- 12.Maestre-Rodríguez O, González-García R, Mateo-Arias J, Moreno-García C, Serrano-Gil H, Villanueva-Alcojol L, et al. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med Oral Patol Oral Cir Bucal. 2009;14:e601–4. doi: 10.4317/medoral.14.e601. [DOI] [PubMed] [Google Scholar]
- 13.Basely M, Bonnel S, Maszelin P, Verdalle P, Bussy E, de Jaureguiberry JP, et al. A rare presentation of metastatic renal clear cell carcinoma to the tongue seen on FDG PET. Clin Nucl Med. 2009;34:566–9. doi: 10.1097/RLU.0b013e3181b06ad7. [DOI] [PubMed] [Google Scholar]
- 14.Makos CP, Psomaderis K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an Epulis-like metastasis. J Oral Maxillofac Surg. 2009;67:653–60. doi: 10.1016/j.joms.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Altınel D, Etİt D, Tan A, Bayol Ü, Bulut V, Gökçöl-Erdoǧan I, et al. Metastatic renal cell carcinoma initially presented as a tongue mass. Turk J Pathol. 2010;26:261–3. [Google Scholar]
- 16.Syryło T, Syryło A, Jurkiewicz D, Zieliński H, Pietka T. An upper lip tumour as the presenting symptom of metastatic renal cancer. Otolaryngol Pol. 2010;64:318–9. doi: 10.1016/S0030-6657(10)70614-4. [DOI] [PubMed] [Google Scholar]
- 17.Eivazi Ziaei J, Fakhrgoo A, Estakhri R. Gingival metastasis of renal cell carcinoma. Iran J Cancer Prev. 2011;4:44–7. [Google Scholar]
- 18.Yoshitomi I, Kawasaki G, Mizuno A, Nishikido M, Hayashi T, Fujita S, et al. Lingual metastasis as an initial presentation of renal cell carcinoma. Med Oncol. 2011;28:1389–94. doi: 10.1007/s12032-010-9596-y. [DOI] [PubMed] [Google Scholar]
- 19.Morvan JB, Veyrières JB, Mimouni O, Cathelinaud O, Allali L, Verdalle P, et al. Clear-cell renal carcinoma metastasis to the base of the tongue and sphenoid sinus: Two very rare atypical ENT locations. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:91–4. doi: 10.1016/j.anorl.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Ganini C, Lasagna A, Ferraris E, Gatti P, Paglino C, Imarisio I, et al. Lingual metastasis from renal cell carcinoma: A case report and literature review. Rare Tumors. 2012;4:e41. doi: 10.4081/rt.2012.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Julio H, Vázquez-Alonso F, Fernández-Sánchez AJ, Puche-Sanz I, Flores-Martín JF, Cózar JM, et al. Metastasis of renal cell carcinoma to the buccal mucosa 19 years after radical nephrectomy. Case Rep Oncol Med. 2012;2012:823042. doi: 10.1155/2012/823042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghazali N, Davis C, Barrett AW, Tighe JV. Bilateral asynchronous renal cell carcinoma with metastatic involvement of the tongue. Case Rep Pathol. 2012;2012:729642. doi: 10.1155/2012/729642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray A, Bhattacharya J, Ganguly S. Renal cell carcinoma presenting with oral tongue metastasis: A rare case presentation. J Cancer Res Ther. 2013;9:117–8. doi: 10.4103/0973-1482.110392. [DOI] [PubMed] [Google Scholar]
- 24.Mazeron R, Fenoll L, Mathieu MC, Dumas I, Haie-Meder C. Brachytherapy for isolated tongue metastasis of renal clear cell carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130:149–51. doi: 10.1016/j.anorl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Sikka S, Sikka P, Kaur G, Shetty DC. A review of histopathological and immunohistochemical parameters in diagnosis of metastatic renal cell carcinoma with a case of gingival metastasis. J Cancer Res Ther. 2013;9:105–7. doi: 10.4103/0973-1482.110395. [DOI] [PubMed] [Google Scholar]
- 26.Abbaszadeh-Bidokhty H, Motallebnejad M, Rajabi-Moghaddam M. Metastatic renal cell carcinoma presenting as a clear-cell tumor in tongue: A case report. Iran J Otorhinolaryngol. 2014;26:185–90. [PMC free article] [PubMed] [Google Scholar]
- 27.Milner P, Janas A, Grzesiak-Janas G. Clear cell renal carcinoma metastasis to the oral cavity – Case report. J Preclin Clin Res. 2014;8:127–9. [Google Scholar]
- 28.Okita M, Hariya Y, Sekido K, Harada M, Sekiguchi T, Nakayama E, et al. A case of renal cell carcinoma metastasis to the soft palate. J Jpn Soc Oral Oncol. 2014;26:123–9. [Google Scholar]
- 29.Altuntaş O, Petekkaya İ, Süslü N, Güllü İ. Renal cell carcinoma metastatic to the tongue: A case report and review of the literature. J Oral Maxillofac Surg. 2015;73:1227–30. doi: 10.1016/j.joms.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Ali RA, Mohamed KE. Metastatic clear cell renal cell carcinoma presenting with a gingival metastasis. Clin Pract. 2016;6:847. doi: 10.4081/cp.2016.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirazian S, Bahrami N. An oral metastatic carcinoma guiding to discovery of a renal carcinoma: A case report. J Craniomax Res. 2016;3:230–4. [Google Scholar]
- 32.Guimarães DM, Pontes FS, Miyahara LA, Guerreiro MY, de Almeida MC, Pontes HA, et al. Metastatic renal cell carcinoma to the oral cavity. J Craniofac Surg. 2016;27:E533–4. doi: 10.1097/SCS.0000000000002738. [DOI] [PubMed] [Google Scholar]
- 33.Neville BW, Damm DD, Allen CM, Chi AC. Oral and Maxillofacial Pathology. 4th ed. St Louis (MO): Elsevier; 2016. [Google Scholar]
- 34.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. A distinct histopathological variant of a malignant melanoma with perivascular pseudorosettes: A case report. Oncol Lett. 2013;6:673–5. doi: 10.3892/ol.2013.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]