Abstract
The serrated neoplasia pathway is thought to account for up to 30% of sporadic colorectal cancer, but its role in inflammatory bowel disease (IBD)-related colorectal cancer is still not well elucidated. Hyperplastic polyps are not thought to impart an increased risk of colorectal cancer; however, sessile serrated adenomas/polyps and traditional serrated adenomas may have malignant potential. From the limited research currently available, this appears to hold true for IBD patients as well. IBD patients do not seem to be at a higher risk of typical serrated colorectal lesions than the general population, but it is still not known if they have a quicker progression to colorectal cancer. Serrated epithelial change is a newly described finding in patients with longstanding colitis that may increase the risk of colorectal cancer in IBD patients. Overall, serrated lesions are not uncommon in the IBD population, and further research is needed to understand the role that serrated lesions play in the development of colorectal cancer.
Keywords: Crohn’s disease, ulcerative colitis, inflammatory bowel disease, colorectal cancer, serrated polyps, serrated epithelial change
Inflammatory bowel disease (IBD) patients have a higher risk of colorectal cancer, which increases with the duration, extent, and degree of inflammation. Most colorectal cancer in IBD patients is thought to arise from the inflammation-dysplasia-carcinoma sequence.1 The serrated neoplasia pathway is now thought to account for 30% of sporadic colorectal cancer; however, the role of this pathway in IBD-related colorectal cancer is still lacking robust data. This article will review the available, albeit limited, literature on the effects of serrated colorectal lesions in IBD patients.
Serrated Lesions of the Colon
Serrated lesions of the colon are classified by the World Health Organization (WHO) into 3 categories: hyperplastic polyps (HPs; 70%-95%), sessile serrated adenomas/polyps (SSA/Ps; 5%-25%), and traditional serrated adenomas (TSAs; <2%).2 HPs are characterized by straight crypts, serrations limited to the upper half of the crypts, and minimal cytologic atypia. These lesions are further divided into 3 groups based upon their epithelial lining: microvesicular HPs (right colon predominant), goblet cell–rich HPs (left colon predominant), and mucin-poor HPs (left colon predominant). SSA/Ps are most commonly found in the right colon, are characterized by branching and disorganized crypt patterns, have serrations throughout the length of the crypt, and have varying degrees of cytologic atypia. Therefore, SSA/Ps are further classified histologically by the presence or absence of cytologic dysplasia (either adenoma-like or serrated). SSA/Ps are typically flat and coated with excess mucin, making them difficult to identify endoscopically and possibly contributing to interval cancers.3 TSAs are histologically complex with prominent serrations of glands, villiform and protuberant growth, and abundant eosinophilic cytoplasm. TSAs are most commonly located in the left colon and contain cytologic dysplasia (either serrated or adenomatous).4 Frequently, the dysplasia is adenomalike, although serrated dysplasia has also been described.2
In addition, serrated epithelial change (SEC) is a newly described (non-WHO–sanctioned) histologic finding in patients with colitis characterized by disorganized crypt architecture and serrations throughout the crypt but no evidence of cytologic atypia.5 The risk of progression to dysplasia and colorectal cancer is still unknown. Table 1 summarizes the histologic similarities and differences between all serrated lesions and SEC.
Table 1.
Histopathologic Characteristics of Serrated Lesions of the Colon
| Hyperplastic Polyps | Sessile Serrated Adenomas/Polyps | Traditional Serrated Adenomas | Serrated Epithelial Changea | |
|---|---|---|---|---|
| Serrations | Limited to top half of crypt | Top and bottom of crypt | Limited to top half of crypt | Top and bottom of crypt |
| Basal Crypt Architecture | Narrow, straight, tubular | Distorted, branching crypts dilated at base; inverted L or T shape | Ectopic crypts that are narrow, straight, and tubular with protuberant villiform architecture | Distorted; some crypts do not reach muscularis mucosae |
| Nuclear Features | Unremarkable | Small foci of pseudostratification, occasional mitosis in the upper crypt | Elongated nuclei, mild pseudostratification, occasional mitosis in the upper crypt | Unremarkable |
| Epithelium | Hypermature, microvesicular | Gastric-like mucin prominent | Eosinophilic | Goblet cell–rich |
Serrated epithelial change is not a widely recognized histopathologic finding and does not have sanctioned World Health Organization criteria; in contrast, hyperplastic polyps, sessile serrated adenomas/polyps, and traditional serrated adenomas have criteria sanctioned by the World Health Organization.
The term serrated polyp unclassified (SPU) has emerged to describe serrated lesions that do not neatly fit into the categories previously described due to overlapping features.2 Serrated lesions of the colon tend to have moderate interobserver variation among pathologists, likely due to the evolving definitions and significance of these lesions.6-10
The exact risk and rate of progression to colorectal cancer associated with serrated lesions is still not well elucidated. There are some emerging data that SSA/Ps are associated with synchronous polyps or colorectal cancer, particularly in lesions larger than 1 cm.11,12 Another study found an increased risk of metachronous cancer in patients with SSA/Ps compared to patients with HPs or tubular adenomas.13 Serrated adenocarcinomas are thought to have a worse 5-year survival than conventional colorectal cancer, possibly due to difficult detection endoscopically, incomplete resection of frequently flat lesions, and more aggressive cytology.2,14 The current guidelines suggest more aggressive surveillance in patients with findings of SSA/Ps or TSAs based upon the number and size of the lesions (Table 2).2
Table 2.
Colonoscopy Screening Guidelines After Finding Serrated Colorectal Lesions
| Finding | Interval (years) |
|---|---|
| HP <10 mm, rectosigmoid, any number | 10 |
| HP ≤5 mm, proximal to sigmoid, ≤3 in number | 10 |
| HP ≥4 in number, proximal to sigmoid | 5 |
| HP >5 mm, proximal to sigmoid | 5 |
| SSA/P or TSA <10 mm | 5 |
| SSA/P or TSA ≥10 mm, or ≥3 SSA/P or TSA | 3 |
| SSA/P with dysplasia | 1-3 |
| Serrated polyposis | 1 |
HP, hyperplastic polyp; SSA/P, sessile serrated adenoma/polyp; TSA, traditional serrated adenoma.
Serrated Lesions in Inflammatory Bowel Disease
There are limited studies examining serrated lesions within the IBD population. Jackson and colleagues performed a retrospective analysis to characterize synchronous and metachronous lesions of 134 IBD patients with serrated polyps: 25 patients had SSA/Ps (3 contained cytologic dysplasia), 97 patients had HPs, and 12 patients had SPUs.15 A cohort of 139 non-IBD patients with SSA/Ps was used to compare the risk of metachronous neoplasia in IBD patients. SSA/Ps were more likely to be located in the right colon compared to HPs and SPUs (76%, 28%, and 42%, respectively; P=.002). Rates of synchronous dysplasia (defined as within 1 year of the index serrated lesion) were not different between IBD patients with SSA/Ps and those with HPs; however, rates of multifocal synchronous dysplasia were significantly higher with SSA/Ps (16%) compared to HPs (3%). Metachronous dysplasia was found in 8 of the 13 patients with index SSA/Ps (61.5%): 5 adenomas and 3 SSA/Ps. IBD patients with SSA/Ps (n=13) did not have a greater risk of metachronous dysplasia compared to non-IBD patients with SSA/Ps (n=139); however, the number of patients was very small. There was no difference in the rates of synchronous or metachronous dysplasia in serrated lesions found in inflamed vs noninflamed mucosa, which calls into question whether inflammation is a factor in serrated lesions. Not surprisingly, larger SSA/Ps were associated with higher rates of subsequent dysplasia. It should be noted that this study was limited by the size of the population and the fact that the majority of patients had HPs. The rate of metachronous dysplasia in HPs was not reported. No comparison was made between the rates of serrated lesions in IBD patients compared to non-IBD patients. Based on these data, the authors could not conclude whether IBD patients with serrated lesions have a higher risk of colorectal cancer compared to non-IBD patients.
Another small retrospective study examined 115 IBD patients with serrated lesions from 2002 to 2010. The majority of patients had HPs, and only 7 patients had SSA/Ps. No IBD patients with TSAs were found in this pathology search. Synchronous dysplasia (at index colonoscopy) was found in 14% of SSA/Ps and 6% of HPs, which was not a statistically significant difference. Metachronous conventional dysplasia, defined as dysplasia or adenocarcinoma, was found in 0% of SSA/Ps and 4% of HPs, which is not significantly different. The rate of metachronous conventional dysplasia was 29% in SSA/Ps compared to 3% in HPs. Similar to the previous study, 25% of serrated polyps occurred outside of an area of inflammation.16
In a third study, Ko and colleagues studied serrated colorectal polyps in IBD patients.17 From 2000 through 2013, the authors reported a 1.2% prevalence rate of serrated polyps in IBD patients undergoing surveillance colonoscopy. A total of 78 patients were included: 35 patients had serrated polyps negative for dysplasia consistent with the WHO criteria for SSA/Ps, 25 patients had serrated polyps with low-grade dysplasia (LGD) consistent with the WHO definition for TSAs, and 18 patients had serrated polyps with features indefinite for dysplasia. No polyps were identified that histologically resembled SSA/Ps with cytologic dysplasia. Serrated polyps with LGD or indefinite dysplasia were more likely to be left-sided and have a male predominance. In contrast, serrated polyps negative for dysplasia were more likely to be right-sided and have a female predominance. IBD patients with serrated polyps containing LGD had significantly higher rates of prior synchronous or metachronous dysplasia (76%) compared to IBD patients with serrated polyps without dysplasia (12%), serrated polyps with indefinite dysplasia (39%), and HPs (21%). There was a high degree of location concordance (74%) of metachronous dysplasia following the index serrated polyp.
Among 77 patients with index serrated polyps (including HPs), incident advanced neoplasia (high-grade dysplasia [HGD] and adenocarcinoma) developed in 0 of 27 patients with serrated polyps negative for dysplasia, 4 of 21 patients with serrated polyps positive for LGD, 1 of 11 patients with serrated polyps indefinite for dysplasia, and 1 of 18 patients with HPs. Compared to a reference population with baseline LGD, there was no statistical difference in 10-year rates of advanced neoplasia between serrated polyps with LGD or indefinite dysplasia. Rates of advanced neoplasia were similar between patients with index serrated polyps negative for dysplasia, index HPs, and a reference cohort without dysplasia at baseline. The authors concluded that serrated polyps without dysplasia did not appear to have an increased risk of incident dysplasia or colorectal cancer.17
It should be noted that among these 3 studies of serrated lesions in IBD, there is significant difference in patient selection criteria, methodologies, histopathologic terminology, and outcome measures. Additionally, prior conventional dysplasia is a major risk factor for subsequent colorectal cancer and must be controlled for in future studies. Larger studies are also needed to better characterize the risks of serrated lesions in IBD patients and to determine whether they convey a different risk of developing advanced neoplasia compared to serrated lesions in non-IBD patients.
Serrated Epithelial Change
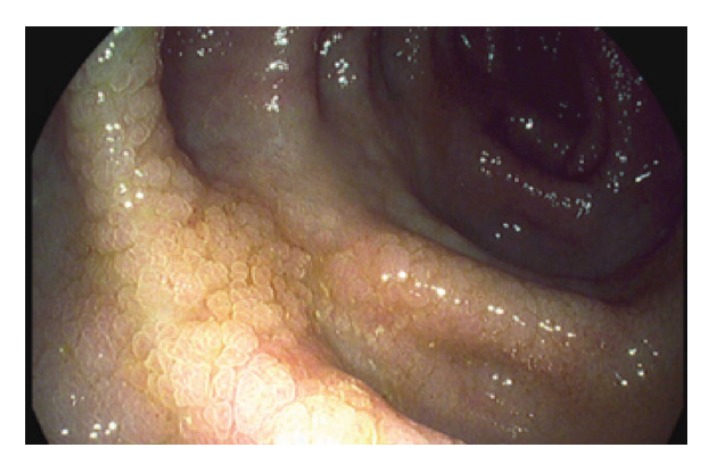
SEC is a histologic finding in patients with long-standing colitis. SEC is not consistently recognized endoscopically and is not widely accepted as a histopathologic finding. However, there is growing literature on this topic, with 3 published manuscripts and 2 nationally presented abstracts (2 from the same group of researchers).5,18-21 SEC is typically found on flat mucosa during routine biopsies. When visualized, SEC is flat or nodular mucosa without a discrete polyp (Figure 1). SEC is synonymous with flat serrated change and hyperplastic-like mucosal change, which are mentioned in prior publications.18,19 However, SEC is distinct from SSA/Ps. The role of SEC in IBD is not fully known. Several studies suggest that the finding of SEC in IBD patients may be associated with higher rates of colonic neoplasia.5,18,19,21
Figure 1.
An endoscopic example of serrated epithelial change during high-definition endoscopy.
Reproduced from Parian et al.5
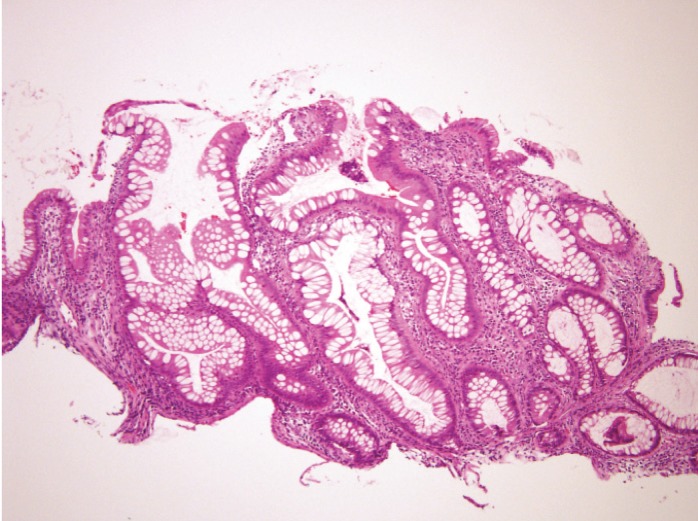
SEC is characterized histologically by serrated epithelium throughout the entirety of the crypt and distorted crypt architecture with crypts no longer perpendicular or extending down to the muscularis mucosae. Also present are normal surface maturation, normal basal nuclei, and goblet rich cells in the apices (Figure 2).
Figure 2.
A histologic example of serrated epithelial change with serrations throughout the length of the crypt and crypts no longer reaching the muscularis mucosae.
Reproduced from Parian et al.5
The first report of SEC in the literature was in 2000 in an article by Kilgore and colleagues that described hyperplastic-like mucosal change, histologically equivalent to SEC, in 60 Crohn’s disease colectomy specimens.18 There was a significantly higher rate of SEC in colectomy specimens with colorectal cancer (33%) compared to colectomy specimens without colorectal cancer (10%). The histopathology finding of SEC was both adjacent to and distant from the findings of adenocarcinoma.
In abstract form, Atwaibi and colleagues presented their experience of 94 IBD patients with flat serrated change, histologically equivalent to SEC, compared to 187 IBD controls.19 There was an increased rate of subsequent dysplasia among IBD patients with SEC compared to IBD patients without SEC (12.8% vs 4.3%; P=.013). On multivariate analysis, adjusting for different rates of surveillance, age, and sex, the odds ratio was 2.53 (P=.081). This study may not have been adequately powered to detect a difference due to the small number of patients.
Johnson and colleagues were the first to publish a full manuscript examining the progression of SEC to dysplasia.20 The authors identified 79 IBD patients with SEC (incidence rate of 10/1000 among IBD patients) between 2006 and 2012. Twenty-seven patients had a history of dysplasia, while 52 patients did not. Thirty-six of 52 patients without a history of dysplasia had at least 1 follow-up colonoscopy, compared to 76 controls with IBD and no history of SEC. Among patients with SEC and no history of dysplasia, the median age was 58 years, the median IBD duration was 19 years, 83% had extensive disease, and the median follow-up time was 31 months. There was no significant difference in these indices compared to controls without a history of dysplasia. Among patients without a history of dysplasia, the 1- and 3-year risk of developing dysplasia was 6% and 17%, respectively, compared to 0% and 2%, respectively, for controls (P=.11). Subsequent lesions in the SEC group included unifocal LGD, multifocal LGD, TSAs, and SSA/Ps; no cases of cancer were observed. In the control group, lesions included unifocal LGD, SSA/Ps, and 1 colorectal cancer. Because a large proportion of patients in the study had prior dysplasia, the study was likely underpowered to show a statistically significant effect to support the progression of SEC to new dysplasia.
Parian and colleagues examined the association between SEC and dysplasia in the largest group of IBD patients with SEC to date.5 This was a retrospective, observational study of 187 IBD patients (52% with ulcerative colitis, 39% with Crohn’s disease, 9% with IBD unclassified) with SEC but without prior dysplasia or colorectal cancer from 2000 to 2008. The mean age at SEC diagnosis was 48.4 years with a median disease duration of 16.0 years. The median follow-up time was 28 months, and patients had pathology results from, on average, 2 colonoscopies. Most of the findings of SEC (72%) were identified on nontargeted biopsies of the colon. Fifteen patients (8%) had synchronous dysplasia that was concordant with the location of the SEC 60% of the time. Of the 112 patients without prior or synchronous dysplasia and at least 1 follow-up colonoscopy, 24 (21%) had new metachronous dysplasia with a similar rate (52%) of concordance. The overall dysplasia rate was 60 per 1000 patient-years. Approximately 6% of SEC patients (11/187) developed HGD or colorectal cancer with a rate of 17 per 1000 patient-years. Nine of the 11 cases of HGD/colorectal cancer were in ulcerative colitis patients. Older patients and those who had at least 1 metachronous SEC were at higher risk of developing dysplasia.
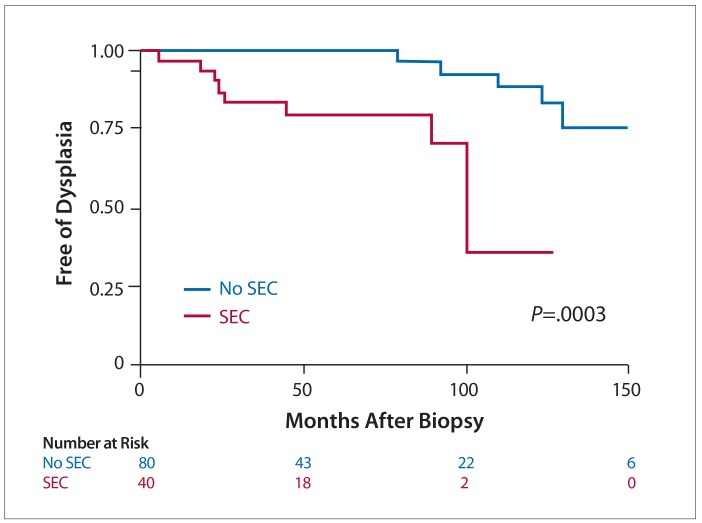
Given the high rates of dysplasia observed in their retrospective, observational study, Parian and colleagues further examined whether SEC plays a role in the development of dysplasia in a case-control study that was presented in abstract form in 2017.21 Because the high-risk lesions were mainly found in the setting of ulcerative colitis, the study was limited to ulcerative colitis patients with or without SEC. Sixty-four ulcerative colitis patients with SEC were matched to 113 ulcerative colitis patients without SEC. Exclusion criteria included any prior dysplasia or colorectal cancer, missing disease characteristics from electronic medical records, or the inability to match at least 1 control. Patients were matched by age (± 5 years), disease duration (± 5 years), and disease extent (E1=proctitis, E2=left-sided, E3=extensive). Ulcerative colitis patients with SEC had a higher rate of any dysplasia compared to ulcerative colitis patients without SEC (23.4% vs 5.3%; P<.001). The percentage of HGD/colorectal cancer was higher among ulcerative colitis patients with SEC compared to ulcerative colitis patients without SEC (9.4% vs 1.8%; P=.03). The time to dysplasia was significantly shorter among patients with SEC who developed dysplasia vs those without SEC who developed dysplasia (22.2 months vs 116.3 months; P<.001; Figure 3).
Figure 3.
A Kaplan-Meier survival curve showing a higher risk and shorter time to dysplasia in ulcerative colitis patients with SEC than ulcerative colitis patients without SEC.a
SEC, serrated epithelial change.
aThese findings have only been presented in abstract form.
Reproduced from Parian et al.21
Genetics of Serrated Colorectal Lesions
The genetics of serrated colorectal lesions can help distinguish them from adenomas and better classify them. At the present time, there are no recommendations to routinely order genetic testing on polyps aside from patients with polyposis syndrome or colorectal cancer. However, with more affordable assays, genetic testing of colonic tissue may help distinguish patients at higher risk of dysplasia in the future.
KRAS and BRAF mutations are mutually exclusive in serrated lesions, both resulting in constitutive activation of the MAPK pathway leading to uncontrolled cell proliferation, survival, and invasion. Microsatellite instability is another important factor in serrated lesions that involves the loss of mismatch repair genes and, interestingly, is found in serrated cancers but rarely in precursor lesions.22
The epigenetic CpG island methylator phenotype (CIMP) is characterized by vast hypermethylation of promoter CpG island sites, resulting in the inactivation of several tumor suppressor genes, which allows these cells to grow in an unregulated fashion. CIMP is strongly associated with BRAF mutations and frequently found in right-sided colorectal lesions. It is considered to be a major mechanism driving the serrated pathway to colorectal cancer. Although many colorectal cancers have some hypermethylation of genes, it is the global methylation that distinguishes CIMP-high tumors.22
Microvesicular HPs most commonly contain a BRAF mutation up to 75% of the time and frequently are CIMP+ (41%-73%). Goblet cell–rich HPs tend to have KRAS mutations up to 55% of the time and infrequently are CIMP+ (8%-18%). SSA/Ps frequently contain BRAF mutations (32%-83%) and are CIMP+ (44%-77%), whereas TSAs can have either BRAF (45%-55%) or KRAS (25%) mutations but the majority are still CIMP+ (43%-80%).22
In a study of the genetics of serrated lesions in IBD patients, Ko and colleagues found BRAF mutations in 65% of serrated polyps negative for dysplasia (histologically synonymous with SSA/Ps), compared to 18% of serrated polyps with LGD (histologically synonymous with TSAs) and 0% of serrated polyps indefinite for dysplasia.17 In contrast, KRAS mutations were seen in 45% of serrated polyps with LGD (histologically synonymous with TSAs) and 25% of serrated polyps with indefinite dysplasia, compared to only 17% of serrated polyps negative for dysplasia (histologically synonymous with SSA/Ps).
A study by Johnson and colleagues found increasing degrees of aberrant methylation on colonic samples from controls with IBD, IBD patients with LGD, IBD patients with SEC, and IBD patients with SSA/Ps, respectively.23 SEC, but not LGD or SSA/Ps, was associated with higher levels of mutant KRAS. The BRAF V600E mutation was found in 40% of SSA/Ps compared to 9% of SEC.
Most of the data have supported inflammation as the primary driver of colorectal cancer in IBD patients with frequent findings of p53 mutations, and CIMP+ cancers account for only approximately 5% of cases.24 However, with the increasing use of high-definition endoscopy and better visualization of the mucosa, serrated lesions may be found to play a larger role in the development of colorectal cancer in IBD patients. Further studies investigating the genetics and genetic precursors of IBD cancers are needed to better elucidate this issue.
Conclusion
Serrated colorectal lesions are understudied in the IBD patient population. The few studies that have been published on the risk of colorectal cancer associated with serrated lesions in IBD suggest that serrated lesions with dysplasia increase the risk of incident advanced neoplasia. However, it remains unknown whether the risk of neoplasia in IBD patients with serrated lesions is higher than in non-IBD patients with serrated lesions. SEC is infrequently recognized and reported by expert pathologists likely due to its unknown significance. Currently, there is only moderate agreement between expert pathologists when classifying serrated lesions; thus, better classification systems are needed. A concrete histologic definition of SEC is also needed, and larger studies are required to evaluate the extent that SEC is involved in the development of dysplasia in the IBD patient population. The genetic and molecular markers of serrated lesions should be further studied to better identify patients at highest risk in order to better stratify surveillance recommendations.
References
- 1.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315–1329. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Parian A, Koh J, Limketkai BN, et al. Association between serrated epithelial changes and colorectal dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2016;84(1):87–95.e1. doi: 10.1016/j.gie.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology. 2015;66(1):49–65. doi: 10.1111/his.12564. [DOI] [PubMed] [Google Scholar]
- 7.Wong NA, Hunt LP, Novelli MR, Shepherd NA, Warren BF. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology. 2009;55(1):63–66. doi: 10.1111/j.1365-2559.2009.03329.x. [DOI] [PubMed] [Google Scholar]
- 8.Ensari A, Bilezikçi B, Carneiro F, et al. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch. 2012;461(5):495–504. doi: 10.1007/s00428-012-1319-7. [DOI] [PubMed] [Google Scholar]
- 9.Gill P, Wang LM, Bailey A, East JE, Leedham S, Chetty R. Reporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009-2012) J Clin Pathol. 2013;66(8):655–658. doi: 10.1136/jclinpath-2013-201608. [DOI] [PubMed] [Google Scholar]
- 10.Bretagne JF, Hamonic S, Piette C, Viel JF, Bouguen G. Interendoscopist variability in proximal colon polyp detection is twice higher for serrated polyps than adenomas. World J Gastroenterol. 2016;22(38):8549–8557. doi: 10.3748/wjg.v22.i38.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139(5):1503–1510, 1510.e1-e3. doi: 10.1053/j.gastro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Messick CA, Church J, Casey G, Kalady MF. Identification of the methylator (serrated) colorectal cancer phenotype through precursor serrated polyps. Dis Colon Rectum. 2009;52(9):1535–1541. doi: 10.1007/DCR.0b013e3181afbe05. [DOI] [PubMed] [Google Scholar]
- 13.Lu FI, van Niekerk W, Owen D, Tha SP, Turbin DA, Webber DL. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010;34(7):927–934. doi: 10.1097/PAS.0b013e3181e4f256. [DOI] [PubMed] [Google Scholar]
- 14.García-Solano J, Pérez-Guillermo M, Conesa-Zamora P, et al. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41(10):1359–1368. doi: 10.1016/j.humpath.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Jackson WE, Achkar JP, Macaron C, et al. The significance of sessile serrated polyps in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(9):2213–2220. doi: 10.1097/MIB.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Gibson JA, Schulte S, et al. Clinical, pathologic, and outcome study of hyperplastic and sessile serrated polyps in inflammatory bowel disease. Hum Pathol. 2015;46(10):1548–1556. doi: 10.1016/j.humpath.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Ko HM, Harpaz N, McBride RB, et al. Serrated colorectal polyps in inflammatory bowel disease. Mod Pathol. 2015;28(12):1584–1593. doi: 10.1038/modpathol.2015.111. [DOI] [PubMed] [Google Scholar]
- 18.Kilgore SP, Sigel JE, Goldblum JR. Hyperplastic-like mucosal change in Crohn’s disease: an unusual form of dysplasia? Mod Pathol. 2000;13(7):797–801. doi: 10.1038/modpathol.3880138. [DOI] [PubMed] [Google Scholar]
- 19.Atwaibi M, Batts KP, Weinberg DI, McCabe RP. Flat serrated change: does it predict the development of colonic mucosal dysplasia in inflammatory bowel disease? Gastroenterology. 2012;142(5) suppl 1:S-665. Abstract Mo1705. [Google Scholar]
- 20.Johnson DH, Khanna S, Smyrk TC, et al. Detection rate and outcome of colonic serrated epithelial changes in patients with ulcerative colitis or Crohn’s colitis. Aliment Pharmacol Ther. 2014;39(12):1408–1417. doi: 10.1111/apt.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parian AM, Chowdhury R, Rubin DT, et al. Serrated epithelial change in ulcerative colitis patients is associated with high rate of colonic dysplasia. Gastroenterology. 2017;152(5) suppl 1:S76. Abstract 300. [Google Scholar]
- 22.Yamane L, Scapulatempo-Neto C, Reis RM, Guimarães DP. Serrated pathway in colorectal carcinogenesis. World J Gastroenterol. 2014;20(10):2634–2640. doi: 10.3748/wjg.v20.i10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DH, Taylor WR, Aboelsoud MM, et al. DNA methylation and mutation of small colonic neoplasms in ulcerative colitis and Crohn’s colitis: implications for surveillance. Inflamm Bowel Dis. 2016;22(7):1559–1567. doi: 10.1097/MIB.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez JA, Dejulius KL, Bronner M, Church JM, Kalady MF. Relative role of methylator and tumor suppressor pathways in ulcerative colitis-associated colon cancer. Inflamm Bowel Dis. 2011;17(9):1966–1970. doi: 10.1002/ibd.21526. [DOI] [PubMed] [Google Scholar]