Abstract
The VWA8 gene was first identified by the Kazusa cDNA project and named KIAA0564. Based on the observation, by similarity, that the protein encoded by KIAA0564 contains a Von Willebrand Factor 8 domain, KIAA0564 was named Von Willebrand Domain-containing Protein 8 (VWA8). The function of VWA8 protein is almost unknown. The purpose of this study was to characterize the tissue distribution, cellular location, and function of VWA8. In mice VWA8 protein was mostly distributed in liver, kidney, heart, pancreas and skeletal muscle, and is present as a long isoform and a shorter splice variant (VWA8a and VWA8b). VWA8 protein and mRNA were elevated in mouse liver in response to high fat feeding. Sequence analysis suggests that VWA8 has a mitochondrial targeting sequence and domains responsible for ATPase activity. VWA8 protein was targeted exclusively to mitochondria in mouse AML12 liver cells, and this was prevented by deletion of the targeting sequence. Moreover, the VWA8 short isoform overexpressed in insect cells using a baculovirus construct had in vitro ATPase activity. Deletion of the Walker A motif or Walker B motif in VWA8 mostly blocked ATPase activity, suggesting Walker A motif or Walker B motif are essential to the ATPase activity of VWA8. Finally, homology modeling suggested that VWA8 may have a structure most confidently similar to dynein motor proteins.
Keywords: VWA8, KIAA0564, baculovirus, ATPase activity, homology modeling
1. Introduction
The Von Willebrand Factor A Domain Containing 8 (VWA8) gene originally was identified by the Kazusa cDNA project and given the designation KIAA0564. Sequence analysis showed that the protein encoded by the KIAA0564 gene likely contains a VWA domain in its C-terminus. VWA8 has two predicted isoforms, a long (VWA8a) and short (VWA8b) isoform. The human VWA8 gene is localized to Chromosome 13 while the mouse VWA8 gene is on Chromosome 14. The human and mouse VWA8 amino acid sequences are 86 and 89% identical for the long and short isoforms, respectively. Although VWA8 protein is essentially uncharacterized, the VWA8 gene appears as a possible signal in three genome wide association studies, for serum calcium concentrations [1], autism [2], and bipolar disorder with comorbid migraine [3]. The promoter of the VWA8 gene also is differentially methylated in acute myeloid leukemia [4]. One proteomics study found that VWA8 associates with TRβ2 (Thyroid hormone receptor β2), but not TRβ1 [5]. We previously found, in an unbiased proteomic screening of livers from high fat fed, obese, insulin resistant mice compared to lean, standard diet fed control C57BL/6J mice that VWA8 protein abundance was increased in livers from the obese mice [6]. As an initial step in determining whether VWA8 protein might be involved in dysregulation of metabolism in obesity, this study was undertaken to characterize the tissue distribution, cellular location, and potential function of VWA8.
2. Materials and methods
2.1. Animals, diets, and adenovirally driven expression of VWA8 in mouse liver
All procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (Protocol No. A38109). Male C57BL/6J mice were purchased from Harlan Teklad (Houston, TX) and housed under controlled temperature (23°C) and lighting (10 h light:14 h dark) conditions with free access to water and food. Details of diets and mice are as previously described [6]. To produce diet-induced obesity and fatty livers, 6 week C57BL/6J mice were fed an irradiated rodent diet (10% fat diet D12450B2018S; or 60% fat diet D12492, at libitum) for 10 weeks. VWA8 also was expressed ectopically in mouse liver in vivo for further functional analysis. For these studies, 10 week C57BL/6J mice were infected via retro orbital injection with empty vector (Ad-Vec) or an adenoviral vector construct encoding the short isoform of VWA8 (Ad-VWA8b). Three days after injection, the mice were sacrificed and livers were harvested and homogenized [6] for further analysis.
2.2. Real Time PCR for VWA8 mRNA expression
Quantitative real time PCR analyses for VWA8a (long) and VWA8b (short) isoform mRNAs were performed as described [7]. Total mRNA was isolated from livers of low fat diet and high fat diet mice (RNeasy Mini Kit, cat.no.74104, QIAGEN) and then converted into cDNA. For VWA8a, the primers were 5′-GGCTGACCAAGGGATTATCA-3′ (Forward) and 5′-TCTGGCAGAGGAAACTCCTT-3′ (Reverse). For VWA8b, the primers were 5′-GGCTGACCAAGGGATTATCA-3′ (Forward) and 5′-GTTCTACCAATAGTGCGTTTCCTTAC-3′ (Reverse).
2.3. VWA8 cloning and expression constructs
Total RNA was isolated from the liver of a 10 week old C57BL6/J mouse (RNeasy Mini Kit, cat.no.74104, QIAGEN). The RT–PCR approach was utilized to convert mRNA into single-stranded cDNA (LongRange 2Step RT-PCR Kit, cat.no. 205922, QIAGEN). Total cDNA was used to amplify specific cDNA with specific primers by PCR and then the amplified cDNA was inserted into a vector. For cloning of mouse VWA8 long isoform cDNA, the sense primer was 5′ GATCGCCTCCTGCCCGGTCCAGGGACTG-3′ and antisense primer 5′-GCTGGACATGGCACAGGTCGGCTTGGTCG-3′. For cloning of mouse VWA8 short isoform cDNA, the sense primer was 5′-GATCGCCTCCTGCCCGGTCCAGGGACTG-3′ and antisense primer 5′-GGACAATGCTCAAGGTTCTACCAATAGTG-3′. The sequencing results revealed that the cloned mouse VWA8a cDNA exactly matched NM_027906.1 and VWA8b matched NM_173758.3.
Mouse VWA8a and VWA8b cDNA were subcloned into pCMV6-AC-HA plasmid (Origene #PS100004) to generate pCMV-mVWA8a-HA and pCMV-mVWA8b-HA. A deletion of the first 34 amino acids in N-terminus of long isoform from its wild type pCMV-mVWA8a-HA by PCR with primers 5′-GCTAGCGATCGCCATGGGCGGGGACCAGCAGCGG -3′ and 5′-GCTAGCGGCCGCGTGATACTGGACAGCATGGTGG-3′ generated a construct pCMV-mVWA8a (35-1905)-HA. A deletion of GGKGCGKT (Walker A motif) from the wild type pCMV-mVWA8b-HA by PCR with primers 5′-GTTCTTAGCGATGACAATTAAGCATATGTC-3′ and 5′-GACATATGCTTAATTGTCATCGCTAAGAAC-3′ generated a construct pCMV-mVWA8b-(ΔGKT)-HA. A deletion of LVLLDG (Walker B motif) from the wild type pCMV-mVWA8b-HA by PCR with primer 5′-GTTGACGCGGTGGATTTTGCCTTCCCGGGC-3′ and 5′-GCCCGGGAAGGCAAAATCCACCGCGTCAAC-3′ generated a construct pCMV-mVWA8b-(ΔLVL)-HA. All of the constructs and mutant were verified by DNA sequencing.
2.4. Generation of adenovirus and baculovirus vectors
Ad-mVWA8a with an HA tag, Ad-mVWA8b with an HA tag and an adenovirus empty vector Ad-Vec (as a control), were created by following a protocol described previously [8]. The baculovirus Bac-mVWAb with a His tag was produced using the Bac-to-Bac Baculovirus Expression System (Invitrogen #10359-016). Mouse VWA8b cDNA with a His tag was subcloned into pFastBac 1 vector and the construct was transformed into DH10Bac competent cells (containing Bacmid and Helper) to obtain recombinant Bac-mVWA8b. The recombinant Bacmid DNA Bac-mVWA8b was used to transfect Sf21 insect cells to express the protein. The expressed protein mouse VWA8b with a His tag was purified using Ni-NTA Agarose (Invitrogen #R901-15) and eluted.
2.5. Cell Culture Transfection and Immunoprecipitation
AML12 cells were purchased from ATCC and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Hela cells were purchased from ATCC and grown in EMEM (ATCC #30-2003), also supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Transfection and immunoprecipitation were performed as described previously [8].
2.6. Immunofluorescence staining
AML 12 cells were cultured on glass cover slips in 6-well dishes. Cells were live-stained with 200 nM MitoTracker™ Red (Invitrogen #M7512) for 30 min before the fixation with 3% paraformaldehyde in PBS for 20 min. Cells then were permeabilized with 0.5% triton X-100 in PBS for 5 min, quenched with 100 mM glycine in PBS for 20 min, and blocked with 1% BSA in PBS for 1h. Cells were then exposed to primary antibodies for 2 h at room temperature. Following three washes with PBS, the cells were treated for 1 h with Alexa Fluor secondary antibodies (Invitrogen #A21202) diluted 1:1000. Samples were mounted on glass slides with Vectashield mounting medium and examined under an inverted confocal microscope.
2.7. ATPase Activity Assay and Western Blotting
ATPase activity was measured using an ATPase Assay Kit (Fisher #60-101-20) according to the manufacturer’s instructions. Expressed VWA8b with an HA tag from mouse liver or Hela cells was immunoprecipitated by anti-HA antibody and Protein A beads. Immunoprecipitates were added to SB Mix (containing ATP) to incubate at 37°C for 15 minutes. After 15 minutes, beads were spun down and supernatant was transferred to a new tube. The reaction was stopped and colored by adding Gold Mix. Absorbance was measured at 600 nm. Proteins bound to beads were eluted by heating at 95°C for 4 min in SDS sample loading buffer. Eluted proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and detected by Western blotting with primary antibody followed by horseradish peroxidase-conjugated secondary antibodies. This ATPase assay also was used for baculovirus-expressed mVWA8b solution eluted from Ni-NTA.
2.8 Bioinformatics and homology modeling
VWA8 amino acid sequences were analyzed for the presence of a mitochondrial targeting sequence using MitoProt [9]. For homology modeling, VWA8a and VWA8b primary amino acid sequences were analyzed using the Phyre2 server [10].
3. Results
3.1. VWA8 is expressed in liver, kidney, heart, pancreas, and muscle
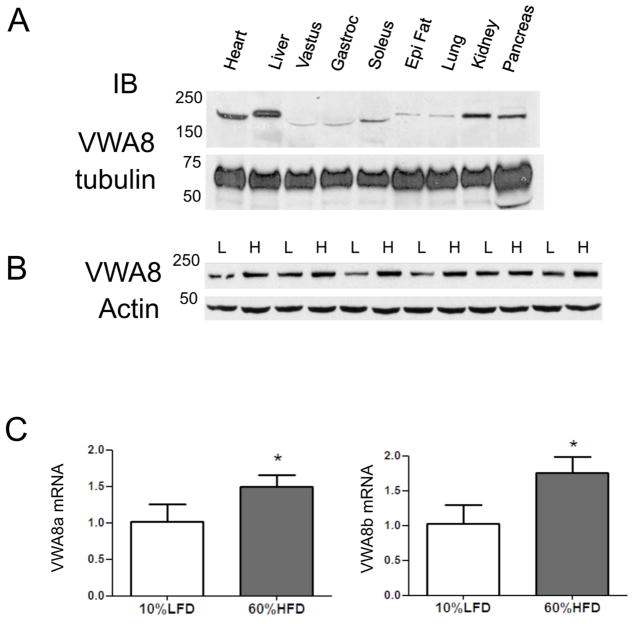
Since VWA8 is uncharacterized, we determined its tissue distribution. Fig 1A shows the tissue distribution of VWA8 protein in C57BL/6J mouse tissues. VWA8a was present most abundantly in liver, kidney, pancreas, heart, and skeletal muscle. Only results from VWA8a are shown due to the lack of availability of an antibody directed against VWA8b. Because the heavy myosin band in skeletal muscle interferes with detection of proteins that migrate with similar molecular weight, VWA8 mRNA expression in human tissues was examined in the GTEx Gene Expression database. These data show that VWA8 is most highly expressed in skeletal muscle, followed by EBV-transformed lymphocytes, bladder, adrenal and heart, with lesser expression in liver, kidney, and pancreas (https://gtexportal.org/home/gene/VWA8#geneExpression).
Figure 1. VWA8 protein is expressed widely and VWA8 protein and mRNA are elevated in response to high fat feeding.
A. Lysates of indicated mouse tissues were subjected to immunoblot analysis using an antibody directed against VWA8a, with tubulin as a loading control. B. Livers from six C57BL/6J mice fed a low fat diet for 10 weeks (L), and six C57BL/6J mice fed a high fat diet were homogenized, and lysates were subjected to immunoblot analysis with anti-VWA8a antibody or β-actin (control). C. Total mRNA from the same two groups (LFD, low fat diet; HFD, high fat diet) of 6 mice each was subjected to Q-rtPCR analysis of mRNA for VWA8a and VWA8b. Values for individual mRNA levels were expressed relative to the average for the low fat diet group (*P<0.05 vs. 10% LFD).
3.2 VWA8 protein and mRNA were elevated in response to high fat feeding
Our previous proteomics study showed that mouse liver VWA8 in abundance increases in response to high fat feeding [6]. To confirm this, immunoblot analysis was applied to homogenates of liver from high fat and standard chow fed mice. Fig 1B shows the immunoblot analysis confirming that VWA8 protein increased in livers from mice fed a high fat diet, consistent with previous results obtained using proteomics techniques. To determine if increased VWA8 protein abundance was accompanied by higher mRNA expression, Q-rt-PCR was used to measure mRNA expression for VWA8a and VWA8b. Fig 1C shows that mRNA expression for both isoforms is higher in livers from high fat fed mice.
3.3 VWA8 is targeted to mitochondria
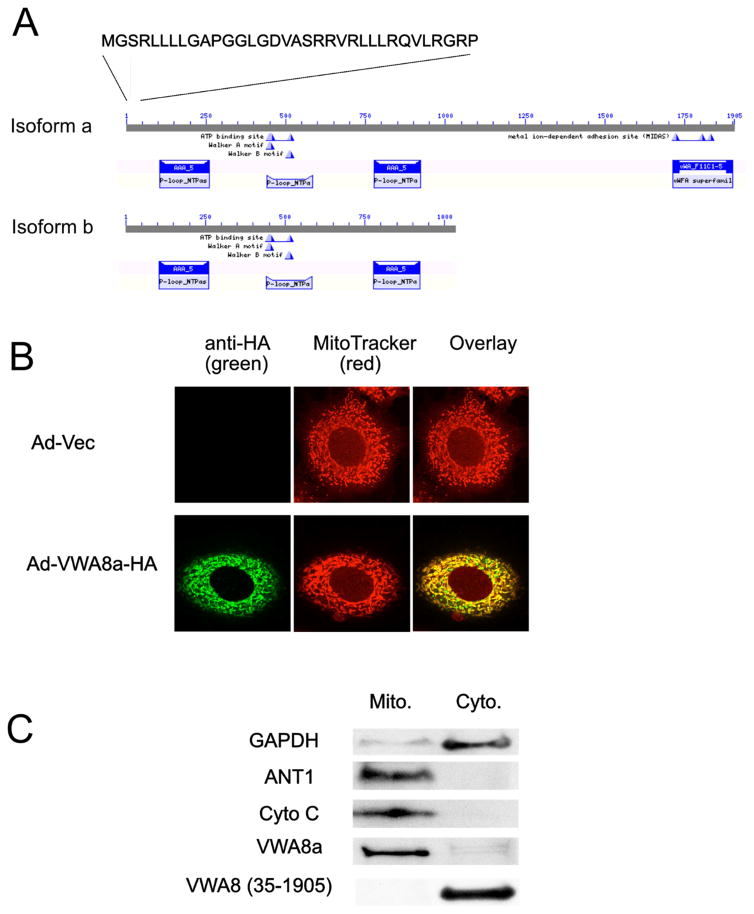
Bioinformatics analysis using MitoProt software predicted that VWA8 has an 89% probability of VWA8 localization to mitochondria, with the N-terminal 34 amino acids predicted to be a mitochondrial target sequence (Fig 2A). To test this, AML12 were infected with Ad-mVWA8a-HA and Ad-Vec (as a control). The infected cells were subject to immunofluorescence staining with MitoTracker Red and anti-HA antibody. The overlay of these images (Fig. 2B) shows that the majority of VWA8 is localized in mitochondria. Subcellular fractionation experiments also showed that ectopically expressed VWA8 was targeted to mitochondria (Fig. 2C left). To test if the predicted 34 amino acid targeting sequence is necessary for VWA8 export to mitochondria, AML12 cells were transfected with pCMV-mVWA8a-(35-1905)-HA, a construct with the N-terminal putative mitochondrial targeting sequence deleted. Cells expressing the 1–34 N-terminal deletion were fractionated into cytosol and mitochondrial subcellular fractions, and these fractions were subjected to immunoblot analysis using an anti-HA antibody. Deletion of first 34 amino acids in the N-terminus results in a shift of VWA8 localization from the mitochondrial to cytosolic fractions (Fig. 2C right).
Figure 2. VWA8 has ATPase domains, a mitochondrial targeting sequence, and is a mitochondrial protein.
A. Domain architecture of VWA8a and VWA8b. Both isoforms have N-terminal mitochondrial targeting sequences and putative ATPase domains. B. Mouse AML12 hepatocytes were infected with adenoviral vectors encoding VWA8a with an HA tag or empty vector (control). Cells were stained with MitoTracker Red and a green fluorescent tagged anti-HA antibody. The overlap shows VWA8a is targeted to mitochondria. C. AML12 hepatocytes were fractionated into cytoplasmic and mitochondrial fractions and immunoblot analysis was used to detect endogenous VWA8a, GAPDH, adenine nucleotide translocase 1(ANT1) and Cytochrome C oxidase (Cyto C). Endogenous VWA8a was present only in the mitochondrial fraction. AML12 cells were transiently transfected with a vector encoding a VWA8a mutant, tagged with HA, and lacking the 1–34 N-terminal amino acid putative mitochondrial targeting sequence, VWA8 (35-1095). Immunoblot analysis with anti-HA antibody showed that this mutant was not targeted to mitochondria.
3.4. VWA has ATPase activity
Human and mouse VWA8 both have two isoforms, VWA8a (1905 amino acids in human and mouse) and VWA8b (1039 and 1308 amino acids in human and mouse, respectively). The long and short isoforms in human and mouse share the identical amino acid sequence in the first 1039 (human) or 1038 (mouse) amino acids. The short isoforms lack the remaining amino acids because, in both species, there are extensions in Exon 26 that introduce a stop codon (TAA).
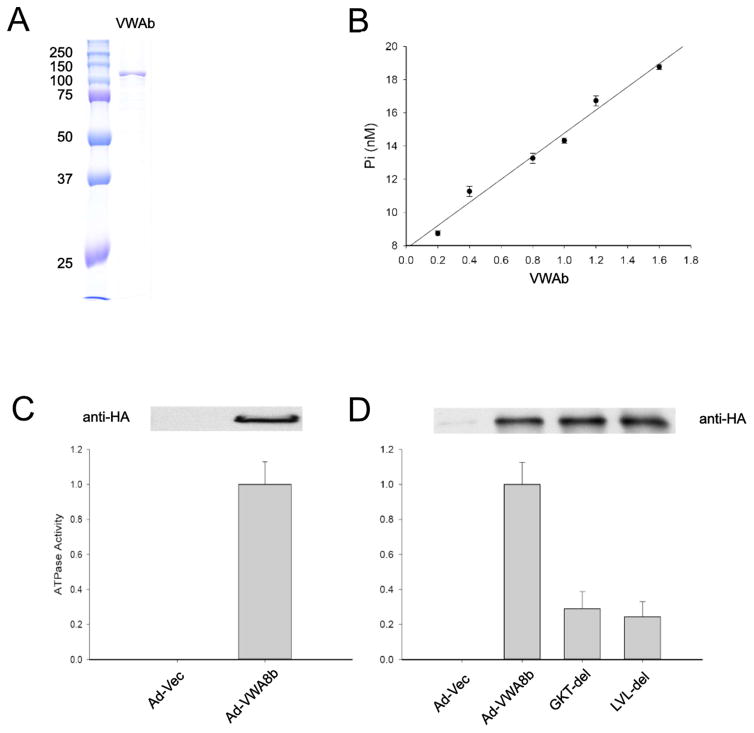
Bioinformatics analysis shows that VWA8 has an AAA (ATPase) domain in the N-terminus (Fig. 2A). The AAA domain contains Walker A and Walker B motifs. The Walker A motif GXXGXGK[S/T] forms a loop that binds to the alpha and beta phosphate moieties of the bound nucleotide. The Walker B motif, containing 4 aliphatic residues followed by 2 negatively charged residues, is important for interacting with Mg2+ ions. The presence of these structures indicates that VWA would be likely to have ATPase activity. To test whether VWA8 has ATPase activity in vitro, we produced relatively pure VWA8 protein using ectopic expression of an mVWA8b-encoding baculovirus vector in insect cells. The lower molecular weight of mVWA8b renders this protein easier to express and purify, and both VWA8a and VWA8b have the domains putatively required for ATPase activity. Therefore, a baculovirus Bac-mVWA8b-His was constructed and Sf21 insect cells were transfected for ectopic protein expression. The expressed protein was purified by Ni-NTA agarose beads and eluted (Fig. 3A). The eluate was subjected to an in vitro ATPase assay. The results show that the VWA8 short isoform has dose-responsive ATPase activity (Fig 3B). To test whether or not VWA8b that is ectopically expressed in mammalian tissues also has ATPase activity in vitro, an adenovirus construct Ad-mVWA8b was used to infect C57BL/6J mice in vivo. VWA8b expressed in mouse liver was immunoprecipitated using an anti-HA antibody, and the immunoprecipitated proteins were subjected to an in vitro ATPase activity assay. Similar to VWA8 expressed in insect cells, VWA8 expressed in mammalian liver also has ATPase activity (Fig 3C).
Figure 3. VWA8b expressed in insect cells or in vivo in mouse liver has ATPase activity in vitro and both Walker A and Walker B motifs are required for this activity.
A. Sf21 insect cells were infected with baculovirus Bac-mVWA8b-His and expressed the mouse short isoform of VWA8b with a His tag. His-tagged VWA8b from Sf21 was purified from cell lysate using Ni-NTA agarose beads and eluted. The purified VWA8b was subjected to SDS-PAGE and the gel was stained with Coomassie Blue. B. Eluted, purified VWA8b was subjected to in vitro ATPase assay at 37°C for 15 min, and a dose response curve was generated using various phosphate concentrations (n = 3). C. Ten week old C57BL/6J mice were infected with Ad-Vec (control) or Ad-VWA8b-HA for 3 days. Livers from infected mice were homogenized, and homogenates were immune-precipitated with anti-HA antibody. The immunoporecipitated proteins were subjected to ATPase assay (n = 4). D. Hela cells were transfected with pCMV6-AC-HA (control), pCMV-mVWA8b-HA, pCMV-mVWA8b-(ΔGKT)-HA, and pCMV-mVWA8b-(ΔLVL)-HA for 28 hours. After transfection and protein expression, cells were lysed and the cell lysates were immunoprecipitated with anti-HA antibody. The immunoprecipitates were subjected to immunoblotting to confirm expression, and to an in vitro ATPase assay (n = 4). The activity of Vector (control) was assigned to 0 and that of VWA8 wild type as 1. The activities of VWA8 wild type and deletions were normalized by the immunoblot band densities. (**P<0.01 vs. WT)
3.5. Walker A and B motifs are essential for ATPase activity of VWA8
To determine if the Walker A and B motifs are essential for the ATPase activity of VWA8, the constructs pCMV-AC-HA vector (negative control), pCMV-mVWA8b-HA (Wild Type, positive control), pCMV-mVWA8b-(ΔGKT)-HA (GGKGCGKT deletion), and pCMV-mVWA8b-(ΔLVL)-HA (LVLLDG deletion), were used to transfect Hela cells to overexpress proteins. The overexpressed proteins were immunoprecipitated by anti-HA antibody and subjected to an ATPase activity assay. ATPase activity of VWA8-S was mostly abrogated by either GGKGCGKT deletion or LVLLDG deletion, suggesting that the Walker A (GGKGCGKT) and Walker B motifs (LVLLDG) are essential for ATPase activity of VWA8 (Fig. 3D).
3.6 Homology modeling
The primary amino acid sequences of VWA8a and VWA8b were submitted to the Phyre2 server for homology modeling of structure and similarity to known protein structures (Kelley LA et al. Nature Protocols 10, 845–858 (2015)). This analysis revealed that 52% of the sequence of VWA8 could be modeled with 100% confidence based on the structure of the cytoplasmic dynein heavy chain (PDB c3ykhB). VWA8a and the dynein heavy chain motor domain have 16% identity at the amino acid level (Table 1). Homology modeling also revealed that 90% of the VWA8b sequence could be modeled with 100% confidence using the cytoplasmic dynein heavy chain (c3vkhb) as a template (Table 1). The known structures matching the VWA8 homology model produced by Phyre2 are given in Table 1 and include a number of dynein related motor proteins.
Table 1.
Homology modeling of VWA8a and VWA8b
| Template | Alignment Coverage | Confidence | % identity | Template Information |
|---|---|---|---|---|
| VWA8a (long isoform) | ||||
|
| ||||
| C3vkhB | 19-1022 (52%) | 100.0 | 16 | PDB header: motor protein Chain: B: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of a functional full-length dynein motor domain |
| C3vkhA | 20-1022 (52%) | 100.0 | 16 | PDB header: motor protein Chain: A: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of a functional full-length dynein motor domain |
| C4rh7A | 19-1022 (52%) | 100.0 | 14 | PDB header: motor protein Chain: A: PDB
Molecule: green fluorescent protein/cytoplasmic dynein 2 heavy
chain PDB Title: crystal structure of human cytoplasmic dynein 2 motor domain in 2 complex with adp.vi |
| C3vkgA | 20-1101 (56%) | 100.0 | 14 | PDB header: motor protein Chain: A: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of an mtbd truncation mutant of dynein motor domain |
| C3vkgB | 19-1022 (52%) | 100.0 | 15 | PDB header: motor protein Chain: B: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of an mtbd truncation mutant of dynein motor domain |
|
| ||||
| VWA8b (short isoform) | ||||
|
| ||||
| C4rh7a | 86-1022 (90%) | 100.0 | 15 | PDB header: motor protein Chain: A: PDB
Molecule: green fluorescent protein/cytoplasmic dynein 2 heavy
chain PDB Title: crystal structure of human cytoplasmic dynein 2 motor domain in2 complex with adp.vi |
| C3vkhb | 86-1022 (90%) | 100.0 | 13 | PDB header: motor protein Chain: B: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of a functional full-length dynein motor domain |
| C4akgB | 86-1022 (90%) | 100.0 | 14 | PDB header: motor protein Chain: B: PDB
Molecule: glutathione s-transferase class-mu 26 kDa isozyme,
dynein PDB Title: dynein motor domain - atp complex |
| C3vkhA | 86-1022 (90%) | 100.0 | 13 | PDB header: motor protein Chain: A: PDB
Molecule: dynein heavy chain, cytoplasmic; PDB Title: x-ray structure of a functional full-length dynein motor domain |
| C4w8fA | 86-1022 (90%) | 100.0 | 14 | PDB header: motor protein Chain: A: PDB
Molecule: dynein heavy chain lysozyme chimera; PDB Title: crystal structure of the dynein motor domain in the amp pnp-bound state |
Primary amino acid sequences of VWA8a and VWA8b were subjected to Phyre2 for homology modeling. Shown are the top 5 models for each isoform.
4. Discussion
The mouse VWA8 gene was first identified by the Kazusa cDNA project and given the terminology KIAA0564, as the protein predicted to be encoded by this gene had no characterized function. More recently this protein was renamed VWA8 after sequence homology analysis predicted that there is a VWA domain in the C-terminus of the long isoform, VWA8a. However, the short isoform, VWA8b, lacks the VWA domain. VWA8b has an extension of Exon 26 that introduces a stop codon (TAA), which terminates the protein translation. In VWA8a, this codon resides in an intron, where there is no interruption of protein translation. Because the short isoform of this protein does not have a VWA8 domain, this may not be the best terminology for these proteins.
Analysis of the tissue distribution of mouse VWA8 shows it is mostly localized to liver, kidney, pancreas, heart, and skeletal muscle, although in muscle, the VWA8a signal is somewhat obscured by the high abundance of myosin. Data from curated expression analysis of VWA8 in human tissues suggests that VWA8 expression is highest in skeletal muscle. All of these organs have high energy requirements and are rich in mitochondria. In fact, our data also show that VWA8 is localized exclusively to mitochondria, and that the predicted N-terminal mitochondrial targeting sequence is necessary for export of VWA8 to this organelle. The presence of VWA8 exclusively in mitochondria raises the possibility that this protein has a role in metabolic regulation or bioenergetic events. This notion is bolstered by the observation that VWA8 protein is elevated in livers of mice fed a high fat diet. Mice fed a high fat diet (at libitum) are obese, have fatty livers, and reduced energy expenditure [11]. Taken together, these observations compel analysis of the function of VWA8.
Inspection of the amino acid sequences of VWA8a and VWA8b revealed that these proteins have a predicted AAA domain in its N-terminus, which is common to both the long and short isoforms. AAA (or called AAA+) domains are ATPases Associated with diverse cellular Activities mainly consists of Walker A motif and Walker B motif. The Walker A motif GGKGCGKT binds to ATP and hydrolyzes ATP to ADP, in a reaction that requires Mg2+. The Walker B motif LVLLDG (4 aliphatic residues followed by 2 negatively charged residues) binds Mg2+. The presence of the AAA domain of VWA8 raised the possibility that VWA8a and VWA8b may have ATPase activity. We used the shorter isoform (VWA8b) to test this, since its lower molecular weight makes it easier to express. In addition, both isoforms have all the same putative ATPase elements. The results show that VWA8b has ATPase activity in vitro in a dose responsive manner. Deletion of either the Walker A or Walker B motif blocked most of the ATPase, suggesting that these motifs are essential to the ATPase activity of VWA8. Although this ATPase activity has a high background level, use of the empty vector negative control and VWA8 wild type as positive control allowed us to evaluate the ATPase activities of the deletions. Because the ATPase domains of VWA8a and VWA8b are identical, we predict that both isoforms have ATPase activity.
There remain questions regarding the cellular function of VWA8. The observation that VWA8 possesses ATPase activity suggests that this protein is involved in an energy consuming biological process. For example, molecules involved in motor activities, like dynein, also have ATPase activity and use the energy released from the hydrolysis of ATP to perform mechanical work. Homology modeling shows that dynein serves as a highly confident template for a putative structure of the N-terminal portion of VWA8a and VWA8b. Cytoplasmic dynein may be involved in mitochondrial motility along microtubules [12, 13]. However, the present results do not provide direct evidence that VWA8 acts as a motor protein. Lack of identification of other protein domains or motifs by sequence analysis also makes it more difficult to assign a biological activity for this protein, although the present results do provide a cellular location and molecular function. The present results also do not allow us to assign distinct functions to the two isoforms of VWA8. Although it is likely that VWA8a, like VWA8b, has ATPase activity, the presence of the additional amino acid sequence may modulate ATPase activity, confer an additional function, or allow VWA8a to associate with different proteins than VWA8b that lacks the VWA domain.
In summary, VWA8 encodes two mitochondrial proteins that have ATPase activity in vitro and are expressed in highly energetic tissues. Changes in VWA8 abundance with high fat feeding in mice suggest a role in metabolic regulation. Homology modeling predicts that VWA8 may have dynein-like properties.
Acknowledgments
This work was supported by National Institutes of Health Grants R01DK47936 and R01DK66483 (L.J.M.).
Footnotes
Disclosure Statement: The authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Seaghdha CM, Wu H, Yang Q, et al. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet. 2013;9:e1003796. doi: 10.1371/journal.pgen.1003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anney R, Klei L, Pinto D, et al. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet. 2010;19:4072–4082. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oedegaard KJ, Greenwood TA, Johansson S, Jacobsen KK, Halmoy A, Fasmer OB, Akiskal HS, Bipolar Genome S, Haavik J, Kelsoe JR. A genome-wide association study of bipolar disorder and comorbid migraine. Genes Brain Behav. 2010;9:673–680. doi: 10.1111/j.1601-183X.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcucci G, Yan P, Maharry K, Frankhouser D, Nicolet D, Metzeler KH, Kohlschmidt J, Mrozek K, Wu YZ, Bucci D, Curfman JP, Whitman SP, Eisfeld AK, Mendler JH, Schwind S, Becker H, Bar C, Carroll AJ, Baer MR, Wetzler M, Carter TH, Powell BL, Kolitz JE, Byrd JC, Plass C, Garzon R, Caligiuri MA, Stone RM, Volinia S, Bundschuh R, Bloomfield CD. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32:548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahm JB, Privalsky ML. Research resource: identification of novel coregulators specific for thyroid hormone receptor-beta2. Mol Endocrinol. 2013;27:840–859. doi: 10.1210/me.2012-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo M, Mengos AE, Stubblefield TM, Mandarino LJ. High Fat Diet-Induced Changes in Hepatic Protein Abundance in Mice. J Proteomics Bioinform. 2012;5:060–066. doi: 10.4172/jpb.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294:E607–614. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 8.Luo M, Reyna S, Wang L, Yi Z, Carroll C, Dong LQ, Langlais P, Weintraub ST, Mandarino LJ. Identification of insulin receptor substrate 1 serine/threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology. 2005;146:4410–4416. doi: 10.1210/en.2005-0260. [DOI] [PubMed] [Google Scholar]
- 9.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 13.Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]