Abstract
Abuse of prescription opioids, such as oxycodone, has markedly increased in recent decades. While oxycodone’s antinociceptive effects have been detailed in several preclinical reports, surprisingly few preclinical reports have elaborated its abuse-related effects. This is particularly surprising given that oxycodone has been in clinical use since 1917. In a novel oral operant self-administration procedure, C57BL/6J mice were trained to self-administer water before introducing increasing concentrations of oxycodone (0.056–1.0 mg/ml) under post-prandial conditions during daily, 3-h test sessions. As the concentration of oxycodone increased, the numbers of deliveries first increased, then decreased in an inverted U-shape fashion characteristic of the patterns of other drugs self-administered during limited access conditions. After post-prandial conditions were removed, self-administration at the highest concentration was maintained suggesting oral oxycodone served as a positive reinforcer. In other mice, using a novel regimen of physical dependence, mice were administered increasing doses of oxycodone (9.0–33.0 mg/kg, s.c.) over 9 days, challenged with naloxone (0.1–10.0 mg/kg, s.c.), and then observed for 30 min. Naloxone dose-dependently increased the observed number of somatic signs of withdrawal, suggesting physical dependence of oxycodone was induced under this regimen. This is the first report demonstrating induction of oral operant self-administration of oxycodone and dose-dependent precipitations of oxycodone withdrawal in C57BL/6J mice. The use of oral operant self-administration as well as the novel physical dependence regimen provides useful approaches to further examine the abuse- and dependence-related effects of this highly abused prescription opioid.
Keywords: Oxycodone, oral self-administration, dependence, mouse, prescription opioid
1. Introduction
The nonmedical use and abuse of prescription opioids has become an increasing concern in recent decades exemplified by the number of overdose deaths attributable to them that now surpass heroin and cocaine combined (Centers for Disease Control and Prevention [CDC], 2011). Oxycodone is one of the most abused of these prescription opioids in the United States as measured by the number of emergency visitations for its nonmedical use (CDC, 2010). The abuse-related effects of oxycodone, however, have been inadequately characterized in preclinical studies, particularly so in mice, and surprisingly so given that it has been in clinical use since 1917 (Kalso, 2005). Intravenous oxycodone self-administration in C57BL/6J mice has been reported (Zhang et al., 2015; Zhang et al., 2014), although the oral route, also an important route of administration through which the majority of prescription opioid abusers may prefer (Kirsh et al., 2012), has not yet been reported. To our knowledge, the oral self-administration of any opiate in the mouse in which an operant contingency was required to obtain drug delivery has been confined to one report and only using the potent benzimidazole opioid, etonitazene (Elmer et al., 1995).
In addition to self-administration, physical dependence is another abuse-related phenomenon associated with oxycodone. Here again few preclinical reports have characterized the physical dependence effects of oxycodone especially in the mouse. Oxycodone has been reported to dose-dependently suppress somatic signs of withdrawal in morphine-dependent rhesus monkeys suggesting cross-dependency to morphine (Beardsley et al., 2004). In addition, characteristic somatic signs of opiate withdrawal (e.g., jumping, body shakes, and diarrhea) have been reported in ICR mice administered a subcutaneous slow-release emulsion mixture of oxycodone to a similar degree as those produced by morphine (Mori et al., 2013). Oxycodone delivered to rats via osmotic minipumps resulted in substantial weight loss upon termination of drug administration after pump removal indicative of physical dependence (Hutchinson et al., 2009). The authors reported that they did not analyze the somatic signs of oxycodone withdrawal because of the "severity of … withdrawal" it produced (Hutchinson et al., 2009). Disruption of operant behavior can also be used to infer a type of “behavioral dependence” even when not accompanied by somatic signs of withdrawal (Schuster and Thompson, 1969). Naloxone-precipitated withdrawal disruption of lever pressing maintained by intracranial self-stimulation in rats indicative of behavioral dependence has also been reported (Wiebelhaus et al., 2016).
The purpose of the present study was to establish an oral oxycodone operant self-administration procedure in C57BL/6J mice to enable further investigations of oxycodone’s abuse-related effects and their treatment using a route of administration that coincides with a popular medical and nonmedical route of its administration. Another objective of this study was to establish a regimen for reliably and efficiently inducing physical dependence upon oxycodone in C57BL/6J mice, and to determine the sensitivity of dependence to precipitated withdrawal. To our knowledge, this is the first report disclosing methods to induce the oral operant self-administration of oxycodone and the induction of its physical dependence in non-modified C57BL/6J mice.
2. Materials and methods
2.1 Subjects
Male C57BL/6J mice were obtained at approximately 8 weeks of age (The Jackson Laboratory, Bar Harbor, ME) and were allowed to acclimate to the vivarium for at least one week prior to commencement of training and testing. Mice were housed in an AALAC-accredited animal facility, kept on a 12-h/12-h light/dark cycle (lights on from 0600-h to 1800-h), and given water ad libitum. Mice in oral self-administration studies were maintained at an 85% free-feeding weight with daily allotments of chow (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories Inc., Indianapolis, IN) prior to the initiation of the training phase, whereas mice in the physical dependence procedure were given the same chow ad libitum throughout the study. All procedures were conducted during the light phase and were in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press, 2011), and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2 Oral operant self-administration
2.2.1 Apparatus
Sixteen mouse operant chambers (MED Associates, Inc., St. Albans, VT) enclosed in sound- and light-attenuating cubicles were used for this study. Each operant chamber contained a house light mounted on the rear wall, a Sonalert® tone-generating device, and panels of cue lights mounted above two response levers between which was positioned a well into which a drinking cup was positioned. Vendor-supplied drinking cups were replaced with fabricated dipper cups into which were soldered stainless steel liquid intake tubes. Attached to each stainless steel intake tube was silicone tubing (0.79 mm ID/ 3.99 mm OD; Helix Medical, Carpinteria, CA) that was routed behind the operant chamber and attached to a syringe that was compressed by a Razel Model R-ES syringe infusion pump (Razel Scientific Instruments, St. Albans, VT). When scheduled, 20 ul of liquid could be delivered into the drinking cups by the syringe pumps. Recording of lever presses, activation of house and cue lights, sonalerts, and syringe pumps were accomplished via computer-controlled circuitry and software (MED-PC IV, MED Associates, Inc., St. Albans, VT).
2.2.2 Procedure
The overall procedure was adapted as previously described by Meisch and collaborators that had been used to establish a highly potent opioid, etonitazene, as an oral reinforcer in rats and mice (Beardsley and Meisch, 1981; Elmer et al., 1995; Meisch and Kliner, 1979). Training and testing proceeded according to the following phases: 1) Post-prandial induction of water reinforcement; 2) Post-prandial induction of increasing concentrations of oxycodone; 3) Maintenance of OXY consumption without prandial induction.
2.2.3 General training and testing conditions
Mice were trained and tested daily in 3-h experimental sessions that began each day between 11:00 and 11:45h. Mice were transported from the vivarium to their testing rooms in their home cages, weighed, and during the 1.5-h period immediately prior to the experimental session, they could be given their daily chow portions depending upon the phase of the study (i.e., enabling testing under post-prandial conditions). During the 1.5-h pre-session period, their home-cage water bottles were either present or absent, also depending upon the phase of the study.
2.2.4 Phase I: Post-prandial induction of water reinforcement
Daily food allotments were provided in the home cages during the 1.5-h pre-session period to induce thirst. Any uneaten chow was placed on the floor of the operant chambers during experimental sessions, and any uneaten chow at the end of the session was returned to the mice when returned to their vivarium home cages. Mice were trained to press the right lever under a fixed-ratio 1 (FR 1) up to a FR 4 schedule of reinforcement for obtaining deliveries of water. Presses of the left lever prior to completion of the fixed ratio contingency reset the ratio requirement, but otherwise were without scheduled consequences. At the commencement of each delivery, the Sonalert® sounded and the cue lights above the right-side lever were illuminated for 6 s. During the 6-s reinforcement period, lever presses were not counted toward completing the FR 4 contingency, but were recorded.
2.2.5 Phase II: Post-prandial induction of increasing concentrations of oxycodone
After performances of the mice had stabilized in which there were no increasing or decreasing trends in the number of water deliveries across three consecutive experimental sessions, water was replaced with increasing concentrations of oxycodone aqueous solutions (0.056, 0.1, 0.3, 0.56, and 1.0 mg/ml) under FR 4 reinforcement and post-prandial conditions. Five experimental sessions were conducted at each concentration before advancing to the next phase. Testing was completed in a consecutive order up until 0.56 mg/ml at which after a holiday break all mice were put through a re-training period and were then tested at the highest concentration, 1 mg/ml, before moving onto the next phase.
2.2.6 Phase III: Maintenance of OXY consumption without prandial induction
Deliveries of 1 mg/ml oxycodone solutions continued to be available according to FR 4 reinforcement contingencies. Any uneaten pre-session chow was no longer provided during experimental sessions, but instead was given post session in the home cages. After five experimental sessions had occurred, pre-session feedings were reduced to 50, 25, and finally 0% of the total daily food allotment with each reduction in effect for five consecutive experimental sessions. Any uneaten chow, and the complement to provide 100% of their total daily food allotment, was provided after experimental sessions in the home cages. Mice were then maintained on 1 mg/ml OXY during test sessions without prior prandial induction.
2.3 Physical dependence
In separate groups of male C57BL/6J adult mice, oxycodone was administered subcutaneously for eight days with increasing doses of oxycodone of 9, 17.8, 23.7, and 33 mg/kg b.i.d. (~7-h separating injections) on days 1–2, 3–4, 5–6, and 7–8, respectively, and then on the morning of the 9th day was administered 33 mg/kg oxycodone followed 2-h later with an injection of either 0.1, 1, 3 or 10 mg/kg s.c. naloxone. Another group of mice was administered saline instead of oxycodone for eight days, and were challenged with 10 mg/kg s.c. naloxone on Day 9. Immediately following naloxone injections, mice were individually placed in Plexiglas cages and were observed and scored for manifestation of different somatic signs of withdrawal including the total number of jumps, wet dog shakes, paw tremors, backing, ptosis and diarrhea for 30-min using methods we have previously reported in testing morphine-dependent mice (Muldoon et al., 2014). Changes in body weight (g) immediately before and 30-min after naloxone injections were also recorded. All testing was conducted in a blind manner.
2.4 Drugs
Oxycodone HCl (Mallinckrodt Inc., St. Louis, MO) was initially prepared in an aqueous sterile stock solution of 10 mg/ml for self-administration studies, which was then diluted with deionized water to make working solutions for oral self-administration tests of 0.056, 0.1, 0.3, 0.56, and 1.0 mg/ml. For physical dependence studies, oxycodone was prepared in a sterile stock solution of 10 mg/ml in non-heparinized 0.9% saline before diluting in sterile saline to make working solutions in the following concentrations: 0.09, 1.78, 2.37, and 3.3 mg/ml. Naloxone HCl (Sigma-Aldrich, St. Louis, MO) was prepared in sterile 0.9% saline to make working solutions in the following concentrations: 0.01, 0.1, 0.3, and 1 mg/ml. All injections were given at a 10 ml/kg injection volume.
2.5 Data analysis
For self-administration tests, numbers of liquid deliveries as well as active and inactive lever presses were the dependent measures. A two-tailed, paired t-test was used to compare number of deliveries before and after complete removal of pre-session feeding. For physical dependence tests, somatic signs and body weight changes indicative of withdrawal were scored quantitatively by a blinded research assistant and used as dependent measures. A two-tailed, unpaired t-test was used to compare the chronic vehicle (saline)-treated group with the chronic oxycodone-treated group for all somatic signs of withdrawal. All data were analyzed and graphed using microcomputer software (Prism 6 for Windows, GraphPad Software, Inc., San Diego, CA), and all types of comparisons were considered statistically significant if P<0.05.
3. Results
3.1 Post-prandial induction of oxycodone self-administration
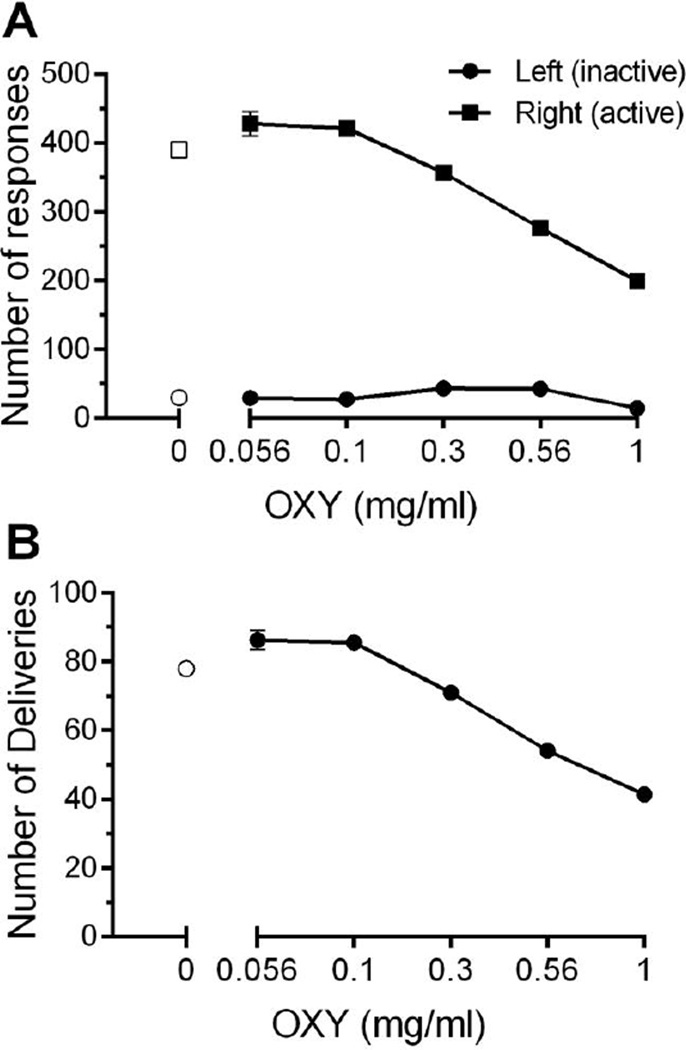
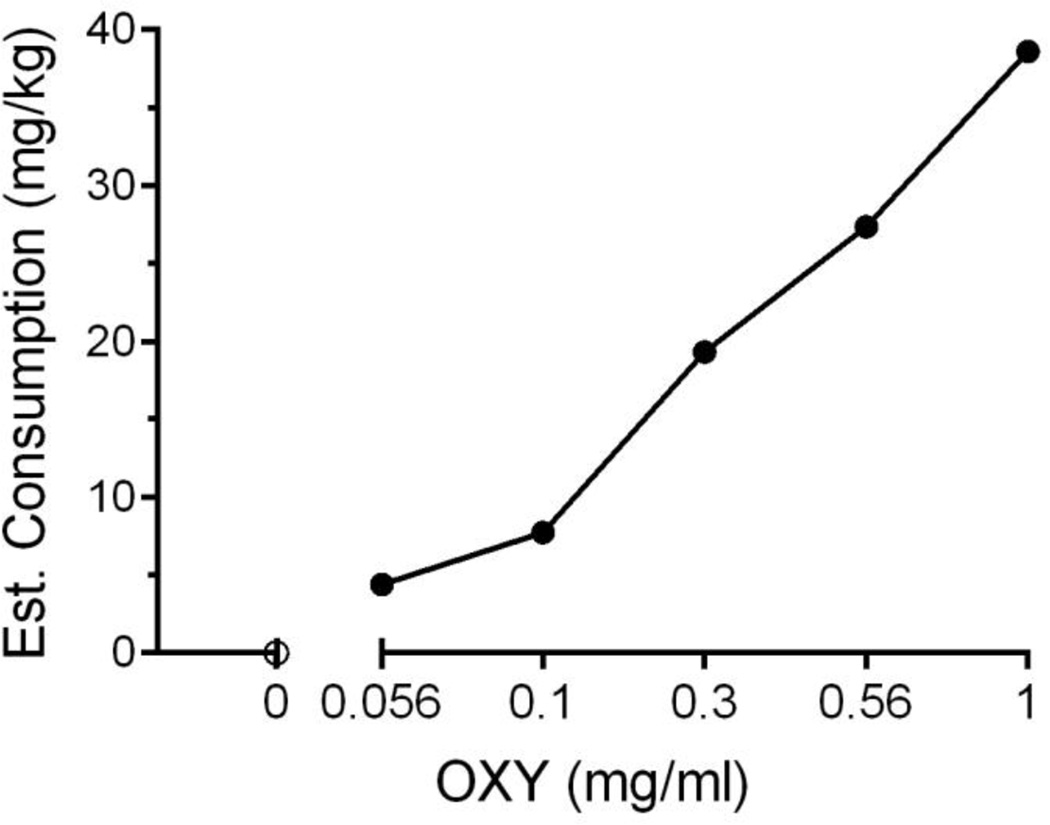
Mice learned to lever press reinforced with water delivery under post-prandial conditions before introduction of oxycodone availability (Fig. 1, empty symbols). Little change in the number of lever presses (Fig. 1A) or in the number of liquid deliveries (Fig 1B) occurred when 0.56 and 0.1 mg/ml of oxycodone was available. With further increases beyond 0.1 mg/ml in oxycodone concentration, numbers of reinforced lever presses and the number of deliveries obtained decreased. Numbers of presses of the left-side (unreinforced) lever were low, and unsystematically related to oxycodone concentration, and never overlapped with the numbers of right-side (reinforced) lever presses (Fig. 1A). Despite decreases in liquid deliveries with increases in oxycodone concentration, estimated OXY consumption (mg/kg body weight) increased (Fig. 2). Behavioral signs characteristic of opiate-like effects in mice, such as hyperlocomotion and presence of Straub tail, were observed at the two highest oxycodone concentrations during routine observations. In addition, during hyperlocomotion events, mice were occasionally observed pressing the reinforced lever without liquid consumption before re-commencing rapid, circular movements within the test chamber.
Fig. 1.
Effects of oral oxycodone obtained deliveries on active and inactive lever discrimination (A) and deliveries (B) across concentrations under post-prandial conditions in 3-h experimental sessions. As concentration increased, lever discrimination was maintained while a decrease in number of active lever responses corresponded with a decrease in number of deliveries obtained. N=15–16 C57BL/6J mice with 2–5 days per concentration. Data are expressed as mean (± S.E.M.).
Fig. 2.
Estimated consumption of oxycodone as a function of available concentration under post-prandial conditions. As concentration increased, estimated consumption of oxycodone also increased. N=15–16 C57BL/6J mice with 2–5 days per concentration. Data are expressed as mean (± S.E.M.), where consumption is estimated based on individual subject’s daily body weight and the total session oxycodone delivered, and expressed in mg/kg.
3.2 Oxycodone self-administration is maintained after withdrawal of prandial induction
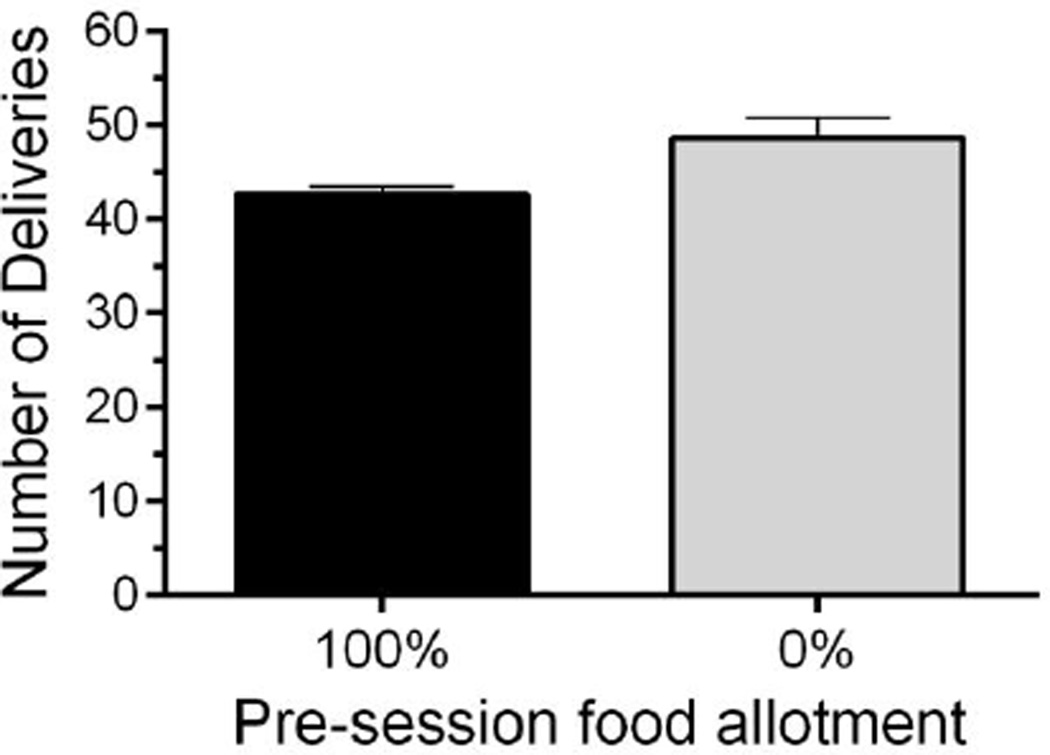
After completing concentration-response tests during post-prandial oxycodone induction, test solutions were maintained at 1 mg/ml OXY for the remainder of the study. Pre-session feedings were then gradually decreased until all daily food allotments were given following test sessions in the home cages. As pre-session feeding was reduced, self-administration of OXY was maintained, and slightly increased once all pre-session feedings were withdrawn (Fig. 3) although this increase was not statistically significant once pre-session feedings were completely withdrawn (t=2.575, df=4, P=0.0616).
Fig. 3.
Effects of removing pre-session feeding on oral oxycodone obtained deliveries in 3-h experimental sessions. Oral oxycodone self-administration was maintained, and slightly increased, in mice after full removal of pre-session feeding. N=14 C57BL/6Jmice. Data are expressed as mean (± S.E.M.).
3.3 Naloxone precipitates oxycodone somatic withdrawal signs in mice
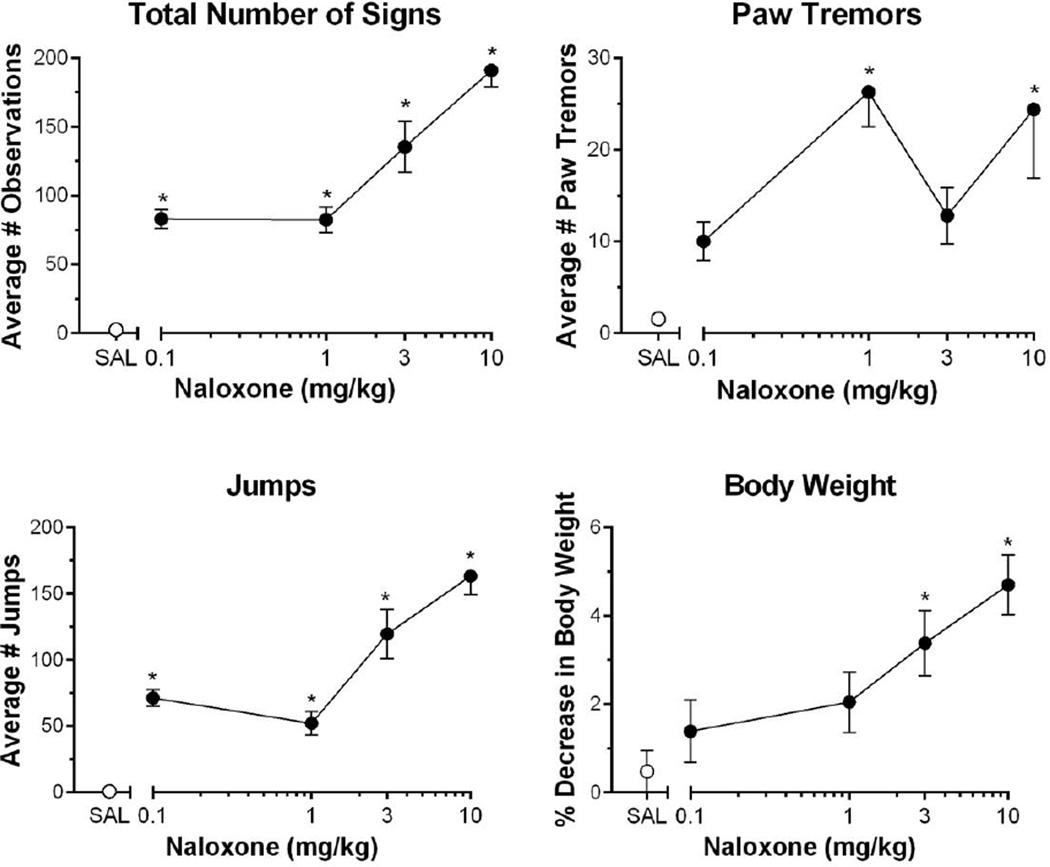
The ability of naloxone (0.1, 1, 3 and 10 mg/kg) to precipitate signs of dependence was examined in groups of mice that were either treated with vehicle or oxycodone. As seen in Figure 4, naloxone precipitated oxycodone somatic withdrawals signs such as jumps, paw tremors, and loss of body weight. Near-zero levels of total number of withdrawal signs (Fig. 4A), paw tremors (Fig. 4B), and jumps (Fig. 4C) were elicited by the 10 mg/kg naloxone dose in control mice administered the saline dosage regimen (empty circles in Fig. 4) and they experienced little change in body weight (Fig. 4D). In contrast, two or more of the tested naloxone doses significantly elevated these measures in oxycodone-treated mice (total number of signs, [F (4,44)=27.37; P<0.0001] (Fig. 4A); paw tremors, [F (4, 44)=5.857; P=0.0007] (Fig. 4B); jumps, [F (4,44)=37.15; P<0.0001] (Fig. 4C); and loss of body weight, [F (4, 44)=6.066; P=0.0006] (Figure 4D). Post-hoc analysis revealed that all four doses of naloxone produced significant increases in total number of signs and jumps, whereas 1 and 10 mg/kg naloxone produced significant increases in paw tremors, and only the two highest doses tested, 3 and 10 mg/kg naloxone, significantly decreased body weight.
Fig. 4.
Naloxone precipitated somatic withdrawal signs in oxycodone chronically injected mice. Behaviors scored were (A) the presence of jumping (B) paw tremors (C) total somatic signs (D) body weight decrease. Naloxone dose-dependently increased the number of somatic withdrawal signs in oxycodone-treated mice. N=9–10 C57BL/6J mice. Data are expressed as mean (± S.E.M.). *P < 0.05, **P < 0.01, ***P< 0.001, ****P< 0.0001 to saline-treated control mice.
4. Discussion
The present study identified experimental conditions in which oxycodone was orally self-administered by mice in which an operant response contingency was required, and this procedure thus enables future studies examining determinants of its self-administration heretofore restricted to intravenous self-administration procedures. In addition, an oxycodone administration protocol (9-day) was identified for inducing physical dependence in mice and thus enables future studies examining mechanisms of the induction of dependence and the modulation of the expression of signs of withdrawal such as in the search for pharmacotherapeutics ameliorating the malaise of withdrawal.
Oxycodone has been previously demonstrated to be intravenously self-administered in rats and mice (Beardsley et al., 2004; Zhang et al., 2015; Zhang et al., 2014; Zhang et al., 2009) while requiring an operant response for its delivery, and the present study now extends self-administration to the oral route of administration in mice. Previous preclinical studies using the oral route have examined oxycodone’s antinociceptive properties or other behaviors, such as in learning and memory tasks, in which oxycodone was administered to laboratory mice or rats via oral gavage (Davis et al., 2010; Nozaki et al., 2006). Gavage techniques to study oral oxycodone’s effects on behavior, pharmacokinetics, or dopamine receptor responses (e.g., Chan et al., 2008; Emery et al., 2015) have been useful as they precisely control the level of oxycodone exposure across subjects. However, the use of volitional oral consumption entailing an operant response (i.e., oral self-administration) is imperative to assess oxycodone’s effects related to its abuse liability because, unlike experimenter-administered oxycodone, the response requirement to obtain oxycodone access can be manipulated to examine motivational levels, the neuropharmacological and genetic effects produced by selfadministered versus experimenter-administered opiate can markedly differ (Jacobs et al., 2003), and selfadministered oxycodone would likely be better predictive of actual oxycodone abuse.
In the present study, as the concentration of oxycodone increased, the number of deliveries obtained slightly increased before decreasing with higher concentrations, whereas the estimated consumption increased reaching an average maximum of approximately 40 mg/kg. In the only other oral opioid, operant self-administration report we know of using mice, consumption of the potent benzimidazole opioid, etonitazene, also increased with increases in concentration in four different strains of mice (Elmer et al., 1995). In regards to the inverted U-shaped relationship between oxycodone infusions and dose, we had also observed a similar relationship in a rat oxycodone intravenous self-administration study (Beardsley et al., 2004) and which is characteristic of other reports of self-administered drugs under limited access conditions (e.g., Moreton et al., 1977; Suzuki et al., 1988). In an intravenous oxycodone self-administration procedure with adolescent and adult C57BL/6J mice, however, it was reported that the number of infusions dose-dependently decreased with increases in dose in both age groups, while intake increased resulting in a maximum intake of 8.25 mg/kg i.v. in adult mice (Zhang et al., 2009). In rats, a similar decrease in number of oxycodone infusions and an increase in total intake was found in an intravenous self-administration procedure examining duration of drug access (Wade et al., 2015). Differences in these reported patterns of self-administered oxycodone might be a function of the range of doses and concentrations tested, in that the ascending limb of the dose-effect curve may be missed if doses not low enough are untested. In the present study, levels of oxycodone intake reached behaviorally active levels inducing mu-opioid receptor-like mediated effects in the mice including hyperlocomotion and Straub tail (Aceto et al., 1969; Hecht and Schiorring, 1979). Likely, in part, attributable to these observed effects, it is important to note that the present self-administration procedure is not without its limitations, specifically regarding the precise measurement of oxycodone consumption. For example, one subject was observed engaging in stereotypic biting/chewing upon the active lever that resulted in oxycodone deliveries that were not consumed. These observations were noted after ~30 min into the test session (not shown), and after a bout of oxycodone consumption had been observed, suggesting that the drug elicited these stereotypic effects. These stereotypic effects have been previously observed in rats that had orally self-administered the highly potent opiate, etonitazene, under food-deprivation conditions (Beardsley and Meisch, 1981; Carroll and Meisch, 1981; Meisch and Kliner, 1979). Interestingly, these stereotypic behaviors were not observed in the present study once pre-session feeding was completely withdrawn and instead given after the test session, suggesting these effects were not simple due to food deprivation.
Once pre-session feedings were withdrawn completely, mice maintained the discrimination between the active and inactive levers (not shown) as well as increased the number of deliveries obtained. This demonstrates that pre-session feedings were no longer needed to induce oral self-administration of oxycodone solutions, and suggests, although does not definitively confirm, that oral oxycodone was serving as a positive reinforcer. Similar maintenance of behavior has been found previously in an oral operant self-administration study in mice with the highly potent opiate, etonitazene, once pre-session feeding was withdrawn (Elmer et al., 1995). In addition to previous mouse intravenous self-administration reports, these results suggest that oxycodone can also be volitionally consumed via the oral route in mice at behaviorally active levels. Overall, this methodology provides a useful, noninvasive technique enabling the study of the determinants of oxycodone self-administration the duration of which may only be limited by a mouse's natural lifespan.
Physical dependence upon oxycodone was induced in C57BL/6J mice after nine days of its b.i.d. subcutaneous administration as inferred by naloxone-precipitated somatic signs of opiate-like withdrawal syndrome. We previously reported that morphine-dependent rhesus monkeys demonstrate cross-dependency to oxycodone (Beardsley et al., 2004), and that naloxone can precipitate disruptions of lever pressing maintained by intra-cranial self-stimulation in rats chronically-treated with oxycodone suggestive of dependence (Wiebelhaus et al., 2016). Thus, oxycodone demonstrates opiate-like dependence effects across species including mice, rats and rhesus monkeys. The signs and their patterns of direction observed in C57BL/6J mice of the current study are similar to those reported in previous studies with other strains of mice that used oxycodone regimens involving continuous drug delivery (Mori et al., 2013; Raehal and Bohn, 2011) or repeated injections (Bhalla et al., 2015) to induce dependence. The regimen to induce physical dependence in the present study was adapted from a previous study that found physical dependence upon morphine to be induced in mice (Muldoon et al., 2014). After naloxone-precipitated withdrawal in morphine-treated wildtype mice, similar numbers of paw tremors but fewer numbers of jumps were observed in comparison to the present study’s results with oxycodone. Moreover, unlike morphine, naloxone precipitated similar degrees of withdrawal in both wildtype and beta-arrestin 2 knockout mice made dependent on oxycodone via osmotic pumps (Raehal and Bohn, 2011). These differences between morphine and oxycodone, in addition to clinical psychopharmacological and analgesic effects, suggest that these two mu-opioid agonists have different pharmacological profiles that warrant further investigation into oxycodone’s abuse-related effects on behavior. Unlike previous studies that only reported tests after one dose of naloxone to precipitate withdrawal, we evaluated a wide range of naloxone doses (0.1 to 10 mg/kg). We observed a naloxone dose-dependent increase in withdrawal severity. The C57BL/6J strain of mice has been observed to consistently display characteristic effects of opiate dependence (e.g., naloxone-precipitated jumping) across various methods of physical dependence induction upon morphine, although at times with less intensity than some other strains such as Swiss-Webster mice (Kest et al., 2002). The regimen used in the present study therefore provides an easy novel method to accurately measure dependence-related effects on future behavioral endpoints.
5. Conclusions
This report has identified novel conditions for establishing the oral self-administration of oxycodone and the induction of its physical dependence in mice. These procedures will help enable the further examination of the determinants of abuse-related effects of oxycodone including the effects of potential pharmacotherapeutics on these effects.
Acknowledgments
Portions of this research were funded by National Institute on Drug Abuse (NIDA) N01DA-12-8904. R.M.E. was supported by NIDA training grant T32DA007027, and some studies within this report were included in her doctoral dissertation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of this report.
References
- Aceto MD, McKean DB, Pearl J. Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol. 1969;36:225–239. doi: 10.1111/j.1476-5381.1969.tb09500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:163–172. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Meisch RA. A precision drinking device for rats tested with water, etonitazene, and ethanol. Pharmacol Biochem Behav. 1981;14:871–876. doi: 10.1016/0091-3057(81)90376-2. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Pais G, Tapia M, Gulati A. Endothelin ETA receptor antagonist reverses naloxone-precipitated opioid withdrawal in mice. Can J Physiol Pharmacol. 2015;93:935–944. doi: 10.1139/cjpp-2015-0022. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Emergency department visits involving nonmedical use of selected prescription drugs-United States, 2004–2008. MMWR: Morbidity and mortality weekly report. 2010;59:705–709. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol. 2008;35:295–302. doi: 10.1111/j.1440-1681.2007.04821.x. [DOI] [PubMed] [Google Scholar]
- Davis CP, Franklin LM, Johnson GS, Schrott LM. Prenatal oxycodone exposure impairs spatial learning and/or memory in rats. Behav Brain Res. 2010;212:27–34. doi: 10.1016/j.bbr.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Goldberg SR, George FR. Opioid operant self-administration, analgesia, stimulation and respiratory depression in mu-deficient mice. Psychopharmacology (Berl) 1995;117:23–31. doi: 10.1007/BF02245094. [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates ML, Wellman PJ, Eitan S. Differential effects of oxycodone, hydrocodone, and morphine on the responses of D2/D3 dopamine receptors. Behav Brain Res. 2015;284:37–41. doi: 10.1016/j.bbr.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Hecht A, Schiorring E. Behavioral effects of low and high acute doses of morphine in solitary mice. Psychopharmacology (Berl) 1979;64:73–79. doi: 10.1007/BF00427348. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain, behavior, and immunity. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kalso E. Oxycodone. J Pain Symptom Manage. 2005;29:S47–S56. doi: 10.1016/j.jpainsymman.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kirsh K, Peppin J, Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother. 2012;26:348–361. doi: 10.3109/15360288.2012.734905. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Kliner DJ. Etonitazene as a reinforcer for rats: increased etonitazene-reinforced behavior due to food deprivation. Psychopharmacology (Berl) 1979;63:97–98. doi: 10.1007/BF00426928. [DOI] [PubMed] [Google Scholar]
- Moreton JE, Meisch RA, Stark L, Thompson T. Ketamine self-administration by the rhesus monkey. J Pharmacol Exp Ther. 1977;203:303–309. [PubMed] [Google Scholar]
- Mori T, Komiya S, Uzawa N, Inoue K, Itoh T, Aoki S, Shibasaki M, Suzuki T. Involvement of supraspinal and peripheral naloxonazine-insensitive opioid receptor sites in the expression of mu-opioid receptor agonist-induced physical dependence. Eur J Pharmacol. 2013;715:238–245. doi: 10.1016/j.ejphar.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, McIntosh JM, Dierssen M, Miles MF, Chen X, De Biasi M, Damaj MI. The alpha3beta4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol. 2014;171:3845–3857. doi: 10.1111/bph.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Saitoh A, Kamei J. Characterization of the antinociceptive effects of oxycodone in diabetic mice. Eur J Pharmacol. 2006;535:145–151. doi: 10.1016/j.ejphar.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245:164–170. [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2015;40:421–428. doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebelhaus JM, Walentiny DM, Beardsley PM. Effects of Acute and Repeated Administration of Oxycodone and Naloxone-Precipitated Withdrawal on Intracranial Self-Stimulation in Rats. J Pharmacol Exp Ther. 2016;356:43–52. doi: 10.1124/jpet.115.228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brownstein AJ, Buonora M, Niikura K, Ho A, Correa da Rosa J, Kreek MJ, Ott J. Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience. 2015;285:34–46. doi: 10.1016/j.neuroscience.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J, Kreek MJ. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl) 2014;231:1277–1287. doi: 10.1007/s00213-013-3306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]