Abstract
Advances in imaging technologies have greatly increased our understanding of cellular and molecular interactions in humans and their corresponding animal models of infectious diseases. In the HIV/SIV field, imaging has provided key insights into mucosal viral transmission, local and systemic virus spread, host–virus dynamics, and chronic inflammation/immune activation and the resultant immunopathology. Recent developments in imaging applications are yielding physical, spatial, and temporal measurements to enhance insight into biological functions and disease processes, while retaining important cellular, microenvironmental, organ, and intact organism contextual details. Taking advantage of the latest advancements in imaging technologies may help answer important questions in the HIV field. The Global HIV Vaccine Enterprise in collaboration with the National Institutes of Health (NIH) sponsored a meeting on May 8 and 9, 2017 to provide a platform to review state-of-the-art imaging technologies and to foster multidisciplinary collaborations in HIV/AIDS research. The meeting covered applications of imaging in studies of early events and pathogenesis, reservoirs, and cure, as well as in vaccine development. In addition, presentations and discussions of imaging applications from non-HIV biomedical research areas were included. This report summarizes the presentations and discussions at the meeting.
Keywords: : HIV, SIV, imaging, lymphocyte trafficking, viral reservoirs, early vaccine responses
Introduction
The major discussion topics addressed during the meeting were organized into four sessions. The “Early Events and Pathogenesis” session focused on the earliest events after viral transmission, that is, which cells are infected first and what are the initial immune responses, early replication sites, and pathways leading to systemic spread. The “Reservoirs and Cure” session was designed to discuss the formation of HIV reservoirs, identification of latently infected cells, and the signaling mechanisms driving latency.
The session on “Vaccines” covered the immune responses to vaccines, including the fate of the immunogen right after vaccination in the context of the complex structure of lymphatic tissues. The session on “Emerging Technologies and Lessons from non-HIV/SIV Models” spanned a wide variety of topics, building on advances in the areas of basic immunology, pharmacology, cancer, as well as introducing recently developed imaging techniques (Tables 1 and 2).
Table 1.
Summary of Ex Vivo Imaging Techniques Presented
| Technique | Description of principle | Examples of applications | Investigators |
|---|---|---|---|
| In situ hybridization | Radioactive | Detection of HIV/SIV RNA and DNA on tissue sections | Haase |
| Chromogenic | Haase | ||
| RNAscope | Detection of HIV/SIV RNA on tissue sections, allows for simultaneous signal amplification and background suppression | Haase, Estes, Connick | |
| DNAscope | Detection of HIV/SIV DNA in situ, technique similar to RNAscope, but for DNA | Estes, Yu, Germain | |
| BASEscope | Detection of multiple spliced RNA in situ, technique similar to RNA scope, but for multiple spliced RNA | Estes | |
| Tyramide signal amplification | Novel technique using a biotinylated tyramine to detect specific proteins or nucleic acid sequences in situ, where tyramide, a phenolic compound, has the ability to bind to the electron-rich surface of targets | Visualization of virions with the light microscope | Haase |
| In situ tetramer staining | Fluorescent detection of epitope-specific T cell receptors | Identification and quantification of antigen-specific CTL in situ | Haase, Skinner |
| Visualizing the virus | Gp160-GFP, mCherry-Vpr, integrase-Ruby, tetracysteine-p24 | Detection of fluorescent or luciferase-labeled reporter viruses | Hope, Connick, Young |
| GFP or ELuc reporter virus | |||
| EM tomography | Correlated Light and EM microscopy (CLEM) | Identification of intracellular HIV reverse transcribing complexes | Hope |
| Antibody labeling | VRC01, VRC01-LS labeling with Cy3, Cy5, and 64Cu | Tracking of labeled antibodies, which reveal antibody distribution and new mechanisms of antibody transport | Hope |
| Multiplexed confocal imaging assays | Combined with RNAscope | Visualization of vaccination or infection-induced germinal centers | Petrovas |
| Histocytometry | For visualizing and quantifying phenotypically complex cell populations directly in tissue sections | In situ multiplex cell phenotyping, quantification, and spatial analysis | Gerner, Yu, Germain, Petrovas |
| Technology is based on multiplexed antibody staining, tiled high-resolution confocal microscopy, voxel gating, volumetric cell rendering, and quantitative analysis | Cell distance mapping | ||
| Analysis of local cellular interactions | |||
| Monitoring immune responses to vaccines | |||
| Expanded to 3D volume imaging | Quantifying cellular distributions of SIV provirus in tissues | ||
| High-throughput imaging and analysis | (1) Automated liquid handling | Nuclear features of Hutchinson–Gilford Progeria syndrome cells | Shachar (Misteli) |
| (2) High-throughput microscopy | |||
| (3) High-content image and data analysis | Measure cellular phenotype of aging | ||
| Immunofluorescence or fluorescence in situ hybridization (FISH)-based | Identification and characterization of novel cellular factors and molecular mechanisms | ||
| Study nuclear organization | |||
| Detect interactions between genes or loci and nuclear bodies | |||
| Measure distances between loci, such as enhancer-promoter |
Table 2.
Summary of In Vivo Imaging Techniques Presented
| Technique | Description of principle | Examples of applications | Investigators |
|---|---|---|---|
| Mucosal exposure to Methylene Blue | Measures dye dissemination | Combined with MR imaging | Keele |
| Tracking of lymphatic dissemination following mucosal exposure | |||
| Molecularly tagged SIVmac239X | SIVmac239 clones tagged with a specific sequence | Combined with laser capture microdissection and proviral sequencing | Keele |
| Measures viral dissemination and evolution in tissues | |||
| Dynamic MRI | Mean specific uptake/cm3 | Generation of high-resolution images of lymph nodes | Schacker |
| 3 Tesla magnet with unique coil | |||
| SPECT/CT | Single-photon emission computed tomography (SPECT) is a nuclear medicine tomographic imaging technique using gamma-emitting radioisotopes. | In vivo imaging of the CD4 pool, following SIV infection or hematopoietic stem cell transplantation in NHPs | Di Mascio |
| It has lower spatial resolution and sensitivity (but also less cost prohibitive) compared with Positron Emission Tomography (PET). | |||
| Computed Tomography (CT), produces anatomic pictures of the organs and structures of the body for precise identification of the regions of interest in the coregistered nuclear medicine images. | |||
| Bioluminescence Imaging | Firefly Luciferase + D-Luciferin = Bioluminescence | Methodology for revealing cell trafficking during ongoing biological processes by detection of light emitted following chemical reaction of luciferase enzyme with its substrate | Young, Mempel |
| HIV-GFP | SIV-GFP-T2A-SSTR2 | ||
| MLV Gag-GFP | SIV-GFP-IRES-Ferritin | ||
| mCherry or GFP labeling of cells | Dual reporter imaging (ELuc and NanoLuc™) in humanized mice | ||
| Multimodality imaging | PET/CT plus near-infrared imaging | Multimodality imaging to harness complementary nature of different modalities by enabling sequential functional and anatomic imaging on integrated imaging platforms | Santangelo, Villinger, Le Grand |
| Positron emission tomography (PET) is a nuclear medicine functional imaging technique that uses positron-emitting radioisotopes. | |||
| Colocalization of delivered probes and anti-CD4 staining | |||
| Near-infrared fluorescence imaging | Optical imaging using near-infrared fluorescence (NIR) light is a new imaging modality that has recently emerged in the field of cancer imaging. It can penetrate several centimeters into tissue. | In vivo imaging of antigen expression | Le Grand |
| Effect of electroporation on HIV DNA vaccine | |||
| Two-photon microscopy | Fluorescence imaging utilizing pulsed lasers that penetrate deep into tissues. | Imaging cellular dynamics in vivo | Victora, Mempel, |
| Brainbow system | Multicolor labeling | Visualization of germinal center clonal dynamics | Victora |
| Monitoring cell–cell interactions in vivo | |||
| Mass spectrometry imaging | Protein and small molecule localization using ion/laser beam combined with mass spectrometry | Highly multiplexed histochemistry, tissue distribution of antiretroviral drugs | Angelo, Kashuba |
Session 1: Early Events and Pathogenesis
Dr. Ashley T. Haase (University of Minnesota, Title: “Image analysis of HIV/SIV pathogenesis: A guide to improving treatment, vaccine development, and cure strategies”) presented an overview of the potential of imaging capabilities in advancing the understanding of initial events in HIV-1 infection and transmission at mucosal surfaces, which can help guide HIV vaccine development and cure strategies.
Studies based on the SIV-rhesus macaque model of mucosal transmission have established the relationship between epithelial signaling and innate immune responses that recruit CD4+ T cells into the transition zone of the ectocervix, endocervix, and adjoining tissues, where small founder populations of infected cells have been consistently detected.1 The analysis of cellular events and chemoattractant profiles preceding CD4+ T cell recruitment in cervical tissues showed rapid induction of chemokine expression in epithelium, including CCL3, CCL20, and CXCL8, and the important role of the resulting chemokine gradient in recruiting CD4+ T cells as targets to fuel local expansion of infected founder populations. The disruption of this gradient in cervical epithelium by SIVmac239Δnef vaccination was important for protection.2
Efforts to identify correlates of protection suggested local production of antibodies to gp41 trimers by plasma cells and within ectopic lymphoid follicles in mucosal tissues,3 and concentration of the antibodies on the path of virus entry by the neonatal Fc receptor (FcRn) in epithelium could block establishment of infection. The concentrated gp41 antibodies also form immune complexes with virus that interact with the inhibitory Fc gamma 2b receptor in epithelium to suppress recruitment of CD4 T cells. Thus, the early events observed in the course of protection by the SIVmac239Δnef vaccine provide insights and guidance on designing strategies to prevent systemic spread of HIV even after establishment of local infection in the mucosa.
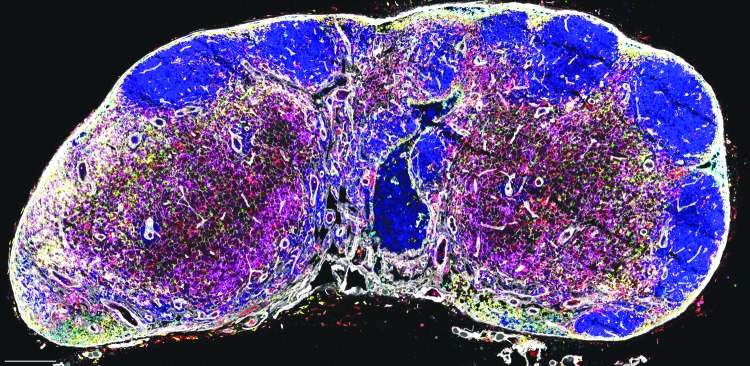
Dr. Haase also provided evidence to confirm the role of lymphoid tissues as primary sites for viral production by fluorescent labeling of HIV RNA producing CD4+ T cells in lymph nodes (Fig. 1). Using these imaging approaches, his group has shown that immune activation in response to infection induces fibrosis of lymphoid tissues mediated by T regulatory (Treg) cells. The deposition of collagen disrupts the fibroblastic reticular cell (FRC) network4 and access of T cells to the survival factor interleukin-7 (IL-7) as they migrate on the network resulting in depletion particularly of naive T cells in infected subjects.
FIG. 1.
Visualizing virus production by resting and activated T cells at transmission and after dissemination to lymphoid tissues.63–65 (A) ISH to detect viral RNA within cells and virions with subsequent TSA reveals virions at the light microscopic level surrounding two kinds of virus-producing cells typed in adjacent tissue sections by combining ISH with staining for cell type and activation state. The long arrow points to a large activated CD4+ T cell (long arrow); the short arrow points to a smaller ostensibly resting T cell lacking markers of activation and proliferation in the cervix of a rhesus macaque following vaginal inoculation of SIV (original magnification 400 × , from reference66). (B). Explosive virus production in a lymph node following dissemination in activated (long arrow) and resting T cells (short arrow). Image suggests clustered virus factories, where virus is efficiently transmitted to susceptible cells in close spatial proximity or through cell-to-cell transmission (long arrow) (original magnification 200 × , image unpublished). Image Credit: Wietgrefe SW and Haase AT. ISH, in situ hybridization; TSA, tyramide signal amplification.
Dr. Thomas J. Hope (Northwestern University, Title: “Image analysis of HIV/SIV pathogenesis: A guide to improving treatment, vaccine development, and cure strategies”) illustrated the events leading to HIV/SIV transmission and prevention in macaque mucosal infection and human tissue explant models. He highlighted his early use of green fluorescent protein (GFP)-labeled HIV virions to visualize the intracellular movement and accumulation of viral particles in the perinuclear region of living cells and the dependence of this process on the microtubule network.5 Dr. Hope demonstrated the utility of photoactivatable fluorophore-labeled virions for defining the interaction of HIV with the mucosal barrier of the female reproductive tract (FRT), confirming the mechanism for HIV transmission as functional diffusive percolation through the squamous epithelium of the FRT.6 Moreover, the presence of naturally produced or injected progestins accelerated the penetration of tagged virions through the columnar epithelium of the FRT,7 increasing the likelihood of encounter between HIV virions and potential target cells. Further studies of nonhuman primate vaginal challenge with an SIV-based dual reporter construct revealed that virions can access target cells throughout the FRT8 and that Th17 lineage, CCR6+ CD4+ T cells represent primary targets for SIV during vaginal transmission.9 These findings suggest that, to provide protection against HIV, immune responses need to be present throughout the entire FRT.
Utilizing direct antibody labeling with Cy5 fluorophores, Dr. Hope's laboratory showed that the mucin protein MUC5AC-IgG complex is able to slow down HIV mobility by trapping immune complexes formed between HIV-1 BaL and anti-gp120 monoclonal antibody VRC01. His work suggests that gaining a greater understanding of the initial steps of virus interaction with mucus, mucosal antibodies, and the cells at the epithelial barrier may be of paramount importance to increase protection from HIV acquisition.
Dr. Brandon F. Keele (Frederick National Laboratory, Title: “Imaging and molecular techniques combined to track mucosal transmission and dissemination”) summarized his work attempting to better understand and track the mucosal transmission and dissemination of HIV. Simulated viral inoculations with Methylene Blue vital dye or Gadofosveset Trisodium contrast dye allowed visualization of the inoculum during luminal exposure as well as lymphatic drainage pathways. After vaginal challenge, the dye revealed the exposure area of the vaginal epithelium and the ectocervix, whereas intrarectal administration identified the lower gastrointestinal tract from the rectum to the distal descending colon as potential sites of viral entry. Mapping the lymphatic drainage pathways in macaques with magnetic resonance imaging (MRI), he identified the internal iliac lymph nodes followed by the common iliac chain to the para-aortic lymph nodes as potential conduits for HIV systemic dissemination after vaginal or rectal challenge.10 In addition, an alternative draining pathway involving the colonic and mesenteric lymph nodes was confirmed after rectal challenge.
Dr. Keele's laboratory pioneered the use of synthetic viral swarms created by mixing variants of SIVmac239 that differ by two or three synonymous mutations that “barcode” viral clones and that are used to identify independent infection events in the same animal.11 Following intrarectal transmission of SIV, detailed dissection of the exposed sites as well as sequencing of the marker regions demonstrated infection by single viral clones and limited local replication in tissue foci. Interestingly, the intravaginal route of viral transmission resulted in infection by multiple viral clones in local tissue followed by systemic dissemination by a single dominant clone along with one or more subdominant clones, contrary to the common notion that a single infectious clone gives rise to the majority of established infections.
The number and the proportion of each transmitted founder lineage were evenly distributed across multiple tissues following systemic dissemination often with dominant and minor variants representing multilog differences in proportion. The observation that some lineages were delayed during early replication suggests that intervention strategies might be developed that can augment conditions to limit or slow viral dissemination.
Dr. Timothy Schacker (University of Minnesota, MN, Title: “Image analysis of HIV-induced lymphoid tissue fibrosis advances our understanding of HIV pathogenesis”) presented the quantitative image analysis of progressive lymphoid tissue fibrosis in HIV infection using Masson's trichrome staining to identify collagen fibers. Damage to lymphoid tissue FRC network and collagen deposition after HIV-1 infection led to the loss of naive T cells due to restricted access to IL-7, a critical factor required for T cell survival. The induced apoptosis within naive T cell populations further resulted in continual depletion of both naive CD4+ and CD8+ T cell populations. However, restoration of the FRC network and reconstitution of naive T cell populations occurred if combination antiretroviral therapy (cART) was initiated in the acute stage of infection, suggesting that early treatment can moderate the pathological consequences of lymphoid tissue fibrosis and improve immune reconstitution.12 A potential mechanism of lymphoid tissue fibrosis involved immune activation driving a premature immunosuppressive response as Treg cells induce collagen formation by overexpression of transforming growth factor beta 1.13
Lymph node imaging also revealed that loss of FRCs attenuates humoral immunity due to reduced B cell viability, abrogated B cell activating factor expression and impaired follicular organization.14 Combining the antifibrotic drug pirfenidone with cART preserved paracortical T cell zone architecture by decreasing fibrosis during acute infection and post-cART reconstitution of peripheral and lymphoid CD4+ T cells,15 supporting a potential role of antifibrotic drug treatment to improve immune reconstitution in HIV-infected people.
Dr. Michele Di Mascio (NIAID, NIH, Title: “Dynamics of CD4 pool repopulation in the whole-body following antiretroviral treatment of SIV infection: an in vivo imaging study”) highlighted the potential of in vivo imaging approaches to study the pathogenesis of HIV infection by detailing the dynamics of CD4+ T cell pool repopulation following initiation of cART after SIV infection or following total body irradiation and hematopoietic stem cell transplantation.16,17 The use of single-photon emission computed tomography (SPECT) combined with CT and monoclonal antibody labeling specific for CD4+ T cells in SHIV-infected nonhuman primates established a correlation between radiotracer uptake in lymphoid tissues (a measure of total CD4+ T cells in the body) and peripheral blood CD4+ T cell counts.16 Furthermore, combining data from in vivo and ex vivo imaging with gamma counter analysis of tissue samples provided a validation of the CD4 pool imaging system in nonhuman primates by identifying the lymphoid organs, in which the association between radiotracer uptake and CD4 content was found, hence legitimating the use of this technology to study SIV disease progression in nonhuman primates.
Those ex vivo data were also used to generate a new estimate of the relative numbers of CD4+ T cells in different organs, challenging the prevalent notion that the vast majority of CD4 T cells reside in the gut mucosa, consistent with minimal probe uptake detected in this anatomic compartment. Dr. Di Mascio showed that increasing the total mass of the probe injected in these earlier studies by 10-fold (from ∼100 μg to ∼1 mg) resulted in a dramatic decrease of relative radiotracer uptake in the spleen as well as a decrease in relative uptake in the liver and bone marrow, concomitant with an increase in relative radiotracer uptake in the kidney and gut. He stressed that additional ex vivo measurements need to be performed to ascertain the specific/nonspecific content of this increase in probe uptake, and whether the dose-dependent increase in probe uptake in the gut is also observed when the F(ab')2-CD4R1 antibody fragment is radiolabeled with different radionuclides.
In his opinion, the optimal probe dose for in vivo imaging of the CD4 pool also needs to be scrutinized with the focus on the effect of probe occupancy on the normal physiology of CD4 T cells in preparation for translation of the technology into human studies. The ability to accurately assess the CD4+ T cell pool from total body imaging may enhance our understanding of the relationship between different physiological and pathophysiological states and CD4+ T cell distribution, depletion, and reconstitution. Further studies based on whole-body SPECT/CT imaging suggested that the CD4+ T cell pool of splenic lymphocytes may increase or decrease by up to 30% within 4 weeks of initiation or interruption of cART, respectively.18 However, reconstitution of lymph node CD4 pools was incomplete in long-term cART-treated animals, which had reduced peripheral lymph node CD4 pools, highlighting an irreversible lymphoid tissue damage. Dr. Di Mascio also advocated the instrumental role of the NIAID Integrated Research Facility as a unique resource to facilitate molecular imaging-based preclinical research on infectious diseases and to perform a comprehensive evaluation of the immune system for the development of prevention and treatment options relevant to human diseases.
Session 2: Reservoirs and Cure
Dr. Robert Siliciano (Johns Hopkins University, Title: “New developments in understanding the latent reservoir”) gave an overview about the HIV reservoir and cure scientific efforts. He described the low frequency of latently infected cells, the low or absent viral gene expression in latently infected cells, and the wide distribution and recirculation of latently infected cells in lymphoid organs and other sites as major challenges for the imaging field. Rarely, HIV-infected cells exit the latent reservoir (<1–57 cells per day), however, they are likely to be widely distributed.19–21 The questions related to imaging these cells are how many target molecules per cell and how many infected cells per unit volume of tissue are required for detection. Dr. Siliciano believes that the main challenge for the imaging field relies in detecting HIV proviruses in deep latency in tissues.
In 1997, Dr. Siliciano's laboratory developed the Quantitative Viral Outgrowth Assay to measure the number of latently infected cells.22,23 It was shown that the decay of latently infected CD4+ T cells was slow with a half-life of 44 months and a time to eradication of >73.4 years. Patients on cART have about 1 HIV RNA copy per ml detectable in plasma. The residual HIV reservoir was sensitive to the current cART regimen, archival, and nonevolving 24–29 with occasional reactivation events and production of noninfectious viral particles. Treatment intensification (i.e., adding a 4th drug) did not decrease the residual reservoir further, indicating that residual viremia is not the result of incomplete viral suppression by cART. In about half of the studied patients, residual viremia at any given time point was dominated by a small number of clones, whose sequences did not show evidence of evolution from the time of cART initiation and which appeared to represent clonal expansion of individual cells.28
His data indicated that while cART is effective at blocking viral replication, it did not eliminate latent HIV. He emphasized that it takes 6–8 months of continuous cART treatment for the latent reservoir to decay to the level of one latently infected cell per million. Therefore, imaging studies of the latent reservoir should only be performed after at least 6–8 months on suppressive cART, when labile populations of infected cells have decayed. When performing the quantitative viral outgrowth assay during long-term cART, a large number of integrated proviruses could not be induced, because they were defective with large deletions or hypermutated regions.30 In addition, a single round of maximal T cell activation did not lead to replication of all intact proviruses; each additional round of stimulation reactivated additional proviruses.30–32 Dr. Ya-chi Ho reconstructed some of the noninduced intact proviruses and found that they all were replication competent in vitro.31 Therefore, the total number of intact proviruses in a given patient sample provides a more accurate upper limit of the reservoir size than standard HIV DNA PCR quantification.
Clonal expansion of HIV-infected cells was examined by integration site analysis.33,34 Proliferation of infected cells can be induced in two ways: (1) Antigen drives T cell proliferation, but also triggers viral gene expression, so productively infected cells die quickly. (2) Cytokines, such as IL-7, can drive homeostatic proliferation of memory T cells, possibly expanding the reservoir, but may also reverse latency. However, latently infected cells can also proliferate in vitro without producing virus.31,32 Predominance of a small number of HIV clones in plasma is evidence that proliferation of HIV-infected cells occur in vivo, and expanded cellular clones account for the majority of the reservoir. In fact, the total HIV reservoir would decrease faster over time, if the loss of infected cells would not be counteracted by clonal proliferation.35,36 Finding ways to block proliferation of latently infected cells is therefore a high priority for advancing HIV cure efforts.
Dr. Jake Estes (Frederick National Laboratory, Title: “Novel in situ imaging approaches to understand viral persistence”) presented his laboratory's use of quantitative in situ imaging to understand viral persistence, to identify the anatomical locations of viral reservoirs, and to conduct a phenotypic assessment of T and “non-T cell” reservoirs that can be difficult to extract from tissues. He compared the sensitivity of radiolabeled in situ hybridization, chromogenic in situ hybridization, and the new next-generation RNAscope platforms for the detection of intracellular HIV/SIV RNA and HIV/SIV RNA trapped on the surface of follicular dendritic cells (FDCs). He concluded that RNAscope is the most sensitive and rapid method, allowing for the visual and spatial discrimination of individual virus particles in tissue sections with the results obtained in just 1 day.37 Using an in vitro SIV serial dilution binding assay, he found a linear correlation between the number of SIV virions per cell detected by quantitative RT-PCR and RNAscope. His laboratory was also able to quantify trapped virus particles on the FDC network ranging from a few particles to >105 per mm2 in B cell follicles. Even rhesus macaques on prolonged cART still had detectable virus particles on the FDC network of their lymph nodes. The false-positive detection rate was low with ∼0.05 viral RNA copies per 105 cells and ∼0.2 virions per 105 cells. The second technique, DNAscope, was first tested and validated on 3D8 cells, which contain one integrated copy of SIV genome per cell.38 Upon RNase treatment, SIV vRNA disappeared, but SIV p17 and SIV vDNA remained, demonstrating the DNA specificity of the application. The technique can be multiplexed with other assays to detect various proteins and other RNA species, for example, it is possible to perform costaining for CD3, vDNA, myeloid macrophages (CD68), and nuclear staining of 4,6-diamidino-2-phenylindole (DAPI).
Another challenge associated with in situ imaging approaches is the difficulty of distinguishing from replication-competent defective proviruses. It has been reported by the Siliciano Laboratory that most proviruses of latently infected CD4+ T cells in chronic infection are defective, with up to 70% of CD4+ T cells that harbor HIV DNA having deletions and hypermutations that span the tat-rev splice junction. Therefore, a new technology, termed BASEscope, has been developed that detects spliced viral RNA using probes specific for the SIV or HIV tat-rev splice junctions, thus increasing the likelihood of detecting viral RNA+ cells in situ with replication-competent viruses. RNAscope can be multiplexed with RNA BASEscope and immunohistochemistry allowing for colocalization studies of total and spliced viral RNA that can be traced to cells expressing specific proteins.
Dr. Francois Villinger (New Iberia Research Center, Title: “Mapping response to treatment and early SIV replication during acute and post-acute infection”) main imaging technique is Immuno-PET (positron emission tomography), which uses antibodies coupled to positron-emitting radionuclides as probes. To validate these in vivo studies, it is important to assure adequate circulation, penetration of tissues, specificity, and uptake of the probes. The disadvantages of using antibodies as probes are high background (especially in the kidney), long circulation time that outlasts the isotope signal, and competition with the host antibodies for infectious targets. The use of F(ab) or F(ab')2-based probes markedly decreased background and improved the signal-to-noise ratio. The probe was a 64Cu-labeled, SIV/SHIV Env-binding, non-neutralizing antibody or antibody fragment administered to SIV-infected rhesus macaques followed by PET/CT imaging typically 36 h postinjection.
In chronically infected viremic macaques, the gut-associated tissues, spleen, lymph nodes, genital tract, and surprisingly nasal cavity lighted up demonstrating gp120 accumulation, a surrogate for active viral replication.39 During cART treatment, the signal decreased, but remained visible. Even after 6 months on suppressive cART, large amounts of virus could be found in the gut of some animals. Dr. Villinger also presented imaging data on animals treated with cART and a rhesusized α4β7-specific antibody as part of a study40 demonstrating that animals receiving α4β7 antibody had a lower SIV signal than the animals treated with control antibody even before cART interruption. After cART interruption, the SIV signal increased especially in the gut of the animals treated with the control antibody, suggesting that these tissues are the major source of the persistent HIV reservoir.
Dr. Villinger also showed imaging data for CD4+ T cells, which were mostly found in the gut, spleen, and lymph nodes in uninfected animals. In late-stage SIV-infected animals, CD4+ T cells were depleted in the gut, especially the colon and spleen. The α4β7 antibody treatment did not prevent acute CD4+ T cell loss. However, when combined with cART, the anti-α4β7 antibody treatment mediated long-term restoration of the CD4 signal in the gut, spleen, and select lymph nodes. In a separate study tracking of viral spread in acutely SIVmac239-infected animals at week 0, 1, 2, and 4 demonstrated that the largest increase of SIV replication in the lymph nodes occurred in the first week postinfection. In the gastrointestinal tract and spleen, the signal for SIV increased in weeks 1 and 2 with a small drop in week 4 postinfection. Immuno-PET coupled with CT, therefore, is useful for tracking viral spread in whole animals in real time.
Dr. Elizabeth Connick (University of Arizona, Title: “Imaging B cell follicles to investigate HIV/SIV persistence”) presented her work on HIV persistence in B cell follicles performed in collaboration with Dr. Pamela Skinner. She showed by in situ hybridization that most HIV replication occurs in secondary lymphoid tissues, including the lymph nodes and spleen. Within the lymph node, most T cells reside in the paracortical area, where antigen responses are initiated. B cell follicles contain germinal centers, where B cells undergo affinity maturation, somatic hypermutation, and differentiate into memory B cells or plasma cells with the help of FDCs and T follicular helper cells (Tfh). HIV replication is concentrated in Tfh cells in B cell follicles.41
Dr. Connick estimated that a CD4+ T cell in the follicle has a 31-fold greater likelihood of being HIV RNA+ than a CD4+ T cell in the extrafollicular region.41 In in vitro infection studies, germinal center Tfh cells (PD-1high, CXCR5+high), are vastly more permissive to HIV than the other subsets of T cells.42 Dr. Connick is trying to understand why cytotoxic T lymphocytes (CTL) are unable to suppress HIV replication in B cell follicles, which may be an immune-privileged site. She presented results showing that CD8+T cells and many antiviral proteins (INF-α, α-defensins 1,2, and 3, RANTES, MIP-1α, MIP-1β, and granzyme A) are less abundant in B cell follicles compared with the extrafollicular region.41
In situ tetramer staining performed by Dr. Pamela Skinner demonstrated that HIV Gag-specific CTL fail to accumulate in large numbers in lymph node follicles of untreated, HIV- infected persons.43 In chronically infected rhesus macaques, SIV RNA+ cells are also more frequent in the follicle than in the extrafollicular space in all secondary lymphoid tissues.44 SIV RNA+ cells remain concentrated in the follicle after adjusting for frequencies of target memory cells. Dr. Skinner demonstrated that SIV-specific tetramer-stained cells are concentrated in the extrafollicular zones. In addition, the CTL to SIV RNA+ cell ratio is more than 10-fold higher in the extrafollicular space than in the follicles.44 The role of CD8+ T cells in the control of SIV replication is confirmed by CD8+ T cell depletion, when SIV RNA increases primarily in the extrafollicular zone.45 CTL likely fail to accumulate in large numbers in the follicles where virus is highly concentrated because few SIV-specific CTL exhibit a follicular homing phenotype (CXCR5+CCR7−).44 Emerging data indicate that follicles are also reservoirs in cART-treated HIV-infected individuals. Drs. Connick and Skinner are developing a strategy for redirecting CTLs to B cell follicles through CXCR5 transduction to eliminate this SIV/HIV reservoir sanctuary.
Dr. Won-Bin Young (University of Pittsburgh, Title: “Visualizing HIV mucosal transmission, viremia rebound and drug-resistance in humanized mice”) discussed the principle of noninvasive bioluminescence imaging through a replication-competent HIV reporter virus expressing firefly luciferase, whereas the anatomical locations are simultaneously confirmed by X-ray. For this purpose, he constructed several SIVmac239 and HIV reporter viruses.46 In comparative studies, Dr. Young found that luciferase bioluminescence is the most sensitive method, followed by PET imaging with SSTR2 as a reporter, then by ferritin-detecting MRI. The Young laboratory also performed dual reporter imaging using enhanced beetle luciferase (eLuc) with D-luciferin as the substrate as well as NanoLuc™ Luciferase with furimazine as the substrate. The combination made it possible to quantify infected cells in mice by in vivo imaging. Using this approach, EcoHIV (MLV-Env in an HIV-1 backbone) was visualized after intraperitoneal inoculation of NCr athymic nude mice, and HIV was visualized in bone marrow–liver–thymus (BLT) mice after intraperitoneal as well as intravaginal challenge. In addition, HIV reservoirs in BLT mice were imaged at different time points after infection as well as during cART treatment (PMPA/FTC/Raltegravir) and after cART treatment interruption. The advantages of in vivo imaging are that the technique is noninvasive for longitudinal studies, provides real-time information, allows quantification, and reveals spatial–temporal dynamics. Dual-reporter imaging with two different Luciferase reporter constructs allows studying trafficking and biodistribution of different cell populations simultaneously, for example, T cells and macrophages.
Session 3: Vaccines
Dr. Dan Barouch (Beth Israel Deaconess Medical Center, Title: “New approaches to HIV vaccines and imaging”) opened the vaccine-focused session of the meeting by highlighting the fact that so far only four HIV vaccine concepts have been tested in human efficacy trials. Currently, a follow-up efficacy trial for one of the four concepts has begun in Africa and a trial of a new concept is set to begin at the end of 2017. The second trial is advancing an adenovirus vector expressing Env, Gag, and Pol (Ad26) prime with an Ad26/Env protein boost candidate developed by Barouch et al.47 The candidate demonstrated efficacy in the macaque model with binding anti-Env antibodies (ELISA) and cytokine-secreting cells (ELISpot) as the primary correlates of protection. Additional factors that appear to contribute to protection included G2S1 glycosylation of antibodies, their complement-binding activity, and recognition of V2 peptides. In an effort to further understand the mechanisms of antibody-mediated protection, Dr. Barouch with collaborators performed detailed necropsies of macaques passively immunized by infusion of the PGT121 broadly neutralizing antibody and challenged with SHIV-SF162P3. They found that despite the apparent sterilizing protection, both viral RNA and DNA were initially found in distal sites, suggesting that the virus spread systemically, but the antibody was able to mediate viral clearance during the first 7 days after infection.48 Dr. Barouch set the stage for the following presentations by identifying key areas where imaging may help advance HIV vaccine research. These included visualizations of recruitment of immune cells to the site of vaccine administration, trafficking of vaccine components, and expression of antigens, all of which need to be better understood for a more rational vaccine design.
Dr. Constantinos Petrovas (NIAID, NIH, MD, Title: “Tissue imaging: Shedding light into immune dynamics in vaccinology and viral infections”) described novel applications of confocal tissue imaging for studying immune responses to vaccines and viral infections. Single-round multiplexed confocal imaging allows simultaneous labeling with up to seven probes. This technique may be of particular value for studying stromal cells that are difficult to isolate from tissues, such as FDCs and reticular cells from lymph nodes, or when it is important to obtain spatial or structural information. For example, by combining the RNAscope technology with other approaches, his laboratory visualized SIV-infected cells in lymph nodes of macaques and showed that their phenotype is CD4low, PD1high. They also adopted the histochemistry technique that allows identification of specific populations of cells that carry a particular set of markers. In another approach aimed to better understand the complexity of lymphoid tissues, the samples were subjected to multiple rounds of immunocytochemistry, in which samples were stained with one set of markers, then “stripped,” and restained with different markers, and the resulting images were stacked to produce a multicolor view of the sample. By applying these techniques to samples from macaques vaccinated with SIV Env proteins, Petrovas' group visualized an increase in germinal center cell proliferation (Ki67) stimulated by adjuvants, as well as dissected adjuvant-specific differences in the number, morphology, and topology of germinal centers. Looking at germinal center formation during SIV infection in macaques, they were able to show a correlation between the number of Tfh in germinal centers and the magnitude of antibody responses. One of the advantages of the multiplexing approach is the ability to obtain data from low-quantity samples. Using tonsillar samples obtained from childhood tonsillectomies, Dr. Petrovas showed mobilization of Tfh cells and downregulation of Treg cells in lymph nodes in response to seasonal influenza vaccination. Application of these new powerful techniques in combination with other approaches allows for an unprecedented level of detail in visualizing immune processes occurring during infection and vaccination.
Dr. Philip Santangelo (Georgia Tech, Title: “Immuno-PET/CT interrogations of SIV and SHIV infections”) described improvements in the immuno-PET approach which, in combination with CT, allowed them to track SIV infection in macaques in the context of detailed anatomical information, including visualization of individual lymph nodes39. Novel improvements to decrease background signals included the use of the radionucleotides 18F or 89Zr and F(ab) and dimeric F(ab')2 fragments that are smaller than full antibodies and have shorter half-lives. Dr. Santangelo also highlighted the need to test antibodies that differ in affinity and specificity of binding; empirical testing of multiple probes is often required to improve the quality of the signal. For example, when developing a probe for detecting SHIV88, PGT121 was found to be superior to five other antibodies that were tested and allowed tracking viral infection in various tissues in live animals. Improving immuno-PET probes also depended on understanding their mode of action. For the gp120-targeting antibodies, Dr. Santangelo showed that binding lead to endocytosis of the complexes and the continued appearance of newly synthesized Env on the surface, which increased the probe's sensitivity by intracellular accumulation. Similar results were obtained for CD4-binding probes. He finished by discussing the potential advantages of combining whole-body imaging with individual cell imaging and characterization, such as near-infrared imaging, microscopy, and flow cytometry. In one example, CD4+ T cells were first localized in vivo using PET/CT and then excised and stained for CD4 to identify the region of interest at single cell resolution, demonstrating the potential for future bridging of these technologies.
Dr. Roger Le Grand (CEA, France, Title: “Imaging changes at vaccine injection site”) summarized the various imaging approaches being developed to visualize tissues, cells, and viruses in nonhuman primate models at the Infectious Diseases Models for Innovative Therapies (IDMIT) research facility. He focused on the attempts to target vaccines by conjugating antigens with antibodies targeting molecules on the surface of dendritic cells. Previous work has shown that such conjugates can be used to deliver an antigen to a specific subset of dendritic cells, such as Langerhans cells.49 Near-infrared fluorescence technology showed how the conjugates traffic from the injection site to a draining lymph node within minutes after injection.
In vivo confocal laser endomicroscopy uses a fiber that can be inserted in tissues of a live animal to visualize conjugate uptake at the cellular level. As one example, Dr. Le Grand showed how this technology confirmed that Anti-Langerin Antibody fused to HIV p24 protein was specifically delivered to Langerhans cells, which subsequently became activated and migrated out of the skin to lymph nodes and boosted antibody response to p24.50,51 In another example, application of endomicroscopy to studies of a DNA vaccine provided direct evidence for enhanced antigen expression when DNA was delivered with electroporation. Imaging of biopsies also revealed local inflammation at the electroporation site and provided evidence for close interaction between antigen-expressing and antigen-presenting cells. Dr. Le Grand finished his presentation by highlighting the latest efforts at the facility to increase the arsenal of imaging technologies that can be used in macaques, such as in vivo two-photon microscopy and PET-CT imaging for pathogens classified at biosafety level 3.
Session 4: Emerging Technologies and Lessons From Non-HIV/SIV Models
Dr. Gabriel Victora (Rockefeller University, Title: “Visualizing the immune response to infection and immunization”) opened the session with a visual analysis of B cell ontogeny in the mouse lymph node using two-photon microscopy combined with “brainbow” combinatorial fluorescent protein tagging of clonal B cell populations. Two-photon microscopy has been widely utilized to observe immune cell trafficking within the tissues of living mice, informing the understanding of the cellular interactions elicited during immune responses. In this talk, Dr. Victora addressed the key question of how one moves from beautiful to meaningful data. He described his work quantifying the B cell response in germinal centers within the lymph node.52 The group first demonstrated that individual germinal centers supported the expansion of dozens or even hundreds of individual B cell clones early in the immune response, contrary to long-standing models that estimated only a handful of clones per germinal center.53 To determine how clonal B cell populations evolve over time, the group utilized a transgenic “brainbow” approach to tag B cell clones. Brainbow refers to a cre/lox recombinase system that, when induced with tamoxifen, randomly recombines four fluorescent protein genes in each cell,53 providing in this case up to ten unique color tags to identify individual B cell clones. The group showed that early in the immune response, multiple B cell clones populated each germinal center, visualized as multicolored follicles. Importantly, manual dissection and sequencing of the germinal centers demonstrated that the individual colored cells indeed represented unique B cell clones. Over time, some germinal centers contained only one or two colors (clones), whereas most of the germinal centers had multiple clones, demonstrating heterogeneity among germinal centers. Interestingly, phylogenetic sequence analysis of single-color germinal centers identified selected clonal “bursts” representing a single clone that hits a “jackpot” of high affinity and takes over an individual germinal center. The group then showed that in Friend murine leukemia virus infection, which elicits very long-lasting germinal centers in response to chronic infection, clonal selection can occur months after initial infection and at the stage when the virus is apparently controlled by the immune system. Although Dr. Victora was careful to point out that Friend murine leukemia virus is not a model for HIV infection, evolution of the B cell response in HIV/AIDS is known to occur over the course of many months and even years. In summary, multiphoton imaging can reveal cell dynamics, cell–cell interactions, signaling, anatomy, and clonal dynamics, among other things. Interpretation of complex imaging data requires creative solutions; ancillary techniques (sequencing, flow) can help interpretation of the visual data.
Dr. Weiming Yu (NIAID, NIH, Title: Histo-cytometry–highly multiplexed quantitative 2D and 3D tissue imaging”) discussed ongoing histocytometry studies being carried out in Dr. Ron Germain's laboratory. Flow cytometry is an indispensable tool for quantitative cell population analysis. However, the analysis requires cells to be extracted from the tissues and, therefore, all the spatial information of the cells of interests is lost. On the other hand, microscopy imaging methods can provide detailed cellular structural and spatial information in the tissue, and the optics of a microscope and a flow cytometer are similar. Histocytometry is a technique that combines both microscopic imaging with flow cytometric-like techniques to derive quantitative cell population analysis. This technique was initially developed for mouse tissue sections54 and has now been extended to human and nonhuman primate tissues and to 3D volume imaging using a tissue clearing technique, Ce3D, developed in the NIAID's Lymphocyte Biology Section. The team can image any number of fluorescently tagged cell markers, up to 14 colors simultaneously, and Dr. Yu presented a set of 14-color human lymph node images.
One of the basic steps in histocytometry is image segmentation to retrieve cellular objects and their spatial coordinates. A number of quantitative analyses can be performed using the spatial information of these cellular subsets. The presentation focused on the quantitative aspects in (1) cell–cell distances using the k-nearest neighbors approach and (2) cellular distributions of SIV provirus in tissues. To meet the challenge of finding and measuring integrated viral DNA, the Germain laboratory used DNAscope, which allows visualization of a single SIV provirus in situ, and combined it with a histocytometry protein staining protocol. Dr. Yu showed some preliminary image data visualizing SIV proviruses together with multiple cell markers in nonhuman primate tissues and quantifications of the number of viral DNA in cells in situ by using the combined technique. He is developing strategies for more efficient and accurate segmentation and measuring provirus-containing cells and viral particles.
Dr. Michael Gerner (University of Washington, Title: “Visualizing immune responses to vaccines”), who originally developed the histocytometry technique,54 utilized it to visualize the spatial orientation of dendritic cell subsets within lymph nodes, and the delivery of soluble and particulate antigens to these dendritic cells (Fig. 2). Not surprisingly, he found that soluble and particulate antigens were distributed within the lymph nodes quite differently, and suggested that the spatial segregation of lymph node-resident dendritic cells may impact CD4+ and CD8+ T cell responses depending on the nature of the antigens. Using multicolor histocytometry, unique dendritic cell subsets were shown to be distributed in distinct regions within lymph nodes. Furthermore, soluble antigens were observed to diffuse rapidly through the lymph node conduits into the deep T cell zone, where CD8+ T cell responses are preferentially activated by the centrally localized dendritic cells. Particulate antigens, on the other hand, tended to be captured from the peripheral lymphatic sinus space by specialized, sinus-resident dendritic cells, leading to preferential CD4+ T cell activation. The study convincingly demonstrated that tissue microanatomy dictates antigen distribution and the location of dendritic cell subsets impacted antigen sampling and induction of CD4+ and CD8+ T cell responses.55 These results help to understand the structural underpinnings of immune responses and may aid in the design of effective vaccination strategies to direct subset-specific immune responses, in particular in multiplatform antigen delivery strategies.
FIG. 2.
Unpublished image of a mouse steady-state lymph node stained with antibodies to detect various innate and adaptive immune populations, as well as stromal structural elements. Mouse steady-state lymph nodes were fixed, sectioned and stained with fluorophore-conjugated antibodies against Collagen Type-IV, B220, MHC-II, CD11c, SIRP-alpha, CD207, and CD301b. Images were acquired using a Leica SP8 laser scanning confocal microscope and visualized in Imaris. In gray, collagen Type-IV structures, encompassing the lymph node capsule, blood endothelial and lymphatic endothelial basement membranes, as well as the lymph node conduit network can be observed. In blue, B cells and B cell follicles can be seen. In magenta, MHC-II bright migratory dendritic cells can be detected. In red, CD11c on lymph node resident dendritic cells are visualized. Cyan staining represents SIRP-alpha, allowing the visualization of different innate cells, including dendritic cells and macrophages. In green and yellow, Langerhans cells and dermal dendritic cells, respectively, are detected. Collectively, by using multiplex high-resolution confocal microscopy on these tissues, various patterns of immune cell spatial organization during health and disease can be discerned with the goal to understand the underlying processes involved in inflammation and the immune response. Image credit: Gerner M.
Dr. Sigal Shachar (NCI, NIH, Title: “Using high-throughput microscopy to study cellular structure and function”) described the high-throughput imaging facility (HiTIF) at the Center for Cancer Research in the National Cancer Institute. The facility consists of three components: an automated sample preparation system, two multiplate automated imaging systems, and downstream image analysis software. It can perform 4-channel, time-resolved 3D imaging of 384-well plates, with a capacity of up to 10,000 wells per day. The image analysis suites can identify dozens of unique cellular features, including nuclear and cytoplasmic structures, cellular membranes, etc. Using machine learning algorithms, more subtle features, such as texture and morphology, can be quantified. Dr. Shachar then presented data from two screens performed in the facility. The first was an siRNA screen to identify cellular proteins involved in Hutchinson Gilford Progeria Syndrome (HGPS), a form of premature aging caused by a truncated Lamin A gene, referred to as progerin. Cells from HGPS patients exhibited “crinkled” nuclei with distinct patterns of nuclear envelope and DNA damage protein expression. A large-scale siRNA screen revealed that knockdown of Cullin-associated NEDD8-dissociated protein-1 (CAND1) suppressed the Progeria defects. CAND1 is known to mediate degradation of Nuclear factor [erythroid-derived 2]-like 2 (Nrf2), a putative regulator of oxidative stress and aging, revealing a new regulatory pathway that could lead to novel therapeutic approaches.56 The second screen utilized a high-throughput fluorescence in situ hybridization (FISH)-based assay to probe the organization of distinct genomic loci inside the nucleus. The approach, High-throughput Imaging Position Mapping (HIPMap), detects the location of any genomic segment (10KB or larger) with respect to another locus or to the nuclear periphery. The technique was used to identify individual loci that associate with the nuclear lamina and, using siRNA knockdowns, showed that the peripheral nuclear localization was dependent on multiple factors, including heterogeneous nuclear ribonucleoprotein K (HNRNPK) expression.57 This approach can be utilized to look at genomic localization with respect to other nuclear structures of interest as well as distance to unique DNA loci, for example enhancer and promoter sequences. Dr. Shachar concluded that high-throughput analysis is a flexible and versatile method to study cellular structure and function. It can be adapted to look at the relationship of protein or DNA components with each other or with cellular structures. The pipeline includes high-throughput phenotyping imaging and image and data analyses that allow screens for rare events in many biological settings, including live cell analysis. Importantly, quantification of many cells provides superior statistical power and facilitates the analysis of complex structural interactions.
Dr. Thorsten Mempel (Harvard University, Title: “Cellular and viral dynamics during HIV infection in humanized mice”) continued the 2-photon intravital imaging theme by presenting visualization of HIV infection in humanized BLT mice. Using a GFP-expressing HIV-1, the group examined T cell trafficking within lymphoid tissues early in infection. The infected cells exhibited distinctly altered mobility, moving more slowly than uninfected T cells and often trailing long cellular extensions. These extensions were shown to result in part from HIV envelope glycoprotein-bound to uninfected CD4-expressing cells. Surprisingly, a significant number of the infected T cells contained two or more nuclei, suggesting that syncytia may be a common feature of HIV infection in vivo. The group went on to examine the molecular basis of slowed migration of infected cells. Specifically, they examined the roles of the HIV proteins Nef and Vpu as well as the respective contributions of altered chemokine receptor expression and cytoskeletal disruption in this phenotype. Since the observed slowed migration of infected cells seems to be counterproductive to cell-mediated viral dissemination, Dr. Mempel hypothesized that altered mobility may favor cell contact-dependent transmission through virological synapses, a presumed pathway of HIV spread within tissues.58,59 He then presented some preliminary data imaging the capture of virions by myeloid cells and subsequent transport across endothelial and epithelial barriers and delivery of infectious particles to target cells within the tissues. Previously, his group had described the role of CD169 in delivering retroviruses to target cells in trans.58,59 In this presentation, he demonstrated that lymph node myeloid cells present at the lymphatic endothelial barrier could be seen to carry virions from the subcapsular sinus lumen into the lymph node cortex, visually demonstrating the importance of dendritic cell localization described earlier in the day by Dr. Gerner. Dr. Mempel concluded that this direct visualization of infection revealed a number of key events in HIV infection: (1) cell-mediated HIV spread is important for viral dissemination within lymphoid tissues; (2) systemically, HIV actively modulates cell motility, which might induce increased virological synapse formation and cell–cell spread, and (3) myeloid cells can shuttle virus across tissue barriers and subsequently transfer infection to target cells.
Dr. Michael Angelo (Stanford University, Title: “High dimensional, subcellular imaging of clinical formalin-fixed paraffin-embedded biopsies using MIBI”) presented a technique named MIBI: Multiplexed ion beam imaging, a platform for imaging dozens of antigens at a time in cells and tissues. Similar to Cytof mass cytometry, MIBI utilizes antibodies labeled with elemental metal isotopes instead of fluorescent markers. In this system, samples fixed to a slide are probed with isotope-tagged antibodies with each antibody having a different mass label. The samples are imaged using a primary ion gun that rasters across the slide much like a confocal beam scan across a fluorescently labeled sample. Instead of reading fluorescence emission, MIBI reads the ion liberated spots in a time-of-flight mass spectrometer (TOF-MS) to read out a pixel-by-pixel mass spectrum. These data are then mapped into a corresponding image to produce an immunofluorescence-like pseudocolored image displaying the signal intensity from the individual antibodies. The image can be further mapped onto as Hematoxylin and Eosin stain, which is read at the same time due to the aluminum in the stain. The system has an impressive sensitivity of one to five antibodies per pixel, has no background signal, which is a common confounder of tissue imaging, and a practical resolution of 0.5–1 μm, although it can be pushed to 0.2 μm or less. The dynamic range is reportedly 100,000, at least an order of magnitude larger than typical optical microscopes, and sample intensity is not attenuated by repeated scanning. The system can also capture the secondary ion channel for electron microscopy imaging, which allows the user to scan the sample in real-time to quickly locate regions of interest. Dr. Angelo showed an example of tissue biopsies from a PD-L1 clinical trial that demonstrated MIBI's utility delineating immune cell subsets and tumor cells using 14 unique markers. He visually demonstrated that multiplexed imaging, while extremely information rich, is also extremely complex, and requires sophisticated analysis to quantify and characterize individual cell populations. The technique is being applied in a variety of experimental settings, including risk stratification of breast cancer progression, analysis of Mycobacterium tuberculosis granulomas, anti-PD1 therapies, and immune toleration of food allergies. The technology is continuing to evolve, with improved reagents, new detection capacity, increased throughput, and improved user interface.
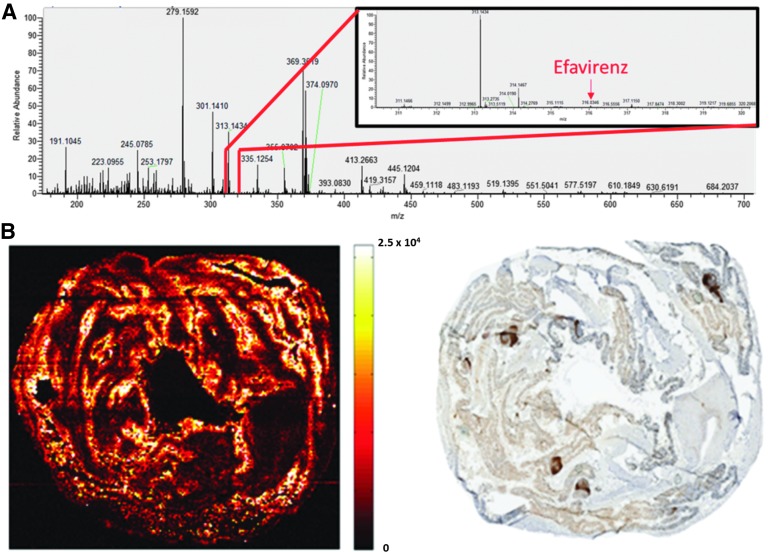
Dr. Angela Kashuba (University of North Carolina, Title: “Using mass spectrometry imaging to visualize drug distribution in putative viral reservoirs”) topped off the day with another mass spectrometry imaging (MSI) approach applied to drug distribution in biological samples. Her research goal is to understand the relationship of pharmacokinetics and pharmacodynamics (PK/PD) in relevant tissues for the purpose of designing rational drug dosage, frequency, and duration60 (Fig. 3). She noted that while plasma may be a surrogate for averaged tissue drug exposure, the heterogeneity of inter- and intratissue distribution (e.g., significant differences in gastrointestinal tract tissues versus lymphoid tissues) makes this measure less informative for understanding true dose–effect relationships. Analysis of homogenized tissue samples provides a direct measure of the total amount of drug. However, this represents an averaged tissue concentration without any finer distribution information. MSI, on the other hand, provides the opportunity to characterize the spatial distribution of compounds and can provide additional information about endogenous compounds and metabolites. Dr. Kashuba's laboratory utilizes Infrared (IR) matrix-assisted laser desorption electrospray ionization (MALDESI), in which an infrared laser ablates discrete regions of a tissue sample as it is rastered under the laser spot, and an electrospray ionizes the material for chemical analysis by mass spectrometry. This method has the benefit of measuring all drugs and metabolites simultaneously. Importantly, MALDESI is performed on frozen tissues under ambient conditions, reducing sample preparation artifacts. LC-MS data have been used to validate the ability of this technique for quantification. Additionally, imaging data from serial sections (e.g., immunohistochemistry and/or in situ hybridization) can be overlaid on the MSI data to determine PK/PD information within a few tissue slices. Dr. Kashuba presented data from several studies, and while the imaging analysis is still in development, it was clear that the approach holds promise to resolve PK/PD information within the lymphoid tissue architecture and even at sites of active HIV replication, identified by Jake Estes' RNAscope technique described above.61,62 Another exciting application of the technique is the measurement of drug distribution in hair samples, which can be used to track adherence to drug regimens over time.
FIG. 3.
Efavirenz distribution in the macaque colon. Frozen cross-sections of colon were thaw mounted and analyzed by MSI according to Thompson et al..61 (A) Using high-resolving power mass spectrometry, on-tissue response to efavirenz (inset) can be sensitively discriminated from the complex endogenous response in each measured mass spectrum. (B) The resulting MSI of efavirenz in a cross-section is shown on the left, with adjacent CD3+ T cell staining of a serial tissue slice on the right. MSI signal intensity is shown next to the image on a concentration-dependent scale. The bottom of the scale (0) represents no efavirenz present, whereas the top of the scale is set to the highest per-voxel efavirenz signal observed. Brighter colors represent higher efavirenz concentrations. Efavirenz is found at higher concentrations in areas of the lamina associated with CD3+ T cell staining. Image credit: Rosen E and Kashuba A. MSI, mass spectrometry imaging.
Discussion
The major focus of discussion following session one was how imaging applications in studies of earliest events after viral transmission are advancing our understanding of HIV disease. The cascade of early events depends on which cells get engaged first. HIV/SIV infection can be detected in the draining lymph nodes very rapidly; however, Tfh cells become infected at a later stage once HIV/SIV is already well distributed throughout the lymphoid tissues. A question was raised how deep labeled antibodies penetrate into tissues, especially into the gut. It was noted that the antibody probes do not only bind to the receptor, but they can also be endocytosed, which can enhance the signal over time. Additional discussion followed on whether there is less immune cell reconstitution in the axillar and inguinal lymph nodes compared with the submandibular nodes following cART treatment, and whether some lymph nodes are not sufficiently penetrated by cART. The exact pathogenesis of the destruction of the FDC network and the reprogramming of the stromal cell populations is also unclear.
For session two, the point was made that it is not possible to distinguish between integrated and unintegrated DNA using in situ hybridization, but it can be determined whether the in situ signal is localized over the nucleus. In acute SIV infection, multiple copies of DNA per cell can be measured, however, in chronic infection or under cART only one DNA copy per cell is generally detected. The question whether infected macrophages constitute a long-lived HIV reservoir is still unresolved. By in situ hybridization, rare RNA-positive macrophages and no DNA-positive macrophages are visualized, indicating that infected T cells are engulfed into macrophages. Another reservoir is the FDC network, which can harbor infectious virus without itself becoming infected. The discussion continued how imaging can be applied to distinguish between latent and productive infection and how to resolve the mechanisms behind clonal proliferation of HIV-infected cells. For HIV reporter viruses, it was stated that the reporter gene is expunged from the HIV construct after several rounds of replication and no longer detectable making it appear that the viral load is decreasing, when in reality the reporter-deleted virus simply cannot be detected.
In session three, it was stated that an accumulation of Tfh cells was only seen in draining, but not in distal lymph nodes. The early events occur in the draining lymph nodes on the ipsilateral vaccination/infection site. Tracking B and T cell responses in vivo after vaccination and also tracking trafficking of cells to mucosal sites and draining lymph nodes after a booster immunization was seen as a gap area. Good imaging resolution is important to study the microanatomy.
The questions that came up in the heterogenous session four were about B cell clonotyping and the early events after vaccination and SIV/HIV infection. After vaccination, there is most likely a massive involvement of lymphatic fluid movement and a re-arrangement of innate and antigen presenting cells right around the follicular region. It was asked how cell trafficking in BLT mice differs from humans. BLT mice have lymph nodes, but no B cell follicles. Human and mouse cells migrate at the same rate. There were also technical questions about the comparison of 2D and 3D analyses for distance measurements, interference issues with antibody multiplexing, and whether tissue preparation artifacts change the distribution of drugs within the tissues. Overall, it was concluded that novel imaging techniques can reveal additional information about HIV pathogenesis, vaccine, and cure research and that another imaging workshop should be held in the near future to discuss advancements in the imaging field.
Acknowledgments
The authors would like to thank the speakers for their excellent presentations, and Jim Bradac, Sandra Bridges, Karl Salzwedel, Diana Finzi, and Cynthia Soriano for their support of the workshop and for helpful discussions.
In addition, the authors would like to thank the Global HIV Vaccine Enterprise and EDJ Associates, Inc., for organizing the workshop and NIAID Meet, in particular, Aaron Robinson and Marilyn Daly, for help with execution of the workshop. The workshop was funded by the Global HIV Vaccine Enterprise and with federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201100001G.
Author Disclosure Statement
The authors have no conflict of interests to declare.
References
- 1.Haase AT: Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464:217–223 [DOI] [PubMed] [Google Scholar]
- 2.Shang L, Duan L, Perkey KE, et al. : Epithelium-innate immune cell axis in mucosal responses to SIV. Mucosal Immunol 2017;10:508–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Zeng M, Duan L, et al. : Live simian immunodeficiency virus vaccine correlate of protection: Local antibody production and concentration on the path of virus entry. J Immunol 2014;193:3113–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng M, Smith AJ, Wietgrefe SW, et al. : Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 2011;121:998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald D, Vodicka MA, Lucero G, et al. : Visualization of the intracellular behavior of HIV in living cells. J Cell Biol 2002;159:441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carias AM, McCoombe S, McRaven M, et al. : Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol 2013;87:11388–11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carias AM, Allen SA, Fought AJ, et al. : Increases in endogenous or exogenous progestins promote virus-target cell interactions within the non-human primate female reproductive tract. PLoS Pathog 2016;12:e1005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stieh DJ, Maric D, Kelley ZL, et al. : Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog 2014;10:e1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieh DJ, Matias E, Xu H, et al. : Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 2016;19:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smedley J, Turkbey B, Bernardo ML, et al. : Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One 2014;9:e92830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Prete GQ, Park H, Fennessey CM, et al. : Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol 2014;88:8077–8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng M, Southern PJ, Reilly CS, et al. : Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog 2012;8:e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estes JD, Li Q, Reynolds MR, et al. : Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis 2006;193:703–712 [DOI] [PubMed] [Google Scholar]
- 14.Cremasco V, Woodruff MC, Onder L, et al. : B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 2014;15:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes JD, Reilly C, Trubey CM, et al. : Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis 2015;211:744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Mascio M, Paik CH, Carrasquillo JA, et al. : Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood 2009;114:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue RE, Srinivasula S, Uchida N, et al. : Discordance in lymphoid tissue recovery following stem cell transplantation in rhesus macaques: An in vivo imaging study. Blood 2015;126:2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Mascio M: Whole-body SPECT in Vivo Imaging reveals delayed reconstitution of lymph-nodes, but not spleen, CD4 pools in long-term cART treated animals (P16). Towards an HIV Cure Symposium Durban, South Africa, 2016 [Google Scholar]
- 19.Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF: Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A 2014;111:13475–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkevych M, Kent SJ, Tolstrup M, et al. : Modeling of experimental data supports HIV reactivation from latency after treatment interruption on average once every 5–8 days. PLoS Pathog 2016;12:e1005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fennessey CM, Pinkevych M, Immonen TT, et al. : Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog 2017;13:e1006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun TW, Carruth L, Finzi D, et al. : Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997;387:183–188 [DOI] [PubMed] [Google Scholar]
- 23.Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 24.Hermankova M, Ray SC, Ruff C, et al. : HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA 2001;286:196–207 [DOI] [PubMed] [Google Scholar]
- 25.Persaud D, Zhou Y, Siliciano JM, Siliciano RF: Latency in human immunodeficiency virus type 1 infection: No easy answers. J Virol 2003;77:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieffer TL, Finucane MM, Nettles RE, et al. : Genotypic analysis of HIV-1 drug resistance at the limit of detection: Virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis 2004;189:1452–1465 [DOI] [PubMed] [Google Scholar]
- 27.Nettles RE, Kieffer TL, Kwon P, et al. : Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA 2005;293:817–829 [DOI] [PubMed] [Google Scholar]
- 28.Bailey JR, Sedaghat AR, Kieffer T, et al. : Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 2006;80:6441–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan TP, Woods JO, Sedaghat AR, Siliciano JD, Siliciano RF, Wilke CO: Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol 2009;83:8470–8481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruner KM, Murray AJ, Pollack RA, et al. : Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016;22:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho YC, Shan L, Hosmane NN, et al. : Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosmane NN, Kwon KJ, Bruner KM, et al. : Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med 2017;214:959–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldarelli F, Wu X, Su L, et al. : HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014;345:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner TA, McLaughlin S, Garg K, et al. : HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014;345:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finzi D, Blankson J, Siliciano JD, et al. : Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999;5:512–517 [DOI] [PubMed] [Google Scholar]
- 36.Siliciano JD, Kajdas J, Finzi D, et al. : Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9:727–728 [DOI] [PubMed] [Google Scholar]
- 37.Deleage C, Wietgrefe SW, Del Prete G, et al. : Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun Spring 2016;1:68–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura Y, Sadjadpour R, Mattapallil JJ, et al. : High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc Natl Acad Sci U S A 2009;106:8015–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santangelo PJ, Rogers KA, Zurla C, et al. : Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods 2015;12:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrareddy SN, Arthos J, Cicala C, et al. : Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science 2016;354:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkvord JM, Armon C, Connick E: Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses 2005;21:363–370 [DOI] [PubMed] [Google Scholar]
- 42.Kohler SL, Pham MN, Folkvord JM, et al. : Germinal center T follicular helper cells are highly permissive to HIV-1 and alter their phenotype during virus replication. J Immunol 2016;196:2711–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connick E, Mattila T, Folkvord JM, et al. : CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 2007;178:6975–6983 [DOI] [PubMed] [Google Scholar]
- 44.Connick E, Folkvord JM, Lind KT, et al. : Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 2014;193:5613–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Folkvord JM, Rakasz EG, et al. : Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol 2016;90:11168–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song J, Cai Z, White AG, et al. : Visualization and quantification of simian immunodeficiency virus-infected cells using non-invasive molecular imaging. J Gen Virol 2015;96:3131–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barouch DH, Alter G, Broge T, et al. : Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015;349:320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Ghneim K, Sok D, et al. : Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 2016;353:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flamar AL, Zurawski S, Scholz F, et al. : Noncovalent assembly of anti-dendritic cell antibodies and antigens for evoking immune responses in vitro and in vivo. J Immunol 2012;189:2645–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salabert N, Todorova B, Martinon F, et al. : Intradermal injection of an anti-Langerin-HIVGag fusion vaccine targets epidermal Langerhans cells in nonhuman primates and can be tracked in vivo. Eur J Immunol 2016;46:689–700 [DOI] [PubMed] [Google Scholar]
- 51.Flamar AL, Contreras V, Zurawski S, et al. : Delivering HIV Gag p24 to DCIR induces strong antibody responses in vivo. PLoS One 2015;10:e0135513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tas JM, Mesin L, Pasqual G, et al. : Visualizing antibody affinity maturation in germinal centers. Science 2016;351:1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Victora GD, Wilson PC. Germinal center selection and the antibody response to influenza. Cell 2015;163:545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN: Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 2012;37:364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 2015;42:172–185 [DOI] [PubMed] [Google Scholar]
- 56.Kubben N, Zhang W, Wang L, et al. : Repression of the antioxidant NRF2 pathway in premature aging. Cell 2016;165:1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shachar S, Voss TC, Pegoraro G, Sciascia N, Misteli T: Identification of gene positioning factors using high-throughput imaging mapping. Cell 2015;162:911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murooka TT, Deruaz M, Marangoni F, et al. : HIV-infected T cells are migratory vehicles for viral dissemination. Nature 2012;490:283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sewald X, Ladinsky MS, Uchil PD, et al. : Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 2015;350:563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bokhart MT, Rosen E, Thompson C, Sykes C, Kashuba AD, Muddiman DC: Quantitative mass spectrometry imaging of emtricitabine in cervical tissue model using infrared matrix-assisted laser desorption electrospray ionization. Anal Bioanal Chem 2015;407:2073–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson CG, Bokhart MT, Sykes C, et al. : Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob Agents Chemother 2015;59:2944–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosen EP, Thompson CG, Bokhart MT, et al. : Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal Chem 2016;88:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, Schuler T, Zupancic M, et al. : Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 1999;286:1353–1357 [DOI] [PubMed] [Google Scholar]
- 64.Li Q, Duan L, Estes JD, et al. : Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005;434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 65.Reilly C, Wietgrefe S, Sedgewick G, Haase A: Determination of simian immunodeficiency virus production by infected activated and resting cells. Aids 2007;21:163–168 [DOI] [PubMed] [Google Scholar]
- 66.Zhang ZQ, Wietgrefe SW, Li Q, et al. : Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A 2004;101:5640–5645 [DOI] [PMC free article] [PubMed] [Google Scholar]