Abstract
A subset of patients experience persistent symptoms after pediatric concussion, and magnetic resonance imaging (MRI) is commonly used to evaluate for pathology. The utility of this practice is unclear. We conducted a retrospective cohort study to describe the MRI findings in children with concussion. A registry of all patients seen at our institution from January 2010 through March 2016 with pediatric sports-related concussion was cross-referenced with a database of radiographical studies. Radiology reports were reviewed for abnormal findings. Patients with abnormal computed tomographies or MRI scans ordered for reasons other than concussion were excluded. Among 3338 children identified with concussion, 427 underwent MRI. Only 2 (0.5%) had findings compatible with traumatic injury, consisting in both of microhemorrhage. Sixty-one patients (14.3%) had abnormal findings unrelated to trauma, including 24 nonspecific T2 changes, 15 pineal cysts, eight Chiari I malformations, and five arachnoid cysts. One child underwent craniotomy for a cerebellar hemangioblastoma after presenting with ataxia; another had cortical dysplasia resected after seizure. The 2 patients with microhemorrhage each had three previous concussions, significantly more than patients whose scans were normal (median, 1) or abnormal without injury (median, 1.5; p = 0.048). MRI rarely revealed intracranial injuries in children post-concussion, and the clinical relevance of these uncommon findings remains unclear. Abnormalities unrelated to trauma are usually benign. However, MRI should be thoughtfully considered in children who present with concerning or atypical symptoms.
Keywords: : brain concussion, magnetic resonance imaging, post-concussion syndrome
Introduction
With an incidence as high as 1.9 million per year in the United States, sports concussion is the most common form of traumatic brain injury (TBI) among children.1 Unlike more-severe forms of TBI, it is characterized by functional disturbance rather than overt structural injury, and symptoms rapidly resolve in most cases.2 Whereas the vast majority of adults are symptomatic for less than 1 week, recovery can be more protracted in children and adolescents. Roughly 25–30% of children with concussion continue to suffer from headaches, irritability, cognitive difficulty, and other symptoms beyond 1 month post-injury.3–5 These persistent post-concussive symptoms (PCS) can be debilitating and are associated with neurocognitive deficits, decreased quality of life, and greater need for educational interventions at school.6,7
The role for neuroimaging in the acute setting post-TBI is well established; the small minority of children with concussion whose injury characteristics or acute clinical presentation are concerning should undergo either computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate for intracranial hemorrhage.2,8–10 However, the utility of these studies in the post-acute phase of injury is less clear. Some researchers recommend neuroimaging to rule out alternative structural etiologies for persistent symptoms, but there is little evidence to guide this practice.11 Two recent studies examined MRI findings in children with PCS and found that between 5 and 13% of scans revealed new trauma-related diagnoses, although the rate of findings not related to trauma was substantially greater.12,13 Although these publications add substantively to our knowledge of conventional imaging in PCS, they suffer from small sample sizes and are subject to referral bias, which may inflate the rate of positive findings.
The aim of this study was to characterize the use and utility of structural brain imaging with MRI in children with persistent symptoms post-concussion. Specifically, we aimed to identify which patients with concussion were selected for MRI and to describe the pathologies identified on these scans. Because comprehensive brain MRI scans are costly and time-intensive, we also sought to test the hypothesis that a limited brain MRI consisting of T2 fluid-attenuated inverse recovery (FLAIR) and susceptibility-weighted imaging (SWI) would achieve a yield for abnormal results similar to that of a full scan.
Methods
This single-center, retrospective cohort study was approved by the Seattle Children's Hospital Institutional Review Board. Patients were identified using a registry of children seen at our institution between January 2010 and March 2016 with health care provider-diagnosed concussion sustained during recreation or sport. Concussions sustained in both high- and low-energy activities, such as physical education class, field sports, and motocross, were included. Because of the retrospective nature of this study and the large number of patients identified, we were not able to confirm the clinical criteria used to diagnose concussion. However, providers at our institution follow the recommendations of the International Conference on Concussion in Sport when diagnosing concussion.2
Our concussion database includes patients seen in all settings, including the emergency department (ED) and outpatient clinics. The following data were obtained from this database: patient demographics; site of first visit for concussion (e.g., ED or clinic); the activity in which the injury was suffered; the mechanism of the injury; history of previous TBI; and the date of the concussion. If the exact date of injury was not known, the first of the month of injury was used as an approximation.
The resulting list of patients was cross-referenced with a separately maintained database of radiology records, from which all head CT and brain MRI reports were extracted. These reports were than manually reviewed to identify the following: the indication for the study; the pertinent findings; and the sequences utilized in all MRI scans. We relied solely on radiology reports generated by board-certified pediatric radiologists and did not manually review the images. MRI scans obtained outside of our hospital system were not included because the reports for these studies were not routinely available. MRI scans were ordered by providers in the subspecialty clinics evaluating these patients. Individuals with any one of the following were excluded: an abnormal CT preceding the MRI; an MRI ordered for reasons other than concussion, such as for tumor surveillance; or an MRI ordered as part of a research study. Children with abnormal CT scans were removed because we aimed to study the yield of MRI in concussion, which consensus statements have defined as a functional disturbance of brain function without imaging evidence of structural pathology.2 Scans that identified normal variants, such as a cavum septum pellucidum, were classified as normal. Abnormal MRI scans were then reviewed by a pediatric neuroradiologist (F.A.P.) blinded to reports from the complete scan to determine whether or not the findings could be identified on FLAIR or SWI without additional sequences.
Statistical analysis
Continuous variables were described with median and interquartile range (IQR) and analyzed using the Wilcoxon rank-sum test or the Kruskal-Wallis test, as appropriate. Categorical variables were summarized with frequencies and proportions and analyzed using Pearson's chi-square test. The threshold for statistical significance was set to α = 0.05. Statistical analysis was performed using Stata/IC software (version 14.1 for Macintosh; StataCorp LP, College Station, TX).
Results
Between January 2010 and March 2016, 3360 patients with pediatric sports-related concussion were identified. CT scans obtained at the time of injury revealed trauma-related findings in 12 patients, including skull fractures in 11, pneumocephalus in 2, and a traumatic subarachnoid hemorrhage in 1. An additional 10 patients had CT scans that revealed five arachnoid cysts, two Chiari I malformations, and a single vascular malformation, as well as other incidental abnormalities. These 22 patients with abnormal CT scans were excluded, leaving a final cohort of 3338 patients. Of these, 427 underwent imaging with brain MRI because of concussive symptoms a median of 32 days post-injury (IQR, 21–45); 90.2% (n = 385) were scanned 14 or more days post-injury. Clinical and demographic characteristics of children who did and did not receive an MRI scan are shown in Table 1. Children receiving MRI scans were older and more likely to be female. They were also more likely to have been first evaluated in the concussion subspecialty clinic rather than urgent care or the ED. All of the patients who were first seen in the ED and who underwent an MRI scan had their MRI obtained subsequent to their ED visit. Twice as many children who had an MRI reported a history of another TBI in the preceding 3 months, and the median number of total previous concussions among the scanned group was slightly greater. There were no differences with regard to insurance status or mechanism of injury.
Table 1.
Demographic and Clinical Characteristics of Children Who Had MRI Compared to Those Who Did Not
| Characteristics | No brain MRI N = 2911 | Brain MRI N = 427 | p value |
|---|---|---|---|
| Male sex (%) | 1726 (59.3) | 222 (52.0) | <0.01 |
| Age, median (IQR) | 14 (11, 15) | 15 (13, 16) | <0.001 |
| Race (%) | 0.071 | ||
| Caucasian | 2017 (69.3) | 314 (73.5) | |
| African American | 144 (4.9) | 15 (3.5) | |
| Asian American | 136 (4.7) | 15 (3.5) | |
| Native American | 51 (1.8) | 13 (3.0) | |
| Other/unknown | 563 (19.3) | 70 (16.4) | |
| Payer (%) | 0.097 | ||
| Private insurance | 2255 (77.5) | 346 (81.0) | |
| Public or no insurance | 656 (22.5) | 81 (19.0) | |
| Setting of first visita (%) | <0.001 | ||
| Concussion clinic | 1248 (42.9) | 333 (78.0) | |
| Rehab medicine clinic | 213 (7.3) | 17 (4.0) | |
| Neurology clinic | 95 (3.3) | 5 (1.2) | |
| Emergency department | 814 (28.0) | 46 (10.8) | |
| Urgent care | 491 (16.9) | 24 (5.6) | |
| Other | 50 (1.7) | 2 (0.5) | |
| Sport (%) | <0.001 | ||
| Baseball/softball | 94 (3.2) | 12 (2.8) | |
| Basketball | 261 (9.0) | 51 (11.9) | |
| Football | 543 (18.7) | 85 (19.9) | |
| Hockey | 46 (1.6) | 8 (1.9) | |
| Soccer | 571 (19.6) | 103 (24.1) | |
| Snowsports | 115 (4.0) | 16 (3.7) | |
| Running/track | 117 (4.0) | 11 (2.6) | |
| PE/recess | 281 (9.7) | 16 (3.7) | |
| Bicycling | 77 (2.6) | 5 (1.2) | |
| Other/unknown | 806 (27.7) | 120 (28.1) | |
| Mechanism (%) | 0.26 | ||
| Collision with individual | 926 (31.8) | 130 (30.4) | |
| Collision with ground/wall | 1075 (36.9) | 149 (34.9) | |
| Collision with object | 638 (21.9) | 112 (26.2) | |
| Other/Unknown | 272 (9.3) | 36 (8.4) | |
| TBI within past 3 months (%) | 284 (9.8) | 78 (18.3) | <0.001 |
| Previous concussions, median (IQR) | 0 (0, 1) | 0 (0, 1) | <0.001 |
The setting of the first visit corresponds to the setting where the patient was first seen in our system for concussion; it may not correspond to the location where the MRI was ordered. Categorical proportions compared using Pearson's χ2 test.
Distributions of continuous variables compared using the Wilcoxon's rank-sum test. MRI, magnetic resonance imaging; IQR, interquartile range; PE, physical education.
Among the 427 children in whom imaging with MRI was performed, 15.7% (n = 63) had abnormal scans (Table 2). Only two of these scans revealed findings plausibly related to trauma; in both cases, the MRI demonstrated evidence of petechial microhemorrhage. These 2 patients had each experienced three previous concussions, significantly more than patients whose scans did not demonstrate intracranial injury (H(2) = 9.95; p < 0.01). They constituted 4.1% of the 49 patients with a history of three or more concussions who underwent MRI, but only 0.5% of all children with MRIs.
Table 2.
Demographic and Clinical Characteristics of Children with Normal and Abnormal MRI Scans
| Characteristics | Normal MRI N = 364 | Nontrauma findings N = 61 | Trauma findings N = 2 |
|---|---|---|---|
| Male sex (%) | 188 (51.6) | 32 (52.5) | 2 (100.0) |
| Age, median (IQR) | 14 (13, 16) | 15 (13, 16) | 16 (15, 17) |
| Race (%) | |||
| Caucasian | 267 (73.4) | 46 (75.4) | 1 (50.0) |
| African American | 14 (3.8) | 1 (1.6) | 0 (0.0) |
| Asian American | 13 (3.6) | 2 (3.3) | 0 (0.0) |
| Native American | 12 (3.3) | 1 (1.6) | 0 (0.0) |
| Other/unknown | 58 (15.9) | 11 (18.0) | 1 (50.0) |
| Payer (%) | |||
| Private insurance | 295 (81.0) | 49 (80.3) | 2 (100.0) |
| Public or no insurance | 69 (19.0) | 12 (19.7) | 0 (0.0) |
| Setting of first visita (%) | |||
| Concussion clinic | 286 (78.6) | 45 (73.8) | 2 (100.0) |
| Rehab medicine clinic | 16 (4.4) | 1 (1.6) | 0 (0.0) |
| Neurology clinic | 4 (1.1) | 1 (1.6) | 0 (0.0) |
| Emergency department | 38 (10.4) | 8 (13.1) | 0 (0.0) |
| Urgent care | 18 (4.9) | 6 (9.8) | 0 (0.0) |
| Other | 2 (0.5) | 0 (0.0) | 0 (0.0) |
| Sport (%) | |||
| Baseball/softball | 10 (2.7) | 2 (3.3) | 0 (0.0) |
| Basketball | 47 (12.9) | 4 (6.6) | 0 (0.0) |
| Football | 65 (17.9) | 19 (31.1) | 1 (50.0) |
| Hockey | 8 (2.2) | 0 (0.0) | 0 (0.0) |
| Soccer | 89 (24.5) | 13 (21.3) | 1 (50.0) |
| Snowsports | 13 (3.6) | 3 (4.9) | 0 (0.0) |
| Running/track | 9 (2.5) | 2 (3.3) | 0 (0.0) |
| PE/recess | 16 (4.4) | 0 (0.0) | 0 (0.0) |
| Bicycling | 5 (1.4) | 0 (0.0) | 0 (0.0) |
| Other/unknown | 102 (28.0) | 18 (29.5) | 0 (0.0) |
| Mechanism (%) | |||
| Collision with individual | 104 (28.6) | 24 (39.3) | 2 (100.0) |
| Collision with ground/wall | 132 (36.3) | 17 (27.9) | 0 (0.0) |
| Collision with object | 98 (26.9) | 14 (23.0) | 0 (0.0) |
| Other/unknown | 30 (8.2) | 6 (9.8) | 0 (0.0) |
| Head injury within past 3 months | 67 (18.4) | 11 (18.0) | 0 (0.0) |
| Previous concussions, median (IQR) | 0 (0, 1) | 1 (0, 1) | 3 (3, 3) |
The setting of the first visit corresponds to the setting where the patient was first seen in our system for concussion; it may not correspond to the location where the MRI was ordered.
MRI, magnetic resonance imaging; IQR, interquartile range; PE, physical education.
MRI findings did not vary with scanner field strength (Table 3). Routine sequences such as T1, T2, FLAIR, and diffusion-weighted imaging (DWI), were utilized in almost all patients. The proportions of scans including either gradient recalled echo (GRE) or SWI were similar across groups, and SWI was not used more commonly in children with abnormal findings. However, contrast-enhanced MRI and constructive interference in steady state (CISS) sequences were more commonly utilized in children whose scans revealed abnormalities not related to trauma.
Table 3.
MRI Field Strength and Imaging Sequences Used
| Normal MRI N = 364 | Nontrauma findings N = 61 | Trauma findings N = 2 | p value | |
|---|---|---|---|---|
| Field strength (%) | 0.21 | |||
| 1.5T | 282 (77.7) | 43 (70.5) | 1 (50.0) | |
| 3T | 81 (22.3) | 18 (29.5) | 1 (50.0) | |
| Any T1 (%) | 364 (100.0) | 61 (100.0) | 2 (100.0) | N/A |
| T1 MPRAGE | 147 (40.4) | 25 (41.0) | 0 (0.0) | 0.71 |
| T2 (%) | 363 (99.7) | 61 (100.0) | 2 (100.0) | 1.00 |
| T2-FLAIR (%) | 360 (98.9) | 61 (100.0) | 2 (100.0) | 1.00 |
| GRE or SWI (%) | 332 (91.2) | 54 (88.5) | 2 (100.0) | 0.57 |
| SWI | 156 (42.9) | 27 (44.3) | 1 (50.0) | 0.89 |
| DWI (%) | 358 (98.4) | 60 (98.4) | 2 (100.0) | 1.00 |
| DTI (%) | 63 (17.3) | 12 (19.7) | 1 (50.0) | 0.34 |
| CISS (%) | 18 (4.9) | 9 (14.8) | 0 (0.0) | <0.05 |
| MRA (%) | 5 (1.4) | 1 (1.6) | 0 (0.0) | 1.00 |
| Contrast (%) | 4 (1.1) | 13 (21.7) | 0 (0.0) | <0.001 |
Proportions compared using Fisher's exact test.
MRI, magnetic resonance imaging; T, Tesla; MPRAGE, magnetization prepared rapid gradient echo imaging; FLAIR, fluid attenuated inversion recovery; SWI, susceptibility weighted imaging; DWI, diffusion weighted imaging; DTI, diffusion tensor imaging; CISS, constructive interference in steady state; MRA, magnetic resonance angiography; N/A, not applicable.
Among children with an abnormal MRI, repeat scans were obtained in 24.6%. Overall, 49.2% of these children were referred to neurosurgery clinic for evaluation, and 18.0% had more than one clinic visit. Only 2 patients required surgery, and only 1 was directly admitted as a result of his scan. This child presented with new-onset ataxia weeks after his concussion and was found to have a cerebellar hemangioblastoma that was resected. The other patient who required surgery experienced a questionable seizure and was found to have a T2 hyperintense mass consistent with either cortical dysplasia or a low-grade glioma; this lesion was resected and pathological examination of the tissue confirmed the diagnosis of cortical dysplasia.
The specific abnormalities identified are listed in Table 4. Of the 64 abnormal findings observed in 63 patients, all could be observed on FLAIR or SWI sequences upon manual review of these scans (Table 4).
Table 4.
MRI Findings and the Sequences on Which They Were Detected Using a Proposed MRI Protocol Optimized for Efficiency
| Finding | n | % | FLAIR | SWI/GRE |
|---|---|---|---|---|
| Microhemorrhage | 2 | 0.5 | ||
| Nontrauma findings | ||||
| T2 WM hyperintensity | 23 | 5.4 | ||
| Hemangioblastoma | 1 | 0.2 | ||
| Cortical dysplasia | 1 | 0.2 | ||
| Cavernous malformation | 1 | 0.2 | ||
| Arachnoid cyst | 4 | 0.9 | ||
| Chiari I malformation | 8 | 1.9 | ||
| Pineal cyst | 15 | 3.5 | ||
| Cholesterol granuloma | 2 | 0.5 | ||
| DVA | 1 | 0.2 | ||
| Heterotopia | 2 | 0.5 | ||
| Nonspecific calcification | 1 | 0.2 | ||
| Pituitary cyst | 2 | 0.4 | ||
| Pituitary microadenoma | 1 | 0.2 | ||
| Total Patients | 427 | 100.0 | ||
Because some patients had more than one abnormality, the total number of findings is greater than the number of abnormal scans. Black, detected reliably in all cases; gray, detected in some but not all cases; white, not detected in any cases.
MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; SWI, susceptibility-weighted imaging; GRE, gradient echo; WM, white matter; DVA, developmental venous anomaly.
Two patients with microhemorrhage
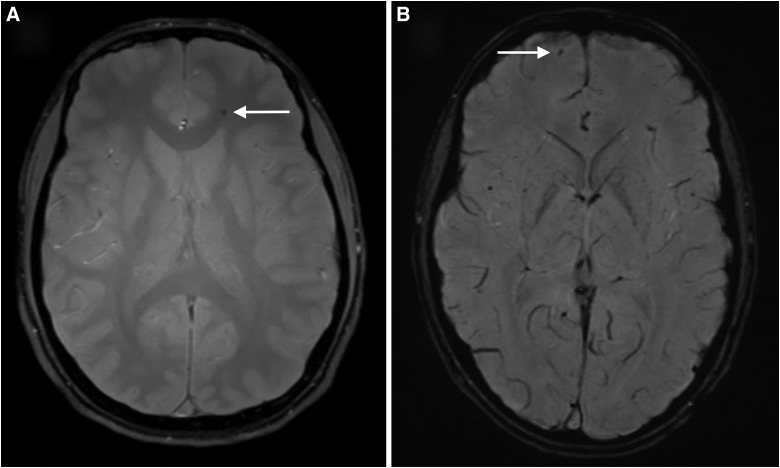
The first patient was a 15-year-old male with a history of three previous concussions who suffered helmeted head-to-head contact with another player during a football game. He continued to play through the match with increasing headache, dizziness, and memory loss. Three weeks post-injury, he presented to the concussion subspecialty clinic complaining of intermittent headaches, impaired concentration, and irritability. An MRI 27 days post-injury demonstrated a solitary focus of SWI signal hypointensity in the white matter of the left frontal lobe, consistent with microhemorrhage (Fig. 1A). Although his neuropsychiatric symptoms improved with time, the headaches did not. As a consequence of his persistent headaches and MRI findings, the recommendation was made that he retire from contact sports. On most recent follow-up 3 years post-injury, he continued to suffer from headaches.
FIG. 1.
Representative slices from SWI sequences in the 2 patients with microhemorrhage. (A) A solitary focus of SWI hypointensity was observed in the white matter of the left frontal lobe in a 15-year-old male football player 27 days after sustaining a concussion in the course of play. (B) Six foci of SWI hypointensity were observed in this 17-year-old male 10 days after sustaining a concussion during a soccer match; shown here is the finding in the left frontal lobe. SWI, susceptibility-weighted imaging.
The second patient was a 17-year-old male with a history of three previous concussions who suffered a kick to the head during a soccer game. The patient suffered a loss of consciousness for approximately 5 min, during which time he had spasms in his legs and irregular respirations that were interpreted as possible seizure activity. When the patient's level of consciousness returned to normal, he was amnestic to the event and complained of headache and difficulty concentrating. He was taken to the ED, where a CT scan of the head was normal. His symptoms persisted for 3 days and then resolved. An MRI was obtained 10 days after his injury attributed to the possible seizure at the time of injury; SWI showed six foci of microhemorrhage in the right parietal, right frontal, and left temporal lobes (Fig. 1B). Four months post-injury, formal neurocognitive testing of verbal and visual-spatial problem solving, fine motor skills, attention, language, emotional function, and other domains demonstrated average to above-average performance with no obvious deficits referable to his TBI. Based on the MRI findings, the recommendation was made that he retire from soccer. The patient did not follow through on these recommendations and continued to play at the collegiate level. Two years post-injury, he still experiences intermittent headaches that are not lifestyle limiting.
Discussion
Position papers and guideline documents have recommended against the routine use of neuroimaging studies in children presenting with an acute concussion because the vast majority of these studies are normal and rarely inform medical decision making.2,9,10 Some researchers advocate neuroimaging in those who are persistently symptomatic, but these recommendations are based only on expert opinion.11 Two recent studies have found that whereas the majority of scans for chronic symptoms are normal, 5–12% of patients had new intracranial injuries identified on MRI.12,13
In this retrospective cohort study, 427 of 3360 children with concussion underwent imaging with MRI. Children who were scanned had experienced a greater number of previous concussions and they were twice as likely to have had a TBI within the preceding 3 months. These historical factors may have led ordering providers to be more concerned about these patients, prompting the decision to image. Alternatively, it is also plausible that these children were more symptomatic because of their repeated injuries, leading to higher MRI utilization.
We also found that children were more likely to be scanned if they were first evaluated for their concussion in our institution's concussion clinic. This finding is not entirely surprising given that a substantial portion of the children seen in this clinic are referred by outside primary care providers for severe or persistent symptoms; by contrast, children first evaluated in urgent care or the ED are more likely to have been evaluated acutely after the injury and thus may be more representative of the “average” concussion patient whose symptoms are short-lived.
Of the 427 children who were imaged with MRI, 85% had normal scans. Only two (0.5%) of these studies revealed findings plausibly related to the patient's TBI, consisting in both of microhemorrhage. Notably, both of these children had three previous concussions, significantly more than those whose scans did not disclose intracranial injuries. Whether the effects of multiple concussions accumulate over time to increase the risk for these bleeds is not clear. It is also unclear whether or not these microhemorrhages are related to the patient's symptoms.
The rate of trauma-related findings in our data is less than one tenth what has been reported previously by others. Ellis and colleagues studied 151 children with concussion, 16 of whom underwent MRI because of persistent symptoms.12 Of these, 1 was found to have a small intraparenchymal hemorrhage and a second was found to have a nonhemorrhagic contusion, yielding an intracranial injury rate of 12.5%. Notably, this study was conducted in a multidisciplinary concussion clinic staffed, in part, by a neurological surgeon, and some of their subjects had their scans obtained before referral. As a consequence, their findings are likely subject to referral bias; patients with abnormal scans may have been more likely to be included, thus inflating the rate of positive results. A separate investigation by Morgan and colleagues reviewed 52 patients with PCS, 19 of whom underwent imaging with MRI.13 One of these patients was found to have multiple foci of microhemorrhage, yielding an intracranial injury rate of approximately 5%.
The clinical ramifications of these MRI findings are unclear. In adults with mild TBI, the presence of a cerebral contusion or four or more foci of microhemorrhage has been associated with worse functional outcomes at 3 months, suggesting that these findings may be helpful in prognostication.14 However, there are little data to guide the incorporation of information generated by these scans into the medical decision-making process. At best, the results can be used to guide return to play recommendations, as was the case in both of our patients with positive scans. However, these recommendations are based on expert opinion or clinical experience rather than objective data.11,15 Although it is known that repeated mild TBIs can predispose to neurocognitive decline later in life,16 we are not aware of any evidence linking microhemorrhage or other forms of intracranial injury to a higher risk of neurocognitive decline with subsequent TBI. Such an association will require further work to prove or disprove, although the apparent rarity of traumatic findings in this population suggests that it may be difficult to adequately power such a study.
Findings unrelated to trauma
Although our data indicate that MRI in children with persistent symptoms post-concussion rarely identifies injuries, 14.3% of scans disclosed an abnormality not related to trauma. Notably, this result is lower than the 18–21% rate of abnormal findings that has been described in healthy, asymptomatic children and adults.17,18
One scan led to the discovery of a hemangioblastoma in a child with new-onset ataxia weeks after his injury, prompting surgical resection. Another underwent craniotomy for what was ultimately found to be cortical dysplasia after she experienced a possible seizure. In both cases, the patients had alarming clinical presentations that are not typical of post-concussive syndrome; imaging would likely have been pursued because of these children's symptoms even in the absence of concussion.
However, the vast majority of these discoveries were truly incidental and did not materially affect patient management beyond neurosurgical consultation and repeat imaging. The most common findings were nonspecific T2 hyperintense lesions in the cerebral white matter (WM), which were noted in 5.4% of our patients. These nonspecific WM changes are observed in 1.3% of asymptomatic children, but are known to be more common in patients with migraine headache.17,19 That migraine itself is associated with a higher risk of persistent symptoms post-concussion likely explains the increased prevalence of these WM abnormalities in our series, although we cannot rule out a causative effect of concussion.4
Arachnoid cysts and pineal cysts were commonly observed in our cohort with rates of 0.9% and 3.5%, respectively. These are common incidental findings on MRI that rarely require intervention beyond simple observation and are unlikely to be related to symptomatology post-concussion.20–22 Although previous studies have identified an increased prevalence of arachnoid cysts in children with PCS compared to the general population, our data are not consistent with such a link7,12,13; the prevalence of arachnoid cysts in children has been found to be between 1.0 and 2.6%, in line with our results.17,23
Magnetic resonance imaging sequences
MRI scans that revealed abnormalities not related to trauma more commonly included high-resolution CISS and contrast-enhanced sequences. At our institution, atypical results are noted as the scan is acquired and generally prompt further investigation with additional sequences to better evaluate the finding. Thus, the higher utilization of these sequences may be a consequence of the abnormality being detected on routine sequences rather than the reason the abnormality was identified in the first place.
All of the abnormal MRI findings in our series could be observed on FLAIR or SWI, including microhemorrhage, the only trauma-related pathology we identified. Although we did not observe any patients with a subarachnoid hemorrhage, subdural hematoma, or cerebral contusion, these may be present in children with TBI and are readily identified with FLAIR and SWI. A limited MRI scan consisting only of these sequences may therefore reliably detect intracranial injuries as well as other abnormalities and can do so with substantially reduced scanner time. At our institution, a complete trauma protocol scan requires 25 min, whereas FLAIR and SWI together require only 7.5 min. However, this advantage may be counterbalanced by the need to bring patients back for complete scans if limited studies identify, but incompletely characterize, abnormal findings. The utility of a limited study may therefore be restricted to centers where scans are reviewed as they are acquired, allowing for addition of further sequences before the patient leaves the scanner if abnormalities are detected. Further study of limited MRI scans is warranted before they are adopted into routine clinical practice.
Limitations
Because of the large number of patients identified with and without MRI scans, we were unable to perform a detailed review to obtain granular information on the severity of symptoms or the specific symptoms suffered in all patients; as a consequence, the precise clinical indications for obtaining MRI scans are not known for the majority of our cohort. We also acknowledge that the minority were imaged with either a 3 Tesla scanner or SWI, which may have reduced the rate of hemorrhagic findings. Further, we did not strictly limit our sample to patients whose scans were obtained 1 month or more post-injury, which may account for some differences with other studies that have focused on such limited populations. Finally, this study included only children who either did not have a previous CT scan or who had a CT scan that was normal; individuals with traumatic or otherwise abnormal findings on CT were not examined and therefore represent a different population to which our results may not generalize.
Although our data demonstrate that evidence of injury is very rare on conventional MRI, we did not examine findings on advanced neuroimaging. Techniques such as diffusion tensor imaging, MR spectroscopy, functional MRI, and MR perfusion commonly show pathological changes in mild TBI that are not observed with more-established macrostructural imaging modalities.24–30 These findings correlate with symptomatology, and further study of these techniques may lead both to a better understanding of the underlying pathophysiology and to advances in patient care.31
Conclusions
Trauma-related findings on MRI are very rare in children who are imaged with conventional methods for persistent symptoms of concussion. In the few cases where trauma-related findings are identified, it is not clear how they should influence decision making. In this cohort, the presence of traumatic intracranial hemorrhage had substantial bearing on return-to-play guidance, prompting providers to recommend cessation of contact sports. Although such guidance is in line with expert opinion,11 there is little objective evidence to support or refute this practice.
In contrast, abnormalities not related to trauma are relatively common, although no more so than in healthy patients without a history of concussion.17,18 The vast majority of these were truly incidental findings that could be managed expectantly with observation. However, identification of abnormalities that are themselves benign can have unanticipated consequences; children who are given the diagnosis of a nonthreatening condition may be seen as vulnerable, leading to unnecessary emergency department visits and lifestyle restrictions.32–34 Because of these risks, providers should be thoughtful when considering an MRI in this population.
Rarely, children may require neurosurgical interventions based on the results of their scans. In our series, these patients presented with concerning symptoms that are not common after concussion, highlighting the importance of vigilance on the part of the provider. Whereas routine neuroimaging does not appear to be useful in children who are persistently symptomatic post-concussion, MRI should be pursued in those with alarming presentations. Special consideration should also be given to the very young and to the developmentally delayed, who may have difficulty explaining their symptoms.
Clinicians caring for children with persistent PCS should consider these factors when deciding whether or not to pursue imaging with conventional MRI. If the decision is made to obtain a scan, limited screening studies with a minimum number of sequences may be of use in the future to shorten scanner time, reduce cost, and minimize patient inconvenience.
Acknowledgments
The authors acknowledge Lauren Anderson and Shannon Higgins for their assistance with data collection.
Author Disclosure Statement
R.H.B. received indirect salary support through an educational grant provided to the University of Washington Department of Neurological Surgery by Codman Neuro. S.R.B. has ownership in Aqueduct Neurosciences Inc, Aqueduct Critical Care Inc, and Navisonics Inc. C.L.M. has provided paid consulting services for Neurotrauma Sciences, LLC, and Sinapis Pharma, Inc. These commercial entities had no role in the design, conduct, or reporting of this research.
References
- 1.Bryan M.A., Rowhani-Rahbar A., Comstock R.D., and Rivara F.; Seattle Sports Concussion Research Collaborative. (2016). Sports- and recreation-related concussions in US youth. Pediatrics 138, e20154635–e20154635 [DOI] [PubMed] [Google Scholar]
- 2.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorák J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg M.A., Meehan W.P., and Mannix R. (2014). Duration and course of post-concussive symptoms. Pediatrics 133, 999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemek R., Barrowman N., Freedman S.B., Gravel J., Gagnon I., McGahern C., Aglipay M., Sangha G., Boutis K., Beer D., Craig W., Burns E., Farion K.J., Mikrogianakis A., Barlow K., Dubrovsky A.S., Meeuwisse W., Gioia G., Meehan W.P., Beauchamp M.H., Kamil Y., Grool A.M., Hoshizaki B., Anderson P., Brooks B.L., Yeates K.O., Vassilyadi M., Klassen T., Keightley M., Richer L., DeMatteo C., and Osmond M.H.; Pediatric Emergency Research Canada (PERC) Concussion Team. (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025 [DOI] [PubMed] [Google Scholar]
- 5.Meehan W.P., Mannix R.C., Stracciolini A., Elbin R.J., and Collins M.W. (2013). Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. J. Pediatr. 163, 721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeates K.O., Kaizar E., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., and and Taylor H.G. (2012). Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch. Pediatr. Adolesc. Med. 166, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tator C.H., Davis H.S., Dufort P.A., Tartaglia M.C., Davis K.D., Ebraheem A., and Hiploylee C. (2016). Postconcussion syndrome: demographics and predictors in 221 patients. J. Neurosurg. 125, 1206–1216 [DOI] [PubMed] [Google Scholar]
- 8.Kuppermann N., Holmes J.F., Dayan P.S., Hoyle J.D.J., Atabaki S.M., Holubkov R., Nadel F.M., Monroe D., Stanley R.M., Borgialli D.A., Badawy M.K., Schunk J.E., Quayle K.S., Mahajan P., Lichenstein R., Lillis K.A., Tunik M.G., Jacobs E.S., Callahan J.M., Gorelick M.H., Glass T.F., Lee L.K., Bachman M.C., Cooper A., Powell E.C., Gerardi M.J., Melville K.A., Muizelaar J.P., Wisner D.H., Zuspan S.J., Dean J.M., and Wootton-Gorges S.L.; Pediatric Emergency Care Applied Research Network (PECARN). (2009). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 374, 1160–1170 [DOI] [PubMed] [Google Scholar]
- 9.Giza C.C., Kutcher J.S., Ashwal S., Barth J., Getchius T.S.D., Gioia G.A., Gronseth G.S., Guskiewicz K., Mandel S., Manley G., McKeag D.B., Thurman D.J., and Zafonte R. (2013). Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 80, 2250–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead M.E., and Walter K.D.; Council on Sports Medicine and Fitness. (2010). American Academy of Pediatrics. Clinical report—sport-related concussion in children and adolescents. Pediatrics 126, 597–615 [DOI] [PubMed] [Google Scholar]
- 11.Cantu R.C., and Register-Mihalik J.K. (2011). Considerations for return-to-play and retirement decisions after concussion. PM R 3, 10 Suppl. 2, S440–S444 [DOI] [PubMed] [Google Scholar]
- 12.Ellis M.J., Leiter J., Hall T., McDonald P.J., Sawyer S., Silver N., Bunge M., and Essig M. (2015). Neuroimaging findings in pediatric sports-related concussion. J Neurosurg. Pediatr. 16, 241–247 [DOI] [PubMed] [Google Scholar]
- 13.Morgan C.D., Zuckerman S.L., King L.E., Beaird S.E., Sills A.K., and Solomon G.S. (2015). Post-concussion syndrome (PCS) in a youth population: defining the diagnostic value and cost-utility of brain imaging. Childs Nerv. Syst. 31, 2305–2309 [DOI] [PubMed] [Google Scholar]
- 14.Yuh E.L., Mukherjee P., Lingsma H.F., Yue J.K., Ferguson A.R., Gordon W.A., Valadka A.B., Schnyer D.M., Okonkwo D.O., Maas A.I.R., and Manley G.T.; TRACK-TBI Investigators. (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 73, 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantu R.C. (2003). Recurrent athletic head injury: risks and when to retire. Clin. Sports Med. 22, 593–603, x. [DOI] [PubMed] [Google Scholar]
- 16.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.-S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim B.S., Illes J., Kaplan R.T., Reiss A., and Atlas S.W. (2002). Incidental findings on pediatric MR images of the brain. Am. J. Neuroradiol. 23, 1674–1677 [PMC free article] [PubMed] [Google Scholar]
- 18.Katzman G.L., Dagher A.P., and Patronas N.J. (1999). Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA 282, 36–39 [DOI] [PubMed] [Google Scholar]
- 19.Swartz R.H., and Kern R.Z. (2004). Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch. Neurol. 61, 1366–1368 [DOI] [PubMed] [Google Scholar]
- 20.Al-Holou W.N., Yew A.Y., Boomsaad Z.E., Garton H.J., Muraszko K.M., and Maher C.O. (2010). Prevalence and natural history of arachnoid cysts in children. J. Neurosurg. Pediatr. 5, 578–585 [DOI] [PubMed] [Google Scholar]
- 21.Al-Holou W.N., Garton H.J.L., Muraszko K.M., Ibrahim M., and Maher C.O. (2009). Prevalence of pineal cysts in children and young adults. Clinical article. J. Neurosurg. Pediatr. 4, 230–236 [DOI] [PubMed] [Google Scholar]
- 22.Al-Holou W.N., Maher C.O., Muraszko K.M., and Garton H.J. (2010). The natural history of pineal cysts in children and young adults. J. Neurosurg. Pediatr. 5, 162–166 [DOI] [PubMed] [Google Scholar]
- 23.Al-Holou W.N., Terman S., Kilburg C., Garton H.J., Muraszko K.M., and Maher C.O. (2013). Prevalence and natural history of arachnoid cysts in adults. J. Neurosurg. 118, 222–231 [DOI] [PubMed] [Google Scholar]
- 24.Lovell M.R., Pardini J.E., Welling J., Collins M.W., Bakal J., Lazar N., Roush R., Eddy W.F., and Becker J.T. (2007). Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery 61, 352–359; discussion, 359–360 [DOI] [PubMed] [Google Scholar]
- 25.Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 26.Yuh E.L., Cooper S.R., Mukherjee P., Yue J.K., Lingsma H.F., Gordon W.A., Valadka A.B., Okonkwo D.O., Schnyer D.M., Vassar M.J., Maas A.I., and Manley G.T.; TRACK-TBI Investigators. (2014). Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J. Neurotrauma 31, 1457–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuh E.L., Hawryluk G.W., and Manley G.T. (2014). Imaging concussion: a review. Neurosurgery 75, Suppl. 4, S50–S63 [DOI] [PubMed] [Google Scholar]
- 28.Smits M., Houston G.C., Dippel D.W.J., Wielopolski P.A., Vernooij M.W., Koudstaal P.J., Hunink M.G., and van der Lugt A. (2011). Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 53, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulsipher D.T., Campbell R.A., Thoma R., and King J.H. (2011). A critical review of neuroimaging applications in sports concussion. Curr. Sports Med. Rep. 10, 14–20 [DOI] [PubMed] [Google Scholar]
- 30.Henry L.C., Tremblay S., Boulanger Y., Ellemberg D., and Lassonde M. (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J. Neurotrauma 27, 65–76 [DOI] [PubMed] [Google Scholar]
- 31.Wintermark M., Coombs L., Druzgal T.J., Field A.S., Filippi C.G., Hicks R., Horton R., Lui Y.W., Law M., Mukherjee P., Norbash A., Riedy G., Sanelli P.C., Stone J.R., Sze G., Tilkin M., Whitlow C.T., Wilde E.A., York G., and Provenzale J.M.; American College of Radiology Head Injury Institute. (2015). Traumatic brain injury imaging research roadmap. AJNR Am. J. Neuroradiol. 36, E12–E23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergman A.B., and Stamm S.J. (1967). The morbidity of cardiac nondisease in schoolchildren. N. Engl. J. Med. 276, 1008–1013 [DOI] [PubMed] [Google Scholar]
- 33.Levy J.C. (1980). Vulnerable children: parents' perspectives and the use of medical care. Pediatrics 65, 956–963 [PubMed] [Google Scholar]
- 34.Kokotos F. (2009). The vulnerable child syndrome. Pediatr. Rev. 30, 193–194 [DOI] [PubMed] [Google Scholar]