Abstract
Purpose: Surveillance for long-term complications related to previous cancer therapy can help diagnose/manage chronic health conditions in childhood cancer survivors and improve survivor quality of life. However, a challenge to delivering long-term care to childhood cancer survivors is loss to follow-up; many patients discontinue care at specialized survivor care centers. The purpose of this study was to examine patterns of loss to follow-up among a cohort of childhood cancer survivors.
Methods: This retrospective study examined follow-up patterns among a nonrandom representative sample of 370 childhood cancer survivors among 1116 patients from a single institution. The median age of patients at diagnosis was 10.2 years (range <1–21). Factors potentially related to follow-up were utilized to evaluate patterns of follow-up across 5-year intervals following completion of active therapy. The association between patient characteristics and follow-up was evaluated using univariate and multivariate binomial regression models.
Results: The probability of follow-up 1–5 years post-treatment was 91.2% (89.7%–92.5%) but dropped to 68.5% (66.2%–70.8%) during years 6–10, 47.7% (45.0%–50.3%) during years 11–15, and continued to steadily decrease over time. Overall, white race, diagnoses at younger ages, patients with lymphomas/leukemias, and decade of diagnosis were each associated with somewhat better rates of follow-up.
Conclusions: These findings highlight the lack of follow-up by adult survivors of childhood cancer with only approximately one-half of patients returning for follow-up 10 years after completion of therapy. Interventions focused on educating both patients and primary care physicians may help to increase long-term follow-up care among this at-risk population.
Keywords: : follow-up care, late effects, long-term follow-up, loss to follow-up, survival, survivorship
Introduction
Over 80% of children treated for childhood malignancies before the age of 20 will survive for 5 years and beyond.1 As a result, the population of childhood cancer survivors is increasing and represents an important group with significant needs for survivorship care. Survivors of childhood cancer are at increased risk for a range of chronic health conditions and late effects related to their malignancy and/or their therapy.2–5 It is estimated that 73% of survivors will have at least one chronic health condition 30 years after cancer diagnosis, with survivors 3.3 times more likely to have chronic conditions compared to their siblings and 9 times more likely than their siblings to have a severe or life-threatening condition.6 Given these findings, specialized follow-up care is essential to survivor's health since follow-up allows for continued surveillance and early diagnosis, rapid intervention, and continued management of late effects.5,7
While long-term follow-up is encouraged by organizations such as the Children's Oncology Group (COG), there are potential barriers. A recent study found that while 88.8% of childhood cancer survivors reported receiving medical care within the prior 2 years, only 17.8% reported receiving “survivor-focused care” that included a discussion about cancer therapy-related risks, screening examinations, and strategies to reduce risks.8,9 Most patients reported receiving healthcare from a primary care physician (PCP), with only 14.6% of these patients receiving care at a cancer center despite studies that find many PCPs lack specific knowledge of treatment complications among childhood survivors.8,10 Even among patients treated at a cancer center, ∼50% of providers failed to perform recommended screening examinations based on risk stratification as per COG Long-Term Follow-up Guidelines.11–13
Another barrier to follow-up is lack of patient knowledge. While 72% of childhood cancer patients accurately reported their cancer diagnosis and 94% and 89% accurately reported receiving chemotherapy and radiotherapy, few knew specific critical details of their treatment. For example, when questioned regarding anthracycline therapy, only 33% and 8% recalled receiving doxorubicin and daunorubicin, respectively.14 As a result, it has been suggested that survivors receive a care plan detailing their disease and therapies to facilitate communication and provide survivorship-focused care.15,16
Published studies of survivorship care focus on patients who have continued with long-term care.1,9 As a result, these studies have likely overestimated the proportion of patients receiving appropriate survivorship care. Given the documented long-term impact of the treatment of childhood cancer, research investigating patterns of care and continued engagement in specialized care settings becomes critical. The purpose of the present investigation is to further this effort by examining patterns of loss-to-follow-up among survivors of childhood cancers who received care at the Roswell Park Cancer Institute (RPCI), a National Cancer Institute (NCI)-designated comprehensive cancer center.
Methods
Design
This study involved a retrospective review of medical records at RPCI.
Study population
Eligibility criteria included (1) cancer diagnosis between 1960 and 2010, (2) age at diagnosis ≤21 years, and (3) treatment primarily at RPCI and/or Women and Children's Hospital of Buffalo, with which RPCI is affiliated. RPCI is a National Cancer Institute-designated comprehensive cancer center providing care to children and adults. The long-term follow-up program for survivors of childhood cancers at RPCI is a multidisciplinary clinic that includes primary care, pediatric hematology/oncology, psychology, and social work; the program has routinely provided patients with survivorship care plans (SCPs). The clinic currently shares space with the pediatrics division, but will soon be transitioning to an institutional survivorship center. To encourage follow-up, survivors are contacted via phone and/or mailings; multiple efforts to re-engage survivors via mailings occur annually.
Information from the RPCI Clinical Data Network was used to ensure completeness of data, including dates of diagnosis and race. A data base generated by a medical records search originally identified 1683 patients with a pediatric malignancy diagnosed at RPCI. A subgroup of these pediatric cases (N = 567) were not treated within the pediatric department (i.e., patients treated by Head and Neck, Gynecology, and Dermatology) and were removed. Of the remaining 1116 patients, a nonrandom representative sample of 370 medical charts (33%) was reviewed; 31 patients were excluded because their essential demographic data and/or treatment information were missing.
A waiver of consent was obtained, and records were de-identified at the time of analysis. Abstraction was completed by a single trained abstractor. As seen in Table 1, our nonrandom representative sample was generally comparable to those not reviewed. Abstracted patients tended to be of younger ages at diagnosis and diagnosed with leukemia or lymphoma; no differences were noted by gender, race, or decade of diagnosis.
Table 1.
Comparison of Reviewed Versus Nonreviewed Cases by Selected Demographics and Clinical Characteristics
| Reviewed (n = 370) n (%) | Not reviewed (n = 691) n (%) | p | |
|---|---|---|---|
| Age at diagnosis | <0.01 | ||
| 0–4 | 88 (23.8) | 149 (21.6) | |
| 5–9 | 95 (25.7) | 133 (19.2) | |
| 10–15 | 107 (28.9) | 173 (25.0) | |
| 16–21 | 80 (21.6) | 236 (34.2) | |
| Gender | 0.060 | ||
| Male | 193 (52.2) | 402 (58.2) | |
| Female | 177 (47.8) | 289 (41.9) | |
| Race | 0.370 | ||
| White | 330 (89.2) | 630 (91.2) | |
| Black | 29 (7.8) | 49 (7.1) | |
| Other | 11 (3.0) | 12 (1.7) | |
| Malignancy | 0.032 | ||
| Leukemia | 115 (31.1) | 166 (24.0) | |
| Lymphoma | 118 (31.9) | 205 (29.7) | |
| CNS | 25 (6.8) | 54 (7.8) | |
| Sarcoma | 57 (15.4) | 122 (17.7) | |
| Other | 55 (14.9) | 144 (20.8) | |
| Decade of diagnosis | 0.225 | ||
| 1960/1970s | 89 (24.1) | 190 (27.5) | |
| 1980s | 95 (25.7) | 196 (28.4) | |
| 1990s | 107 (28.9) | 164 (23.7) | |
| 2000s | 79 (21.4) | 141 (20.4) |
CNS, central nervous system.
Independent variables
Data abstracted from medical records included demographic data (birth date, gender, race), type of malignancy, date of diagnosis, age at diagnosis, treatment history, and date(s) of clinic attendance. Race was grouped into white, black, and other; Hispanic ethnicity was not considered due to the limited numbers in this cohort. Cancer diagnoses were classified into five groups: leukemia, lymphoma, central nervous system (CNS), sarcomas, and other malignancies. Treatment history was recorded as any versus no use of chemotherapy, any versus no use of radiation therapy, and/or any versus no surgery as treatment for the primary malignancy. Procedures such as biopsy, placement and removal of central lines or catheters, bone marrow aspirates, and lumbar punctures were not included as part of the surgical history.
Dependent variables
Dates of follow-up visits through December 31, 2015, were recorded based on clinic visits following completion of active treatment. The main dependent variable was adherence to follow-up visits. Follow-up by year since completion of therapy was initially treated as a continuous variable; however, to clarify analyses, follow-up was grouped into 5-year increments beginning at completion of active therapy. For example, if a patient completed treatment in 2000, they were eligible for the 1–5-, 6–10-, and 11–15-year intervals only, and were excluded from analyses for more extended time periods. Adherence with follow-up during these intervals was defined as one or more clinic visits during each interval. If a patient was known to be deceased, they were no longer considered eligible for follow-up.
Analyses
Descriptive analyses were completed based on follow-up rates within 5-year intervals for gender, race, diagnosis category (leukemia, lymphoma, CNS, sarcoma, other), treatment history (surgery, chemotherapy, radiation), decade diagnosed, and age group at diagnosis subgroups. Patient characteristics are reported using means, medians, and standard deviations for continuous variables; and using frequencies and relative frequencies for categorical variables. Comparisons were made using ANOVA and Pearson chi-square tests for continuous and categorical variables, respectively.
In the overall sample, binomial regression models were used to obtain estimated follow-up rates and odds ratios, with 95% confidence intervals (CIs), for each of the 5-year time periods. The estimated rates represent the probability that a patient attended a follow-up visit during a given year of the defined 5-year period. For each 5-year time period, the univariate association between patient characteristics and follow-up visits was evaluated using binomial regression models. Then, a multivariate binomial regression model was used to evaluate the collective association between patient characteristics (all variables in Table 2) and follow-up visits. Estimated follow-up rates and odds ratios, with 95% CIs, were obtained from model estimates. All analyses were conducted using SAS v9.4 (Cary, NC) at a significance level of 0.05. Therefore, p-values less than 0.05 denote statistically significant results.
Table 2.
Selected Demographic and Clinical Characteristics of Study Population by Category of Cancer
| Overall, N | Leukemia115 (31.1%) | Lymphoma118 (31.9%) | CNS cancer25 (6.8%) | Sarcoma57 (15.4%) | Other cancer55 (14.9%) | Overall370 (100%) | p |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| Mean/Std/N | 7.5/4.6/115 | 12.4/4.4/118 | 9.1/5.1/25 | 11.0/5.6/57 | 8.1/5.3/55 | 9.8/5.3/370 | <0.001 |
| Median/min/max | 6.7/0.3/16.9 | 13.1/0.4/19.7 | 9.4/0.8/20.7 | 10.6/0.0/20.6 | 7.6/0.6/17.9 | 10.2/0.0/20.7 | |
| Gender, n (%) | |||||||
| Male | 59 (51.3) | 68 (57.6) | 12 (48.0) | 28 (49.1) | 26 (47.3) | 193 (52.2) | 0.670 |
| Female | 56 (48.7) | 50 (42.4) | 13 (52.0) | 29 (50.9) | 29 (52.7) | 177 (47.8) | |
| Race, n (%) | |||||||
| Caucasian | 102 (88.7) | 109 (92.4) | 22 (88.0) | 50 (87.7) | 47 (85.5) | 330 (89.2) | 0.428 |
| African American | 8 (7.0) | 9 (7.6) | 1 (4.0) | 5 (8.8) | 6 (10.9) | 29 (7.8) | |
| Other | 5 (4.3) | 2 (8.0) | 2 (3.5) | 2 (3.6) | 11 (3.0) | ||
| Surgery, n (%) | |||||||
| No | 102 (88.7) | 67 (56.8) | 4 (16.0) | 9 (15.8) | 5 (9.1) | 187 (50.5) | <0.001 |
| Yes | 13 (11.3) | 51 (43.2) | 21 (84.0) | 48 (84.2) | 50 (90.9) | 183 (49.5%) | |
| Chemotherapy, n (%) | |||||||
| No | 8 (6.8) | 4 (16.0) | 4 (7.0) | 8 (14.5) | 24 (6.5) | 0.001 | |
| Yes | 115 (100.0) | 110 (93.2) | 21 (84.0) | 53 (93.0) | 47 (85.5) | 346 (93.5) | |
| Radiation, n (%) | |||||||
| No | 87 (75.7) | 45 (38.1) | 3 (12.0) | 31 (54.4) | 32 (58.2) | 198 (53.5) | <0.001 |
| Yes | 28 (24.3) | 73 (61.9) | 22 (88.0) | 26 (45.6) | 23 (41.8) | 172 (46.5) | |
| Decade, n (%) | |||||||
| 1960/70s | 41 (35.7) | 20 (16.9) | 3 (12.0) | 14 (24.6) | 11 (20.0) | 89 (24.1) | 0.004 |
| 1980s | 31 (27.0) | 28 (23.7) | 7 (28.0) | 13 (22.8) | 16 (29.1) | 95 (25.7) | |
| 1990s | 21 (18.3) | 34 (28.8) | 11 (44.0) | 19 (33.3) | 22 (40.0) | 107 (28.9) | |
| 2000s | 22 (19.1) | 36 (30.5) | 4 (16.0) | 11 (19.3) | 6 (10.9) | 79 (21.4) | |
| Follow-up (years) | |||||||
| Mean/Std/N | 24.6/9.8/115 | 21.3/9.9/118 | 21.2/8.0/25 | 23.8/10.8/57 | 23.7/9.7/55 | 23.1/9.9/370 | 0.090 |
| Median/min/max | 26.0/5.0/45.0 | 20.0/6.0/40.0 | 20.0/11.0/40.0 | 22.0/5.0/41.0 | 23.0/5.0/41.0 | 23.0/5.0/45.0 | |
| Age, n (%) | |||||||
| 0–4 | 46 (40.0) | 9 (7.6) | 5 (20.0) | 10 (17.5) | 18 (32.7) | 88 (23.8) | <0.001 |
| 5–9 | 32 (27.8) | 23 (19.5) | 9 (36.0) | 17 (29.8) | 14 (25.5) | 95 (25.7) | |
| 10–15 | 31 (27.0) | 41 (34.7) | 8 (32.0) | 12 (21.1) | 15 (27.3) | 107 (28.9) | |
| 15–21 | 6 (5.2) | 45 (38.1) | 3 (12.0) | 18 (31.6) | 8 (14.5) | 80 (21.6) | |
Bold values represent statistically significant p-values less than 0.05.
Results
As presented in Table 2, clinical characteristics of pediatric cancer survivors were examined by category of cancer diagnosis. The mean age at diagnosis within the sample was 9.8 years (median 10.2, range 0.0–20.7), and mean period of eligibility for follow-up was 23.1 years, with a standard deviation of 9.9 years (median 23.0, range 5.0–45.0) overall. Analysis of variance revealed no significant difference in mean years of eligibility by cancer diagnosis. The low number of CNS survivors in the sample reflects local patterns of clinical care, where CNS malignancies have generally been treated and followed at our regional children's hospital and not at the comprehensive cancer center. Chi-square and analysis of variance testing revealed that the groups differed with respect to age at diagnosis, decade of patient diagnosis, as well as treatments (p < 0.05), but not by gender or race.
The percent of patients who attended a follow-up visit in each year following treatment completion is displayed in Figure 1. There is a clear and steady drop off in the percentage of patients who attend an office visit as time since treatment completion increases; by 5 years out 82% return, by 10 years this drops to 59%, and after 15 years it is 42%.
FIG. 1.
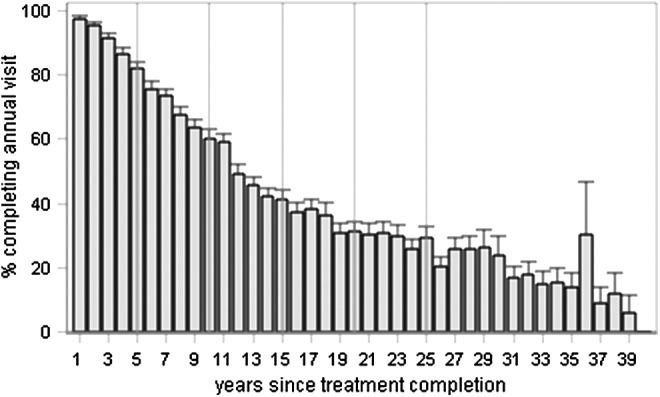
Percent of childhood cancer survivors who attended an annual follow-up visit, by years since completion of treatment. Error bars denote the standard error. y-axis—percent of patients completing annual visit. x-axis—years since treatment completion.
Table 3 presents estimates of the probability of attending a follow-up visit by the 5-year time period since completion of therapy, based on binomial regression models. During the time period of 1–5 years removed from treatment completion, the likelihood that a patient attends a given follow-up visit is quite high, at 91.2% (95% CI 89.7%–92.5%). However, during the interval 11–15 years following completion of therapy, this decreased to 47.7% (45.0%–50.3%) and this probability further dropped to 29.2% (26.3%–32.%3) during the interval 21–25 years removed from treatment.
Table 3.
Unadjusted Probability of Attending a Given Follow-Up Visit by 5-Year Interval Since Completion of Treatment
| Follow-up period, years | Estimated probability of follow-up visit (95% CI) | Odds ratio (95% CI) |
|---|---|---|
| 1–5 | 91.2 (89.7–92.5) | 1.00 (reference) |
| 6–10 | 68.5 (66.2–70.8) | 0.21 (0.17–0.26) |
| 11–15 | 47.7 (45.0–50.3) | 0.09 (0.07–0.11) |
| 16–20 | 34.5 (31.8–37.4) | 0.05 (0.04–0.06) |
| 21–25 | 29.2 (26.3–32.3) | 0.04 (0.03–0.05) |
| 26+ | 20.8 (18.5–23.3) | 0.03 (0.02–0.03) |
The association between selected demographic and clinical characteristics and rate of follow-up is summarized in Table 4. After 6+ years since completion of therapy, patients who were older at time of diagnosis, non-whites, and persons diagnosed in the 1970s through 1990s tended to demonstrate lower rates of follow-up visits.
Table 4.
Probability of Attending a Given Follow-Up Visit by 5-Year Interval Since Completion of Treatment and Selected Demographic and Clinical Characteristics
| 1–5 years | 6–10 years | 11–15 years | 16–20 years | 21–25 years | 26+ years | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 0–4 | 93.6 (90.9–95.6) | 74.4 (70.0–78.3) | 55.9 (50.9–60.7) | 32.7 (28.2–37.6) | 24.8 (20.3–29.9) | 12.7 (9.9–16.1) |
| 5–9 | 92.4 (89.7–94.5) | 72.6 (68.2–76.5) | 51.6 (46.5–56.7) | 39.8 (34.1–45.7) | 27.1 (21.4–33.5) | 16.6 (12.4–21.9) |
| 10–15 | 88.2 (85.2–90.7) | 66.8 (62.6–70.8) | 42.5 (37.9–47.2) | 31.6 (27.0–36.7) | 31.4 (26.0–37.3) | 21.0 (16.9–25.8) |
| 15–21 | 88.5 (85.0–91.3) | 58.7 (53.7–63.6) | 40.3 (34.7–46.1) | 36.6 (30.7–43.1) | 38.1 (31.0–45.7) | 38.1 (31.7–44.9) |
| Gender | ||||||
| Male | 90.6 (88.6–92.3) | 66.9 (63.7–69.8) | 44.9 (41.4–48.4) | 31.2 (27.8–34.8) | 30.3 (26.4–34.4) | 22.3 (19.4–25.5) |
| Female | 90.7 (88.6–92.5) | 70.0 (66.8–73.0) | 51.2 (47.5–54.8) | 38.9 (34.9–43.0) | 28.4 (24.4–32.9) | 16.7 (13.7–20.2) |
| Race | ||||||
| Caucasian | 91.5 (90.1–92.8) | 69.8 (67.4–72.0) | 50.5 (47.8–53.2) | 36.2 (33.5–39.1) | 30.5 (27.5–33.6) | 20.8 (18.6–23.3) |
| African American | 84.1 (77.2–89.2) | 56.2 (47.5–64.5) | 22.7 (15.8–31.5) | 15.8 (9.2–25.8) | 15.8 (7.2–31.1) | 2.9 (0.4–17.9) |
| Other | 81.8 (69.4–89.9) | 54.5 (39.8–68.5) | 23.3 (11.5–41.6) | 16.7 (5.4–41.0) | 0.0 (0.0–100.0) | 0.0 (0.0–100.0) |
| Surgery | ||||||
| No | 92.0 (90.0–93.6) | 72.1 (69.0–75.0) | 52.5 (48.8–56.2) | 40.9 (37.0–44.9) | 33.7 (29.5–38.2) | 18.8 (15.7–22.2) |
| Yes | 89.3 (87.1–91.1) | 64.7 (61.4–67.8) | 43.9 (40.4–47.4) | 28.9 (25.5–32.6) | 25.4 (21.7–29.4) | 21.0 (18.1–24.3) |
| Chemotherapy | ||||||
| No | 78.3 (70.0–84.8) | 54.7 (45.6–63.5) | 36.2 (27.6–45.8) | 21.7 (14.4–31.4) | 16.7 (9.9–26.7) | 9.9 (5.7–16.7) |
| Yes | 91.5 (90.1–92.7) | 69.3 (67.1–71.5) | 48.8 (46.2–51.5) | 35.7 (33.0–38.6) | 30.6 (27.6–33.7) | 21.1 (18.8–23.6) |
| Radiation | ||||||
| No | 91.5 (89.6–93.1) | 69.5 (66.5–72.4) | 47.5 (44.0–50.9) | 35.3 (31.7–39.0) | 29.3 (25.5–33.4) | 13.5 (11.1–16.4) |
| Yes | 89.7 (87.4–91.5) | 67.0 (63.7–70.2) | 48.5 (44.7–52.2) | 34.0 (30.2–38.1) | 29.6 (25.4–34.1) | 27.6 (24.1–31.5) |
| Decade | ||||||
| 1960/70s | 90.8 (87.7–93.2) | 70.9 (66.5–75.0) | 55.4 (50.7–60.0) | 43.6 (39.0–48.3) | 33.3 (29.0–37.8) | 18.3 (16.0–20.9) |
| 1980s | 94.1 (91.6–95.9) | 72.2 (68.0–76.1) | 46.9 (42.5–51.5) | 33.2 (29.1–37.6) | 27.3 (23.3–31.6) | 25.8 (21.0–31.3) |
| 1990s | 89.7 (86.8–92.0) | 62.1 (57.9–66.1) | 40.6 (36.4–45.0) | 24.7 (20.3–29.7) | 15.4 (7.9–27.9) | |
| 2000s | 87.6 (83.9–90.5) | 69.7 (64.3–74.7) | 62.3 (49.6–73.5) | |||
| Diagnosis | ||||||
| Leukemia | 94.1 (91.8–95.7) | 76.7 (72.9–80.1) | 59.5 (55.0–63.9) | 48.8 (44.1–53.6) | 39.9 (34.9–45.1) | 19.7 (16.4–23.5) |
| Lymphoma | 93.4 (91.1–95.1) | 69.9 (65.9–73.5) | 43.4 (38.8–48.1) | 27.9 (23.4–32.8) | 26.4 (21.3–32.2) | 29.3 (24.3–34.8) |
| CNS cancer | 80.0 (72.0–86.1) | 41.6 (33.3–50.4) | 28.7 (20.9–38.0) | 14.3 (8.1–24.0) | 8.0 (3.0–19.5) | 0.0 (0.0–100.0) |
| Sarcoma | 85.6 (81.0–89.2) | 62.9 (56.9–68.5) | 43.0 (36.8–49.4) | 31.1 (25.0–38.0) | 26.8 (20.1–34.7) | 19.7 (15.0–25.6) |
| Other cancer | 87.6 (83.2–91.0) | 66.2 (60.2–71.7) | 47.1 (40.9–53.4) | 27.4 (21.5–34.2) | 18.6 (13.1–25.8) | 9.6 (6.2–14.7) |
Numbers in parentheses identify 95% confidence interval for each estimate.
Predictors of follow-up were also examined for each time interval based on multivariate binomial regression modeling and revealed that age at diagnosis (favoring those diagnosed at younger ages), cancer diagnosis (lymphoma and leukemia), and decade of patient diagnosis (earlier decades) were significant predictors of adherence across all time periods (p < 0.05) seen in Table 5. Patterns of completion of follow-up were variable at specific points during follow-up. For example, gender was not a significant factor in years immediately following treatment completion, but was 6–10, 11–15, and 16–20 years post-treatment. Although no treatment modality (chemotherapy, radiation, surgery) consistently contributed to follow-up adherence, relevant patterns were observed. Consistent with results from univariate models, presence of chemotherapy was a significant model predictor in early follow-up years, whereas radiation appeared to play a role in later follow-up years; specifically, 26+ years since treatment (adjusted odds ratio [OR] = 2.72, 95% CI = 1.9–3.9).
Table 5.
Multivariate Associations Odds Ratios (95% Confidence Interval)
| 1–5 years | 6–10 years | 11–15 years | 16–20 years | 21–25 years | 26+ years | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 5–9 vs. 0–4 | 0.78 (0.45–1.35) | 0.96 (0.69–1.33) | 0.94 (0.69–1.28) | 1.64 (1.15–2.35) | 1.12 (0.73–1.72) | 0.85 (0.53–1.36) |
| 10–15 vs. 0–4 | 0.51 (0.31–0.84) | 0.75 (0.55–1.02) | 0.73 (0.54–0.98) | 1.34 (0.95–1.88) | 1.71 (1.14–2.56) | 1.31 (0.85–2.03) |
| 15–21 vs. 0–4 | 0.48 (0.27–0.84) | 0.45 (0.32–0.64) | 0.62 (0.43–0.88) | 2.01 (1.35–2.99) | 2.77 (1.73–4.43) | 3.01 (1.89–4.78) |
| Gender | ||||||
| Female vs. male | 1.14 (0.82–1.59) | 1.33 (1.08–1.65) | 1.46 (1.17–1.82) | 1.41 (1.10–1.82) | 0.85 (0.62–1.16) | 0.61 (0.44–0.84) |
| Race | ||||||
| African American vs. Caucasian | 0.51 (0.31–0.85) | 0.56 (0.38–0.83) | 0.28 (0.17–0.45) | 0.39 (0.20–0.75) | 0.51 (0.21–1.29) | 0.10 (0.01–0.73) |
| Other vs. Caucasian | 0.48 (0.23–1.01) | 0.55 (0.29–1.04) | 0.32 (0.13–0.79) | 0.31 (0.08–1.18) | 0.00 (0.00–infinity) | 0.00 (0.00–infinity) |
| Surgery | ||||||
| Yes vs. no | 1.58 (1.02–2.44) | 1.13 (0.86–1.49) | 1.04 (0.78–1.38) | 0.97 (0.70–1.35) | 1.39 (0.90–2.16) | 1.41 (0.92–2.17) |
| Chemotherapy | ||||||
| Yes vs. no | 2.47 (1.45–4.21) | 1.46 (0.96–2.22) | 1.34 (0.85–2.11) | 1.36 (0.78–2.36) | 1.74 (0.88–3.42) | 2.05 (1.00–4.18) |
| Radiation | ||||||
| Yes vs. no | 0.93 (0.66–1.33) | 1.17 (0.93–1.48) | 1.59 (1.25–2.02) | 1.50 (1.14–1.98) | 1.40 (1.00–1.95) | 2.72 (1.90–3.90) |
| Diagnosis | ||||||
| Lymphoma vs. leukemia | 1.13 (0.65–1.97) | 0.87 (0.63–1.21) | 0.51 (0.37–0.71) | 0.32 (0.22–0.48) | 0.28 (0.17–0.46) | 0.50 (0.30–0.83) |
| CNS cancer vs. leukemia | 0.23 (0.11–0.45) | 0.21 (0.13–0.34) | 0.22 (0.13–0.38) | 0.16 (0.07–0.34) | 0.09 (0.03–0.28) | 0.00 (0.00–infinity) |
| Sarcoma vs. leukemia | 0.35 (0.19–0.63) | 0.58 (0.39–0.86) | 0.49 (0.33–0.73) | 0.40 (0.25–0.62) | 0.32 (0.18–0.56) | 0.55 (0.32–0.96) |
| Other cancer vs. leukemia | 0.39 (0.21–0.72) | 0.64 (0.43–0.95) | 0.66 (0.44–0.99) | 0.42 (0.27–0.67) | 0.25 (0.14–0.45) | 0.19 (0.10–0.36) |
| Decade | ||||||
| 1980s vs. 1960/70s | 1.80 (1.07–3.03) | 1.20 (0.89–1.62) | 0.79 (0.60–1.04) | 0.67 (0.51–0.90) | 0.81 (0.59–1.10) | 1.37 (0.96–1.95) |
| 1990s vs. 1960/70s | 1.20 (0.74–1.93) | 0.85 (0.63–1.15) | 0.74 (0.56–0.99) | 0.50 (0.36–0.71) | 0.39 (0.17–0.90) | |
| 2000s vs. 1960/70s | 0.84 (0.52–1.37) | 1.22 (0.86–1.74) | 2.14 (1.19–3.87) | |||
Discussion
We are unaware of prior reports that have assessed short-, intermediate-, and long-term follow-up among survivors of childhood cancers. In this study, we explored loss to follow-up and factors influencing patterns of follow-up among childhood cancer survivors following completion of therapy. Based on these analyses, loss to follow-up in our study population occurred rapidly and without evidence of stabilization. After 5 years following completion of therapy, 82% completed a follow-up visit and this proportion decreased to 59% and 42% at 10 and 15 years following completion of therapy. This observation is particularly concerning because it has been shown that incidence of chronic disease and the emergence of late effects related to cancer therapy increase as survivors age; moreover, there is no evidence that the incidence of chronic disease plateaus over time.6,17 Patients are lost to follow-up as time increases from completion of therapy, yet they are also at greater risk of late effects as time passes.
Published studies have noted that many survivors report receiving “general healthcare” in a community setting; however, it is not clear whether these at-risk patients receive survivor-focused care from community-based clinicians.2,12,18 While PCPs, including internists and family physicians, are willing to provide care to childhood cancer survivors, knowledge gaps may exist with regard to surveillance guidelines set forth by the COG and patients may not report their cancer history to their PCP.19,20 In addition, when presented with a hypothetical clinical vignette, physicians could not optimally identify the recommended screening and responded that they would be most comfortable providing care in consultation with a physician in a cancer center or long-term follow-up program. In response to these and related findings, SCPs detailing a patient's diagnosis, treatment, recommended surveillance, and contact information are recommended by the Institute of Medicine.6,15 A survey of PCPs reported that an SCP is a valued tool in providing care for adult survivors of childhood cancer; however, Kadan-Lottick et al. noted that only 15% of patients reported that they had received an SCP.14,21
Regardless of whether or not patients lost to follow-up are likely to be seeing a PCP for further care, there is a demonstrated knowledge deficit in cancer survivors regarding their previous disease and risk of further complications as a result of their malignancy and/or therapy. In one survey, only 72% of patients could report their cancer diagnosis accurately. In addition, this same study reported that only 35% of patients believed that they were at risk for serious health issues as a result of their therapy.14 In order for patients to receive proper care, they must be knowledgeable about their diagnosis and risks to advocate for necessary medical surveillance. Future studies might explore on the use of patient education regarding late effects to see whether that might help to increase rates of follow-up.
Our study also examined several factors to assess whether they impacted rates of follow-up. Patients diagnosed with CNS tumors had the lowest rates of follow-up and patients with leukemia tended to demonstrate better follow-up rates defined as 1 or more visits over a 5-year time interval. We hypothesize that most of the leukemia patients were treated on a chemotherapy regimen administered over an extended period of time, supporting the development of a stronger relationship between the patient/family and the medical care team. Anecdotally, this is supported by our observation that adult survivors are not infrequently accompanied to their long-term follow-up visits by their parents well after the completion of their active treatment.
Patients aged 15–21 years at time of diagnosis were less likely to continue regular follow-up beyond 6–10 years after completing therapy. Interestingly, adolescents and young adults are also less likely to be compliant with cancer therapy, with nonadherence rates ranging from 27% to 63%, and it is possible that similar factors are driving low rates of follow-up care.22–24
We did not observe that gender affected rates of follow-up. However, other studies that have evaluated healthcare in survivor populations found that male survivors are less likely to report receiving overall healthcare than women.2,8,18 Nathan et al. reported that older, male, black, or uninsured patients received less risk-based survivor-focused care than their counterparts.8 Despite limited numbers of non-white patients, we observed lower rates of follow-up among non-whites, with these differences apparent beyond 10 years after completion of therapy.
Since a substantial proportion of survivors of childhood cancer are lost to follow-up, it is important to understand whether it is possible to re-engage survivors back into long-term medical care. Edgar et al. investigated this issue and reported that among survivors who had not followed up in 2 or more years, one-third of patients sent a postal survey re-engaged in follow-up care.25 Of note, about half of the patients who were brought back to the long-term follow-up clinic after a lapse in their attendance were experiencing some late effects and 20% reported having two or more late effects, underscoring the importance of long-term care in this population.25 Moreover, after re-engagement efforts, Edgar et al. found that 38% of survivors returned questionnaires with 35% of the respondents requesting long-term follow-up care appointments. These re-engagement efforts increased the percentage of survivors in active follow-up from ∼54% to 74%.25
Recent literature regarding the St. Jude Lifetime Cohort (SJLIFE) also offers encouraging results related to re-engagement for evaluating and monitoring long-term health outcomes.26 Based on an update provided in 2014–2015, roughly 63% of eligible survivors agreed to complete an initial evaluation at St. Jude's, with nearly 60% completing at least one visit.26 Of note, the SJLIFE protocol utilizes various strategies to promote patient engagement, including covering the costs of transportation, meals, and housing, financial incentives as well as free clinic visits27 and it is not clear whether other centers have similar resources available to achieve these results.
Despite multiple attempts to re-engage childhood cancer survivors at our institution, we have continued to experience challenges. Due to low response rates to mailings, marriages, relocations, and a relatively large geographical catchment area, re-engagement, or even contact, has been an ongoing challenge. In addition to aiding in re-engagement efforts, the current study identifies subpopulations at greater risk of “loss” to follow-up and can help to prevent the observed patterns from continuing.
Our study has some inherent limitations. The results of this single-institutional study may limit the generalizability to other institutions/geographical regions. Furthermore, because it relied on a retrospective review of existing medical records, it did not allow for additional information to be gathered regarding why those lost to follow-up were not participating in surveillance. In addition, it was presumed that patients not returning to RPCI were not getting appropriate care elsewhere. These modest limitations are balanced, however, by noteworthy and unique strengths. RPCI has operated a long-term follow-up clinic for several decades, and as a result, survivor records were available for review dating back into the 1960s. This provided a larger population of survivor records and the opportunity to assess follow-up over an extended time period.
In summary, our study examined follow-up patterns in childhood cancer survivors overall and within subgroups. To our knowledge, this is the first comprehensive assessment tracking short-, intermediate-, and long-term loss to follow-up among a population of childhood cancer survivors. Beyond objective data on the generally lower rates of follow-up, these findings identify differences in rates of follow-up, which warrant further examination. Continued research is needed to determine the potential impact of patient education and PCP education in helping to improve rates of follow-up in the growing at-risk population of survivors of pediatric and young adult cancer.
Acknowledgments
This study was supported, in part, by the Roswell Park Cancer Institute and National Cancer Institute (NCI) grants 3P30CA01605. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This research was approved by the RPCI IRB.
Author Disclosure Statement
Dr. D.A.R. has received research support from Hyundai Hope on Wheels and the Roswell Park Cancer Institute Alliance Foundation related to survivorship research among pediatric cancer patients. Drs. D.A.R., M.C.M., K.A., and M.A.Z., and Ms. C.C. and Ms. J.E.H, all declare that they have no conflicts of interest related to the content of this article.
References
- 1.Howlader NNA, Krapcho M, Garshell J, et al. (Eds). SEER cancer statistics review, 1975–2012, April 2015 edn. Bethesda, MD: National Cancer Institute; 2014 [Google Scholar]
- 2.Casillas J, Oeffinger KC, Hudson MM, et al. Identifying predictors of longitudinal decline in the level of medical care received by adult survivors of childhood cancer: a report from the childhood cancer survivor study. Health Serv Res. 2015;50(4):1021–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skinner R, Wallace WH, Levitt G. Long-term follow-up of children treated for cancer: why is it necessary, by whom, where and how? Arch Dis Child. 2007;92(3):257–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82 [DOI] [PubMed] [Google Scholar]
- 7.Skinner R, Wallace WH, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7(6):489–98 [DOI] [PubMed] [Google Scholar]
- 8.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashore L. Childhood and adolescent cancer survivors' knowledge of their disease and effects of treatment. J Pediatr Oncol Nurs. 2004;21(2):98–102 [DOI] [PubMed] [Google Scholar]
- 10.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians' and oncologists' knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26(12):1403–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood AaYAC, Version 4.0. Monrovia, CA: Children's Oncology Group; 2013. Accessed June20, 2016 from: www.survivorshipguidelines.org [Google Scholar]
- 12.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153(7):442–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH. Improving screening practices in childhood cancer survivors at risk for treatment-related heart failure. J Clin Oncol. 2014;32(35):3923–5 [DOI] [PubMed] [Google Scholar]
- 14.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287(14):1832–9 [DOI] [PubMed] [Google Scholar]
- 15.Hewitt MGS, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006 [Google Scholar]
- 16.Ganz P, Horning SJ. Cancer survivorship: today and tomorrow. New York: Springer; 2007 [Google Scholar]
- 17.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan PC, Daugherty CK, Wroblewski KE, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7(3):275–82 [DOI] [PubMed] [Google Scholar]
- 20.Suh E, Daugherty CK, Wroblewski K, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160(1):11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalom MM, Hahn EE, Casillas J, Ganz PA. Do survivorship care plans make a difference? A primary care provider perspective. J Oncol Pract. 2011;7(5):314–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondryn HJ, Edmondson CL, Hill JW, Eden TO. Treatment non-adherence in teenage and young adult cancer patients: a preliminary study of patient perceptions. Psychooncology. 2009;18(12):1327–32 [DOI] [PubMed] [Google Scholar]
- 23.Kondryn HJ, Edmondson CL, Hill J, Eden TO. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12(1):100–8 [DOI] [PubMed] [Google Scholar]
- 24.Gesundheit BGM, Reuven OR, Gideon K. Drug compliance by adolescents and young adult cancer patients: challenges for the physician. In: Bleyer WA, Barr RD. (Eds). Cancer in adolescents and young adults. Berlin: Springer; 2007; pp.353–63 [Google Scholar]
- 25.Edgar AB, Borthwick S, Duffin K, et al. Survivors of childhood cancer lost to follow-up can be re-engaged into active long-term follow-up by a postal health questionnaire intervention. Eur J Cancer. 2012;48(7):1066–73 [DOI] [PubMed] [Google Scholar]
- 26.Hudson M. LIFEline Newsletter. 2015. Accessed June20, 2016 from: www.stjude.org/content/dam/en_US/shared/www/patient-support/stjude-life/lifeline-winter-2015.pdf
- 27.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60(5):856–64 [DOI] [PMC free article] [PubMed] [Google Scholar]


