Abstract
Uterine fibroids (also known as leiomyomas or myomas) are the most common benign tumors affecting reproductive organs in women. They are monoclonal tumors of the uterine smooth muscle, which spring from myometrium. It is estimated that they occur in 50-60% of the female population and rise to 70% by the age of 50. While mostly asymptomatic, myomas can be connected with several conditions, including abnormal bleeding with subsequent anemia, pelvic masses, pelvic pain, bulk symptoms, unfavorable impact on fertility and obstetric complications. Factors, which predispose the emergence of fibroids are: hormones, Afro-American ethnicity, age, obesity, adverse pregnancy outcome history, early menarche, genetic factors, alcohol, caffeine or eating too much red meat. On the other hand, there are factors, which can decrease this risk: pregnancy, early menopause and tobacco smoking. There are several mechanisms of fertility impairment in females with fibroids: alternations in uterus function (flawed blood supply, increased contractility), changes in the normal uterus anatomy, local hormonal changes induced by fibroids. In this review the connection between fibroids and infertility is analyzed.
Keywords: uterine fibroids, leiomyomas, infertility, fertility impairment
Introduction
Uterine fibroids (leiomyomas, myomas) are the most common benign tumors affecting reproductive organs in women. They are the monoclonal tumors of uterine smooth muscle, which spring from myometrium [1]. Fibroids consist of large amounts of extracellular matrix (ECM), which contains collagen, fibronectin and proteoglycans [2, 3]. It is estimated that they affect 50-60% of the female population and rise to 70% by the age of 50 [4]. While mostly asymptomatic, myomas can be connected with several conditions, including abnormal bleeding with subsequent anemia, pelvic masses, pelvic pain, bulk symptoms, unfavorable impact on fertility and obstetric complications [5]. Despite the progress in the field of medicine, the actual cause of fibroids has not been discovered yet. However, it has been proved recently that estrogens and progesterone have some influence on the leiomyomas etiology. Scientists observed that in women undergoing progesterone therapy or treated with medroxyprogesterone acetate, there was an increase in mitotic and cellular activity [6–8]. Moreover, estrogens are connected with increased proliferation of uterine fibroid smooth muscle cells [9, 10]. There are also other factors which predispose the emergence of fibroids: Afro-American ethnicity, age, obesity, adverse pregnancy history, early menarche, genetic factors (chromothripsis), and excessive use of alcohol, caffeine or eating too much red meat. On the other hand, there are factors which can decrease this risk: pregnancy, early menopause and tobacco smoking [11–13].
Methods
A comprehensive review of literature on uterine fibroids and their influence on fertility impairment was carried out. PubMed and Google Scholar websites were used to find significant articles. Terms such as: “uterine fibroids”, “fibroids”, “leiomyomas”, “myomas” were used in combination with “fertility”, “infertility”, “fertility impairment”. References from these articles were used to identify new sources. The reports written in English and in Polish were included in the literature review. There were no restrictions on the year of publication. The existing knowledge about the connection between leiomyomas and fertility impairment was described.
Results
Donnez in his recent article mentioned several mechanisms of infertility in females with fibroids: alternations in uterus function (flawed blood supply, increased contractility), changes in the normal uterus anatomy, local hormonal changes induced by fibroids [14].
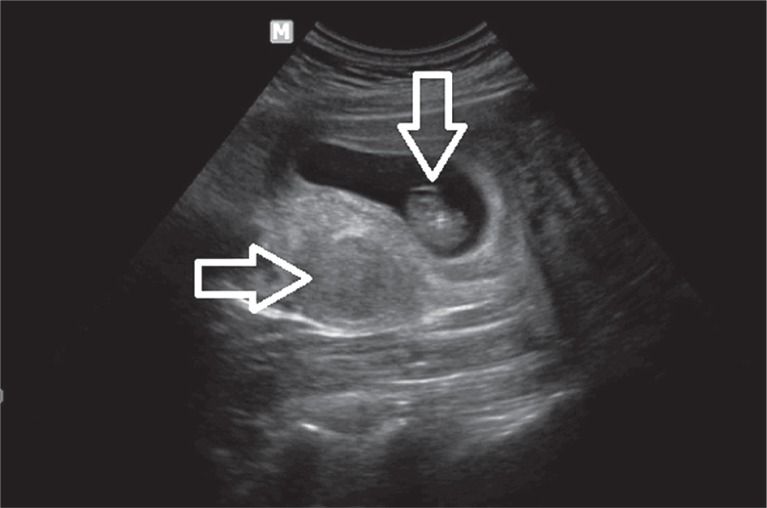
One in ten women seeking fertility treatment has fibroids [15–17]. Their effect on fertility impairment depends on the location of the fibroid. Intramural and submucosal fibroids have the most significant impact. Klatsky et al. in their systematic review stated that patients with submucosal myomas distorting the uterine cavity had lower implantation rates and increased incidence of early pregnancy loss in comparison with women without fibroids [18–21]. Pritts et al. in their meta-analysis showed that women with submucosal myomas had significantly decreased live birth rates, ongoing pregnancies, pregnancy rates and implantation rates. This type of fibroids also had a connection with an increased risk of spontaneous abortion [22]. Intramural fibroids are located within the wall of myometrium. Pritts et al. reported data which showed significantly lower rates of implantation, ongoing pregnancies, live births, and increased rates of spontaneous abortions in women with intramural leiomyomas [22]. An ultrasound image of an early pregnancy in a patient with fibroids is shown on Fig. 1.
Fig. 1.
An early intrauterine pregnancy (gestational sac and embryo – vertical arrow) located in a uterus with an intramural fibroid (horizontal arrow)
Many studies are focused on association between fibroids and in vitro fertilization (IVF) outcomes. The most extensively investigated factor is myoma location. There is a consensus that submucous uterine fibroids or intramural leiomyomas distorting the cavity of the uterus are connected with lower clinical pregnancy rates, delivery rates and higher spontaneous abortion rate after IVF or intracytoplasmic sperm injection (ISCI) treatment [5]. Christopoulos et al. presented data of lower pregnancy rates after IVF in women with non-cavity-distorting myomas [23]. Sagi-Dain et al. published results of a retrospective analysis of 744 ovum donation cycles, which were conducted in two private IVF centers between 2005 and 2012. Transvaginal ultrasound examination including the analysis of the endometrial thickness and grad measurements was performed in all recipients. The recipients with fibroid uteri had a decreased endometrial thickness, lower rates of Grade A and higher rates of Grade C endometrium. Also, in this group significantly higher spontaneous miscarriage rates were observed, which could be explained by the adverse sonographic appearance of the endometrium [24].
On the other hand, there also is contrary data. The Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation clinical trial by the Reproductive Medicine Network found no differences in conception and live birth rates in women with non-cavity-distorting intramural leiomyomas [25]. Additionally, in a study focused on pregnancy rates in the recipients of donor oocytes by Klatsky et al. no differences were found in the implantation or clinical pregnancy rates in women with or without uterine fibroids [26].
As long as conflicting data will be given, the debate about the clinical effect of non-cavity-distorting intramural fibroids will be ongoing. There are suggestions that if a clinical effect is present, it might be unmasked and as a result become more clinically significant for IVF cycles than for ovarian stimulation and intrauterine insemination.
Another question is how the size of a fibroid is associated with the harmful effect on the endometrial functions. Pritts et al. investigated this problem and found no connection between fibroid size or number and endometrial function, yet it need to be noticed that these data were obtained from only 5 studies [22].
It is noteworthy that hysteroscopic myomectomy can improve clinical pregnancy rates with timed intercourse from 21% to 39%, which was shown in a Cochrane review [27].
When discussing implantation disorders, the window of implantation (WOI) has to be defined. WOI is a narrow time period during which endometrium is receptive to implantation of the embryo. It occurs between the 7th and 10th day following the luteinizing hormone (LH) surge. This is the time when endometrium is prepared for the attachment of the blastocyst [28]. Basically, the successful implantation includes apposition, adhesion and invasion, but in practice a complex series of interactions between various processes are essential for these steps to occur. Any small change e.g. aberration could result in infertility, implantation failure or early pregnancy loss.
Previous literature reviews showed the effect of myomas on endometrial function. Submucosal fibroids produce a blunted decidualization response with decreased release of cytokines critical for implantation, such as leukemia inhibitory factor (LIF) and cell adhesion molecules. Additionally, fibroids can alter the expression of genes, which are crucial for implantation, such as glycodelin, bone morphogenetic protein receptor type II (BMPR2). This is performed through paracrine interactions. This phenomenon affects the entire endometrium, not only the part in contact with a fibroid.
Transcription factors known as homeobox genes - homeobox A10 (HOXA10) and homeobox A11 (HOXA11), expressed in the female reproductive system are important for implantation [29]. The expression of HOXA10 is lower in endometrium of women with submucosal myomas. This decreased expression is most influential in the endometrium, which overlies the submucosal fibroid, but can be observed also throughout the endometrium [30]. The decreased expression of the cell adhesion molecule E-cadherin and HOXA10 was described in the endometrium of women with non-cavity-distorting intramural leiomyomas during the WOI [31]. 68.8% of women with uterine fibroids had low mid-secretory phase expression of HOXA10 protein [32]. Bone morphogenetic protein type II (BMP2) mediates HOXA10 expression and if there exists an increased endometrial resistance to BMP2, it may add up to a decreased expression of HOXA 10 in the endometrium of these patients [33].
Ben-Nagi et al. measured levels of interleukin (IL) 6, IL-10, tumor necrosis factor α (TNF-α), osteopontin, glycodelin in uterine flushing of women with and without submucosal myomas during WOI. There were decreased levels of glycodelin and IL-10 in uterine flushing from the mid-luteal endometrium of women with fibroids [34].
The rise of progesterone following ovulation is responsible for decidualization of endometrium marked by the increasing levels of vascular endothelial growth factor (VEGF) and prostaglandins, which results in increased endometrial blood vessel permeability allowing for extravasation of polymorphonuclear cells. These cells also produce cytokines crucial for implantation, including LIF [35].
The LIF-deficient mice showed no chance for implantation because of defective decidualization, but embryos from LIF-deficient mice were able to implant in the endometrium of a wild type mice [36]. In humans, the expression of LIF increased in the luteal phase and its peak falls during WOI. On the other hand, when submucosal fibroids exist, the luteal phase increase in expression of LIF protein is blunted [37]. Deregulation of LIF production in secretory endometrium appears clinically as unexplained fertility impairment and recurrent abortions [38].
Interleukin 11 (IL-11) is crucial to sustain decidualization. The IL-11-deficient mice can start decidualization, but are not able to complete the decidual response, which leads to pregnancy loss by day 8 [39]. In humans, IL-11 is responsible for regulation of trophoblast invasion. Its production is lower during WOI if submucosal myomas exist [37], which may lead to impaired implantation, yet new studies are needed to confirm the clinical correlation.
Progesterone also induces BMP2 secretion and its downstream target wingless-type Mouse Mammary Tumor Virus (MMTV) integration site family, member 4 (WNT) by embryonic stem cells (ESCs). The endometrium in BMP2-deficent mice cannot undergo decidualization due to the absence of BMP2 production. Despite the possibility of embryo attachment in BMP2-deficient mice, defective decidual differentiation results in impaired implantation and pregnancy loss [40]. In spite of the exposition on progesterone, WNT4 knockout mice have abnormal implantation as a result of defective ESC survival and decidualization [41]. BMP2 activation in response to progesterone may be necessary for WNT4 activation and subsequent implantation.
Uterine fibroids can put huge stress and stretch the nearby myometrium and overlying endometrium, proportionate to the size and location of the myoma. This increased uterine stretch is connected with abnormal gene expression [42–44], which is associated with physical existence of fibroids. This usually results in impaired uterine contractility. According to the recent studies, uterine contractility implicates implantation. Abnormal uterine contraction and peristalsis during mid-luteal phase in women with leiomyomas have been observed on cine magnetic resonance imaging [45]. Yoshino et al. in their study suggest that abnormal uterine peristalsis during WOI in women with intramural fibroids may lead to defective implantation and poor pregnancy outcomes in this group of patients [46], yet further studies are needed to confirm this data.
Conclusions
According to the current literature submucosal and mural leiomyomas exert significant effects on functioning and gene expression of endometrium. There is a strong relation between submucosal myomas, which distort the uterine cavity infertility. On the other hand, the clinical significance of intramural fibroids is controversial. Further studies are required to provide firm evidence for the connection of intramural myomas with infertility.
Disclosure
Authors report no conflict of interests.
References
- 1.Kim J, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358:223–231. doi: 10.1016/j.mce.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 3.Sankaran S, Manyonda IT. Medical management of fibroids. Best Pract Res Clin Obstet Gynecol. 2008;22:655–676. doi: 10.1016/j.bpobgyn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Baird DD, Dunson DB, Hill MC, et al. High cumulative invidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 5.Olive DL, Pritts EA. Fibroids and reproduction. Semin Reprod Med. 2010;28:218–227. doi: 10.1055/s-0030-1251478. [DOI] [PubMed] [Google Scholar]
- 6.Woytoń J, Tomiałowicz M, Florjański J. Mięśniaki macicy w ciąży. Ginekol Położ. 2002;73:301–306. [PubMed] [Google Scholar]
- 7.Rein M, Barbieri R, Friedman A. Progesterone: A critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:4–8. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 8.Vij U. Progestin and antiprogestin interactions with progesterone receptors in human myomas. Int J Gynecol Obstet. 1990;31:347–353. doi: 10.1016/0020-7292(90)90913-6. [DOI] [PubMed] [Google Scholar]
- 9.Andersen J, DyReyes VM, Barbieri RL, et al. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 10.Pedeutour F, Quade BJ, Weremowicz S, et al. Localization and expression of the human estrogen receptor beta gene in uterine leiomyomata. Genes Chromosomes Cancer. 1998;23:361–366. doi: 10.1002/(sici)1098-2264(199812)23:4<361::aid-gcc12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Parazzini F, La Vecchia C, Negri E, et al. Epidemiologic characteristics of women with uterine fibroids: a case control study. Obstet Gynecol. 1988;72:853–857. doi: 10.1097/00006250-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Silva PD, Sloane KA. Uterine leiomyomata: an overview. Female Patient. 1992;17:49–56. [Google Scholar]
- 13.Zaloudek C, Norrsis HJ. Mesenchymal tumours of the uterus. In: Kurman, editor. Blaustein S Pathology of the female genital tract. 4 ed. New York: Springer Verlag; 1994. pp. 487–528. [Google Scholar]
- 14.Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665–686. doi: 10.1093/humupd/dmw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook H, Ezzati M, Segars JH, McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62:225–236. [PMC free article] [PubMed] [Google Scholar]
- 16.Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39:521–533. doi: 10.1016/j.ogc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surrey ES, Minjarez DA, Stevens JM, et al. Effect of myomectomy on the outcome of assisted reproductive technologies. Fertil Steril. 2005;83:1473–1479. doi: 10.1016/j.fertnstert.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Klatsky PC, Tran ND, Caughey AB, et al. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198:357–366. doi: 10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Casini ML, Rossi F, Agostini R, et al. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22:106–109. doi: 10.1080/09513590600604673. [DOI] [PubMed] [Google Scholar]
- 20.Eldar-Geva T, Meagher S, Healy DL, et al. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcomes of assisted reproductive technology treatment. Fertil Steril. 1998;70:687–691. doi: 10.1016/s0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 21.Farhi J, Ashkenazi J, Feldberg D, et al. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. doi: 10.1093/oxfordjournals.humrep.a135748. [DOI] [PubMed] [Google Scholar]
- 22.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Christopoulos G, Vlismas A, Salim R, et al. Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using extensive matching criteria. BJOG. 2017;124:615–621. doi: 10.1111/1471-0528.14362. [DOI] [PubMed] [Google Scholar]
- 24.Sagi-Dain L, Ojha K, Bider D, et al. Pregnancy outcomes in oocyte recipients with fibroids not impinging uterine cavity. Arch Gynecol Obstet. 2017;295:497–502. doi: 10.1007/s00404-016-4273-9. [DOI] [PubMed] [Google Scholar]
- 25.Styer AK, Jin S, Liu D, et al. Association of uterine fibroids and pregnancy outcomes after ovarian stimulation-intrauterine insemination for unexplained infertility. Fertil Steril. 2017;107:756–762.e3. doi: 10.1016/j.fertnstert.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatsky PC, Lane DE, Ryan IP, et al. The effect of fibroids without cavity involment on ART outcomes independent of ovarian age. Hum Reprod. 2007;22:521–526. doi: 10.1093/humrep/del370. [DOI] [PubMed] [Google Scholar]
- 27.Bosteels J, Kasius J, Weyers S, et al. Hysteroscopy foe treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2013;(1):CD009461. doi: 10.1002/14651858.CD009461.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Achache H, Revel A. Endometrial receptivity makers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 29.Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 30.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makker A, Goel MM, Nigam D, et al. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod Sci. 2017;24:435–444. doi: 10.1177/1933719116657196. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki S, Canis M, Darcha C, et al. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24:3180–3187. doi: 10.1093/humrep/dep306. [DOI] [PubMed] [Google Scholar]
- 33.Doherty LF, Taylor HS. Leiomyoma-derived transforming growth factor-beta impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil Steril. 2015;103:845–852. doi: 10.1016/j.fertnstert.2014.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Nagi J, Miell j, Marvelos D, et al. Endometrial implantation factors in women with submucous uterine fibroids. Reprod Biomed Online. 2010;21:610–615. doi: 10.1016/j.rbmo.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 36.Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa E, Ito H, Hasegawa F, et al. Expression of leukemia inhibitory factor in the endometrium in abnormal uterine cavities during the implantation window. Fertil Steril. 2012;97:953–958. doi: 10.1016/j.fertnstert.2012.01.113. [DOI] [PubMed] [Google Scholar]
- 38.Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple filures of implantation. Am J Reprod Immunol. 1998;39:137–142. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 39.Robb L, Li R, Hartley L, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Kannan A, Das A, et al. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–e11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payson M, Malik M, Siti-Nur Morris S, et al. Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development. Fertil Steril. 2009;92:748–755. doi: 10.1016/j.fertnstert.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orisaka M, Kurokawa T, Shukunami K, et al. A comparision of uterine peristalts in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2007;135:111–115. doi: 10.1016/j.ejogrb.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 46.Yoshino O, Hayashi T, Osuga Y, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum Reprod. 2010;25:2475–2479. doi: 10.1093/humrep/deq222. [DOI] [PubMed] [Google Scholar]