Abstract
Background
Schizophrenia is associated with lower intelligence and poor educational performance relative to the general population. This is, to a lesser degree, also found in first-degree relatives of schizophrenia patients. It is unclear whether bipolar disorder I (BD-I) patients and their relatives have similar lower intellectual and educational performance as that observed in schizophrenia.
Methods
This cross-sectional study investigated intelligence and educational performance in two outpatient samples (494 BD-I patients, 952 schizophrenia spectrum (SCZ) patients), 2,231 BD-I and SCZ relatives patients, 1,104 healthy controls and 100 control siblings. Mixed-effects- and regression models were used to compare groups on intelligence and educational performance.
Results
BD-I patients were more likely to have completed the highest level of education (OR=1.88 [1.66–2.70]) despite having a lower IQ compared with controls (β=−9.09, SE=1.27, p<0.001). In contrast, SCZ patients showed both a lower IQ (β= −15.31, SE=0.86, p<0.001) and lower educational levels compared with controls. Siblings of both patient groups had significantly lower IQ than control siblings, but did not differ on educational performance. IQ scores did not differ between BD-I parents and SCZ parents, but BD-I parents had completed higher educational levels.
Conclusions
Although BD-I patients had a lower IQ than controls, they were more likely to have completed the highest level of education. This contrasts with SCZ patients, who showed both intellectual and educational deficits compared to healthy controls. Since relatives of BD-I patients did not demonstrate superior educational performance, our data suggest that high educational performance may be a distinctive feature of bipolar disorder patients.
Keywords: Intelligence, IQ, educational performance, cognition, familial vulnerability, bipolar disorder, schizophrenia
1. Introduction
Cognitive deficits are a core feature of schizophrenia (Elvevag and Goldberg, 2000; MacCabe, 2008; Kahn and Keefe, 2013). Lower intelligence is already present before illness manifestation (Woodberry et al. 2008; Dickson et al. 2012) and poor scholastic achievement is associated with an increased risk for developing schizophrenia (van Oel et al. 2002; MacCabe et al. 2008). Several studies report that clinically unaffected first-degree relatives of schizophrenia patients also show lower Intelligence Quotient (IQ) and worse educational performance than healthy controls (Kremen et al. 1998; Cannon et al. 2000; Hughes et al. 2005; McIntosh et al. 2005), implying that an increased familial vulnerability to schizophrenia is associated with intellectual deficits.
Whether intellectual deficits also occur in euthymic bipolar disorder (BD) patients is unclear. Although deficits in specific cognitive domains, e.g. executive function, attention and verbal memory, are frequently reported (Martinez-Aran et al. 2004; Robinson et al. 2006; Torres et al. 2007), results from individual studies on global intelligence in BD patients are equivocal. Whereas some studies show a lower IQ in BD patients compared to controls (Toulopoulou et al., 2006; McIntosh et al., 2005; Frantom et al. 2008; Eric et al. 2013), others demonstrate similar IQ scores (Frangou et al. 2005; Coffman et al., 1990; Pirkola et al., 2005). Furthermore, some studies show comparable IQ scores in BD patients and schizophrenia patients, but failed to include a control group in their study (McClellan et al. 2004; Reichenberg et al. 2009). These inconsistent findings on IQ in BD patients are possibly the result of methodological deficiencies: although there have been large register-based studies on the association between premorbid IQ and BD, studies that focused on IQ in BD patients have been small (n<90) (McIntosh et al. 2005; Toulopoulou et al. 2006), and in some cases lacked a control group (McClellan et al. 2004; Reichenberg et al. 2009). Four meta-analyses compared IQ of first-episode bipolar disorder patients with that of controls, using combined samples (of sizes between 237 and 298 BD patients and 218 to 615 controls) and found lower IQ in the BD patients (with an average effect size of 0.16–0.45 (Robinson et al., 2006; Arts et al., 2008; Bora et al., 2009; Bora and Pantelis, 2015).
What is known is that, in contrast to findings in schizophrenia, intellectual deficits do not appear to be present before onset of BD (Reichenberg et al. 2002; Zammit et al. 2004); and intelligence in first-degree relatives of BD patients appears to be preserved (Balanza-Martinez et al. 2008). In fact, recent studies suggest that high premorbid intelligence (Gale et al. 2013) and excellent scholastic performances (MacCabe et al. 2010) are associated with an increased risk of developing BD. However, since intelligence after disease onset has not been examined in these studies, the question remains whether BD patients also had a higher IQ.
Since research in schizophrenia patients demonstrates clear evidence for general IQ deficits (Kahn and Keefe, 2013) and robust associations have been found for IQ and other disease-related measures such as brain morphology (Deary et al., 2010) and genetic background (Davies et al., 2011; McIntosh et al., 2013), this study focuses on general IQ in both BD and schizophrenia patients.
Furthermore, given the strong genetic influence in schizophrenia (Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011) and BD (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011) and some degree of genetic overlap between them (Purcell et al. 2009), it is also interesting to investigate the contribution of a familial vulnerability to differences in intelligence and educational performance. Additionally, delineating intelligence and educational performance is important, since other factors besides intelligence are predictive of educational performance (Chamorro-Premuzic and Furnham, 2003; Deary et al. 2007).
We investigate IQ after onset of disease and educational performance in schizophrenia and BD patients and healthy controls. We include parents and siblings of these patient groups and siblings of controls to investigate IQ and educational performance and the association of a familial vulnerability with the disorders.
2. Methods
2.1 Study design
Data were collected by three Dutch studies: Bipolar Genetics (BiG), Dutch Genetic Risk and Outcome in Psychosis (GROUP) and CannabisQuest. All studies were approved by the medical ethical committee and all participants gave written informed consent.
BiG is an ongoing case-control study that started in June 2011 and is part of a collaboration between the University of California Los Angeles and the Dutch health care institutes University Medical Center Utrecht (UMC Utrecht), GGZ Altrecht, GGz InGeest, University Medical Center Groningen, Delta Center for Mental Health Care, Dimence, Parnassia (PsyQ) and Reinier van Arkel Group. BiG investigates genetic and phenotypic information of patients with bipolar disorder type I (BD-I), first-degree relatives and controls. Patients were recruited via clinicians, the Dutch patient association, pharmacies and advertisements. First-degree relatives were invited through the patients who participated. Controls were recruited via advertisements and among individuals who previously participated in scientific studies and agreed to be contacted for new research. Inclusion criteria for all participants were 1. Age 18 years or older 2. At least three Dutch-born grandparents 3. A good understanding of Dutch language. Patients with a somatic illness that could have influenced the diagnosis of BD were excluded. Participants were considered euthymic when they did not meet DSM-IV criteria for a mood episode in the last month according to the Structured Clinical Interview for DSM-IV (SCID-I). Participants that were not euthymic were excluded from the analyses.
The GROUP study is an ongoing longitudinal study that investigates gene-environment interaction and resilience in schizophrenia spectrum disorder (SCZ) patients, first-degree relatives, controls and siblings of controls (Korver et al. 2012). Supplementary Table 1 demonstrates the diagnoses of the SCZ patients. The GROUP study is conducted by four Dutch university departments of psychiatry (Amsterdam, Groningen, Maastricht and Utrecht). Patients were included when they were between 16 and 50 years of age, had a diagnosis of a non-affective psychotic disorder according to the DSM-IV and a good comment of Dutch language. First-degree relatives were invited through the patients. The CannabisQuest study is a cross-sectional study that included adolescents and young adults from the general population (Schubart et al. 2011; Vinkers et al. 2013). Participants filled out an online questionnaire and were subsequently assessed by a psychiatric interview and neuropsychological tests at the UMC Utrecht.
2.1.1 Participants
For the current study, we included BD-I patients and first-degree relatives of BD-I patients (participants of BiG), SCZ patients and first-degree relatives of SCZ patients (participants of GROUP) and unrelated community controls. Controls were included from all three studies (BiG, GROUP, CannabisQuest), siblings of controls were included by the GROUP study only. Participants were older than 15 years of age. The three studies used identical neuropsychological tests and educational assessment. All psychiatric and neuropsychological assessments occurred at the UMC Utrecht or one of their academic collaborative institutes. To avoid inclusion of an unrepresentative population, we did not exclude controls with a psychiatric diagnosis other than a psychotic disorder or BD. Controls and their siblings with a diagnosis of BD or a psychotic disorder or with a first-degree relative with BD or a psychotic disorder were excluded. After excluding participants who did not meet the inclusion criteria, data were available for 494 BD-I patients, 952 SCZ patients, 135 parents of BD-I patients (BD-I parents), 896 parents of SCZ patients (SCZ parents), 161 siblings of BD-I patients (BD-I siblings), 1,039 siblings of SCZ patients (SCZ siblings), 1,104 controls and 100 siblings of controls (control siblings). Table 1 shows the characteristics of the participants included in the analyses. For an overview of the excluded participants, see Supplementary Figure 1.
Table 1.
Demographics: Participants included in IQ analyses
| Patients BD-I n=494 |
Parents BD-I n=135 |
Siblings BD-I n=161 |
Patients SCZ n=952 |
Parents SCZ n=896 |
Siblings SCZ n=1,039 |
Controls n=1,104 |
Control siblings n=100 |
BD-I patients vs. SCZ patients vs. controls |
BD-I siblings vs. SCZ siblings vs. controls |
BD-I parents vs. SCZ parents |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender male n (%)* | 217 (43.9%) | 57 (42.2%) | 49 (30.4%) | 741(77.8%) | 380 (42.4%) | 480 (46.2%) | 538 (48.7%) | 41 (41.0%) | BD-I=controls<SCZ | BD-I=control=SCZ | BD-I=SCZ |
| Age mean (SD)* | 49.3 (12.1) | 65.0 (7.1) | 54.4 (11.3) | 27.2 (7.0) | 54.8 (6.8) | 28.0 (8.3) | 28.9 (12.5) | 30.3 (11.6) | BD-I>controls>SCZ | BD-I>controls>SCZ | BD-I>SCZ |
| Age at onset mean (SD)* | 32.0 (10.5) | 22.5(6.5) | BD-I >SCZ | ||||||||
| Disease history n (%)*1 | 31 (23.0%) | 63 (39.1%) | 195 (21.8%) | 176 (16.9%) | 274 (24.8%) | 12 (12.0%) | BD-I>control=SCZ | BD-I=SCZ | |||
| IQ mean (SD)* | 96.8 (14.1) | 102.2 (15.1) | 106.3 (16.1) | 95.0 (15.8) | 102.9 (17.0) | 102.8 (15.7) | 107.8 (15.2) | 111.2 (15.1) | BD-I<controls>SCZ | BD-I<controls>SCZ | BD-I=SCZ |
Significant difference between groups at p<0.01
Lifetime psychiatric diagnosis. Controls with BD or a psychotic disorder or with first-degree relatives with BD or a psychotic disorder are not included.
2.2 Diagnosis
BD-I patients were diagnosed using the Structured Clinical Interview for DSM-IV (SCID-I) (First et al. 1997). In addition, we also used the SCID-I to establish lifetime history of psychotic symptoms in BD-I patients. Relatives of BD-I patients and controls included by the BiG study were diagnosed by the Mini-International Neuropsychiatric Interview (Sheehan et al. 1998). Participants included by the GROUP study were assessed through the Schedules for Clinical Assessment in Neuropsychiatry 2.1 (Wing et al. 1990) or the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al. 1992). Controls that participated in the CannabisQuest study were diagnosed by the SCID-I. Disease history was considered positive when lifetime criteria for any psychiatric disorder were met. We obtained family history of psychiatric diseases through the Family Interview Genetic Studies (Maxwell, 1992). For SCZ patients, age at onset was assessed by the Life Chart Schedule (Sartorius et al., 1996) as previously reported (Apeldoorn et al. 2014). For BD-I patients, age at onset was defined as age of first medication as reported in the Dutch version of the Questionnaire Bipolar Disorders (Leverich et al. 2001; Suppes et al. 2001), given the insidious onset of BD-I and the high probability of recall bias in the retrospective assessment of first reported symptoms. When age at first reported symptoms was used in the models instead of age at first medication, the reported results did not change.
2.3 Intelligence Quotient
To estimate IQ we used four subtests of the Dutch version of the Wechsler Adult Intelligence Scale-III (WAIS-III) (Wechsler D., 1997) consisting of the subtests ‘Information’, ‘Block Design’, ‘Digit Symbol Coding’ and ‘Arithmetic’. Each subtest belongs to one of the four indices (‘Information’ to the Verbal Comprehension Index; ‘Block Design’ to the Perceptual Organization Index; ‘Digit Symbol Coding’ to the Processing Speed Index; ‘Arithmetic’ to the Working Memory Index). The combination of these subtests has been shown to most fully account for full-scale IQ in both schizophrenia patients (R2=0.90) and controls (R2=0.86) (Blyler et al. 2000).
2.4 Educational performance
In the Netherlands, most schools are state schools which are ordinally organized into primary, secondary and tertiary education tiers. From 4 until 12 years of age, all children receive primary education. After 12 years, children are streamed into four levels of secondary education (low, intermediate, high preparatory vocational and pre-university), each level requiring greater intellectual and scholastic abilities (Vonk et al. 2012). After passing the examinations in secondary education, there are three levels of tertiary education possible (intermediate professional education, higher professional education and university). After achieving a master’s degree at university, it is possible to enroll in a doctoral program. The Dutch school system, with different educational levels and similar quality of education across schools, allows a detailed insight into intellectual and scholastic ability.
We asked participants to record their highest completed level of education. To have approximately equally distributed groups, we delineated six categories: Level 1: Low (no education, primary education and low secondary education); Level 2: Intermediate secondary education; Level 3: Intermediate professional education; Level 4: High preparatory vocational and pre-university; Level 5: Bachelor degree (Higher professional education) and Level 6: University (Master’s degree or PhD degree).
2.5 Statistical analyses
We studied the association of group status (five comparisons: 1. BD-I patients versus controls and SCZ patients versus controls 2. BD-I patients versus SCZ patients 3. BD-I siblings versus control siblings and SCZ siblings versus control siblings 4. BD-I siblings versus SCZ siblings 5. BD-I parents versus SCZ parents) with two outcome variables: IQ and educational performance. Since we did not include parents of controls, we compared BD-I parents with SCZ parents. The statistical analyses were carried out using the R package for statistical computing (R core team, 2014) and SPSS 20.0. Assumptions were checked before analysing data. Subjects with missing data were excluded listwise from the analyses. We used ANOVA and t-tests to compare the patient, control and parent groups for age and age at onset and χ2-tests to compare these groups for gender and disease history. Furthermore, we compared BD-I patients with and without a lifetime history of psychotic symptoms on IQ and educational performance using a t-test and a χ2-test. To estimate whether BD-I patients with a lifetime history of psychotic symptoms had a greater cognitive decline than BD-I patients without a history of psychotic features, we calculated standardized residuals of IQ scores by adjusting for educational performance. We compared these standardized residuals using a t-test.
We used mixed-effects models with ‘family’ (indicating the family a person belonged to, used to account for relatedness within the sibling samples) as random factor to compare the three sibling groups on age, gender and disease history. We checked whether selective drop-out had occurred for missing values by comparing the participants included in the IQ analyses with the participants included in the analyses on educational performance. We conducted χ2-tests for gender, disease history and frequency of subjects within the analyses and performed t-tests for age, age at onset and IQ.
First, we studied the association of the disorders with IQ. To investigate whether patients differed from controls, we used a linear mixed-effects model with two dummy variables (BD-I versus controls and SCZ versus controls) as main determinants and IQ as outcome. We included a random factor ‘study’ (indicating the study a participant belonged to) to control for bias that may occur when samples from different studies are compared. To investigate whether siblings of patients differed from control siblings on IQ we used a linear mixed-effects model with two dummy variables (BD-I siblings versus control siblings and SCZ siblings versus control siblings) as main determinants, IQ as outcome and ‘study’ and ‘family’ as random factors.
Since the indicator ‘study’ was identical for the patient, parent and sibling groups, we were unable to use this indicator as random factor for comparisons between BD-I and SCZ patients and BD-I and SCZ relatives. Therefore, we conducted two separate linear models with IQ as outcome and patient (BD-I patients versus SCZ patients) and parent (BD-I parents versus SCZ parents) groups as main determinants. To compare BD-I siblings with SCZ siblings, we conducted a linear mixed-effects model with sibling group as main determinant and ‘family’ as random factor.
Subsequently, we studied the association of the disorders with educational performance. We applied five different thresholds for the six categories for level of education and compared participants that completed an educational level and above with the remaining group (threshold 1: completing ‘Intermediate secondary education’ or higher versus completing ‘Low’; threshold 2: completing ‘Intermediate professional education’ or higher versus below; threshold 3: completing ‘High preparatory vocational and pre-university’ or higher versus below; threshold 4: completing ‘Bachelor’ or higher versus below; threshold 5: completing ‘University’ versus below). We used logistic regression analyses with dummy coding for patients and controls to investigate the differences in educational performance between patients and controls. Furthermore, we conducted logistic regression analyses to investigate educational performance between patient groups and parent groups. In addition, to estimate differences between parents and controls in absence of a group of parents of controls, we ran 10,000 permutation tests in which we compared the parent groups with a random sample of 600 controls. We used this resampling method to reduce the chance of selecting a biased subsample of the control group. We analysed educational performance of siblings with a generalized mixed model with ‘family’ as random factor. Since we used five thresholds as outcome variables, we corrected for multiple testing by applying a Bonferroni correction and considered p-values <0.01 as statistically significant.
Additionally, to account for illness effects in siblings, we excluded 14 BD-I and 50 SCZ siblings with a psychotic disorder or BD and repeated the analyses of IQ and educational performance. We also repeated the IQ and educational performance analyses in BD-I patients, SCZ patients and controls older than 34 years of age to rule out that age differences between these groups influence the results.
To investigate whether BD-I patients with high educational performance but a low IQ constitute a subgroup of patients with rapid cognitive decline, we compared the characteristics of those patients with other BD-I patients with high educational performance. We compared patients with an IQ below 95 (1/3 standard deviation below the average of 100) who had completed University with normal IQ BD-I patients who had completed University.
Age and gender were covariates in all models and age at onset was a covariate in the comparison of BD-I patients and SCZ patients. To be sure that our results were not biased by the different measurements of age at onset in BD and SCZ patients, we repeated the analyses excluding age at onset as covariate. We calculated Cook’s Distances to investigate potential outliers. For two thresholds in the sibling comparisons (‘Intermediate secondary education’ and ‘University’) we could not calculate Cook’s Distance due to non-convergence of the mixed-effects model, so we calculated Cook’s Distance without a random factor in a linear model.
3. Results
We included 4,881 participants. Data on educational performance were missing for 24 BD-I patients, 8 SCZ patients, 5 BD-I parents, 54 SCZ parents, 6 BD-I siblings, 17 SCZ siblings and 56 controls. Listwise exclusion of these participants resulted in a sample of 4,714 participants included in the analyses of educational performance. There was no evidence of selective drop-out for missing values; the frequency of subjects within each group did not change (Patients and controls: χ2=0.54, p=0.76; Patients: χ2=0.27, p=0.60; Parents: χ2=0.03, p=0.85; Siblings and control siblings: χ2=0.05, p=0.98; Siblings: χ2=0.03, p=0.86) and there were no differences in demographic characteristics between the participants included in the IQ analyses and the participants included in the analyses of educational performance (Gender: χ2=0.004, p=0.95; Age: t=0.38, p=0.70; IQ: t=−0.60, p=0.55; Age at onset: t=0.25, p=0.80; Disease history: χ2=0.08, p=0.78).
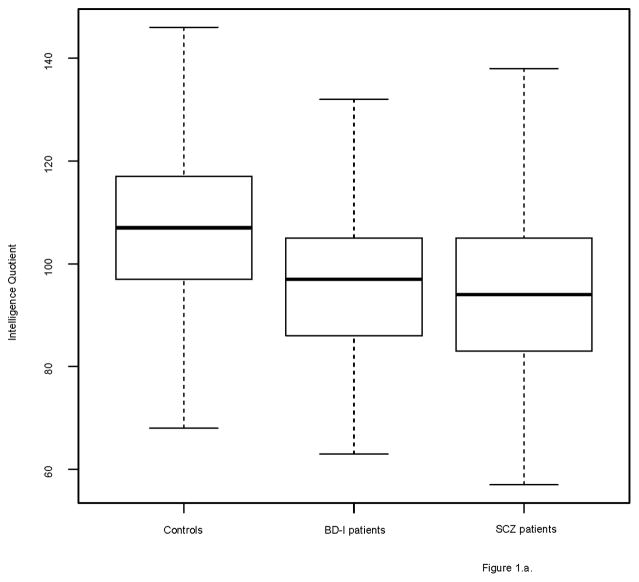
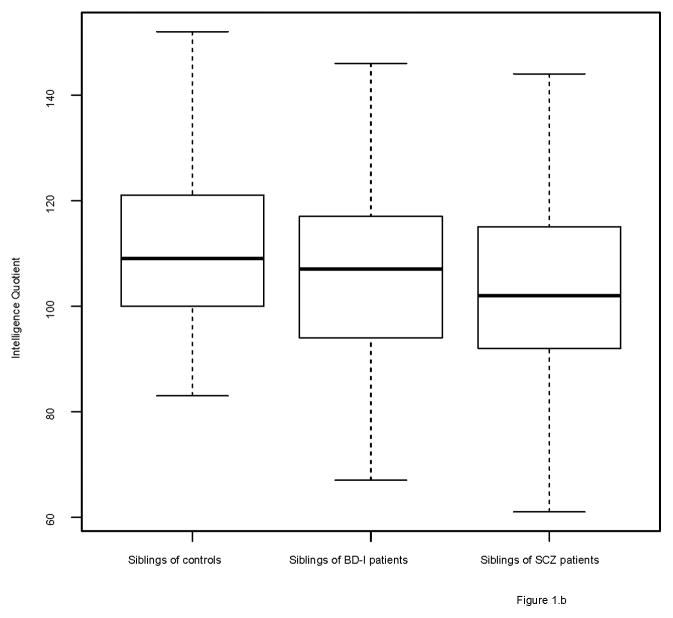
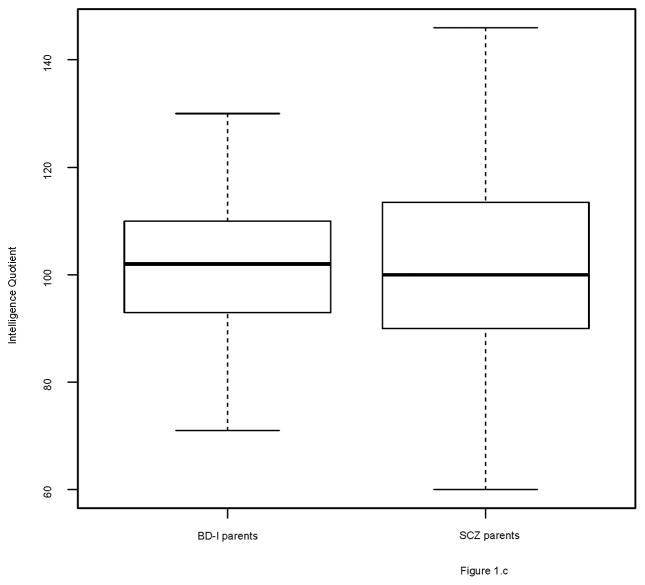
The groups differed on several demographic factors (see Table 1). Figures 1 and 2 show the distribution of IQ and educational performance by group.
Figure 1.
Boxplots of IQ scores
1a. Controls, BD-I and SCZ patients
1b. Control siblings, BD-I siblings and SCZ siblings
1c. BD-I parents and SCZ parents
Figure 2.
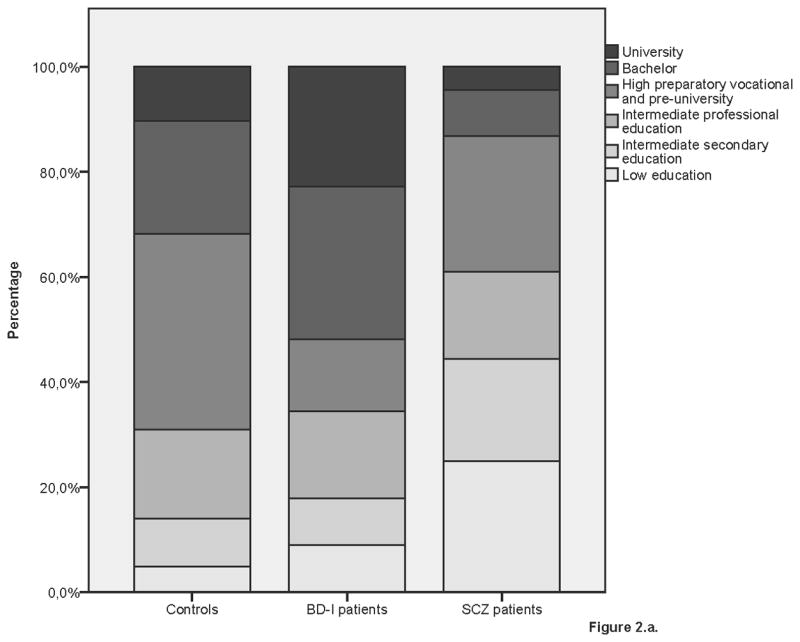
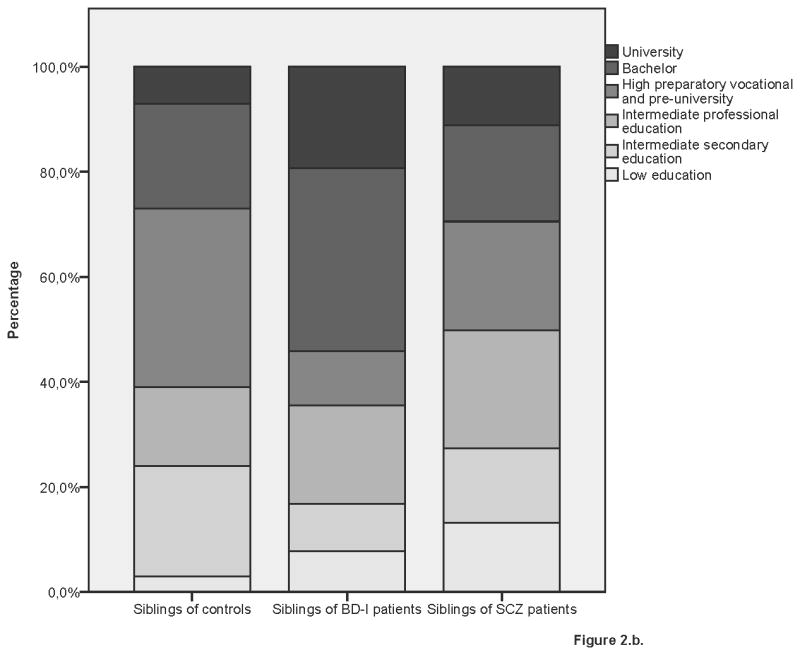
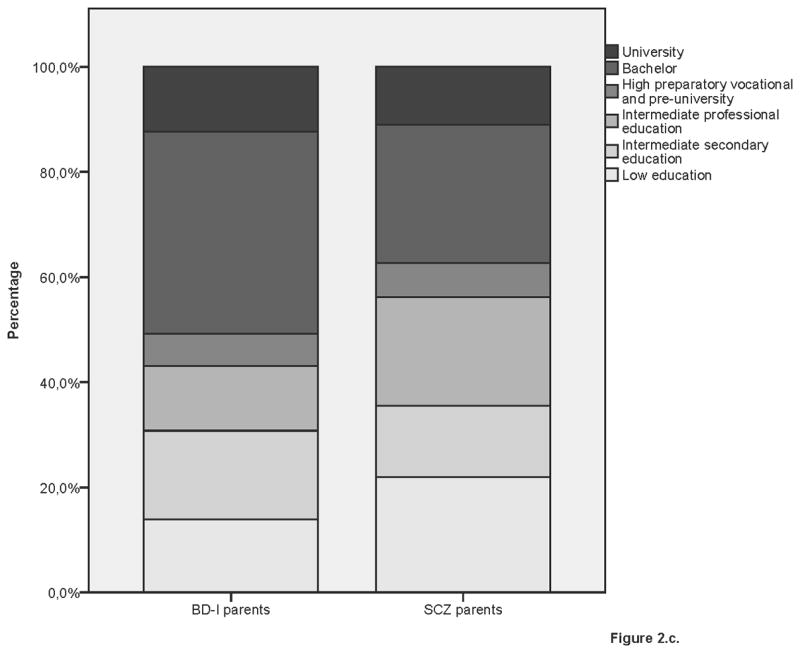
Educational performance by group
2a. Controls, BD-I and SCZ patients
2b. Control siblings, BD-I siblings and SCZ siblings
2c. BD-I parents and SCZ parents
3.2 BD-I and SCZ patients versus controls
Both BD-I and SCZ patients had a significantly lower IQ than controls (BD-I: β= −9.09, SE=1.27, p<0.001; SCZ: β= −15.31, SE=0.86, p<0.001). BD-I patients had a significantly higher IQ than SCZ patients (β=4.57, SE=1.34, p=0.001).
SCZ patients had completed lower educational levels than BD-I patients and controls. In contrast, BD-I patients were more likely to have completed the highest educational level than controls. Table 2 shows the results of the analyses of educational performance in patients and controls. In addition, excluding age at onset as covariate did not change the results in the patient analyses (data not shown).
Table 2.
The association of disorder with educational performance
| Threshold | Highest completed education | BD-I versus SCZ OR and 95% CI |
BD-I versus controls OR and 95% CI |
SCZ versus controls OR and 95% CI |
|---|---|---|---|---|
| 1 | Intermediate secondary education | 4.13 [3.40–7.22]* | 0.76 [0.64–1.24] | 0.16 [0.14–0.22]* |
| 2 | Intermediate professional education | 3.22 [2.79–4.93]* | 0.70 [0.62–0.99] | 0.22 [0.20–0.27]* |
| 3 | High preparatory vocational and pre-university | 2.82 [2.49–4.07]* | 0.97 [0.88–1.28] | 0.30 [0.28–0.36]* |
| 4 | Bachelor | 3.92 [3.42–5.81]* | 1.10 [1.00–1.44] | 0.38 [0.35–0.48]* |
| 5 | University | 5.98 [4.95–10.37]* | 1.88 [1.66–2.70]* | 0.43 [0.38–0.63]* |
Does not include unity
BD-I patients with a history of psychotic symptoms did not differ from BD-I patients without such a history on IQ (t=−0.49, p=0.63) and educational performance (χ2=4.97, p=0.42), nor did they show a more rapid cognitive decline (t=0.46, p=0.65).
We also compared patients with a large IQ decline who had completed University (n=15) with normal IQ BD-I patients who had completed University (n=92). We found no differences between the groups for age (mean=52.73, SD=11.14 vs mean=47.25, SD=11.37) (t=1.74, p=0.09), age at onset (mean 34.00, SD=9.26 vs mean=31.98, SD=9.73) (t=0.75, p=0.46) and gender (6 males (40%) vs 56 males (60.9%)) (χ2=2.31, p=0.13).
To account for the effect of age difference between the patients and controls, we repeated the IQ and educational performance analyses in patients and controls older than 34 years of age. We found very similar results as in the main analyses; BD-I patients and SCZ patients had a lower IQ than controls (BD-I: Beta=−8.08, SE=1.45, p<0.001; SCZ: Beta=−15.81, SE=1.66, p<0.001) and BD-I patients had a higher IQ than SCZ patients (Beta=5.77, SE=1.73, p=0.001). Furthermore, SCZ patients had lower educational performance than BD-I patients and controls, whereas BD-I patients were more likely to have completed University (See Supplementary table 2).
3.3 BD-I and SCZ siblings versus control siblings
BD-I and SCZ siblings had a similar IQ (β=3.53, SE=2.00, p=0.08), which was significantly lower than that of control siblings (BD-I: β= −5.73, SE=2.41, p=0.02; SCZ: β= −8.33, SE=1.68, p<0.01). Moreover, although Figure 2 suggests that BD-I siblings are higher educated than SCZ siblings and control siblings, the three sibling groups did not significantly differ in educational performance after adjusting for age and gender.
Analysis of (healthy) siblings without BD-I or a psychotic disorder revealed lower intelligence for SCZ siblings (β= −8.19, SE=1.67, p<0.001) but not for BD-I siblings (β= −4.69, SE=2.44, p=0.05) as compared with controls. Instead, BD-I siblings had a higher IQ than SCZ siblings (β=4.53, SE=2.06, p=0.03). The results for educational performance did not change: the three sibling groups did not significantly differ on educational performance (see Supplementary Table 3). Table 3 shows the results of the analyses of educational performance in relatives of patients and controls.
Table 3.
The association of familial vulnerability with educational performance
| Siblings | Parents | ||||
|---|---|---|---|---|---|
| Threshold | Highest completed Education | BD-I siblings versus SCZ siblings OR and 95% CI |
BD-I siblings versus controls siblings OR and 95% CI |
SCZ versus controls OR and 95% CI |
BD-I parents versus SCZ parents OR and 95% CI |
| 1 | Intermediate secondary education | 1.87 [0.80–21.80]1 | 0.44 [0.08–58.89]1 | 0.24 [0.05–17.63]1 | 2.00 [1.64–3.56] |
| 2 | Intermediate professional education | 0.51 [0.40–1.08] | 0.42 [0.31–1.03] | 0.87 [0.71–1.58] | 1.48 [1.27–2.33] |
| 3 | High preparatory vocational and pre-university | 1.25 [1.03–2.19] | 0.76 [0.60–1.51] | 0.62 [0.53–1.00] | 1.88 [1.63–2.87]* |
| 4 | Bachelor | 0.63 [0.52–1.16] | 0.88 [0.68–1.83] | 1.40 [1.16–2.43] | 1.83 [1.58–2.80]* |
| 5 | University | 0.43 [0.18–5.68] | 0.90 [0.26–33.74] | 2.04 [0.72–41.98] | 0.96 [0.77–1.84] |
Age was left out due to problems converging the models
Does not include unity
3.4 BD-I parents versus SCZ parents
BD-I parents had a similar IQ as SCZ parents (β= −1.21, SE=1.70, p=0.48), but were more often higher educated (see table 3). The results of 10,000 permutation tests revealed that BD-I parents had similar educational performance as random (unrelated) controls. SCZ parents had lower educational performance than unrelated controls (see Supplementary table 4).
3.5 Analysis of outliers and ethnicity
None of the participants fulfilled the criteria for outliers by Cook’s Distance. Since not all SCZ patients, SCZ relatives and controls were Dutch, we analysed the data from the Caucasian participants only (SCZ patients: n=756; SCZ parents: n=798; SCZ siblings: n=867; controls: n=1067; control siblings: n=92). The results of these analyses did not differ from the results we found for the entire group of participants (data not shown).
4. Discussion
In the largest cross-sectional study on IQ and educational performance in BD-I and SCZ patients and relatives to date, patients with established BD-I had a lower IQ, but superior educational performance relative to healthy controls. By contrast, SCZ patients had lower IQ as well as inferior educational performance compared to controls, suggesting that IQ in schizophrenia is affected in an earlier stage of the illness. The fact that educational performance of BD-I siblings and BD-I parents was comparable to that of controls while in patients it was higher, raises the question as to whether high educational performance is associated with bipolar disorder itself rather than with a familial vulnerability to develop the illness.
Cognitive deficits in BD-I patients have been reported previously (McIntosh et al. 2005; Toulopoulou et al. 2006) and our findings are, to some extent, in line with a recent meta-analysis that also showed broad cognitive deficits in 1,026 euthymic BD patients (Mann-Wrobel et al. 2011). This meta-analysis included 2 out of the 4 subtests we used for IQ estimation, and found lower performance on one of them (Digit Symbol). In addition, cognitive function of BD patients was not compared with that of SCZ patients.
Lower IQ in BD-I patients appears to be associated with the illness itself, as healthy BD-I siblings do not show a significantly reduced IQ compared with control siblings. In contrast, healthy SCZ siblings had a lower IQ than control siblings, indicating that lower IQ may be associated with a familial vulnerability for SCZ. The current study emphasizes that IQ may not fully account for educational performance, as we show different results for both domains in BD-I patients. Moreover, our results confirm findings by previous register-based studies that low educational performance is associated with schizophrenia and high educational performance is associated with BD (MacCabe et al. 2008; MacCabe et al. 2010).
One of the explanations for the contradictory finding regarding high educational performance and lower intelligence in BD-I patients is that intelligence may have been higher before onset of BD, but decreased after illness onset (Trotta et al. 2014) possibly as a result of number of hospitalizations (Robinson and Ferrier, 2006), traumatic experiences (Aas et al. 2011) or long-term medication use (Pachet and Wisniewski, 2003; Senturk et al. 2007; Wingo et al. 2009; Vreeker et al. in press). Additionally, prodromal symptoms like elevated energy (Egeland et al. 2000) may have contributed to high educational performance.
Interestingly, BD-I siblings and BD-I parents did not have higher educational performance than controls, which suggests that high educational performance is associated with the illness itself, rather than with a familial vulnerability to BD. This is partly in line with earlier findings showing fewer completed years of education for non-bipolar co-twins, but not for bipolar co-twins, as compared with controls (Vonk et al. 2012). From an evolutionary perspective our results raise the question whether high (educational) achievement could be a result of an adaptive advantage of the disorder, associated with benefits in leadership (Akiskal and Akiskal, 2005).
Limitations
The current study has several limitations. First it should be noted that the cross-sectional design of our study does not allow us to conclude that there has been a decline in IQ in BD-I patients. Also, BD-I patients were significantly older than SCZ patients and controls which may have resulted in a greater opportunity for BD-I patients to attend higher education. However, analyses of patients and controls older than 34 years of age, yielded very similar results. Furthermore, younger people tend to be higher educated than older people in general (Barro and Lee, 2013) and the fact that BD-I patients were higher educated despite their higher age could also underscore their superior educational performance.
Despite careful analysis that incorporate multilevel analysis, sensitivity analysis and exploration of a range of possible confounders, residual confounding may remain as we are unable to adjust for all possible factors (e.g. medication use, comorbid disorders) that could have influenced IQ scores. Particularly, information on parental socioeconomic status is absent and we cannot rule out the possibility that differences in parental socioeconomic status may have influenced our results. However, since tuition fees are low and income differences are substantially reduced by social security and taxes in the Netherlands we do not expect that differences in socioeconomic status may have accounted for our results on educational performance.
Furthermore, although we accounted for the effect of clustering by including ‘family’ as random factor, the absence of genetic data precluded the use of genetic proximity as a more precise measure of dependencies within families.
A final limitation is that, despite our large sample, we cannot be sure that our populations are representative; inherent to clinical cohorts is Berkson’s bias (bias toward those willing to participate and those under treatment) (Regeer et al. 2009).
Conclusions
We show that despite BD-I patients having a lower IQ relative to controls, they are more likely to have completed the highest level of education. This contrasts with our findings in SCZ patients, who demonstrate both lower IQ and lower educational performance. The fact that BD-I patients, but not BD-I parents or BD-I siblings, had more often completed the highest level of education compared with controls suggests that high educational performance may be a distinctive feature of the illness itself.
Supplementary Material
Figure S1. Flowchart of participants
Table S1. Diagnosis of SCZ patients
Table S2. Educational performance of BD-I patients, SCZ patients and controls older than 34 years of age
Table S3. The association of familial vulnerability with educational performance in siblings without BD or a psychotic illness
Table S4. Educational performance of parents compared to 600 random controls
Acknowledgments
We are grateful for the generosity of time and effort by the patients and their families, healthy subjects, and all the researchers who make the BiG, CannabisQuest and GROUP project possible. We thank the patient association ‘Vereniging voor Manisch Depressieven en Betrokkenen’ and the pharmacy network ‘UPPER’ for their assistance in recruiting participants and Willemijn van Gastel, Christian Schubart, Diandra Bouter, Ellen Bleijenberg, Diane Ramakers, Tim Zandbelt and Yoon Jung for their efforts in collecting and managing the data. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Financial support
This work was supported by several Grants. The infrastructure for the GROUP study is funded through the Geestkracht programme of the Dutch Health Research Council (ZON-MW, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGZ Eindhoven en de kempen, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia psycho-medical center (The Hague). Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, Riagg Amersfoort and Delta.)
The BiG study is funded by the National Institute of Mental Health, Grant number: R01 MH090553 (to Prof. dr. R. A. Ophoff).
The CannabisQuest was financially supported by a Grant of the NWO (Netherlands Organization for Scientific Research), Grant number: 91207039.
Appendix
*GROUP investigators are: Richard Bruggeman, MD, PhD1; Wiepke Cahn, MD, PhD2; Lieuwe de Haan, MD, PhD3; René S. Kahn, MD, PhD2; Carin J. Meijer, PHD3; Inez Myin-Germeys, PhD4; Jim van Os, MD, PhD4,5; Durk Wiersma, PhD1
Footnotes
University Medical Center Groningen, Department of Psychiatry, University of Groningen, The Netherlands
University Medical Center Utrecht, Department of Psychiatry, Brain Center Rudolf Magnus, Utrecht, The Netherlands
Academic Medical Centre, Department of Psychiatry, University of Amsterdam, Amsterdam, The Netherlands
Maastricht University Medical Center, South Limburg Mental Health Research and Teaching Network Euron, Maastricht, The Netherlands
King’s College London, King’s Health Partners, Department of Psychosis Studies, Institute of Psychiatry, London, United Kingdom
Conflict of interest
None.
Reference List
- Aas M, Dazzan P, Fisher HL, Morgan C, Morgan K, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM. Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophrenia Research. 2011;129:12–19. doi: 10.1016/j.schres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Akiskal KK, Akiskal HS. The theoretical underpinnings of affective temperaments: implications for evolutionary foundations of bipolar disorder and human nature. Journal of Affective Disorders. 2005;85:231–239. doi: 10.1016/j.jad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Apeldoorn SY, Sterk B, van den Heuvel ER, Schoevers RA, Islam MA, Bruggeman R, Cahn W, de Haan L, Kahn RS, Meijer CJ, Myin-Germeys I, van Os J, Wiersma D. Factors contributing to the duration of untreated psychosis. Schizophrenia Research. 2014;158:76–81. doi: 10.1016/j.schres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sanchez-Moreno J, Salazar-Fraile J, Vieta E, Tabares-Seisdedos R. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neuroscience Biobehavioral Reviews. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Barro RJ, Lee JW. A new data set of educational attainment in the world, 1950–2010. Journal of Development Economics. 2013;104:184–198. [Google Scholar]
- Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophrenia Research. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophrenia bulletin. 2015 doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. The American Journal of Human Genetics. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Premuzic T, Furnham A. Personality predicts academic performance: Evidence from two longitudinal university samples. Journal of Research in Personality. 2003;37:319–338. [Google Scholar]
- Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biological psychiatry. 1990;27:1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, Ke X, Le HS, Christoforou A, Luciano M, McGhee K, Lopez L, Gow AJ, Corley J, Redmond P, Fox HC, Haggarty P, Whalley LJ, McNeill G, Goddard ME, Espeseth T, Lundervold AJ, Reinvang I, Pickles A, Steen VM, et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry. 2011;16:996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychological Medicine. 2012;42:743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14:1–21. [PubMed] [Google Scholar]
- Eric YW, Halari R, Cheng KM, Leung SK, Young AH. Cognitive performance is impaired in euthymic Chinese patients with Bipolar 1 Disorder. Journal of Affective Disorders. 2013 doi: 10.1016/j.jad.2013.05.070. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders (SCID I) New York: Biometric Research Department; 1997. [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biological Psychiatry. 2005;58:838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Frantom LV, Allen DN, Cross CL. Neurocognitive endophenotypes for bipolar disorder. Bipolar Disorders. 2008;10:387–399. doi: 10.1111/j.1399-5618.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Gale CR, Batty GD, McIntosh AM, Porteous DJ, Deary IJ, Rasmussen F. Is bipolar disorder more common in highly intelligent people? A cohort study of a million men. Molecular Psychiatry. 2013;18:190–194. doi: 10.1038/mp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Kumari V, Das M, Zachariah E, Ettinger U, Sumich A, Sharma T. Cognitive functioning in siblings discordant for schizophrenia. Acta Psychiatrica Scandinavica. 2005;111:185–192. doi: 10.1111/j.1600-0447.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L. Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. International Journal of Methods in Psychiatric Research. 2012;21:205–221. doi: 10.1002/mpr.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Faraone SV, Seidman LJ, Pepple JR, Tsuang MT. Neuropsychological risk indicators for schizophrenia: a preliminary study of female relatives of schizophrenic and bipolar probands. Psychiatry Research. 1998;79:227–240. doi: 10.1016/s0165-1781(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, Denicoff KD, Suppes T, Altshuler LL, Kupka R, Kramlinger KG, Post RM. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. Journal of Affective Disorders. 2001;67:33–44. doi: 10.1016/s0165-0327(01)00430-x. [DOI] [PubMed] [Google Scholar]
- MacCabe JH. Population-based cohort studies on premorbid cognitive function in schizophrenia. Epidemiologic Reviews. 2008;30:77–83. doi: 10.1093/epirev/mxn007. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Lambe MP, Cnattingius S, Sham PC, David AS, Reichenberg A, Murray RM, Hultman CM. Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. British Journal of Psychiatry. 2010;196:109–115. doi: 10.1192/bjp.bp.108.060368. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Lambe MP, Cnattingius S, Torrang A, Bjork C, Sham PC, David AS, Murray RM, Hultman CM. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychological Medicine. 2008;38:1133–1140. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disorders. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Family Interview for genetic studies (FIGS): A manual for FIGS. Bethesda, MD: NIMH: Clinical Neurogenetics Branch; 1992. [Google Scholar]
- McClellan J, Prezbindowski A, Breiger D, McCurry C. Neuropsychological functioning in early onset psychotic disorders. Schizophrenia Research. 2004;68:21–26. doi: 10.1016/S0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Gow A, Luciano M, Davies G, Liewald DC, Harris SE, Corley J, Hall J, Starr JM, Porteous DJ, Tenesa A, Visscher PM, Deary IJ. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;15:938–43. doi: 10.1016/j.biopsych.2013.01.011. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. British Journal of Psychiatry. 2005;186:378–385. doi: 10.1192/bjp.186.5.378. [DOI] [PubMed] [Google Scholar]
- Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology (Berl) 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biological Psychiatry. 2005;58:930–936. doi: 10.1016/j.biopsych.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R core team. R: A language and environment for statistical computing. 2014. [Google Scholar]
- Regeer EJ, Krabbendam L, De Graaf R, Have MT, Nolen WA, van Os J. Berkson’s bias and the mood dimensions of bipolar disorder. International Journal of Methods in Psychiatric Research. 2009;18:279–286. doi: 10.1002/mpr.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. American Journal of Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disorders. 2006;8:103–116. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Sartorius N, Gulbinat W, Harrison G, Laska E, Siegel C. Long-term follow-up of schizophrenia in 16 countries. A description of the International Study of Schizophrenia conducted by the World Health Organization. Soc Psychiatry Psychiatr Epidemiology. 1996;31:249–258. doi: 10.1007/BF00787917. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart CD, van Gastel WA, Breetvelt EJ, Beetz SL, Ophoff RA, Sommer IE, Kahn RS, Boks MP. Cannabis use at a young age is associated with psychotic experiences. Psychological Medicine. 2011;41:1301–1310. doi: 10.1017/S003329171000187X. [DOI] [PubMed] [Google Scholar]
- Senturk V, Goker C, Bilgic A, Olmez S, Tugcu H, Oncu B, Atbasoglu EC. Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disorders. 2007;9(Suppl 1):136–144. doi: 10.1111/j.1399-5618.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, McElroy SL, Rush AJ, Kupka R, Frye MA, Bickel M, Post RM. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. Journal of Affective Disorders. 2001;67:45–59. doi: 10.1016/s0165-0327(01)00432-3. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatrica Scandinavica Suppl. 2007:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Quraishi S, McDonald C, Murray RM. The Maudsley Family Study: premorbid and current general intellectual function levels in familial bipolar I disorder and schizophrenia. Journal of Clinical and Experimental Neuropsycholy. 2006;28:243–259. doi: 10.1080/13803390500360513. [DOI] [PubMed] [Google Scholar]
- Trotta A, Murray RM, MacCabe JH. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychological Medine. 2014:1–14. doi: 10.1017/S0033291714001512. [DOI] [PubMed] [Google Scholar]
- van Oel CJ, Sitskoorn MM, Cremer MP, Kahn RS. School performance as a premorbid marker for schizophrenia: a twin study. Schizophrenia Bulletin. 2002;28:401–414. doi: 10.1093/oxfordjournals.schbul.a006949. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, van Gastel WA, Schubart CD, Van Eijk KR, Luykx JJ, Van Winkel R, Joels M, Ophoff RA, Boks MP, Bruggeman R, Cahn W, De Haan L, Kahn RS, Meijer CJ, Myin-Germeys I, van Os J, Wiersma D. The effect of childhood maltreatment and cannabis use on adult psychotic symptoms is modified by the COMT Val158Met polymorphism. Schizophrenia Research. 2013;150:303–311. doi: 10.1016/j.schres.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Vonk R, van der Schot AC, van Baal GC, van Oel CJ, Nolen WA, Kahn RS. Premorbid school performance in twins concordant and discordant for bipolar disorder. Journal of Affective Disorders. 2012;136:294–303. doi: 10.1016/j.jad.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Vreeker A, Van Bergen AH, Kahn RS. Cognitive enhancing agents in schizophrenia and bipolar disorder. European Neuropsychopharmacology. doi: 10.1016/j.euroneuro.2015.04.014. in press. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III:Wechsler Adult Intelligence Scale (3rd ed.) Administration and Scoring Manual. Psychological Corporation/Harcourt Brace; San Antonio, TX: 1997. [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. Journal of Clinical Psychiatry. 2009;70:1588–1597. doi: 10.4088/JCP.08r04972. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of General Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of participants
Table S1. Diagnosis of SCZ patients
Table S2. Educational performance of BD-I patients, SCZ patients and controls older than 34 years of age
Table S3. The association of familial vulnerability with educational performance in siblings without BD or a psychotic illness
Table S4. Educational performance of parents compared to 600 random controls