Abstract
Background
Radiotheragnostics makes use of the same molecular targeting vectors, labeled either with a diagnostic or therapeutic radionuclide, ideally of the same chemical element. The matched pair of scandium radionuclides, 44Sc and 47Sc, satisfies the desired physical aspects for PET imaging and radionuclide therapy, respectively. While the production and application of 44Sc was extensively studied, 47Sc is still in its infancy. The aim of the present study was, therefore, to investigate and compare two different methods of 47Sc production, based on the neutron irradiation of enriched 46Ca and 47Ti targets, respectively.
Methods
47Sc was produced by thermal neutron irradiation of enriched 46Ca targets via the 46Ca(n,γ)47Ca → 47Sc nuclear reaction and by fast neutron irradiation of 47Ti targets via the 47Ti(n,p)47Sc nuclear reaction, respectively. The product was compared with regard to yield and radionuclidic purity. The chemical separation of 47Sc was optimized in order to obtain a product of sufficient quality determined by labeling experiments using DOTANOC. Finally, preclinical SPECT/CT experiments were performed in tumor-bearing mice and compared with the PET image of the 44Sc labeled counterpart.
Results
Up to 2 GBq 47Sc was produced by thermal neutron irradiation of enriched 46Ca targets. The optimized chemical isolation of 47Sc from the target material allowed formulation of up to 1.5 GBq 47Sc with high radionuclidic purity (>99.99%) in a small volume (~700 μL) useful for labeling purposes. Three consecutive separations were possible by isolating the in-grown 47Sc from the 46/47Ca-containing fraction. 47Sc produced by fast neutron irradiated 47Ti targets resulted in a reduced radionuclidic purity (99.95–88.5%). The chemical purity of the separated 47Sc was determined by radiolabeling experiments using DOTANOC achievable at specific activities of 10 MBq/nmol. In vivo the 47Sc-DOTANOC performed equal to 44Sc-DOTANOC as determined by nuclear imaging.
Conclusion
The production of 47Sc via the 46Ca(n,γ)47Ca nuclear reaction demonstrated significant advantages over the 47Ti production route, as it provided higher quantities of a radionuclidically pure product. The subsequent decay of 47Ca enabled the repeated separation of the 47Sc daughter nuclide from the 47Ca parent nuclide. Based on the results obtained from this work, 47Sc shows potential to be produced in suitable quality for clinical application.
Electronic supplementary material
The online version of this article (doi:10.1186/s41181-017-0024-x) contains supplementary material, which is available to authorized users.
Keywords: 47Sc, Matched pairs, Theragnostics, Radionuclide production, 46Ca, 47Ti, Thermal and fast neutrons, SPECT/CT imaging
Background
Over the past few years, the concept of personalized medicine, where patient treatment is performed according to an individually tailored treatment regime, has gained much recognition (Kraeber-Bodere and Barbet 2014). In nuclear medicine, this approach is realized by exploiting diagnostic techniques, such as non-invasive imaging by means of Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT), together with individualized radiotherapeutic treatment (Velikyan 2012). The resulting combination became known as the theragnostic approach and comprises the use of the same molecular targeting vectors, labeled either with a diagnostic or therapeutic radionuclide (Baum and Kulkarni 2012). Ideally, the employed radionuclides represent a matched pair, where both are radioisotopes of the same chemical element. Only a limited number of matching radionuclides entail suitable decay characteristics for radiotheragnostic application (Rösch and Baum 2011); of those the radionuclides of scandium, 44Sc/43Sc and 47Sc, are interesting candidates. Based on the physical and chemical characteristics, the β−-emitter 47Sc is particularly interesting for radionuclide therapy, while the decay characteristics of 44Sc and 43Sc are well-suited for diagnostic PET imaging (Table 1) (Rösch 2012; Müller et al. 2014a, 2014b; Walczak et al. 2015).
Table 1.
Nuclear data of theragnostic radionuclides for therapy and PET imaging
| Therapeutic radionuclide | Diagnostic radionuclide (positron emitter) | ||||||
|---|---|---|---|---|---|---|---|
| Half-life [d] | Eβ− av [keV] | Eγ [keV] (Iγ [%]) | Half-life [h] | Eβ+ av [keV] (I[%]) | Eγ [keV] (Iγ [%]) | ||
| 177Lu | 6.65 | 134 | 113 (6.4) 208 (11.0) | 68Ga | 1.13 | 830 (89) | 1077 (3.0) |
| 47Sc | 3.35 | 162 | 159 (68.3) | 44Sc | 4.04 | 632 (94) | 1157 (99.9) |
| 43Sc | 3.89 | 476 (88) | 372 (23.0) | ||||
Intensities less than 5% were not considered
This matched pair would present an attractive alternative to 68Ga and 177Lu, which are currently used in clinics for PET imaging and therapy, respectively (Oh et al. 2011). Ga(III) and Lu(III) can be coordinated by 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-complexes (Majkowska-Pilip and Bilewicz 2011), however, they do not share the same coordination chemistry. Lu(III) is coordinated by all carboxyl groups of the octadentate DOTA (Viola-Villegas and Doyle 2009; Parus et al. 2015), while Ga(III) has a preference for the coordination number six, leaving two uncoordinated carboxyl groups in the Ga-DOTA-complex (Viola-Villegas and Doyle 2009; Majkowska-Pilip and Bilewicz 2011). As a result, these structural differences may have an influence on the radioconjugate’s chemical properties and, consequently, on the in vivo kinetics and receptor binding affinity (Reubi et al. 2000; Majkowska-Pilip and Bilewicz 2011). By using chemically identical radionuclides such as 44Sc/47Sc–known to form stable complexes with DOTA–this limitation could be addressed.
The physical half-life of 44Sc of 3.97 h (recently re-determined as 4.04 h (Garcia-Torano et al. 2016), is almost 4-fold longer than that of 68Ga (T1/2 = 68 min) and, hence, allows its use with biomolecules with slower kinetics. Due to the possibility of shipping 44Sc-radiopharmaceuticals over long distances, it can also facilitate logistics as it would allow centralized production and distribution to remote hospitals (Chakravarty et al. 2014; van der Meulen et al. 2015). The increased availability of 44Sc has initiated a number of preclinical in vitro and in vivo studies with DOTA-conjugated biomolecules (Müller et al. 2013a, 2013b; Hernandez et al. 2014) and, recently, labeling of NODAGA (1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid)-functionalized peptides and DTPA (N-[(R)-2-amino-3-(para-isothiocyanato-phenyl)propyl]trans-(S,S)-cyclohexane-1,2-diamine N,N,N’,N”N”-pentaacetic acid)-functionalized antibodies was also demonstrated (Chakravarty et al. 2014; Domnanich et al. 2016).
The emission of low-energy β−-particles from 47Sc (Eβ− av = 162 keV, Table 1) is particularly interesting for targeted radionuclide therapy of small tumors and cancer metastases, similar to the clinically-established 177Lu (Eβ− av = 134 keV, T1/2 = 6.65 d, Table 1). Moreover, the shorter half-life of 47Sc (T1/2 = 3.35 d) would encourage its use, in conjunction with small molecules, with relatively fast pharmacokinetic profiles. In analogy to 177Lu, the decay of 47Sc is characterized by the co-emission of γ-rays with an ideal energy (Eγ = 159 keV, Table 1) for SPECT imaging (Müller et al. 2014a).
The availability of high 47Sc activity with adequate purity becomes a crucial issue for the realization of more detailed preclinical investigations and future clinical applications. So far, successful production of 47Sc was described by two different neutron induced reactions: 47Ti(n,p)47Sc and 46Ca(n,γ)47Ca → 47Sc (Fig. 1) (Bartoś et al. 2012; Müller et al. 2014a). To produce 47Sc from 47Ti, fast neutrons (En > 1 MeV) are required, while the 46Ca(n,γ)47Ca reaction is induced by thermal neutrons (En = 0.025 eV) (Bartoś et al. 2012). Proton irradiation of enriched 48Ti targets made 47Sc available via the 48Ti(p,2p)47Sc nuclear reaction, however, too much of the long-lived 46Sc was co-produced (Srivastava 2012). An alternative 47Sc production route considers photonuclear reactions on enriched 48Ti and 48Ca targets, respectively (Yagi and Kondo 1977; Mamtimin et al. 2015; Rane et al. 2015; Starovoitova et al. 2015). So far only the former route was studied in detail with enriched targets (Yagi and Kondo 1977), while for the latter only natural target material was used for initial benchmark experiments.
Fig. 1.
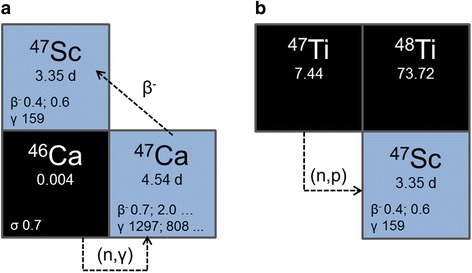
Nuclear reactions for production of 47Sc from 46Ca via 46Ca(n,γ)47Ca → 47Sc (a) and from 47Ti via 47Ti(n,p)47Sc (b)
The aim of the present study was to optimize the parameters of the previously-reported production process for 47Sc from enriched 46Ca targets (Müller et al. 2014a) in order to reproducibly obtain increased 47Sc yields in a formulation allowing direct preclinical application after radiolabeling and to compare it with the method using 47Ti as target material. 47Sc labeling experiments with DOTANOC were performed as part of the quality control. In the second part of the study the stability of 47Sc-labeled DOTANOC was investigated and SPECT/CT imaging studies were performed in tumor-bearing mice to compare the performance of 47Sc-DOTANOC with the previously obtained PET images of the 44Sc-labeled counterpart. Moreover, comparison of SPECT images obtained with mice injected with 177Lu-DOTANOC were also performed.
Methods
Chemicals
Enriched 46CaCO3 (83.09% 40Ca, 1.19% 42Ca, 0.36% 43Ca, 8.55% 44Ca, 5.00 ± 0.50% 46Ca, 1.81% 48Ca, Trace Sciences International, USA) was used as target material for thermal neutron irradiation. Enriched 47TiO2 (0.41% 46Ti, 95.7 ± 0.3% 47Ti, 3.61% 48Ti, 0.15% 49Ti, 0.13% 50Ti, Isoflex, USA) was reduced to 47Ti metal and used as target material for fast neutron irradiation. Prior to irradiation, a precursory scan for trace metals by ICP-OES (Perkin Elmer Optima 3000) was performed.
The chemical separation of Sc from Ca was performed on a N,N,N’,N’-tetra-n-octyldiglycolamide, non-branched resin (DGA, particle size 50–100 μm, TrisKem International, France). SCX cation exchange cartridges (100 mg Bond Elut SCX, particle size 40 μm, Agilent Technologies Inc., USA) or DGA extraction chromatographic resin were used for the preconcentration of Sc. Chemical separations were performed with MilliQ water, hydrochloric acid (HCl, 30% Suprapur, Merck KGaA, Germany) and sodium chloride (NaCl, Trace Select, ≥99.999%, Fluka Analytical, Germany). For the 47TiO2 reduction process, calcium hydride (CaH2, metals basis, Mg <1%, Alfa Aesar, Germany), argon (Ar, 99.9999%, Linde, Germany) and acetic acid (CH3COOH, 100% Suprapur, Merck KGaA, Germany) were used. Nitric acid (HNO3, 65% Suprapur, Merck KGaA, Germany) was required for the preparation of the 46Ca targets. DOTANOC acetate was obtained from ABX GmbH, advanced biochemical compounds, Germany.
Production of 47Sc from enriched 47Ti
The reduction of 47TiO2 was performed at Helmholtz Center for Heavy Ion Research (GSI) in Darmstadt as described elsewhere (Lommel et al. 2014). Briefly, the enriched 47TiO2 was combined with 40% surplus of calcium hydride and the reduction process was performed under constant argon flow at 900 °C for 1 h. Dilute acetic acid was used for the isolation of the reduced 47Ti metal from the co-produced calcium oxide.
To prepare the targets, 0.6–19.9 mg reduced 47Ti powder was placed in a quartz glass ampoule (Suprasil, Heraeus, Germany) and sealed. The targets were irradiated with neutrons at the spallation-induced neutron source, SINQ, at Paul Scherrer Institut (PSI) at a fast neutron flux (>1 MeV) of 3.3–3.5 × 1011 n cm−2 s−1 for 1.5–18.9 days and in the BR2 reactor at SCK.CEN, Mol, Belgium in a reflector channel at a fast neutron flux (>1 MeV) of 5.7 × 1013 n cm−2 s−1 for 7 days. 47Sc was formed via the 47Ti(n,p)47Sc nuclear reaction with fast neutrons.
Production of 47Sc from enriched 46Ca
To prepare the targets, 65–91 mg enriched 46CaCO3 powder was dissolved in concentrated nitric acid and evaporated to complete dryness at 60–70 °C. The 46Ca(NO3)2 residue was taken up in dilute nitric acid (~1 M HNO3) and an aliquot of the aqueous solution (0.14–0.35 mg 46Ca) transferred into a quartz glass ampoule, evaporated to dryness and sealed.
47Sc was produced by the irradiation of the described 46Ca targets with thermal neutrons at the high flux reactor of Institut Laue-Langevin (ILL) in Grenoble, France at a thermal neutron flux of 1.0–1.4 × 1015 n cm−2 s−1, for 4 to 11 days, and at the BR2 reactor at SCK.CEN, Mol, Belgium at a thermal neutron flux of 3.2 × 1014 n cm−2 s−1 for 7 days, respectively. 47Sc was generated by the decay of the formed 47Ca (T1/2 = 4.54 d) occurring during the irradiation, but also after removal of the ampoule from the reactor.
Separation of 47Sc from 46Ca and 47Ca
The irradiated 46Ca ampoules were delivered to PSI several days post-irradiation (2.6–12.4 d) and the 47Sc separation was performed immediately, similarly to previously reported (Müller et al. 2014a). Each ampoule was transferred into a hot cell and the glass surface was cleaned twice with ~20 mL 1.0 M HCl and rinsed twice with ~20 mL MilliQ water. The crushing of the quartz glass ampoule was performed within a plastic target tube in a separate hot cell. Subsequently, the target tube containing the crushed ampoule was attached to the separation panel with the aid of manipulators. The design of the separation panel, including the adaptation of its operation inside the hot cell, was a crucial part of the method development (Fig. 2). The 46Ca(NO3)2 (~10–25 mg) from the ampoule was dissolved in 4 mL 3.0 M HCl and transferred from the target tube to the reaction vessel. A system of syringes, peristaltic pumps and three-way valves (see schematic of the panel in Fig. 2) was used to transfer the reagents from outside into the hot cell. To ensure complete dissolution of the target material, the solution was pumped from the target tube to the reaction vial and back several times. The solution was loaded on a pre-conditioned DGA column (1 mL cartridge filled with 50–70 mg of DGA resin). A second rinse cycle of the crushed glass ampoule with 2.5 mL 3.0 M HCl ensured collection of final traces of the 47Sc activity, which were subsequently sorbed onto the DGA resin column. Radioactivity detection probes were attached in the vicinity of the target tube and the DGA column to follow the transfer of the 47Sc radioactivity. Further application of 2 mL 3.0 M HCl removed the stable 46Ca and radioactive 47Ca from the DGA resin. The entire Ca-containing effluent was collected in a separate vessel and kept for consecutive separation of further in-growing 47Sc from the decaying 47Ca. The sorbed 47Sc was eluted from the resin column with 4 mL 0.1 M HCl and sorbed on a second column containing SCX cation exchange resin (Method A). Alternately, the 47Sc-containing eluate was collected, acidified to yield a 3.0 M HCl solution and sorbed on a second, smaller DGA resin column (1 mL cartridge filled with 20–25 mg DGA resin) at a slow flow rate of ~0.3 mL/min (Method B), as described by Domnanich et al. (Domnanich et al. 2016). The elution of 47Sc from the second column was performed with 700 μL 4.8 M NaCl/0.1 M HCl (for Method A) and with 1.7 mL 0.05 M HCl (for Method B) via a separate valve. In order to collect 47Sc in a small volume, the 0.05 M HCl (Method B) was fractionized into three Eppendorf vials; the first contained ~700 μL and the other two ~500 μL each. Fractionized collection was not necessary for Method A, as the highest proportion of the 47Sc radioactivity was trapped in a low quantity of eluate.
Fig. 2.
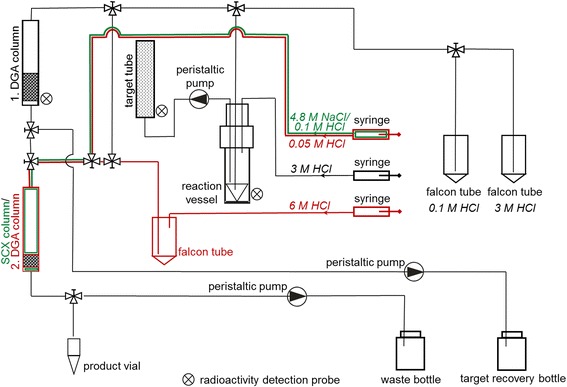
Schematic diagram of the 47Sc production panel. The components drawn in green are used only to perform separations according to Method A, the parts required for Method B are shown in red, while black indicates the apparatus components used for both methods
The renewed generation of 47Sc, by the decay of radioactive 47Ca in the Ca-containing fraction (47Ca and stable 46Ca), enabled subsequent separations after a minimum in-growth time of 3 days.
Radionuclidic purity
To identify the nuclide inventory of the samples, γ-ray spectrometry with an N-type high-purity germanium (HPGe) coaxial detector (EURISYS MESURES, France) and the Ortec InterWinner 7.1 software were employed. The aliquot of 47Sc eluate was in the range of 3–15 MBq, while the entire neutron irradiated glass ampoules containing the 47Ti were used for the measurements. The counting time was determined by ensuring the measurement error was <4%. To determine small activities of long-lived radionuclidic impurities, γ-spectrometry measurements of the same samples were performed with an extensive counting time several days post-irradiation.
Radiolabeling for quality control of the produced 47Sc
After quantitative determination of the 47Sc activity in the eluate with a dose calibrator (ISOMED 2010, Nuclear-Medizintechnik Dresden, GmbH, Germany), the required activity for radiolabeling in 0.05 M HCl was withdrawn from the product vial and 0.5 M sodium acetate solution (pH 8) was added to the 47Sc eluate to obtain a pH value of ~4.5. DOTANOC (0.7 mM solution in MilliQ water) was added to the 47Sc solution (~50 MBq) to obtain a specific activity of 10–25 MBq/nmol (2–5 nmol DOTANOC). The reaction mixture was incubated at 95 °C for 15 min. The preparation of 177Lu-DOTANOC (25 MBq/nmol) was carried out under standard labeling conditions (pH 4.5, 95 °C) using no carrier-added 177Lu (purchased from Isotope Technologies Garching GmbH, Germany) (Müller et al. 2013a).
High-performance liquid chromatography (HPLC, Merck Hitachi, LaChrom) with a C-18 reversed-phase column (XterraTM MS, C18, 5 μm, 150 × 4.6 mm; Waters) was used for determination of the radiolabeled fraction of DOTANOC. The detection was performed with a UV (LaChrom L-7400) and radiodetector (Berthold, HPLC Radioactivity Monitor, LB 506B). The mobile phase consisted of MilliQ water containing 0.1% trifluoracetic acid (A) and acetonitrile (B) with a gradient of 95% A and 5% B to 20% A and 80% B, over a period of 15 min, at a flow rate of 1.0 mL/min.
In vitro stability of 47Sc- and 177Lu-labeled DOTANOC
The in vitro stability of 47Sc- and 177Lu-labeled DOTANOC (radiochemical purities >95%) was investigated in phosphate buffered saline (PBS, pH 7.4). An activity of 50 MBq of 47Sc- or 177Lu-DOTANOC was diluted with PBS (pH 7.4) to a total volume of 500 μL and incubated at room temperature for 3 days. Once every 24 h an aliquot was withdrawn to determine the integrity of the labeled compound by means of HPLC.
SPECT/CT imaging with 47Sc- and 177Lu-DOTANOC
In vivo experiments were approved by the local veterinarian department and conducted in accordance with the Swiss law of animal protection. Female, athymic nude mice (CD-1 nude) at the age of 5–6 weeks were obtained from Charles River Laboratories, Sulzfeld, Germany. AR42J cells (rat exocrine pancreatic tumor cells, European Collection of Cell Cultures ECACC, Salisbury, U.K.) were suspended in PBS (5 × 106 cells in 100 μL) and subcutaneously inoculated on each shoulder. SPECT/CT experiments were performed about 2 weeks after tumor cell inoculation, when the tumor reached a size of about 400 mm3.
Imaging studies were performed using a small-animal SPECT camera (NanoSPECT/CT™, Mediso Medical Imaging Systems, Budapest, Hungary) as previously reported (Müller et al. 2014b). The energy peaks for the camera were set at 159.4 keV (± 10%) for the scans with 47Sc and 56.1 keV (± 10%), 112.9 keV (± 10%) and 208.4 keV (± 10%) for the scans with 177Lu. SPECT/CT scans were followed by CT scans. The images were acquired using Nucline Software (version 1.02, Bioscan Inc., Poway, California, US). The reconstruction was performed iteratively with HiSPECT software (version 1.4.3049, Scivis GmbH, Göttingen, Germany). SPECT and CT data were automatically co-registered and the fused datasets were analyzed with the VivoQuant post-processing software (version 2.50, inviCRO Imaging Services and Software, Boston, USA).
The mice were injected intravenously with 47Sc-DOTANOC (12 MBq, 1.2 nmol, 100 μL) and 177Lu-DOTANOC (40 MBq, 1.2 nmol, 100 μL), respectively. The in vivo SPECT/CT scans of 35 min duration were acquired 3 h after injection of 47Sc-DOTANOC. During the scans, the mice were anesthetized by inhalation of a mixture of isoflurane and oxygen. Post-mortem scans of 1.3–3.5 h were performed 6 h after injection of 47Sc- and 177Lu-DOTANOC. The SPECT acquisitions were performed in such a manner to obtain the same total number of counts for each scan.
Results
Production of 47Sc from 47Ti via the (n,p) reaction
The irradiation of enriched 47Ti targets at both SINQ and the BR2 reactor resulted in the formation of 0.07–4.9 MBq 47Sc at the end of irradiation (EOI). The respective 47Sc saturation yields were determined to be between 1.8 and 10.0 MBq 47Sc/mg 47Ti 10−13 n cm−2 s−1 (summarized in Table 2) by taking the irradiation time, mass of enriched 47Ti and fast neutron flux into consideration. γ-spectrometry measurements of the neutron-irradiated 47Ti ampoules revealed that, other than 47Sc, the long-lived radionuclidic impurity 46Sc was formed. The amount of generated 46Sc was influenced by the irradiation period and the neutron energy (Bokhari et al. 2010; Zerkin 2016) and ranged from 3.8 to 11.5% 46Sc/for the irradiations at SINQ (Additional file 1: Figure S3). Considerably less 46Sc (0.05%) was produced by the irradiation at the BR2 reactor, however. In view of the high percentage of co-produced 46Sc and the relatively low 47Sc production yield at both facilities, the production of sufficiently high 47Sc activities for radiopharmaceutical applications was not considered feasible and, thus, chemical isolation of 47Sc from neutron irradiated 47Ti targets was not performed.
Table 2.
Activity and yield of 47Sc at the end of irradiation (EOI) with fast neutrons (>1 MeV) at SINQ (irradiations PSI 1, PSI 2 and PSI 3) and at the BR2 reactor (irradiation SCK.CEN)
| Irradiation | tirr [d] | m (47Ti) [mg] | A (47Sc) at EOI [MBq] | A (47Sc)saturation [MBq/mg 47Ti 10−13 n cm−2 s−1] | 46Sc activity at EOI [%] |
|---|---|---|---|---|---|
| PSI 1 | 10.9 | 19.03 | 3.9 | 6.6 | 7.8 |
| PSI 2 | 18.9 | 15.11 | 4.9 | 10.0 | 11.5 |
| PSI 3 | 1.5 | 1.31 | 0.07 | 6.9 | 3.8 |
| SCK.CEN | 7.0 | 0.58 | 4.7 | 1.8 | 0.05 |
Production of 47Sc from 46Ca via the (n,γ) reaction
The irradiation of 46Ca targets with thermal neutrons resulted in the formation of 47Ca, which decayed to 47Sc and yielded 210–2140 MBq 47Sc at the time the separation was performed. The 47Sc saturation yield was determined by taking the mass of 46Ca, the irradiation time (tirr), the decay time after EOI (twait) and the thermal neutron flux (Φth) into account and was within the range of 85–98 MBq 47Sc/mg 46Ca 10−13 n cm−2 s−1, which is comparable with the calculated 47Sc saturation yield of 92 MBq 47Sc/mg 46Ca 10−13 n cm−2 s−1 (summarized in Table 3). 47Sc is formed during the irradiation but also, however, for some time after the end of irradiation by the decay of 47Ca. This implies that both the irradiation time (tirr = 7.0–11.0 d) and the elapsed post-irradiation time until the start of the separation (twait = 3.0–12.5 d) are part of the yield-determining factors. The highest 47Sc activities under the applied irradiation conditions become accessible at an optimal post irradiation waiting time (topt) and are represented as relative activity a(47Sc)opt in Table 3. The measured relative 47Sc activities (a(47Sc)meas) are lower than the optimal relative 47Sc activities (a(47Sc)opt), as the separations were performed several days after the optimal waiting time. The variable f(47Sc) describes the ratio of the 47Sc activity at topt and the 47Ca activity at EOI. It can be considered as a measure for the maximal obtainable 47Sc activity, since both activities are referred to the time point of their maximum. The formulae used for the calculation of A(47Sc)calc, topt, a(47Sc)opt and f(47Sc) are given in Additional file 1: Figure S1 a-d.
Table 3.
Activity of 47Sc at the time of separation (A(47Sc)), comparison of the calculated and measured 47Sc saturation yield (A(47Sc)calc and A(47Sc)meas) and of the optimal and measured relative 47Sc activity (a(47Sc)opt and a(47Sc)meas) after irradiation with thermal neutrons at ILL (irradiations ILL 1–5) and BR2 (irradiation SCK.CEN)
| Irradiation | tirr [d] | twait [d] | topt [d] | m(46Ca) [mg] | A(47Sc) at separation [MBq] | A(47Sc)calc | A(47Sc)meas | a(47Sc)opt | a(47Sc)meas | f(47Sc) |
|---|---|---|---|---|---|---|---|---|---|---|
| [MBq/mg 46Ca 10−13 n cm−2 s−1] | ||||||||||
| ILL 1 | 7.0 | 6.7 | 2.8 | 0.17 | 690 | 92 | 86 | 0.43 | 0.34 | 0.65 |
| ILL 2 | 7.2 | 6.7 | 2.7 | 0.35 | 1390 | 92 | 85 | 0.44 | 0.34 | 0.66 |
| ILL 3 | 8.4 | 12.5 | 2.4 | 0.14 | 470 | 92 | 98 | 0.50 | 0.25 | 0.69 |
| ILL 4 | 11.0 | 6.9 | 1.8 | 0.35 | 2140 | 92 | 95 | 0.62 | 0.49 | 0.76 |
| ILL 5 | 7.0 | 6.8 | 2.8 | 0.35 | 1440 | 92 | 93 | 0.43 | 0.37 | 0.65 |
| SCK.CEN | 7.0 | 3.0 | 2.8 | 0.17 | 200 | 92 | 90 | 0.43 | 0.42 | 0.65 |
The measured relative 47Sc activities (a(47Sc)meas) of the irradiations ILL 1, ILL 5 and SCK.CEN are represented by single data points in Fig. 3. After an optimal post irradiation waiting time (topt) of 2.8 days, the relative 47Sc activity (a(47Sc)opt) reaches the maximum with 0.43. The separations ILL 1 and ILL 5 were performed after waiting times of 6.7 and 6.8 days, resulting in lower relative 47Sc activities of 0.34 and 0.37, respectively. The waiting time after the irradiation at SCK.CEN (3.0 days) is close to topt, thus the obtained relative 47Sc activity of 0.44 is comparable with the optimum value.
Fig. 3.
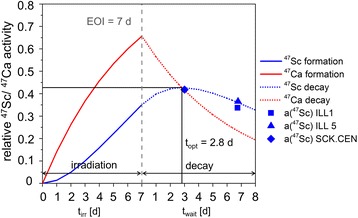
The relative 47Sc activities (a(47Sc)meas) of the irradiations ILL 1, ILL 5 and SCK.CEN accessible (and measured) at the time of separation (single data points). The activation functions of 47Sc and 47Ca at an irradiation period of 7.0 days (solid blue and red line) and their subsequent decay functions after the EOI (dashed blue and red line) are calculated for the respective irradiations
Separation of 47Sc from 46Ca and 47Ca
After the ampoule was crushed, it was moved to the hot cell containing the production panel (Fig. 2), where the target material was dissolved by repeated application of 6.5 mL 3.0 M HCl. The 47Ca and 47Sc activity was transferred from the crushed glass ampoule to the DGA column, leaving only 1.1 ± 0.5% 47Ca and 3.3 ± 0.4% 47Sc attached to the glass. Direct application of 2 mL 3.0 M HCl quantitatively removed the Ca (47Ca and stable Ca isotopes) from the resin. The collected Ca fraction contained 99.8 ± 0.2% of the total 47Ca activity. The 47Sc activity was eluted from the DGA column with 4 mL 0.1 M HCl and only 1.5 ± 0.6% of the 47Sc activity remained on the column. Using Method A, the solution was concentrated on the SCX cation exchange resin (used as the second column) and eluted with 700 μL 4.8 M NaCl/0.1 M HCl solution (pH 0–0.5), collecting 94.8 ± 2.1% of the total 47Sc activity. When using Method B, the molarity of the 47Sc eluate was increased from 0.1 to 3.0 M HCl and the resulting solution adsorbed on a second smaller DGA column and eluted with 1.7 mL 0.05 M HCl. Fractionized collection revealed that about ~90% of the eluted 47Sc activity was obtained in the first 700 μL (pH ~0).
With the installation of the chemical separation system in a hot cell, yields of up to 1.9 GBq 47Sc could be isolated from the irradiated 46Ca target. The renewed generation of 47Sc from the β−-decay of 47Ca (T1/2 = 4.54 d) in the Ca-containing fraction, reached the maximum 47Sc activity after an in-growth period of 5.6 days (Fig. 4) and, thus, enabled repeated separations. As a result of experimental conditions, separations were performed after an in-growth time of 3–7 days. The separation process was successfully repeated 2–4 times, until the eluted 47Sc activity was ~100 MBq.
Fig. 4.
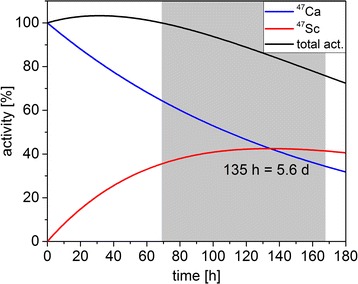
The radioactive decay of the parent nuclide 47Ca (T1/2 = 4.54 d) to the daughter nuclide 47Sc (T1/2 = 3.35 d) reaches the maximum of 47Sc activity after 135 h (5.6 d). The grey-shaded area indicates the time frame wherein the next separation was performed
Radionuclidic purity of 47Sc produced from 46Ca via (n,γ) reaction
The γ-ray spectrum of the neutron-irradiated 46Ca target material (Additional file 1: Figure S2a) showed, exclusively, the γ-lines of 47Sc (159 keV) and the parent nuclide 47Ca (489, 808 and 1297 keV). After chemical separation and concentration of 47Sc on SCX resin (Method A), the radionuclidic purity of the final 47Sc eluate was 99.6 ± 0.7%. When the second DGA column (Method B) was used, the radionuclidic purity increased to 99.99 ± 0.03% (Additional file 1: Figure S2b). The long-lived radionuclidic impurity 46Sc was only present in the eluate obtained from the first separation at a maximum of 0.005% and could be only detected by performing long-term γ-spectrometry measurements several days post separation. The isolated 47Sc which was generated by the decay of 47Ca in the Ca-containing fraction did not contain any 46Sc, due to its entire removal within the first separation.
Radiolabeling and stability of 47Sc labeled DOTANOC
Radiolabeling of 47Sc was reproducible at a specific activity of 10 MBq 47Sc per nmol DOTANOC, with >96% radiochemical purity. Depending on the activity concentration of the 47Sc solution, it was also possible to label at higher specific activity of up to 25 MBq/nmol.
The stability of 47Sc-labeled DOTANOC in PBS (pH 7.4) was investigated over a period of 3 days and compared to the stability of the 177Lu-labeled analogue (Fig. 5). Directly after the radiosynthesis of 47Sc with DOTANOC, the amount of intact radiolabeled product was 96.6–99.0% with less than 2% of radiolysis products visible as pre-peaks on the HPLC chromatogram. After 1 day at room temperature, the amount of intact radiolabeled 47Sc-DOTANOC decreased to 81.3%, while 18.3% were subjected to radiolysis. Over the whole investigation period of 3 days, the percentage of radiolysis products increased to 44.4%, however, the amount of free 47Sc was always below 2.1%. The stability of 47Sc-labeled DOTANOC was found to be comparable with the clinically-used analogue 177Lu-DOTANOC. After 3 days, the amount of intact 47Sc-DOTANOC (54.1%) was similar to the amount of intact 177Lu-DOTANOC (43.2%).
Fig. 5.
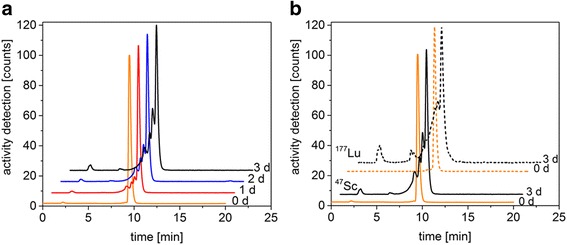
Stability of 47Sc-DOTANOC (a) and the comparison of the stability of 47Sc- and 177Lu-DOTANOC (b) in PBS (pH 7.4) investigated at room temperature over a 3-day period after radiolabeling. The retention times of free 47Sc and 177Lu are 2.2 ± 0.1 min and for 47Sc- and 177Lu-DOTANOC 9.5 ± 0.1 min and 9.3 ± 0.2 min, respectively
Imaging with 47Sc-DOTANOC in comparison to 44Sc-DOTANOC and 177Lu-DOTANOC
SPECT/CT experiments performed with AR42J tumor-bearing mice allowed excellent visualization of the accumulated 47Sc-DOTANOC in tumor xenografts, which express the somatostatin receptor (Fig. 6a). Activity accumulation was also observed in the kidneys, which was due to renal excretion of the radiopeptide. The SPECT/CT image obtained with 47Sc-DOTANOC showed an equal activity distribution profile as was previously demonstrated by a PET/CT scan of an AR42J tumor-bearing mouse 3 h after injection of 44Sc-DOTANOC (Fig. 6b) (Domnanich et al. 2016).
Fig. 6.
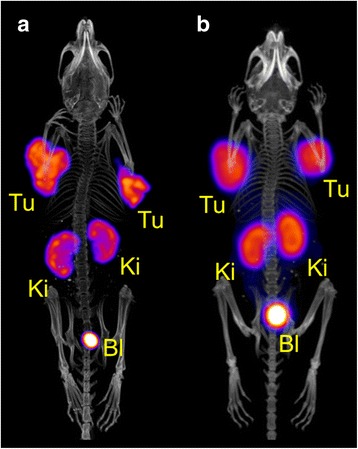
In vivo SPECT/CT scan of a tumor-bearing mouse 3 h after injection of 47Sc-DOTANOC (~12 MBq, ~1.2 nmol) (a). In vivo PET/CT scan of a tumor-bearing mouse 3 h after injection. 44Sc-DOTANOC (~10 MBq, ~1 nmol), image reproduced from Domnanich et al. 2016 (Domnanich et al. 2016) (b). The scan durations were 35 min and 20 min, respectively (Tu = AR42J tumor xenograft, Ki = kidney, Bl = urinary bladder)
To compare the image quality of 47Sc with the clinically-employed 177Lu, mice with AR42J tumors were injected with either 47Sc- or 177Lu-labeled DOTANOC and scanned 6 h after injection (Fig. 7). Both images visualized the uptake of the radiopeptides in tumor xenografts located on each shoulder of the mouse and in the kidneys. The distribution pattern was equal for both radiopeptides, as expected, based on similar coordination of 47Sc and 177Lu when using DOTA.
Fig. 7.
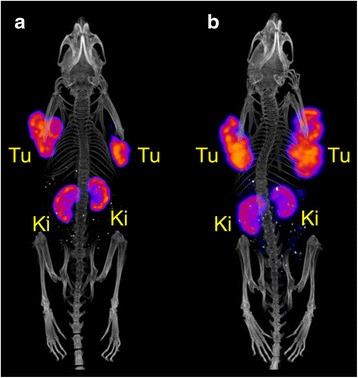
Post-mortem SPECT/CT scans of tumor-bearing mice 6 h after injection of the corresponding radiopeptide. Mouse injected with 47Sc-DOTANOC (~12 MBq, ~1.2 nmol) (a) and mouse injected with 177Lu-DOTANOC (~40 MBq, ~1.2 nmol) (b). The scan durations were 3.5 h and 1.3 h, respectively. (Tu = AR42J tumor xenograft, Ki = kidney)
Discussion
Recently, the use of 47Sc for therapeutic purposes, as part of the 44Sc/47Sc theragnostic radionuclide pair, has attracted considerable attention in the field of nuclear medicine (Rösch and Baum 2011). In the present study we report, to our knowledge, the first reproducible production of MBq to GBq activities of 47Sc by the irradiation of enriched 46Ca target material with thermal neutrons. Concurrently, an alternative production route was investigated, using 47Ti as target material and, by irradiation with fast neutrons, 47Sc was formed via the (n,p) nuclear reaction. Irradiation of enriched 47Ti at the spallation source SINQ (PSI, Switzerland) and the BR2 reactor (SCK.CEN, Mol, Belgium) resulted in the formation of 1.8–10.0 MBq 47Sc/mg 47Ti 10−13 n cm−2 s−1. The production of 47Sc by neutron irradiation of enriched 47Ti target material was performed previously by Mausner and Kolsky in the fission neutron spectrum of the HFIR reactor in Oak Ridge National Laboratory (ORNL) and Brookhaven National Laboratory (BNL) (Kolsky et al. 1998, Mausner, Kolsky et al. 1998) and by Bartoś et al. at the Maria reactor in Świerk, Poland (Bartoś et al. 2012). The reported activity of produced 47Sc was within the range of 1.4–4.2 MBq 47Sc/mg 47Ti 10−13 n cm−2 s−1, which is comparable with the 47Sc radioactivity obtained in our experiment at BR2. The irradiations at SINQ generated higher 47Sc activities, due to the larger proportion of fast neutrons (> 1 MeV) at the spallation source (Lehmann 2016) than at the reactor (Chrysanthopoulou et al. 2014). The production of 85–98 MBq 47Sc/mg 46Ca 10−13 n cm−2 s−1 was feasible via the 46Ca(n,γ)47Ca → 47Sc route with thermal neutrons from nuclear reactors at ILL and BR2, however. The nearly ten-fold higher accessible 47Sc activity from 46Ca irradiations can be attributed to the higher nuclear cross section of the 46Ca(n,γ)47Ca reaction (σ = 0.7 barn) (Magill et al. 2015) in comparison to the 47Ti(n,p)47Sc nuclear reaction (Additional file 1: Figure S4), as well as to an increased flux of thermal neutrons in comparison to fast neutrons. Lower measured relative 47Sc activities than the optimal activities were obtained for the irradiations ILL 1–ILL 5, because the post-irradiation waiting period was too long and the built up 47Sc already started to decay. Shorter waiting periods were prevented due to logistical issues. The waiting period after the irradiation at SCK.CEN, however, was close to the optimum time period, thus the measured relative 47Sc activity obtained from the irradiation at SCK.CEN is in good correlation with the optimal value.
γ-spectra of the irradiated 46Ca indicated the presence of 47Ca and 47Sc, however, due to high dead time a precise determination of both activities before separation was not possible. After chemical separation a product of high radionuclidic purity, containing only 0.005% 46Sc, was obtained (Additional file 1: Figure S2). The acquired radionuclide inventory of 47Ti targets, neutron irradiated at the spallation source SINQ, indicated a higher percentage of 46Sc (Additional file 1: Figure S3), which increased proportionally with irradiation time (3.8–11.5% 46Sc for 1.5–18.9 days irradiation), whereas a significantly smaller amount of 46Sc (0.05%) was produced after 7 days of irradiation at the BR2 reactor (SCK.CEN). Trace activities of 122Sb and 124Sb were identified in the γ-ray spectrum of the irradiated 47Ti ampoule at SINQ (Additional file 1: Figure S3), conceivably produced by neutron activation reactions on natural Sb impurities (Additional file 1: Table S5). The presence of 22Na and 7Be can be attributed to spallation reactions with the capsulation material. The scan for trace metals revealed impurities of Ca, Sr, Sb and Zr in the reduced 47Ti metal (Additional file 1: Table S5), which were probably introduced by the reduction process. Neutron activation reactions were only observed with Sb, but not with any of the other determined impurities, however.
The formation of 46Sc from 47Ti via the 47Ti(n,n + p)46Sc nuclear reaction is known to be only induced by very fast neutrons above the threshold of 10.7 MeV (Additional file 1: Figure S4) (Zerkin 2016). The considerably decreased 46Sc impurity of the sample irradiated at the BR2 reactor can, therefore, be attributed to the lower proportion of very energetic neutrons in the fission spectrum of the BR2 reactor (Chrysanthopoulou et al. 2014), compared to the spallation neutron spectrum in the SINQ (Lehmann 2016). The percentage of 46Sc obtained from the 47Ti irradiation at the BR2 reactor was in agreement with those from previous experiments at ORNL, BNL and the Maria reactor, which was reported to be 0.06–0.64% 46Sc (Kolsky et al. 1998; Mausner et al. 1998; Bartoś et al. 2012). With respect to radiopharmaceutical applications, the ten-fold higher 47Sc production from 46Ca targets, together with the absence of long-lived radionuclidic impurities, intensified our research towards the further development of the more attractive 46Ca route.
In order to meet the requirements for radiopharmaceutical applications, the obtained 47Sc eluate needed to be of high chemical purity and concentrated into a small volume of moderately acidic eluate to facilitate efficient radiolabeling and subsequent in vivo application. Initially, SCX cation exchange resin (Method A) was used and 94.8% ± 2.1% of the total 47Sc activity was recovered in only 700 μL eluate (4.8 M NaCl/0.1 M HCl). The use of this resin is already established for the concentration of the 68Ga eluate from the 68Ge generator (Mueller et al. 2013); however, direct preclinical in vivo application is not feasible due to the high osmolarity of the obtained eluate. In a modification of the separation procedure, a second, smaller DGA column was used (Method B), allowing the elution of ~90% using 700 μL 0.05 M HCl. This enabled labeling and preclinical application as previously shown with 44Sc (Domnanich et al. 2016).
The labeling of DOTANOC with 47Sc was performed to verify a consistent chemical purity of the obtained eluate. Our results demonstrated reproducible radiosynthesis of 47Sc-DOTANOC at specific activities of 10 MBq/nmol, whereas radiolabeling at 25 MBq/nmol proved possible. The obtained results indicated good quality of the produced 47Sc achieving radiolabeling yields, in agreement with the previously-performed 47Sc-radiolabeling of a DOTA-folate conjugate (Müller et al. 2014a).
In PBS 47Sc remained stably coordinated by the DOTA-chelator over 3 days (<6% release), a result which was comparable to the 177Lu-labeled peptide. 47Sc- and 177Lu-DOTANOC were, however, affected by radiolytic decomposition, which decreased the amount of intact product over time. It is likely the radiolytic stability could be enhanced by the addition of radical scavengers, such as ascorbic or gentisic acids, which were previously successfully employed for the stabilization of 90Y- and 177Lu-labeled DOTA-peptides (Liu and Edwards 2001; Liu et al. 2003).
In a proof-of-concept study, 47Sc-DOTANOC was utilized for SPECT/CT imaging of AR42J tumor-bearing mice. The equal distribution profile of 47Sc-DOTANOC and 44Sc-DOTANOC, previously demonstrated using PET/CT, demonstrated the successful realization of the “matched pair” principle using scandium radionuclides. Moreover, the in vivo distribution of 47Sc-DOTANOC was comparable to 177Lu-DOTANOC. Due to the higher percentage of emitted γ-radiation in the case of 47Sc, it is expected that less activity of 47Sc-labeled compounds would be necessary for clinical SPECT as compared to the activity necessary for 177Lu-labeled counterparts.
Conclusions
The reproducible production of activities of up to 2 GBq 47Sc at high radionuclidic purity via the 46Ca(n,γ)47Ca nuclear reaction in the thermal neutron flux of a reactor was demonstrated. The subsequent decay of 47Ca to 47Sc creates a “pseudo-generator” system, which enables the repeated separation of the 47Sc daughter nuclide from the 47Ca parent nuclide. Together with the high radionuclidic purity and the superior yield of the isolated 47Sc activity, the 46Ca production route bears significant advantages over the 47Ti production route with fast neutrons. Even though the high price of enriched 46Ca represents a drawback, implementation of a suitable recovery method will limit the expenses. Based on the results obtained from this proof-of-concept study, 47Sc has the potential to be produced in a suitable quality for clinical applications, however, the quantity of radioactivity still needs to be expanded to meet the requirements for radionuclide therapy.
Additional file
Formula for the calculation of the 47Sc activity in Bq (s−1), accessible under the applied irradiation conditions. σ = nuclear cross section of the 46Ca(n,γ)47Ca reaction in cm−2, NT = number of 46Ca atoms, Φth = thermal neutron flux in n * cm−2 * s−1, λSc and λCa = decay constants of 47Sc and 47Ca in s−1, tirr = irradiation time and twait = post irradiation waiting time in s. b Formula for the calculation of the optimal post irradiation waiting time (topt) in s, accessible at the applied irradiation time (tirr in s). The decay constants of 47Sc (λSc) and 47Ca (λCa) are given in s−1. c Formula for the calculation of the optimal relative 47Sc activity (a(47Sc)opt) (dimensionless), accessible under the applied irradiation conditions. σ = nuclear cross section of the 46Ca(n,γ)47Ca reaction in cm−2, NT = number of 46Ca atoms, Φth = thermal neutron flux in n * cm−2 * s−1, λSc and λCa = decay constants of 47Sc and 47Ca in s−1. d Formula for the maximal obtainable 47Sc activity (dimensionless). The irradiation time (tirr) is given in s and the decay constants of 47Sc (λSc) and 47Ca (λCa) in s−1. Figure S2. γ-Ray spectra of 47Sc and 47Ca from the neutron-irradiated 46Ca ampoule, obtained 71 h after the end of irradiation (measurement time: 10 s) (a) and of the pure 47Sc eluate after separation (Method B), obtained 1 h after the end of separation (measurement time: 250 s) (b). Figure S3. γ-Ray spectrum of the neutron-irradiated 47Ti ampoule at SINQ, obtained 21 d after the end of irradiation (measurement time: 9600 s). Figure S4. Measured cross section values (squares, retrieved from the EXFOR-database) (Zerkin 2016) as well as the theoretical calculations from the TENDL-2015 library (straight line) (Koning, Rochman et al. 2015) for the 47Ti(n,p)47Sc (blue) and the 47Ti(n,p + n)46Sc (black) nuclear reactions. Table S5. Trace metal analysis of the reduced 46Ti metal by ICP-OES. Only the elements determined at a concentration higher than the detection limit are listed below. (DOCX 415 kb)
Acknowledgments
We thank Raffaella Schmid, Renata Farkas, Walter Hirzel and Muhamet Djelili for technical assistance.
Funding
The research was funded by the Swiss National Science Foundation (CR23I2_156852).
Authors’ contributions
KAD developed the production and separation process, performed the separation experiments of 47Sc, did the radiolabeling and the stability experiments, analyzed the data and drafted the manuscript. CM was responsible for the performance of the in vitro and in vivo studies and revised the manuscript. MB supported the 47Sc separation process and reviewed the manuscript. SH performed the in vivo studies. UK and BP were responsible for the irradiation of 46Ca targets and 47Ti targets at ILL and SCK.CEN, respectively. AT and RD assisted with the yield and irradiation calculations, while AT also reviewed the manuscript. RS reviewed the manuscript. NvdM was responsible for the development of the production and separation process of 47Sc, supervised the whole study and finalized the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Katharina A. Domnanich, Email: katharina.domnanich@psi.ch
Cristina Müller, Email: cristina.mueller@psi.ch.
Martina Benešová, Email: martina.benesova@psi.ch.
Rugard Dressler, Email: steffihaller@gmx.ch.
Stephanie Haller, Email: rugard.dressler@psi.ch.
Ulli Köster, Email: koester@ill.fr.
Bernard Ponsard, Email: bernard.ponsard@sckcen.be.
Roger Schibli, Email: roger.schibli@psi.ch.
Andreas Türler, Email: andreas.tuerler@psi.ch.
Nicholas P. van der Meulen, Phone: +41-56-310 50 87, Email: nick.vandermeulen@psi.ch
References
- Bartoś B, Majkowska A, Kasperek A, Krajewski S, Bilewicz A. New separation method of no-carrier-added 47Sc from titanium targets. Radiochim Acta. 2012;100(7):457–61. doi: 10.1524/ract.2012.1938. [DOI] [Google Scholar]
- Baum RP, Kulkarni HR. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy - The Bad Berka Experience. Theranostics. 2012;2(5):437–47. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari TH, Mushtaq A, Khan IU. Separation of no-carrier-added radioactive scandium from neutron irradiated titanium. J Radioanal Nucl Chem. 2010;283(2):389–93. doi: 10.1007/s10967-009-0370-6. [DOI] [Google Scholar]
- Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, Cai W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with 44Sc-Labeled Cetuximab Fab Fragment. Bioconjug Chem. 2014;25(12):2197–204. doi: 10.1021/bc500415x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthopoulou N, Savva P, Varvayanni M, Catsaros N. “Compilation of Existing Neutron Screen Technology”. Science and Technology of Nuclear Installations. 2014. [Google Scholar]
- Domnanich KA, Müller C, Farkas R, Schmid RM, Ponsard B, Schibli R, Türler A, Van der Meulen N. 44Sc for labeling of DOTA- and NODAGA- functionalized peptides: preclinical in vitro and in vivo investigations. EJNMMI Radioanal Chem. 2016;8(1):19. doi: 10.1186/s41181-016-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Torano E, Peyres V, Roteta M, Sanchez-Cabezudo AI, Romero E, Martinez Ortega A. Standardisation and precise determination of the half-life of 44Sc. Appl Radiat Isot. 2016;109:314–8. doi: 10.1016/j.apradiso.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, Cai W. 44Sc: an attractive isotope for peptide-based PET imaging. Mol Pharm. 2014;11(8):2954–61. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsky KL, Joshi V, Mausner LF, Srivastava SC. Radiochemical purification of no-carrier-added Scandium-47 for radioimmunotherapy. Appl Radiat Isot. 1998;49(12):1541–9. doi: 10.1016/S0969-8043(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Kraeber-Bodere F, Barbet J. Challenges in nuclear medicine: innovative theranostic tools for personalized medicine. Front Med (Lausanne) 2014;1:16. doi: 10.3389/fmed.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, E. 2016. “https://www.psi.ch/nis/parameters.” Accessed 2 Dec 2016.
- Liu S, Edwards DS. Stabilization of 90Y-labeled DOTA-biomolecule conjugates using gentisic acid and ascorbic acid. Bioconjug Chem. 2001;12(4):554–8. doi: 10.1021/bc000145v. [DOI] [PubMed] [Google Scholar]
- Liu S, Ellars CE, Edwards DS. Ascorbic acid: useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. Bioconjug Chem. 2003;14(5):1052–6. doi: 10.1021/bc034109i. [DOI] [PubMed] [Google Scholar]
- Lommel B, Beusch A, Hartmann W, Hubner A, Kindler B, Steiner J, Yakusheva V. Reduction of isotopically enriched 50Ti-dioxide for the production of high-intensity heavy-ion beam. J Radioanal Nucl Chem. 2014;299(2):977–80. doi: 10.1007/s10967-013-2615-7. [DOI] [Google Scholar]
- Magill J, Pfenning G, Dreher R, Soti Z. Karlsruher Nuklidkarte/Chart of the Nuclides. Eggenstein-Leopoldshafen: Nucleonica GmbH; 2015. [Google Scholar]
- Majkowska-Pilip A, Bilewicz A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem. 2011;105(2):313–20. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Mamtimin M, Harmon F, Starovoitova VN. Sc-47 production from titanium targets using electron linacs. Appl Radiat Isot. 2015;102:1–4. doi: 10.1016/j.apradiso.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Mausner LF, Kolsky KL, Joshi V, Srivastava SC. Radionuclide development at BNL for nuclear medicine therapy. Appl Radiat Isot. 1998;49(4):285–94. doi: 10.1016/S0969-8043(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Mueller D, Klette I, Baum RP. Purification and labeling strategies for 68Ga from 68Ge/68Ga generator eluate. Recent Results Cancer Res. 2013;194:77–87. doi: 10.1007/978-3-642-27994-2_5. [DOI] [PubMed] [Google Scholar]
- Müller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Turler A, Schibli R. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent β−-emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J Nucl Med. 2013;54(12):2168–74. doi: 10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- Müller C, Struthers H, Winiger C, Zhernosekov K, Schibli R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J Nucl Med. 2013;54(1):124–31. doi: 10.2967/jnumed.112.107235. [DOI] [PubMed] [Google Scholar]
- Müller C, Bunka M, Haller S, Köster U, Groehn V, Bernhardt P, van der Meulen N, Türler A, Schibli R. Promising prospects for 44Sc-/47Sc-based theragnostics: application of 47Sc for radionuclide tumor therapy in mice. J Nucl Med. 2014;55(10):1658–64. doi: 10.2967/jnumed.114.141614. [DOI] [PubMed] [Google Scholar]
- Müller C, Reber J, Haller S, Dorrer H, Bernhardt P, Zhernosekov K, Türler A, Schibli R. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur J Nucl Med Mol Imaging. 2014;41(3):476–85. doi: 10.1007/s00259-013-2563-z. [DOI] [PubMed] [Google Scholar]
- Oh S, Prasad V, Lee DS, Baum RP. Effect of Peptide Receptor Radionuclide Therapy on Somatostatin Receptor Status and Glucose Metabolism in Neuroendocrine Tumors: Intraindividual Comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. 2011;2011:524130. doi: 10.1155/2011/524130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parus JL, Pawlak D, Mikolajczak R, Duatti A. Chemistry and bifunctional chelating agents for binding 177Lu. Curr Radiopharm. 2015;8(2):86–94. doi: 10.2174/1874471008666150312160440. [DOI] [PubMed] [Google Scholar]
- Rane S, Harris JT, Starovoitova VN. 47Ca production for 47Ca/47Sc generator system using electron linacs. Appl Radiat Isot. 2015;97:188–92. doi: 10.1016/j.apradiso.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Macke HR. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27(3):273–82. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- Rösch F. Scandium-44: benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr Radiopharm. 2012;5(3):187–201. doi: 10.2174/1874471011205030187. [DOI] [PubMed] [Google Scholar]
- Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans. 2011;40(23):6104–11. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- Srivastava SC. Paving the way to personalized medicine: production of some promising theragnostic radionuclides at Brookhaven National Laboratory. Semin Nucl Med. 2012;42(3):151–63. doi: 10.1053/j.semnuclmed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Starovoitova VN, Cole PL, Grimm TL. Accelerator-based photoproduction of promising beta-emitters 67Cu and 47Sc. J Radioanal Nucl Chem. 2015;305:127–32. doi: 10.1007/s10967-015-4039-z. [DOI] [Google Scholar]
- van der Meulen NP, Bunka M, Domnanich KA, Müller C, Haller S, Vermeulen C, Türler A, Schibli R. Cyclotron production of 44Sc: From bench to bedside. Nucl Med Biol. 2015;42(9):745–51. doi: 10.1016/j.nucmedbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Velikyan I. Molecular imaging and radiotherapy: theranostics for personalized patient management. Theranostics. 2012;2(5):424–6. doi: 10.7150/thno.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola-Villegas N, Doyle RP. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N, N’, N”, N”’-tetraacetic acid (H4DOTA): Structural overview and analyses on structure-stability relationships. Coord Chem Rev. 2009;253(13-14):1906–25. doi: 10.1016/j.ccr.2009.03.013. [DOI] [Google Scholar]
- Walczak R, Krajewski S, Szkliniarz K, Sitarz M, Abbas K, Choinski J, Jakubowski A, Jastrzebski J, Majkowska A, Simonelli F, Stolarz A, Trzcinska A, Zipper W, Bilewicz A. Cyclotron production of 43Sc for PET imaging. EJNMMI Physics. 2015;2(1):10. doi: 10.1186/s40658-015-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Kondo K. Preparation of Carrier-free 47Sc by the 48Ti(γ, p) Reaction. Int J Appl Radiat Isot. 1977;28(5):463–8. doi: 10.1016/0020-708X(77)90178-8. [DOI] [Google Scholar]
- Zerkin V. “Experimental Nuclear Reaction Data (EXFOR).”. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Formula for the calculation of the 47Sc activity in Bq (s−1), accessible under the applied irradiation conditions. σ = nuclear cross section of the 46Ca(n,γ)47Ca reaction in cm−2, NT = number of 46Ca atoms, Φth = thermal neutron flux in n * cm−2 * s−1, λSc and λCa = decay constants of 47Sc and 47Ca in s−1, tirr = irradiation time and twait = post irradiation waiting time in s. b Formula for the calculation of the optimal post irradiation waiting time (topt) in s, accessible at the applied irradiation time (tirr in s). The decay constants of 47Sc (λSc) and 47Ca (λCa) are given in s−1. c Formula for the calculation of the optimal relative 47Sc activity (a(47Sc)opt) (dimensionless), accessible under the applied irradiation conditions. σ = nuclear cross section of the 46Ca(n,γ)47Ca reaction in cm−2, NT = number of 46Ca atoms, Φth = thermal neutron flux in n * cm−2 * s−1, λSc and λCa = decay constants of 47Sc and 47Ca in s−1. d Formula for the maximal obtainable 47Sc activity (dimensionless). The irradiation time (tirr) is given in s and the decay constants of 47Sc (λSc) and 47Ca (λCa) in s−1. Figure S2. γ-Ray spectra of 47Sc and 47Ca from the neutron-irradiated 46Ca ampoule, obtained 71 h after the end of irradiation (measurement time: 10 s) (a) and of the pure 47Sc eluate after separation (Method B), obtained 1 h after the end of separation (measurement time: 250 s) (b). Figure S3. γ-Ray spectrum of the neutron-irradiated 47Ti ampoule at SINQ, obtained 21 d after the end of irradiation (measurement time: 9600 s). Figure S4. Measured cross section values (squares, retrieved from the EXFOR-database) (Zerkin 2016) as well as the theoretical calculations from the TENDL-2015 library (straight line) (Koning, Rochman et al. 2015) for the 47Ti(n,p)47Sc (blue) and the 47Ti(n,p + n)46Sc (black) nuclear reactions. Table S5. Trace metal analysis of the reduced 46Ti metal by ICP-OES. Only the elements determined at a concentration higher than the detection limit are listed below. (DOCX 415 kb)


