Summary
Mediator is a highly conserved transcriptional coactivator organized into four modules, namely Tail, Middle, Head, and Kinase (CKM). Previous work suggests regulatory roles for Tail and CKM, but an integrated model for these activities is lacking. Here, we analyzed the genome-wide distribution of Mediator subunits in wild-type and mutant yeast cells in which RNA polymerase II promoter escape is blocked, allowing detection of transient Mediator forms. We found that although all modules are recruited to upstream activated regions (UAS), assembly of Mediator within the pre-initiation complex is accompanied by the release of CKM. Interestingly, our data show that CKM regulates Mediator-UAS interaction rather than Mediator-promoter association. In addition, although Tail is required for Mediator recruitment to UAS, Tailless Mediator nevertheless interacts with core promoters. Collectively, our data suggest that the essential function of Mediator is mediated by Head and Middle at core promoters, while Tail and CKM play regulatory roles.
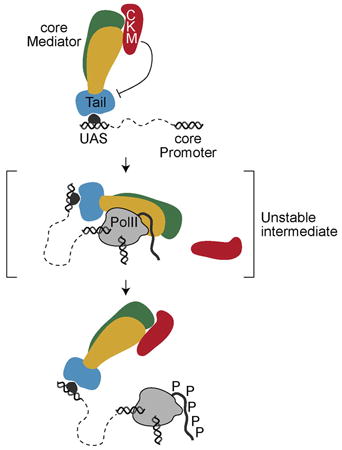
Graphical abstract
Jeronimo et al. describe a dynamic model for the interaction of Mediator modules with genes. CKM negatively regulates Mediator-UAS interactions and dissociates from Mediator upon integration of Mediator into the PIC. Tailless Mediator integrates PIC through an alternative pathway.
Introduction
Mediator is an essential, highly conserved transcriptional coactivator that regulates different aspects of transcription (Allen and Taatjes, 2015; Ansari and Morse, 2013; Malik and Roeder, 2010; Poss et al., 2013). The best characterized function of Mediator is to promote pre-initiation complex (PIC) assembly, a function that appears to be conserved from yeast to human. Mediator was also shown to regulate post-initiation events such as elongation, mRNA splicing, transcription termination, DNA looping, chromatin structure, as well as histone and DNA methylation (Allen and Taatjes, 2015; Ansari and Morse, 2013; Malik and Roeder, 2010; Poss et al., 2013; Yin and Wang, 2014). Most of these additional roles have been shown in metazoans and often involve metazoan-specific Mediator subunits suggesting that Mediator acquired new functions through evolution.
Mediator is made of 25 (yeast) to 30 (human) proteins organized into four modules named Head, Middle, Tail, and Kinase (CKM). The Tail, Middle and Head modules are held together via the scaffold subunit Med14 (Cevher et al., 2014; Plaschka et al., 2015; Robinson et al., 2015; Tsai et al., 2014), while CKM is more loosely associated (Allen and Taatjes, 2015; Ansari and Morse, 2013; Poss et al., 2013). The Head and Middle modules are essential for viability and for the expression of virtually all protein-coding genes (Holstege et al., 1998; Kemmeren et al., 2014; van de Peppel et al., 2005). They interact with RNA polymerase II (RNAPII) and other components of the PIC (Malik and Roeder, 2010). Although the expression “core Mediator” is often used to refer to the Head, Middle, and Tail domains, recent re-constitutions of sub-Mediator complexes from recombinant parts identified the minimal functional complex to be essentially made of Head and Middle subunits held together by the Med14 scaffold (Cevher et al., 2014; Plaschka et al., 2015). As suggested by these two groups, we will refer to Head/Middle/Med14 as the core Mediator, notably because the work presented here provides in vivo support for a tripartite architecture for Mediator where the Head/Middle/Med14 core is regulated by the Tail and CKM modules.
The Tail module is composed of Med2, Med3, Med5, Med15, and Med16, anchored to core Mediator via the C-terminal region of the Med14 subunit (Dotson et al., 2000; Li et al., 1995; Robinson et al., 2015). The main function of the Tail module is to connect Mediator to sequence-specific transcription factors, as most activator-Mediator interactions described to date involve Tail subunits (Brzovic et al., 2011; Herbig et al., 2010; Lee et al., 1999; Myers et al., 1999; Natarajan et al., 1999; Park et al., 2000; Thakur et al., 2008, 2009; Zhang et al., 2004). Interestingly, none of the Tail subunits are essential for viability, and their deletions, alone or in combination, only lead to expression defects for subsets of genes (Ansari et al., 2012; Kemmeren et al., 2014; van de Peppel et al., 2005). These genes are biased toward being more dependent on the SAGA than on the TFIID coactivator, suggesting a gene class-specific function for the Tail module. These data imply that Mediator can be recruited to some genes by activators contacting non-Tail subunits, as shown in metazoans (Malik and Roeder, 2010) or by directly interacting with PIC components (Ansari et al., 2012), but this has never been thoroughly investigated.
Although Head, Middle, and Tail subunits play positive roles in transcription, several lines of evidence suggest that CKM is a negative transcription regulator. First, although CKM-less Mediator stimulates transcription in vitro (Myers et al., 1998; Spahr et al., 2003), human Mediator complexes containing CKM repress transcription under similar conditions (Taatjes et al., 2002). Second, on the basis of gene expression profiling, CKM mutants primarily lead to upregulation of gene expression (Holstege et al., 1998; Kemmeren et al., 2014; van de Peppel et al., 2005). Finally, biochemical and structural work has shown that CKM and RNAPII interact with Mediator in a mutually exclusive manner (Elmlund et al., 2006; Knuesel et al., 2009; Myers et al., 1998; Näär et al., 2002; Samuelsen et al., 2003; Spahr et al., 2003; Tsai et al., 2013), suggesting that CKM may negatively regulate transcription by preventing the interaction between Mediator and RNAPII. Although appealing, this model suffers from the lack of evidence for an RNAPII-Mediator complex, free of CKM, on chromatin. Other mechanisms have been proposed for the negative role of CKM in gene expression, all involving the phosphorylation of specific substrates by Cdk8, the kinase subunit of CKM. These substrates include sequence-specific transcription factors (Chi et al., 2001; Fryer et al., 2004; Gonzalez et al., 2014; Nelson et al., 2003; Poss et al., 2016), the Tail subunit Med3 (Gonzalez et al., 2014; van de Peppel et al., 2005), the general transcription factor TFIIH (metazoan only) (Akoulitchev et al., 2000), and the RNAPII C-terminal domain (CTD) (Hengartner et al., 1998). The relative contributions of kinase-dependent mechanisms and (kinase-independent) interference of CKM with Mediator-RNAPII interaction on the function of CKM have yet to be investigated in vivo.
We and others previously used mutations which block RNAPII promoter escape to reveal that Mediator interacts with promoters (Jeronimo and Robert, 2014; Wong et al., 2014). In wild-type (WT) cells, Mediator is detected at upstream activating sequences (UAS), while in TFIIH kinase (Kin28)-compromised cells, Mediator also accumulates at promoters. These data can be explained by a dynamic model in which Mediator is recruited to the UAS and subsequently interacts transiently with the promoter during PIC formation; this interaction is terminated on promoter escape by phosphorylation of the RNAPII CTD on serine 5. Here, we profiled the genome-wide distribution of 12 Mediator subunits in both WT and Kin28-compromised cells, as well as in cells lacking CKM or Tail Mediator subunits. In conjunction with recent structural data (Plaschka et al., 2015; Robinson et al., 2015; Tsai et al., 2014; Wang et al., 2014), our data suggest a detailed model of the dynamic association of Mediator with genes. We show that although Mediator recruitment to UAS is strictly dependent on the Tail module, the transient association of Mediator with the PIC is largely Tail independent. Our work also reveals that eviction of CKM from Mediator occurs upon PIC assembly and that CKM regulation of Mediator occurs at the UAS, prior to interaction with the promoter.
Results
CKM-Free Mediator Is Present at Promoters
In vitro evidence suggests that CKM and RNAPII interact with Mediator in a mutually exclusive manner (Elmlund et al., 2006; Knuesel et al., 2009; Myers et al., 1998; Näär et al., 2002; Samuelsen et al., 2003; Spahr et al., 2003; Tsai et al., 2013). However, numerous published chromatin immunoprecipitation (ChIP)-chip and ChIP sequencing (ChIP-seq) data sets show remarkable overlap among all Mediator modules (Andrau et al., 2006; Jeronimo and Robert, 2014; Paul et al., 2015; Venters and Pugh, 2009; Wong et al., 2014; Zhu et al., 2006). More recently, however, we and others have shown that Mediator, as detected by ChIP in WT cells, corresponds to the activator-recruited (UAS-bound) population, while another form of Mediator, occupying core promoters, is detected only under conditions in which RNAPII CTD phosphorylation by the TFIIH kinase Kin28 is compromised (Jeronimo and Robert, 2014; Wong et al., 2014). To determine whether the subunit composition of these two forms of Mediator is different, we extended our genome-wide ChIP analysis of Mediator (Jeronimo and Robert, 2014) to nearly half of its subunits, including two to four subunits of each Mediator module in WT cells and those in which Kin28 activity is abrogated through the use of an ATP analog-sensitive allele (kin28as) following treatment with 6 μM of the ATP analog 1-Naphthyl PP1 (NAPP1) for 15 min.
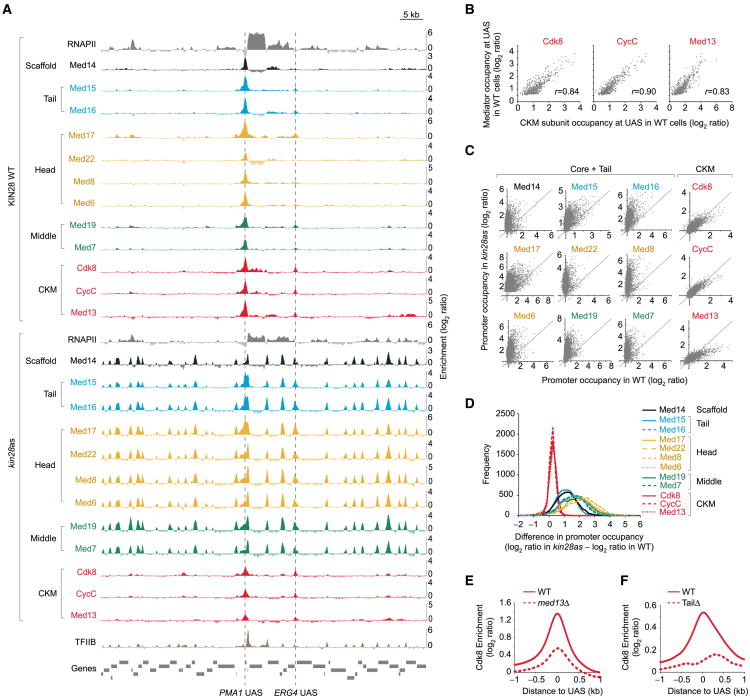
Visual inspection of the genome-wide ChIP-chip data in WT cells clearly shows that all Mediator module subunits bound to the same UAS regions (Figure 1A, top tracks; see dashed vertical lines). This includes CKM subunits, which occupancy at UAS regions correlated with other Mediator subunits (Figure 1B). However, in kin28as cells—conditions in which Mediator accumulates at core promoters—only Tail, Middle, and Head subunits show additional peaks at core promoters (Figure 1A, bottom tracks). Indeed, CKM subunits invariably associated with UAS regions in both WT and kin28as cells (Figure 1A). In order to quantify this observation genome-wide, we plotted, for each Mediator subunit, ChIP signal over core promoters in kin28as versus in WT cells. This analysis clearly highlights that Head, Middle, and Tail subunit occupancy increased at core promoters in kin28as cells, whereas CKM subunit occupancy did not (Figure 1C). This is also evident when looking at the distribution of the kin28as/WT promoter occupancy ratio for each Mediator subunit (Figure 1D) and correlations between the various data sets (Figure S1). These data are consistent with the model that CKM has to be ejected from Mediator in order for Mediator to integrate into the PIC. Alternatively, CKM may be recruited to UAS regions independently of Mediator. However, we ruled out this possibility, because CKM occupancy was radically decreased in med13Δ cells (Figure 1E), in which CKM interaction with Mediator is crippled (Tsai et al., 2013), and virtually abolished in med3Δ/med15Δ cells (Figure 1F, TailΔ), in which Mediator is no longer recruited to UAS regions (see below). Collectively, our data are consistent with a dynamic model in which complete Mediator is recruited to the UAS, followed by dissociation of CKM and interaction of Mediator within the PIC. These data provide the first evidence for a transient CKM-free Mediator-RNAPII complex on chromatin and are consistent with previous in vitro studies.
Figure 1. Mediator CKM Subunits Do Not Associate with Core Promoters in Living Cells.
(A) TFIIB (tan), RNAPII (Rpb3, light gray), Mediator scaffold subunit Med14 (dark gray), Mediator Tail subunits Med15 and Med16 (blue), Mediator Head subunits Med17, Med22, Med8, and Med6 (gold), Mediator Middle subunits Med19 and Med7 (green), and Mediator CKM subunits Cdk8, CycC, and Med13 (red) occupancy (normalized ChIP-chip log2 ratio of tagged versus no-tagged samples) in KIN28 WT (top tracks) and kin28as (bottom tracks) cells, all treated with NAPP1, along a segment of chromosome VII. Vertical dashed lines indicate the position of the two UAS bound by Mediator in this region. Genes (gray boxes) are shown at the bottom. Data for TFIIB, RNAPII, and Med15 in this and other figures are from (Jeronimo and Robert, 2014).
(B) Scatterplots showing the occupancy of Mediator (the average of all subunits profiled) versus that of the indicated CKM subunits over 498 UAS regions in WT cells.
(C) Scatterplots showing the occupancy of various Mediator subunits at core promoters (n = 5,128) in kin28as versus WT cells, all treated with NAPP1.
(D) Distribution of the differential promoter occupancy in WT and kin28as cells for various Mediator subunits, all treated with NAPP1.
(E) Average Cdk8 occupancy around UAS regions (n = 498) in WT (solid trace) and in med13Δ cells (dashed trace).
(F) Average Cdk8 occupancy around UAS regions (n = 498) in WT (solid trace) and in med3Δ/med15Δ (TailD, dashed trace) cells. The coordinates of the UAS used in this and other figures were determined using our Mediator ChIP-chip data (see Experimental Procedures). See also Figure S1.
CKM Regulates Mediator-UAS Interactions Rather than Mediator-RNAPII Interactions
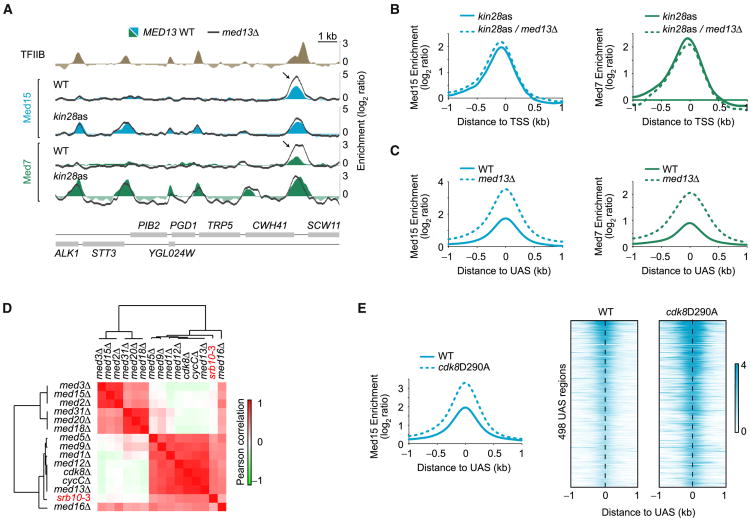
The finding that Mediator at promoters is CKM-free is consistent with ejection of CKM from Mediator being a regulatory step for Mediator-RNAPII interaction. To test this model, we analyzed the distribution of Mediator subunits in med13Δ mutant cells in which CKM dissociates from Mediator (Tsai et al., 2013). Contrary to expectations, Med15 (Tail) and Med7 (Middle) occupancy at promoters was only modestly affected in kin28as/med13Δ cells (Figures 2A, 2B, and S2A). This result suggests that the ejection of CKM from Mediator is not a rate-limiting step at most genes, although we cannot rule out the possibility that this step is rate limiting at specific genes. In contrast, however, the association of both Med15 and Med7 was drastically increased at UAS regions genome-wide in med13Δ cells (Figures 2A and 2C). These data can be interpreted in two ways. On one hand, CKM may be essential to trigger the transition from UAS to promoter binding, perhaps through its kinase activity. In the absence of this trigger, the Mediator cycle would be blocked, and Mediator would accumulate at the UAS. Such a model, however, would imply that CKM primarily plays a positive role in transcription, which would be inconsistent with a large body of literature (see Introduction). Alternatively, CKM may negatively regulate Mediator-UAS interactions. This interpretation is consistent with epistasis analyses of gene expression profiling showing that CKM antagonizes Tail module function (van de Peppel et al., 2005). It is also consistent with the fact that the CKM kinase Cdk8 phosphorylates several transcription factors (Poss et al., 2016), often leading to their degradation (Alarcón et al., 2009; Fryer et al., 2004; Nelson et al., 2003; Raithatha et al., 2012). In addition, a recent study showed that the Tail subunit Med3 is degraded consequently to Cdk8-mediated phosphorylation, leading to downregulation of a subset of genes (Gonzalez et al., 2014). We therefore entertained the possibility that Cdk8-dependent phosphorylation of various substrates, as opposed to kinase-independent Mediator-PIC interactions interference, is the dominant mechanism by which CKM mediates its function. This model is supported by the fact that the gene expression profile of catalytically inactive cdk8 cells (srb10-3) (Liao et al., 1995) is highly correlated with profiles from CKM subunit deleted strains (cdk8Δ, cycCΔ, med12Δ, and med13Δ) (Figures 2D and S2B). In order to directly test whether the effect of CKM on Mediator-UAS interactions is mediated by the kinase activity of Cdk8, we looked at Mediator occupancy in Cdk8 kinase-deficient cells (cdk8D290A). As shown in Figure 2E, Med15 shows increased UAS occupancy in cdk8D290A cells, analogously to what is observed in med13Δ cells (Figure 2C). Collectively, our data show that CKM regulates Mediator-UAS interactions via the Cdk8 kinase activity.
Figure 2. Mediator Accumulates at UAS Regions in the Absence of CKM.
(A) Med15 (blue) and Med7 (green) occupancy in WT and kin28as cells, all treated with NAPP1, along a segment of chromosome VII. Dark gray traces show the effect of deleting MED13 in these strains. The Med15 and Med7 peaks appearing in kin28as cells correspond to promoter peaks and are not enhanced when deleting MED13 (med13Δ, dark gray trace). Arrows are pointing toward a UAS Mediator peak, enhanced upon MED13 deletion.
(B) Average Med15 (blue) and Med7 (green) occupancy around the transcription start site (TSS) of transcriptionally active genes (n = 165, see Experimental Procedures) in kin28as cells (solid traces) and in kin28as/med13Δ cells (dashed traces). See also Figure S2A.
(C) Average Med15 (blue) and Med7 (green) enrichment around UAS regions (n = 498) in WT (solid traces) and med13Δ (dashed traces) cells.
(D) Heatmap representation of the hierarchical clustering of Pearson correlations between expression profiles of various Mediator subunit mutants. Data for srb10-3, a strain with a point mutation (D290A) in the catalytic domain of the CDK8 gene (Liao et al., 1995), were from Holstege et al. (1998), while all other data sets are from Kemmeren et al. (2014). See also Figure S2B.
(E) Average (left) and heatmap representation (right) of Med15 occupancy around UAS regions (n = 498) in WT (solid traces) and cdk8D290A (dashed traces) cells.
The Tail Module Is Required for Mediator Recruitment to UAS Regions but Dispensable for the Assembly of Mediator within the PIC
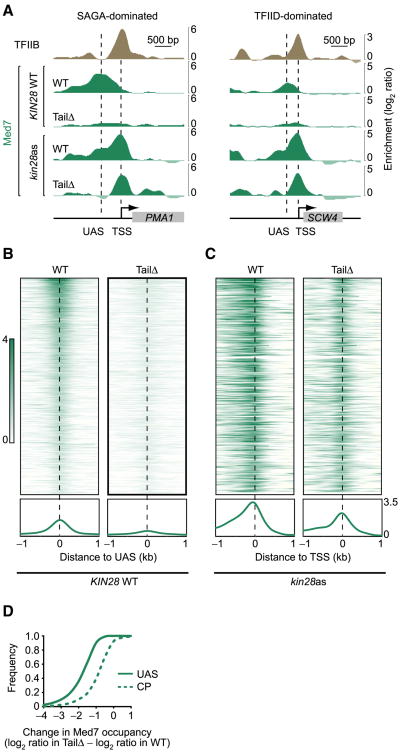
We next profiled Mediator occupancy in cells lacking the Tail subunits Med15 and Med3. This double mutant was previously shown to lead to a loss of Tail module functions (Ansari et al., 2012, 2014; Paul et al., 2015; Zhang et al., 2004), so we will refer to these cells (med3Δ/med15Δ) as TailΔ. Very strikingly, Mediator occupancy at UAS regions was reduced to background level in TailΔ cells (Figures 3A and 3B, KIN28 WT). In fact, we did not find a single Mediator peak above background level in these cells. This result was surprising, becasue these cells are viable, relatively healthy and characterized by rather minor gene expression defects (Ansari et al., 2012). Note that we observed no differences in Tail dependence for SAGA-versus TFIID-dominated genes (Figures 3A and S3), contrary to a previous report (Ansari et al., 2012).
Figure 3. The Tail Domain Is Required for Mediator-UAS Interactions but Dispensable for Mediator to Associate with Core Promoters.
(A) Med7 (green) occupancy at the SAGA-dominated gene PMA1 (left) and the TFIID-dominated gene SCW4 (right) in WT and med3Δ/med15Δ (TailΔ) cells, both in a KIN28 WT and kin28as backgrounds. All samples were treated with NAPP1. TFIIB (tan) is shown as a placeholder for core promoters. Vertical dashed lines indicate the position of the UAS and TSS.
(B) Heatmap representation of Med7 occupancy in WT (left) and med3Δ/med15Δ (TailΔ, right) cells around UAS regions (n = 498, see Experimental Procedures) sorted by decreasing Mediator occupancy.
(C) Heatmap representation of Med7 occupancy in kin28as (WT, left) and kin28as/med3Δ/med15Δ (TailΔ, right) cells around the TSS of genes associated to the UAS regions used in (B). Traces at the bottom of each heatmap from (B) and (C) show the average occupancy across these loci.
(D) Cumulative plot of the change in Med7 occupancy in WT versus med3Δ/med15Δ (TailΔ) cells at UAS (solid trace) and at core promoters (CP, dashed traces). UAS and core promoter traces were calculated from experiments performed in KIN28 WT and kin28as cells treated with NAPP1 respectively, using the same loci as in (B) and (C).
See also Figure S3.
How can a TailΔ Mediator achieve its essential function if it is no longer recruited to chromatin? A solution to this conundrum would be if TailΔ Mediator could find its way to core promoters in the absence of prior recruitment to UAS regions. In order to test this prediction, we profiled Mediator in TailΔ/kin28as cells. Consistent with this prediction, Mediator associated with core promoters in these cells (Figures 3A and 3C). Mediator occupancy in TailΔ/kin28as cells was not quite to the same level as in kin28as cells but the decrease was modest (less than 2-fold for the majority of promoters) contrasting the virtual absence of Mediator observed at UAS regions (Figure 3D). This result has at least three important implications: (1) Mediator can associate with core promoters without recruitment to a UAS (i.e., there are at least two pathways for Mediator recruitment), (2) the essential function of Mediator is achieved at core promoters when Mediator interacts with the PIC, and (3) a looped structure linking UAS and promoter regions is not inherently required for Mediator function.
The Tail Module Is a Modulator of Mediator Function
The data shown above suggest that the main function of the Tail module is, via the recruitment of Mediator at the UAS, to increase the local concentration of Mediator in the vicinity of the core promoter, therefore promoting Mediator-PIC interactions, increasing PIC assembly/stability and transcription initiation. We therefore tested whether deletion of the Tail module affected PIC assembly/stability. Promoter occupancy by the general transcription factor (GTF) TFIIB was used as a surrogate of PIC assembly/stability. As shown in Figure 4A, Tail module deletion led to reduced TFIIB occupancy at promoters (dashed trace). This effect, however, was relatively modest compared with that of depleting the Head subunit Med18 from the nucleus using the anchor-away technique (Haruki et al., 2008) (Figure 4A, solid trace). Note that this result represents an underestimation of the contribution of Med18 on TFIIB occupancy, because nuclear depletion is often incomplete using this technique (Jeronimo and Robert, 2014; Jeronimo et al., 2015) and because TFIIB may not have had enough time to completely turn over from the promoter during the short time used for nuclear depletion (90 min). Taken together, these data suggest that the Tail module positively contributes to, but is not absolutely required for, the positive role of Mediator in PIC assembly/stability.
Figure 4. The Tail Module Contributes to PIC Assembly/Stability to a Similar Extent in SAGA- and TFIID-Dominated Genes.
(A) Cumulative plot of the change in TFIIB promoter occupancy in med18-AA cells after a 90 min treatment with rapamycin (solid trace) and in med3Δ/med15Δ cells (TailΔ, dashed trace), relative to their respective WT. All genes with a TFIIB log2 ratio > 2 were used in this analysis.
(B) Cumulative plot of the change in TFIIB promoter occupancy in med3Δ/med15Δ cells (TailΔ) cells, relative to WT, for the SAGA-dominated (red) and TFIID-dominated (blue) genes with a TFIIB log2 ratio > 2.
(C) Cumulative plot of the change in TFIIB promoter occupancy in med18-AA cells after a 90 min treatment with rapamycin, relative to WT, for the SAGA-dominated (red) and TFIID-dominated (blue) genes with a TFIIB log2 ratio > 2 (high TFIIB, solid traces) or for genes having equivalent TFIIB levels between the SAGA- and TFIID-dominated gene groups (equivalent TFIIB, dashed traces).
(D) Distribution of TFIIB occupancy at the promoter of SAGA-dominated genes (red) and TFIID-dominated genes (blue).
See also Figure S4.
Yeast genes are often classified in two broad categories depending on their expression being more dependent on TFIID or SAGA, two different TBP and TAFs-containing complexes (Huisinga and Pugh, 2004). Although SAGA-dominated genes (which are enriched for regulated genes) generally contain a canonical TATA box, TFIID-dominated genes (mainly housekeeping genes) have been reported to contain a TATA-like sequence (Rhee and Pugh, 2012b). A recent study showed that the Tail module is important for the recruitment of Mediator and for TBP occupancy at a selection of SAGA-dominated genes but not at the TFIID-dominated genes tested (Ansari et al., 2012). Furthermore, gene expression profiling showed that genes affected by Tail deletion were enriched for SAGA-dominated genes (Ansari et al., 2012). This work led to the idea that the Tail module is more critical for Mediator function at SAGA-dominated genes than at TFIID-dominated genes. Our analysis of Mediator occupancy in TailΔ cells, however, clearly shows strong Tail-dependence for binding of Mediator to the UAS of virtually all genes in KIN28 WT cells (Figure 3B), with not obvious bias toward any gene classes (Figure S3). Manual inspection of our data for the TFIID-dominated genes confirmed that Mediator binding to these UAS regions, when detected, requires the Tail module (see Figure 3A for an example; SCW4 gene). We next investigated the contribution of the Tail module to PIC assembly/stability at SAGA- and TFIID-dominated genes. In agreement with Ansari et al. (2012), deleting the Tail had a greater impact on TFIIB occupancy at SAGA-dominated genes than at TFIID-dominated genes (Figure 4B). When looking at the effect of the Head module subunit Med18, however, the same trend was observed (Figure 4C, solid traces) suggesting that SAGA-dominated genes may be more dependent on Mediator as a whole, rather than being specifically dependent on the Tail module. The fact that tampering with Mediator has a more profound effect on TFIIB occupancy at SAGA- versus TFIID-dominated genes, however, must be interpreted cautiously. Indeed, we found very strong correlations between TFIIB occupancy levels in WT cells and the difference in its occupancy upon deletion of the Tail module or anchoring away Med18 (Pearson's r = −0.61 and −0.80, respectively). Because most genes with high TFIIB occupancy are within the SAGA-dominated gene group (Figure 4D), it appears likely that the greater effect observed at SAGA-dominated genes is simply reflecting their expression levels. In support of this interpretation, the difference between SAGA- and TFIID-dominated genes with respect to the effect of Med18 nuclear depletion on TFIIB occupancy was almost completely abolished when considering genes with similar TFIIB levels in WT conditions (Figure 4C, dashed traces). Similarly, although Mediator occupancy is generally higher at SAGA-dominated than at TFIID-dominated genes (Figure S4A), this difference is abolished when considering genes at similar expression levels (Figure S4B). In sum, our data suggest that Mediator promotes PIC assembly/stability at all genes, and that the Tail module stimulates this function, regardless of gene classes.
A Topological Model for the Mediator-Associated PIC in Living Cells
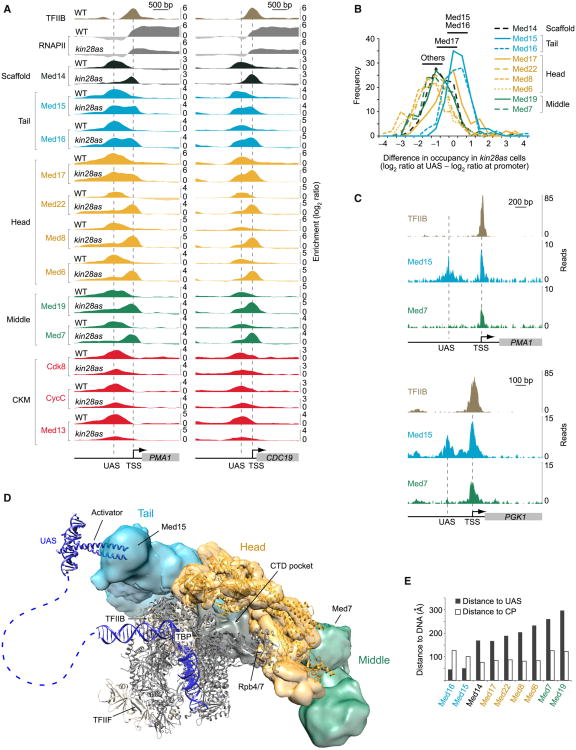
Our analysis is consistent with a dynamic model in which Mediator is recruited to the UAS in a Tail-dependent manner and subsequently interacts with the PIC at core promoter. We sought to determine whether our temporal model is consistent with the topology and structure of Mediator. Our model is most easily explained if the same Mediator complex contacts the UAS and, subsequently, the promoter, forming a small loop. An alternative scenario is that Mediator is recruited to the UAS and subsequently translocates to the promoter, potentially allowing a second Mediator to bind the UAS. These models are distinguished by the presence of a single Mediator (“looping model”), occupying both UAS and promoter, or two Mediators (“translocation model”), one at the UAS and a second at the promoter. To distinguish the models, we analyzed the pattern of crosslinking of different Mediator subunits at UAS and core promoters in WT and kin28as cells. Med15, Med16, and Med17 subunits persisted at UAS in kin28as cells, while other subunits (Med6, Med7, Med8, Med14, Med19, and Med22) showed decreased ChIP signal at UAS regions. This result can be observed at individual genes such as PMA1 and CDC19 (Figure 5A) and can be quantified using all genes where Mediator occupancy at the UAS and at the core promoter can be resolved (genes in which the distance between the UAS and the core promoter is greater than 300 bp) (Figure 5B). To better resolve UAS from promoter regions, we performed ChIP-exo for Med15 and Med7 in kin28as cells. These high-resolution experiments confirmed our ChIP-chip data and clearly showed that the Tail subunit Med15 cross-linked equally well to UAS and promoters in kin28as cells, while the Middle subunit Med7 was found mainly associated with core promoters (Figure 5C). This decrease in ChIP signal at the UAS when the PIC is stabilized by tampering with Kin28 is not consistent with two different Mediators simultaneously occupying the UAS and the promoter in kin28as cells, because all core Mediator subunits are reliably detectable at UAS regions in WT cells (see Figure 1). Instead, the increase in ChIP signal at promoters and decrease at UAS regions are consistent with, although do not directly prove, a change in the topology of the promoter-bound Mediator as predicted for the looping model.
Figure 5. A Topological Model for Mediator Interactions at the UAS and Promoter.
(A) TFIIB, RNAPII, and various Mediator subunits (as in Figure 1A) occupancy in WT and kin28as cells, all treated with NAPP1, at the PMA1 (left) and CDC19 (right) loci. Vertical dashed lines indicate the position of the UAS and TSS, respectively defined as the peak summit of Mediator and TFIIB in WT cells.
(B) Distribution of the difference in occupancy at the UAS versus the core promoter in kin28as cells treated with NAPP1, for various core Mediator subunits. All gene-UAS pairs separated by more than 300 bp are used (n = 108).
(C) Read density for TFIIB (tan), Med15 (blue), and Med7 (green) ChIP-exo experiments performed in kin28as cells, treated with NAPP1, at the PMA1 (top) and PGK1 (bottom) loci. Vertical dashed lines indicate the position of the UAS and TSS.
(D) A model for the topology of core Mediator, transiently interacting with the PIC. The topological model was built using data from Plaschka et al. (2015), onto which the various Mediator subunits were positioned using data from previous Mediator models (Robinson et al., 2015; Tsai et al., 2014) (see Experimental Procedures). Transparent surfaces colored in shades of blue (Tail), gold (Head), and green (Middle) show the positions of the Mediator modules based on Robinson et al. (2015). Ribbons for GTFs TBP, TFIIB, and TFIIF are shown in tan, for RNAPII are shown in gray, and for Head mediator subunits are shown in gold based on Plaschka et al. (2015). A cavity in the structure that coincides with the position were the CTD emerges from Rpb1 is indicated by an arrow. The structure of the Gcn4 DNA-binding domain bound to the UAS (Keller et al., 1995) (PDB accession number 2DGC) is shown as an example of an activator interacting with the Tail subunit Med15.
(E) The minimal distance between the center of mass of each core Mediator subunits and the predicted position of the UAS DNA (gray) and core promoter DNA (CP; white) based on the structural model shown in (D).
See also Figure S5.
Recent cryo-electron microscopy (EM) and protein crosslinking work from the Cramer lab has led to a topological model of a “mini-PIC” containing the Head and Middle (minus Med1) modules, RNAPII, a subset of GTFs and promoter DNA (Plaschka et al., 2015). Thanks to previous structural work from several groups, and in particular the integrative Mediator model recently published by Kornberg and colleagues (Robinson et al., 2015), most Mediator subunits can be positioned in this model. Figure 5D shows a composite topological model based on this previous work (see also Figure S5A). The position of the Tail module, which is absent from the mini-PIC model (Plaschka et al., 2015), was modeled on the basis of its position in previously described Mediator models (Robinson et al., 2015; Tsai et al., 2014) (see Experimental Procedures). Note that an equivalent structural model was recently reported by the Cramer laboratory (Plaschka et al., 2016). Noteworthy, the position of the Tail module in these models is in agreement with a recent structure of the Mediator-containing PIC by the Kornberg group (Robinson et al., 2016).To test whether the model shown in Figure 5D, built solely from structural work, supports our in vivo ChIP data, we calculated the shortest distance between the center of mass of each Mediator subunit and both core promoter DNA and predicted UAS region from the structural model (see Figure S5B and Experimental Procedures). Quite strikingly, these distances match the ChIP pattern: subunits for which crosslinking at the UAS decreased in kin28as cells (Med6, Med7, Med8, Med14, Med19, and Med22) (Figure 5B) are in closer proximity to core promoter DNA relative to the UAS in the model (Figure 5E), while subunits that crosslink equally to UAS and core promoter regions (Med15 and Med16) (Figure 5B) are close in distance to both the UAS and the core promoter in the structural model (Figure 5E). Interestingly, the large architectural subunit Med17 (Robinson et al., 2015) showed intermediate behaviors in both our ChIP data (Figure 5B) and distance measurements (Figure 5E). This analysis provides further evidence for the UAS-promoter looping model and suggests that the structure of the mini-PIC determined in vitro (Plaschka et al., 2015), reflects the structure of the PIC in living cells.
Discussion
The work reported here provides a detailed topological and temporal model for the association of Mediator with genes in budding yeast and shows that CKM dissociates from Mediator upon the association of Mediator with RNAPII and the PIC at the core promoter. What regulates the dissociation of CKM from Mediator will require additional work but may involve the degradation of Med13 as proposed previously (Davis et al., 2013). Contrary to previous expectations, our data does not support the idea that CKM negatively regulates transcription by blocking Mediator-RNAPII interactions (Elmlund et al., 2006; Knuesel et al., 2009; Näär et al., 2002; Samuelsen et al., 2003; Tsai et al., 2013). Instead, our data are consistent with CKM negatively regulating Mediator-UAS interactions via Cdk8-dependent phosphorylation of various substrates such as transcription factors or Mediator subunits such as Med3. Another surprising finding is that Mediator can mediate its essential function even when its recruitment to UAS is completely abolished such as in cells carrying deletions of both MED3 and MED15. These data suggest that recruitment by activators is not inherently required for Mediator function. The business end of Mediator therefore appears to reside in the Head and Middle modules, interacting with PIC components at core promoters, leaving Tail and CKM as accessory modules. We propose that Tail and CKM essentially function as positive and negative regulators of Mediator function, respectively promoting and impairing Mediator-UAS interactions, which in turn impact Mediator-PIC interactions.
The Roeder (Cevher et al., 2014) and Cramer (Plaschka et al., 2015) groups recently suggested redefining “core Mediator” as the minimal, essential and functional Mediator complex. On the basis of their in vitro evidence, core Mediator would contain the Head and Middle modules held together by the Med14 scaffold. This definition excludes the Tail module from core Mediator, which was found to be dispensable for minimal Mediator activity in vitro by both groups (Cevher et al., 2014; Plaschka et al., 2015). The Tail module is generally considered as part of the core Mediator, although one of the original Mediator purification papers from the Hahn laboratory also used the expression “core” to define Tailless form of Mediator (Liu et al., 2001). Our in vivo data support this definition as we found the Tail and CKM modules to play regulatory roles by mediating core Mediator recruitment and function. On the basis of this evidence, we propose that Mediator is better defined as a tripartite architecture composed of an essential core, regulated by the Tail and Kinase modules (Cevher et al., 2014; Plaschka et al., 2015).
The crosslinking behavior of the different Mediator subunits in our ChIP data using WT and kin28-compromised cells is consistent with the mini-PIC model proposed by the Cramer group (Plaschka et al., 2015), with the addition of the Tail module to a location similar to where it is found in free Mediator (Robinson et al., 2015; Tsai et al., 2014; Wang et al., 2014), as recently reported (Plaschka et al., 2016; Robinson et al., 2016). Such positioning of the Tail would allow for contacting the UAS. This topology is different from the one proposed for the RNAPII holoenzyme, where the Tail module localizes near the DNA binding cleft of RNAPII (Robinson et al., 2015). It is therefore tempting to speculate that the RNAPII holoenzyme, as usually purified, is not representative of promoter-bound RNAPII-Mediator. Instead, it may represent other populations of RNAPII-Mediator complexes in the cell, perhaps as a reservoir of transcription machinery components.
Does Mediator function the same way in higher eukaryotes? Several features described here are consistent with data in other organisms. First, in metazoan, the recruitment of Mediator to enhancers and the looping between enhancers and core promoters are well-described phenomena (Allen and Taatjes, 2015; Levine et al., 2014). In these organisms, both the core subunit Med1 and the CKM subunit Cdk8 are generally better detected by ChIP at enhancers than they are around core promoters (Kagey et al., 2010; Pelish et al., 2015; Whyte et al., 2013), similar to what we describe here in yeast. Whether inhibiting Cdk7 kinase (the mammalian ortholog of Kin28) would enhance Mediator detection at core promoters in metazoans remains to be tested, but these experiments may be difficult to interpret because of the prevalence of promoter-proximal pausing in these organisms. Indeed, in metazoans most of the RNAPII ChIP signal detected in promoter areas likely represents paused polymerases as opposed to PIC-associated polymerases. Because both Cdk7 (Nilson et al., 2015) and Mediator (Donner et al., 2010; Galbraith et al., 2013; Takahashi et al., 2011) were shown to regulate pausing, it may be difficult to tease apart promoter escape versus pause release in Cdk7-compromised cells. Although there is currently no data allowing testing whether CKM ejects from core Mediator upon PIC assembly in organisms other than yeast, biochemical and ChIP data from human Mediator suggest that this phenomenon is most likely conserved (Knuesel et al., 2009; Malik et al., 2005; Pavri et al., 2005; Samuelsen et al., 2003; Tsai et al., 2013, 2014). Also, inhibition of Cdk8/Cdk19 in mammalian cells leads to further activation of super-enhancers (Pelish et al., 2015), suggesting that the role of Cdk8 as a kinase-mediated negative regulator of Mediator function at UAS/enhancers is a conserved phenomenon. Finally, all Mediator gene knockout mice attempted to date showed embryonic lethality (reviewed in Yin and Wang, 2014), highlighting the key role of Mediator in gene expression in mammals. Interestingly, however, embryonic stem cells or fibroblasts derived from Tail subunit knockout embryos do generally survive (Ito et al., 2002; Stevens et al., 2002; Wang et al., 2005), while those from Head or Middle subunit knockouts either die (Tudor et al., 1999) or show severe proliferation defects (Risley et al., 2010). These data suggest that, like in yeast, the Head and Middle domains in higher eukaryotes are ubiquitously required for gene expression while the Tail domain plays more specialized regulatory roles, notably in the establishment of developmental programs.
In summary, the work presented here details a dynamic Mediator cycle, driven by its regulatory CKM and Tail modules, that may represent the most fundamental or ancient function of Mediator. In multi-cellular organisms, the role of Mediator in gene expression is likely more complex in part because of the addition and diversification of Mediator subunits.
Experimental Procedures
For detailed experimental procedures, see Supplemental Experimental Procedures.
Yeast Strains and Growth Conditions
Genotypes for the yeast strains used in this study are listed in Table S1. All tagging and deletions were performed using transformation and homologous recombination of appropriate PCR cassettes. cdk8D290A strains were generated by clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 using the plasmid pML107 (Laughery et al., 2015). All yeast cells were grown to an optical density at 600 nm (OD600) of 0.6–0.8 at 30°C following standard procedures. kin28as strains and their controls were treated with 6 mM of 1-Naphthyl PP1 (NAPP1; Tocris Bioscience) for 15 min prior to crosslinking. For ChIP experiments involving med13Δ and cdk8D290A strains, cells were incubated under higher agitation (330 rpm) with the addition of glass beads (4 mm diameter) in order to reduce flocculation as previously described (van de Peppel et al., 2005).
ChIP-Chip and Data Analysis
ChIP-chip experiments were performed in duplicates and normalized as previously described (Jeronimo and Robert, 2014). The microarrays were custom designed by Agilent Technologies and contain a total of about 180,000 Tm-adjusted 60-mer probes covering the entire yeast genome with virtually no gaps between probes (Jeronimo and Robert, 2014). All metagene analyses were performed using VAP 1.1 (Brunelle et al., 2015; Coulombe et al., 2014). Individual Mediator subunit ChIP-chip data sets from experiments performed in WT and kin28as cells were combined into MetaMediator data sets (Data S1 and Data S2, respectively) by averaging normalized data sets from individual Mediator subunits. Coordinates corresponding to MetaMediator maxima in WT cells that were above three were extracted, manually curated, and used as coordinates for UAS regions (n = 498; Table S2). The metagene used in Figure 2B excludes all genes associated to any of the 498 UAS regions (n = 165).
ChIP-Exo Assay and Data Analysis
ChIP-exo experiments for TFIIB (Sua7-TAP), Med15-9×MYC, and Med7-13×MYC were performed in duplicate and carried out essentially as previously described (Rhee and Pugh, 2012a), but using Illumina adaptors and platform. Uniquely aligned sequence tags were mapped to the yeast genome (sacCer3) using BWA-MEM (version 0.7.9a) (Li, 2013). In order to better reflect the crosslinking coordinates, tags were shifted in the 3′ direction by 6 bp, and strand information removed. Epitope-tagged data sets were normalized using the isogenic no-tag strain.
Structure Alignments and Distance Calculations
The EM electron density map of the core initiation transcription complex with core Mediator (cITC-cMed complex; Plaschka et al., 2015; EM Database accession number 2786) and the associated coordinate model (Protein Data Bank [PDB] accession number 4V1O) were aligned to the EM density map of a Mediator complex (Tsai et al., 2014; EM Database accession number 2634) and a recent integrative model of Mediator from Kornberg and colleagues (Robinson et al., 2015; http://salilab.org/mediator/). The center of mass for specific Mediator subunits was determined on the basis of their molecular envelopes (Robinson et al., 2015), and the shortest distance was calculated from the center of mass to the following two locations: (1) a plane defining the edge of the Mediator Tail module, representing the UAS, and (2) the backbone atoms of the promoter DNA in 4V1O, representing the core promoter.
Supplementary Material
Highlights.
A dynamic model for Mediator module interactions with genes
CKM is release upon integration of Mediator into the pre-initiation complex
Mediator can integrate the PIC without prior recruitment to UAS by the Tail module
CKM regulates Mediator-UAS interactions rather than Mediator-PIC association
Acknowledgments
This work was funded by grants from the Canadian Institutes of Health Research (CIHR) to F.R. (MOP-133 648) and J.M.P. (MOP-142 354) and a grant from the National Institutes of Health to B.F.P. (GM059055). B.F.P. has a financial interest in Peconic, which uses the ChIP-exo technology implemented in this study and could potentially benefit from the outcomes of this research. We are grateful to Nicole Francis for helpful discussions and her critical reading of the manuscript. We thank Kevin Struhl for discussing unpublished data. We also thank Richard A. Young, Kevin Struhl, Julie Soutourina, and Alan G. Hinnebusch for providing strains.
Footnotes
Accession Numbers: The accession numbers for the ChIP-chip data sets and ChIP-exo sequencing data reported in this paper are GEO: GSE81107 and GEO: GSE81127, respectively.
Supplemental Information: Supplemental Information includes Supplemental Experimental Procedures, five figures, two tables, and two data files and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2016.09.002.
Author Contributions: C.J. and F.R. designed the study, with contributions from M.-F.L., A.R.B., J.M.P., and B.F.P. C.J. conducted most of the experiments. A.R.B. performed the ChIP-exo experiments described in Figure 5C. M.-F.L. built the topological model shown in Figure 5D and performed distance measurements shown in Figure 5E. F.R. performed the bioinformatic analyses. F.R. and C.J. wrote the manuscript, with input from M.-F.L., A.R.B., J.M.P., and B.F.P. All authors commented on the manuscript.
References
- Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–106. doi: 10.1038/35024111. [DOI] [PubMed] [Google Scholar]
- Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16:155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrau JC, van de Pasch L, Lijnzaad P, Bijma T, Koerkamp MG, van de Peppel J, Werner M, Holstege FCP. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol Cell. 2006;22:179–192. doi: 10.1016/j.molcel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Ansari SA, Morse RH. Mechanisms of Mediator complex action in transcriptional activation. Cell Mol Life Sci. 2013;70:2743–2756. doi: 10.1007/s00018-013-1265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari SA, Ganapathi M, Benschop JJ, Holstege FC, Wade JT, Morse RH. Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J. 2012;31:44–57. doi: 10.1038/emboj.2011.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari SA, Paul E, Sommer S, Lieleg C, He Q, Daly AZ, Rode KA, Barber WT, Ellis LC, LaPorta E, et al. Mediator, TATA-binding protein, and RNA polymerase II contribute to low histone occupancy at active gene promoters in yeast. J Biol Chem. 2014;289:14981–14995. doi: 10.1074/jbc.M113.529354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle M, Coulombe C, Poitras C, Robert MA, Markovits AN, Robert F, Jacques PE. Aggregate and heatmap representations of genome-wide localization data using VAP, a versatile aggregate profiler. Methods Mol Biol. 2015;1334:273–298. doi: 10.1007/978-1-4939-2877-4_18. [DOI] [PubMed] [Google Scholar]
- Brzovic PS, Heikaus CC, Kisselev L, Vernon R, Herbig E, Pacheco D, Warfield L, Littlefield P, Baker D, Klevit RE, Hahn S. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol Cell. 2011;44:942–953. doi: 10.1016/j.molcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevher MA, Shi Y, Li D, Chait BT, Malik S, Roeder RG. Reconstitution of active human core Mediator complex reveals a critical role of the MED14 subunit. Nat Struct Mol Biol. 2014;21:1028–1034. doi: 10.1038/nsmb.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001;15:1078–1092. doi: 10.1101/gad.867501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe C, Poitras C, Nordell-Markovits A, Brunelle M, Lavoie MA, Robert F, Jacques PE. VAP: a versatile aggregate profiler for efficient genome-wide data representation and discovery. Nucleic Acids Res. 2014;42:W485–W493. doi: 10.1093/nar/gku302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Larimore EA, Fissel BM, Swanger J, Taatjes DJ, Clurman BE. The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev. 2013;27:151–156. doi: 10.1101/gad.207720.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol. 2010;17:194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson MR, Yuan CX, Roeder RG, Myers LC, Gustafsson CM, Jiang YW, Li Y, Kornberg RD, Asturias FJ. Structural organization of yeast and mammalian mediator complexes. Proc Natl Acad Sci U S A. 2000;97:14307–14310. doi: 10.1073/pnas.260489497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlund H, Baraznenok V, Lindahl M, Samuelsen CO, Koeck PJ, Holmberg S, Hebert H, Gustafsson CM. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc Natl Acad Sci U S A. 2006;103:15788–15793. doi: 10.1073/pnas.0607483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, Espinosa JM. HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell. 2013;153:1327–1339. doi: 10.1016/j.cell.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Hamidi N, Del Sol R, Benschop JJ, Nancy T, Li C, Francis L, Tzouros M, Krijgsveld J, Holstege FC, Conlan RS. Suppression of Mediator is regulated by Cdk8-dependent Grr1 turnover of the Med3 coactivator. Proc Natl Acad Sci U S A. 2014;111:2500–2505. doi: 10.1073/pnas.1307525111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- Herbig E, Warfield L, Fish L, Fishburn J, Knutson BA, Moorefield B, Pacheco D, Hahn S. Mechanism of Mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol Cell Biol. 2010;30:2376–2390. doi: 10.1128/MCB.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege FCP, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- Huisinga KL, Pugh BF. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell. 2004;13:573–585. doi: 10.1016/s1097-2765(04)00087-5. [DOI] [PubMed] [Google Scholar]
- Ito M, Okano HJ, Darnell RB, Roeder RG. The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development. EMBO J. 2002;21:3464–3475. doi: 10.1093/emboj/cdf348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C, Robert F. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol. 2014;21:449–455. doi: 10.1038/nsmb.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C, Watanabe S, Kaplan CD, Peterson CL, Robert F. The histone chaperones FACT and Spt6 restrict H2A.Z from intragenic locations. Mol Cell. 2015;58:1113–1123. doi: 10.1016/j.molcel.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W, König P, Richmond TJ. Crystal structure of a bZIP/DNA complex at 2.2 A: determinants of DNA specific recognition. J Mol Biol. 1995;254:657–667. doi: 10.1006/jmbi.1995.0645. [DOI] [PubMed] [Google Scholar]
- Kemmeren P, Sameith K, van de Pasch LA, Benschop JJ, Lenstra TL, Margaritis T, O'Duibhir E, Apweiler E, van Wageningen S, Ko CW, et al. Large-scale genetic perturbations reveal regulatory networks and an abundance of gene-specific repressors. Cell. 2014;157:740–752. doi: 10.1016/j.cell.2014.02.054. [DOI] [PubMed] [Google Scholar]
- Knuesel MT, Meyer KD, Bernecky C, Taatjes DJ. The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function. Genes Dev. 2009;23:439–451. doi: 10.1101/gad.1767009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughery MF, Hunter T, Brown A, Hoopes J, Ostbye T, Shumaker T, Wyrick JJ. New vectors for simple and streamlined CRISPR-Cas9 genome editing in Saccharomyces cerevisiae. Yeast. 2015;32:711–720. doi: 10.1002/yea.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Park JM, Min S, Han SJ, Kim YJ. An activator binding module of yeast RNA polymerase II holoenzyme. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Cattoglio C, Tjian R. Looping back to leap forward: transcription enters a new era. Cell. 2014;157:13–25. doi: 10.1016/j.cell.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 2013 arXiv:1303.3997v1 [q-bio.GN] [Google Scholar]
- Li Y, Bjorklund S, Jiang YW, Kim YJ, Lane WS, Stillman DJ, Kornberg RD. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc Natl Acad Sci U S A. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Zhang J, Jeffery DA, Koleske AJ, Thompson CM, Chao DM, Viljoen M, van Vuuren HJ, Young RA. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ranish JA, Aebersold R, Hahn S. Yeast nuclear extract contains two major forms of RNA polymerase II mediator complexes. J Biol Chem. 2001;276:7169–7175. [PubMed] [Google Scholar]
- Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Baek HJ, Wu W, Roeder RG. Structural and functional characterization of PC2 and RNA polymerase II-associated subpopulations of metazoan Mediator. Mol Cell Biol. 2005;25:2117–2129. doi: 10.1128/MCB.25.6.2117-2129.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LC, Gustafsson CM, Bushnell DA, Lui M, Erdjument-Bromage H, Tempst P, Kornberg RD. The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes Dev. 1998;12:45–54. doi: 10.1101/gad.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LC, Gustafsson CM, Hayashibara KC, Brown PO, Kornberg RD. Mediator protein mutations that selectively abolish activated transcription. Proc Natl Acad Sci U S A. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näär AM, Taatjes DJ, Zhai W, Nogales E, Tjian R. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 2002;16:1339–1344. doi: 10.1101/gad.987602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Jackson BM, Zhou H, Winston F, Hinnebusch AG. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator. Mol Cell. 1999;4:657–664. doi: 10.1016/s1097-2765(00)80217-8. [DOI] [PubMed] [Google Scholar]
- Nelson C, Goto S, Lund K, Hung W, Sadowski I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature. 2003;421:187–190. doi: 10.1038/nature01243. [DOI] [PubMed] [Google Scholar]
- Nilson KA, Guo J, Turek ME, Brogie JE, Delaney E, Luse DS, Price DH. THZ1 Reveals Roles for Cdk7 in Co-transcriptional Capping and Pausing. Mol Cell. 2015;59:576–587. doi: 10.1016/j.molcel.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Kim HS, Han SJ, Hwang MS, Lee YC, Kim YJ. In vivo requirement of activator-specific binding targets of mediator. Mol Cell Biol. 2000;20:8709–8719. doi: 10.1128/mcb.20.23.8709-8719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul E, Zhu ZI, Landsman D, Morse RH. Genome-wide association of mediator and RNA polymerase II in wild-type and mediator mutant yeast. Mol Cell Biol. 2015;35:331–342. doi: 10.1128/MCB.00991-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri R, Lewis B, Kim TK, Dilworth FJ, Erdjument-Bromage H, Tempst P, de Murcia G, Evans R, Chambon P, Reinberg D. PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol Cell. 2005;18:83–96. doi: 10.1016/j.molcel.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pelish HE, Liau BB, Nitulescu II, Tangpeerachaikul A, Poss ZC, Da Silva DH, Caruso BT, Arefolov A, Fadeyi O, Christie AL, et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature. 2015;526:273–276. doi: 10.1038/nature14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschka C, Larivière L, Wenzeck L, Seizl M, Hemann M, Tegunov D, Petrotchenko EV, Borchers CH, Baumeister W, Herzog F, et al. Architecture of the RNA polymerase II-Mediator core initiation complex. Nature. 2015;518:376–380. doi: 10.1038/nature14229. [DOI] [PubMed] [Google Scholar]
- Plaschka C, Nozawa K, Cramer P. Mediator architecture and RNA polymerase II interaction. J Mol Biol. 2016;428:2569–2574. doi: 10.1016/j.jmb.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Poss ZC, Ebmeier CC, Taatjes DJ. The Mediator complex and transcription regulation. Crit Rev Biochem Mol Biol. 2013;48:575–608. doi: 10.3109/10409238.2013.840259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss ZC, Ebmeier CC, Odell AT, Tangpeerachaikul A, Lee T, Pelish HE, Shair MD, Dowell RD, Old WM, Taatjes DJ. Identification of Mediator kinase substrates in human cells using cortistatin A and quantitative phosphoproteomics. Cell Rep. 2016;15:436–450. doi: 10.1016/j.celrep.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raithatha S, Su TC, Lourenco P, Goto S, Sadowski I. Cdk8 regulates stability of the transcription factor Phd1 to control pseudohyphal differentiation of Saccharomyces cerevisiae. Mol Cell Biol. 2012;32:664–674. doi: 10.1128/MCB.05420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. ChIP-exo method for identifying genomic location of DNA-binding proteins with near-single-nucleotide accuracy. Curr Protoc Mol Biol. 2012a;Chapter 21:Unit 21.24. doi: 10.1002/0471142727.mb2124s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature. 2012b;483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley MD, Clowes C, Yu M, Mitchell K, Hentges KE. The Mediator complex protein Med31 is required for embryonic growth and cell proliferation during mammalian development. Dev Biol. 2010;342:146–156. doi: 10.1016/j.ydbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Trnka MJ, Pellarin R, Greenberg CH, Bushnell DA, Davis R, Burlingame AL, Sali A, Kornberg RD. Molecular architecture of the yeast Mediator complex. eLife. 2015;4:e08719. doi: 10.7554/eLife.08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Trnka MJ, Bushnell DA, Davis RE, Mattei PJ, Burlingame AL, Kornberg RD. Structure of a complete medi-ator-RNA polymerase II pre-initiation complex. Cell. 2016;166:1411–1422. doi: 10.1016/j.cell.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CO, Baraznenok V, Khorosjutina O, Spahr H, Kieselbach T, Holmberg S, Gustafsson CM. TRAP230/ARC240 and TRAP240/ARC250 Mediator subunits are functionally conserved through evolution. Proc Natl Acad Sci U S A. 2003;100:6422–6427. doi: 10.1073/pnas.1030497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr H, Khorosjutina O, Baraznenok V, Linder T, Samuelsen CO, Hermand D, Mäkela TP, Holmberg S, Gustafsson CM. Mediator influences Schizosaccharomyces pombe RNA polymerase II-dependent transcription in vitro. J Biol Chem. 2003;278:51301–51306. doi: 10.1074/jbc.M306750200. [DOI] [PubMed] [Google Scholar]
- Stevens JL, Cantin GT, Wang G, Shevchenko A, Shevchenko A, Berk AJ. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science. 2002;296:755–758. doi: 10.1126/science.1068943. [DOI] [PubMed] [Google Scholar]
- Taatjes DJ, Näär AM, Andel F, 3rd, Nogales E, Tjian R. Structure, function, and activator-induced conformations of the CRSP coacti-vator. Science. 2002;295:1058–1062. doi: 10.1126/science.1065249. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, et al. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 2008;452:604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- Thakur JK, Arthanari H, Yang F, Chau KH, Wagner G, Näär AM. Mediator subunit Gal11p/MED15 is required for fatty acid-dependent gene activation by yeast transcription factor Oaf1p. J Biol Chem. 2009;284:4422–4428. doi: 10.1074/jbc.M808263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KL, Sato S, Tomomori-Sato C, Conaway RC, Conaway JW, Asturias FJ. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat Struct Mol Biol. 2013;20:611–619. doi: 10.1038/nsmb.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KL, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, Asturias FJ. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell. 2014;157:1430–1444. doi: 10.1016/j.cell.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M, Murray PJ, Onufryk C, Jaenisch R, Young RA. Ubiquitous expression and embryonic requirement for RNA polymerase II co-activator subunit Srb7 in mice. Genes Dev. 1999;13:2365–2368. doi: 10.1101/gad.13.18.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Venters BJ, Pugh BF. A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res. 2009;19:360–371. doi: 10.1101/gr.084970.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol Cell. 2005;17:683–694. doi: 10.1016/j.molcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun Q, Ding Z, Ji J, Wang J, Kong X, Yang J, Cai G. Redefining the modular organization of the core Mediator complex. Cell Res. 2014;24:796–808. doi: 10.1038/cr.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Jin Y, Struhl K. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell. 2014;54:601–612. doi: 10.1016/j.molcel.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JW, Wang G. The Mediator complex: a master coordinator of transcription and cell lineage development. Development. 2014;141:977–987. doi: 10.1242/dev.098392. [DOI] [PubMed] [Google Scholar]
- Zhang F, Sumibcay L, Hinnebusch AG, Swanson MJ. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol Cell Biol. 2004;24:6871–6886. doi: 10.1128/MCB.24.15.6871-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wirén M, Sinha I, Rasmussen NN, Linder T, Holmberg S, Ekwall K, Gustafsson CM. Genome-wide occupancy profile of mediator and the Srb8-11 module reveals interactions with coding regions. Mol Cell. 2006;22:169–178. doi: 10.1016/j.molcel.2006.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.