Abstract
We have begun developing an innovative ultra-fast single-photon counting imager which comprises a mega-pixel CMOS array and a newly-designed Image Intensifier. It is expected to have single photon sensitivity with 100 psec time resolution, operational at a total counting rate exceeding 1MHz. The readout is based on dead-time-free flash ADC, running at 1–2GS/s, followed by a FPGA for real-time parallel data processing. Such a device has not been realized before and is expected to revolutionize time-resolved fluorescence imaging and spectroscopy from a single-molecule to whole animal level. To evaluate the design principle, an Image Intensifier with a GaAsP photocathode (>40% quantum efficiency at 400–600 nm) followed by double MCP was evaluated together with an existing CMOS camera. In our future design, the image from CMOS Camera will be combined with the MCP output, followed by a set of FPGA and CPU for real time data processing. This stream line method will allow ultra fast single-photon counting with 100 psec time resolution and 20 µm position resolution (1M pixel imaging). In this paper, we present the design principle and preliminary results on its performance. Our future plan and the design goals are also described.
Keywords: Photon Counting, Single-Molecule Imaging, CMOS Camera, MCP, GaAsP Photocathode, Image Intensifier
1. INTRODUCTION
During the past few decades, fluorescence photon detection techniques and measurement of their properties (wavelength, decay time and polarization) have progressed tremendously, thanks to technological advances in microscopes, lasers, photon detectors and data acquisition/analysis electronics. Fluorescence spectroscopy is now a major technique to study the structure and dynamics of biological molecules, their function in live cells, and to investigate disease location and progression in animal models. To name a few, techniques such as FCS (Fluorescence Correlation Spectroscopy), TCSPC (Time-Correlated Single Photon Counting), and FLIM (Fluorescence Lifetime Imaging Microscopy) are now well established and commercially available to the scientific community. In particular, FRET (Fluorescence Resonance Energy Transfer) has been developed into a powerful tool to study the structure and dynamics of biomolecules. (For more details on recent developments in this area, see Refs.1 – 6.)
Currently, most approaches that require a precise measurement of fluorescence decay have used point-detectors such as SPAD (Single Photon Avalanche Diode) or PMT (photomultipliers). To image a specimen, such detectors require a point-by-point scanning of the whole observed area, resulting in a very inefficient and slow process. Large area detectors such as CCD cameras combined with the appropriate optics allow on the other hand the high-resolution imaging of areas of any dimension (from microscopic ones using a microscope, to macroscopic ones, such a mouse, using macro-lenses). However, except for the time-gated intensified camera, these detectors do not allow precise timing of photon arrival (even when they can actually detect single photons, which is rather rare and a very inefficient way of using these detectors anyway).
For a fair comparison of various fast non-imaging and slow imaging techniques, key parameters are compiled in Table 1 below. Here, as examples of point-detectors, SPAD and PMT are listed with their typical properties. As examples of sensitive imagers, ICCD (Intensified CCD) and EMCCD (Electron Multiplied CCD) are listed. (For more details and a systematic comparison of various photon detectors, see Refs.7 – 8).
Table 1.
Summary table of various properties of our proposed photon counting imager. For comparison, the properties of today’s standard photon detectors are listed side by side. As shown here, the proposed detector has the ultra fast timing capability of SPAD and PMT. At the same time, it can achieve the same position resolution as CCD-based imagers.
| Proposed Single-Photon Counting Imager |
Current Single-Photon Counting |
Current High Sensitivity Imaging |
|||
|---|---|---|---|---|---|
|
|
|
||||
| SPAD (Single Photon Avalanche Diode) |
PMT (Photomultiplier) |
ICCD (Intensified CCD) |
EMCCD (Electron Multiplied CCD) |
||
|
| |||||
| Type | Hybrid (Vacuum + Solid State) | Solid State | Vacumn | Hybrid (Vacumn + Solid State) | Solid State |
| Photon Counting | Yes | Yes | Yes | Possible | Maybe |
| Imaging | Yes | No | No | Yes | Yes |
|
| |||||
| Photocathode | GaAsP | (Silicon) | Multi Alkali | Multi Alkali | (Silicon) |
| QE (at 600 nm) | 50% | 80% | 6% | 6% | 90% |
| Gain | 106 | 106 | 106 | 106 | 1,000 |
| Time Resolution | 100 ps | 100 ps | 100 ps | 100 ms | 100 ms |
| Count Rate (Total) | 1 MHz | 10 MHz | 10 MHz | 1 MHz | 50 MHz |
| Count Rate (Local) | 100 kHz | 10 MHz | 10 MHz | 100 kHz | 1 MHz |
|
| |||||
| No of Pixels | 1280 × 1024 | 1 | 1 | −1024 × 1024 | −1024 × 1024 |
| Position Resolution | 20 µm | None | None | 20 µm | 20 µm |
| Readout Speed | 1–100 kHz | 1 MHz | 1 MHz | 20 Hz | 20 Hz |
Obviously, a detector that would combine both capabilities (i.e. large area imaging with high-resolution and time-resolved photon counting capabilities) would be a tool of choice for many scientific disciplines concerned with the detection of low light level with high temporal resolution. In the past, to our knowledge, four detectors combining spatial and temporal capabilities have been commercialized and used:
PIAS detector from Hamamatsu Corp (USA)9 (now discontinued)
Mepsicron developed by Quantar Technology Inc (Santa Cruz, CA, USA)10,11
TSCSPC detector from Europhoton Gmbh (Berlin, DE, EU)12
IPD detector from Photek Ltd (St Leonards-on-Sea, UK, EU)
These detectors have a similar design based on (i) a multi-alkali photocathode (<20 % QE in the visible) followed by (ii) one or more electron multiplying micro-channel plates (MCP) and (iii) a plain resistive anode13 or quadrant capacitive anode14 to record the position of the electron cloud proximity-focused onto it. Their acquisition rate is typically limited to ~<100 kHz due mainly to constraints in the readout electronics. A faster position sensitive anode based on cross-delay lines15 has been used in a recently developed detector with a maximum of ~500 kHz global count rate, but this detector also uses a multi-alkali photocathode of limited QE in the visible16.
Can we really combine the imaging capability of CCD with the fast speed and sensitivity of SPAD? To answer this challenge, we have recently invented a concept of ultra-fast imager combining a new type of image intensifier and a CMOS camera, which in principle can meet the following specifications:
| • Single-Photon Sensitivity | |
| • Quantum Efficiency (QE): 50% from 400 to 700 nm | |
| • Time Resolution: | 100 ps (for each single photo-electron) |
| • Image Resolution: | 1280 × 1024 pixels (12 µm × 12 µm pixel size) |
| • Frame Rate: | 1 –100k per second (100 µsec – 1 msec per frame) |
| • Count Rate: | up to 1 MHz (Local count rate > 100 kHz) |
These values are also listed on Table 1 under the column “Proposed Single-Photon Counting Imager”. As is shown here, our proposed device is basically a CCD-like imager but with the advantages of an ultra-fast device such as PMT’s. The following section describes how such an ideal performance can be realized. In the next section, the basic concept of the camera itself and the readout electronics will be described in detail.
2. CONCEPT OF PROPOSED IMAGER
In a nutshell, the proposed imager comprises several key state-of-the-art technologies listed below:
A GaAsP photocathode with 50% quantum efficiency (at wavelengths between 400–700 nm).
An image intensifier (consisting of three layers of MCP with a gain of 106) for single-photon sensitivity.
MCP (Micro Channel Plate) outputs with 80 ps intrinsic time resolution.
1 GS/s flash ADC for MCP outputs, followed by a FPGA (Field-Programmable Gate Array), for dead-time free data processing.
1M pixel CMOS array with high-speed full-frame readout (1k frame/sec).
For even faster speed readout of the CMOS array (<100 µs/frame), addressing of CMOS sub-arrays by Region of Interest (ROI) signals, triggered by MCP-Out + FPGA analysis.
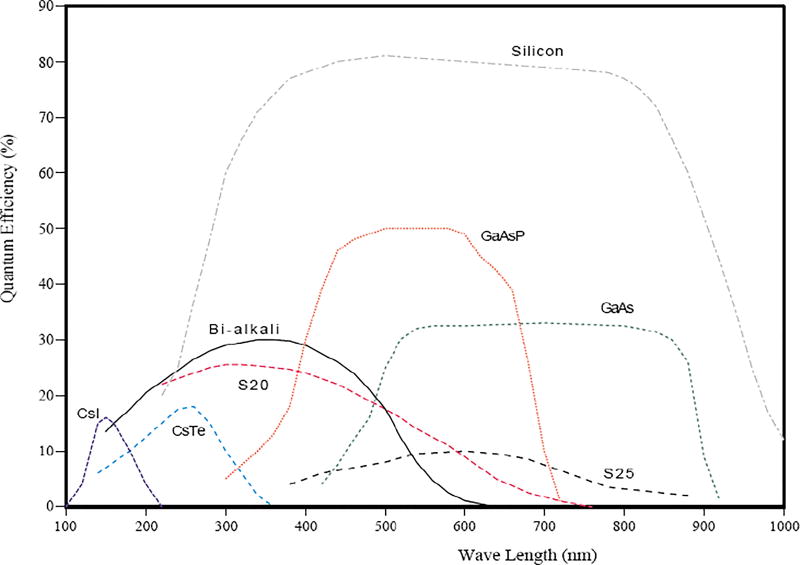
First, we propose to adopt the newly developed GaAsP photocathode with >40% QE. As shown in Figure 1, this photocathode has five times larger QE than conventional multi-alkali photo-cathodes (such as S20 or S25). This high QE photocathode is followed by the image intensifier (consisting of three layers of MCP with a gain of 106), which is further followed by a CMOS camera. The CMOS camera itself does not have fast timing information. However by combining its signals with the fast MCP signal, as shown in Figure 2, 100 ps time resolution and 20 µm position resolution can be obtained for each single photon.
Figure 1.
Typical Quantum Efficiency (QE) of Various Photocathodes7. Generally speaking, Silicon-based solid-state devices such as PIN Photodiode (PD), Avalanche Photodiode (APD) and CCD have ~80% QE in a wide wavelength range from UV to Near Infrared (NIR). On the other hand, standard vacuum devices (such as PMT and MCP-PMT) have alkali-based photocathode with only 10–20% QE. New development of solid-state photocathode (such as GaAsP and GaAs) allows high QE reaching 50% even for vacuum devices.
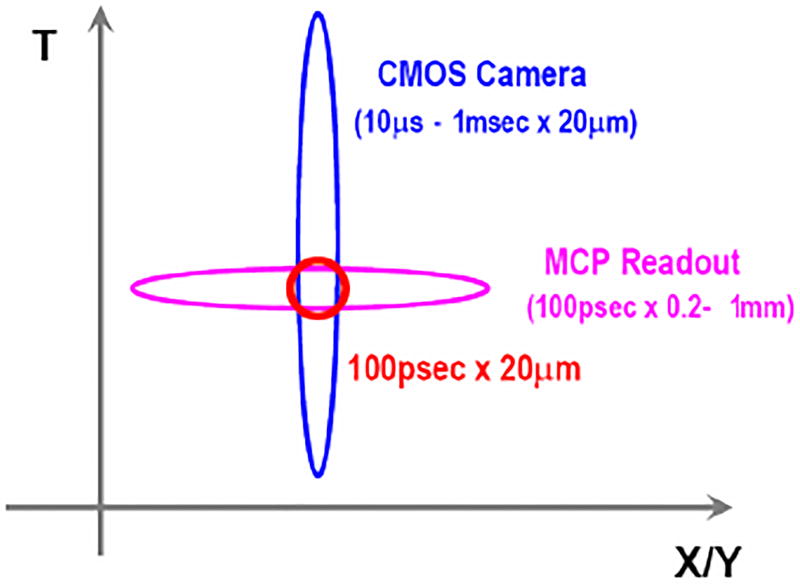
Figure 2.
This figure shows how 100 ps time resolution and 20 µm position resolution can be obtained for each single photon. The time resolution comes from the superior TTS (Transit Time Spread) of the MCP and the position resolution comes from the small CMOS pixel size.
One of the critical issues is how to get coarse position information from the MCP for this purpose. Figure 3 shows the basic concept to realize this. Various possibilities have been investigated by Hamamatsu Photonics Co. in Japan, and they have successfully completed a feasibility study. A more detailed description of the readout scheme is given later.
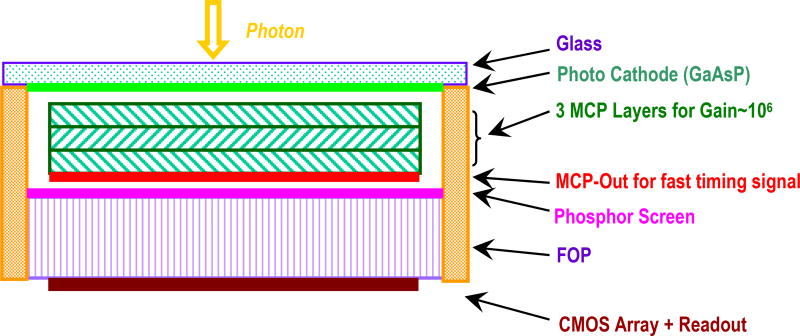
Figure 3.
Cross-sectional view of the proposed detector. For high quantum efficiency, a GaAsP photocathode is adopted. After three layers of MCP, MCP-Out provides the ultra-fast timing signal. A Mega-pixel CMOS image sensor is attached after the exit of the fiber optic window (FOP) for precise imaging.
3. CONCEPT OF READOUT ELECTRONICS
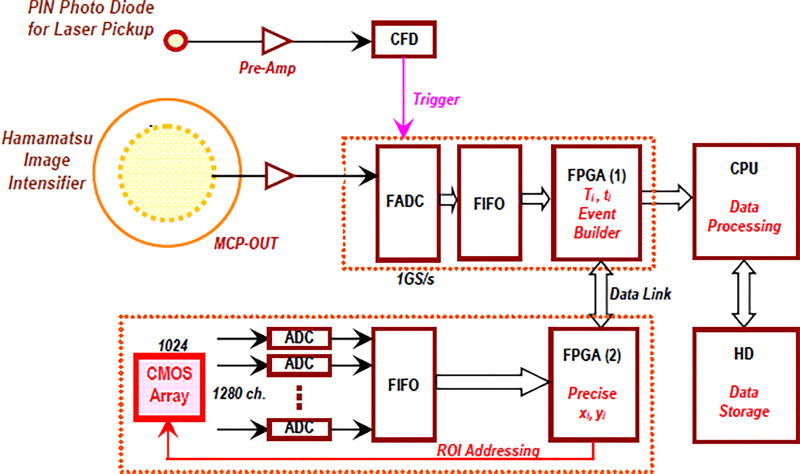
Once the photon detector is manufactured, the readout electronics must be developed to meet our design specifications. After extensive research, we have identified all the required components. Figure 4 below shows the systematic diagram of the entire system, including ultra-fast signals from MCP and precise imaging information from the CMOS camera. These are processed by the internal FPGA. Processed data are transferred via an Optical Data Link (ODL).
Figure 4.
Systematic diagram of the readout system. The macro-time Ti (timing of the laser pulse) is obtained by the chain of PIN Photo Diode, FADC and FPGA (1). The nano-time ti (fluorescence decay time) are obtained by the readout of MCP-OUT and Phosphor screen, followed by 1 GS/s FADC and FPGA (1). Finally the precise position, xi and yi, will be determined by the CMOS Sensor followed by FPGA (2). All these observables of the i’th photon (Ti, ti, xi and yi) will be combined as a single event in FPGA (1) which is acting as the event builder. Then the event is transferred to the CPU for further online data processing.
3.1. CMOS Camera and Data Processing
For high speed imaging purpose, we have adopted a CMOS camera instead of a CCD. The CMOS camera allows x–y addressing of ROI (Region of Interest). This method greatly enhances the readout speed, when the regions of CMOS array to read out are already known. CMOS arrays have poor S/N ratio compare to today’s based CCD arrays (such as cooled CCD or EMCCD). However, in our case, input signals are already amplified by the Image Intensifier. Therefore poor S/N ratio is not a problem at all.
To fully exploit the ROI capability in real time, CMOS output signals are directly fed into a FPGA for immediate data processing. By this method, we intend to determine the initial location of the light spots (generated by quantum dots, for example) by scanning the entire image in the first few frames only, and then define the Region of Interest (ROI) for the rest of the readout process to speed up the operation of the CMOS array. Such image processing is also necessary to reduce the amount of data flow downstream for further off-line analysis.
3.2. Readout and Data Processing of MCP Signals
Signals from the MCP have an intrinsic timing resolution of ~80 psec. In order to maintain and to record this ultra-fast timing information, we have adopted a state-of-the-art FADC at 1 GHz sampling rate, followed by another FPGA. By exploring the full power of FPGA, we plan to determine the precise timing and coarse position of each photo-electron with an accuracy of ~100 psec and a fraction of a mm. Fast communication must be established between the FPGA after the CMOS array and the FPGA after the FADC. For this purpose, the standard protocol of ODL (Optical Data link) is adopted as shown in Figure 4.
3.3. Summary
Our proposed detector development is based on the latest start-of-the-art technologies by industrial leaders in three areas: photon detectors, cameras and ultra-fast readout electronics. Therefore, once it is combined, it should provide the ideal single-photon imager for various applications.
This development is also quite unique for the following reasons:
The heart of the photon detection is done by a custom designed Image Intensifier.
The front-end readout electronics is based on the experience of high rate, state-of-the-art high energy experiments such as the ones at LHC (Large Hadron Collider), running at 50 MHz, parallel processing of massive data flow.
The electronics is all based on today’s industrial standards, therefore it is very effective, well modularized and expandable.
Once it is fully developed, it is straightforward to commercialize such a device.
To successfully achieve the necessary R&D milestones for this detector, we have already identified all the hardware components and most of them are in our laboratory. We expect to complete the hardware assembly in Summer 2006, and the system development including FPGA programming is expected to be finished by the end of 2006.
4. PRELIMINARY RESULTS FROM PROTOTYPE
Based on the proposed scheme mentioned above, we have assembled the first prototype, consisting of an already existing Image Intensifier from Hamamatsu and a CMOS camera. This Image Intensifier consists of a GaAsP photocathode with two layers of MCP (instead of our specs of three layers) and P-43 phosphor with 1 msec decay time (instead of our specs of P-47 with 100 nsec decay time). The photocathode has a QE >40% at 400–600 nm as expected. However, the gain of MCP is of the order of 104 which is not high enough to detect a single photo-electron by the MCP-Out signal for fast timing processing. The phosphor is also not fast enough to speed up the CMOS readout beyond 1 kHz, due to the slow decay time of P-47.
Therefore, we decided to evaluate only the slow imaging capability of low intensity emission by quantum dots (qdots) for the time being. Our first setup is shown in Figure 5. The Image Intensifier is attached to the microscope, and coupled to the CMOS Camera via a 1:1 coupling lens assembly. The CMOS output signal is directly dumped into a computer via a USB port (without image processing by FPGA). The readout system is based on Figure 4. However the Optical Data Link (ODL) has not been established yet and FPGA is currently not fully used for on-fly data analysis. In other words, we are dumping all the incoming data into the computer for offline analysis.
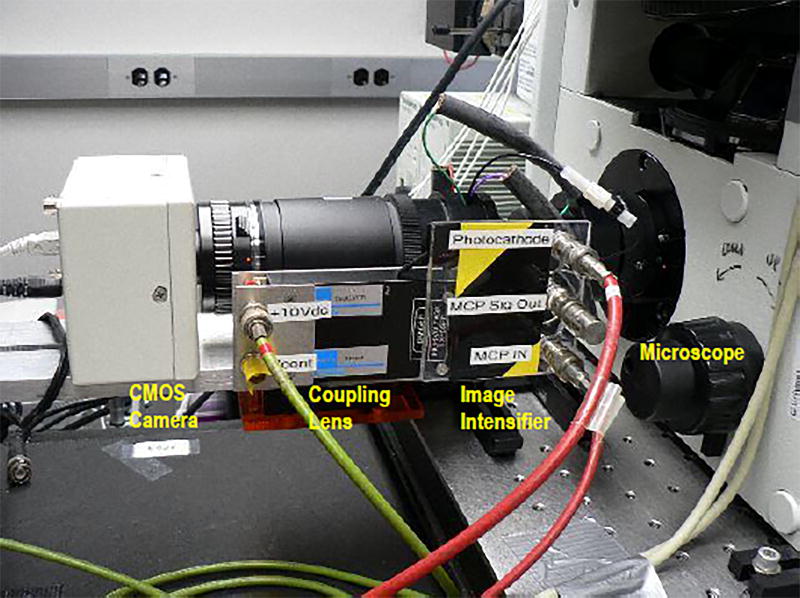
Figure 5.
A picture of the first prototype, assembled at UCLA. The microscope (Olympus, IX71) is on the right side. The image is captured by the Image Intensifier (Hamamatsu, GaAsP photo cathode, 2 MCP, P-43 phosphor). After a 1:1 optical coupling lens, the intensified image is recorded by the CMOS Camera.
As a first step, we have acquired pictures of quantum dots (whith emission peaked at 565 nm). The qdots were excited by a continuous (CW) Argon ion laser line (488 nm) in total internal reflection (TIR) mode, as we were not testing the fast timing of MCP signal yet. Figure 6 shows the first image, taken at slow frame rate (10 Hz). So far, the sensitivity appears similar to that of a typical EMCCD camera. We are still investigating the optimum operating condition of the Image Intensifier, and we are trying to speed up the CMOS readout by incorporating on-fly image processing algorithm in FPGA.
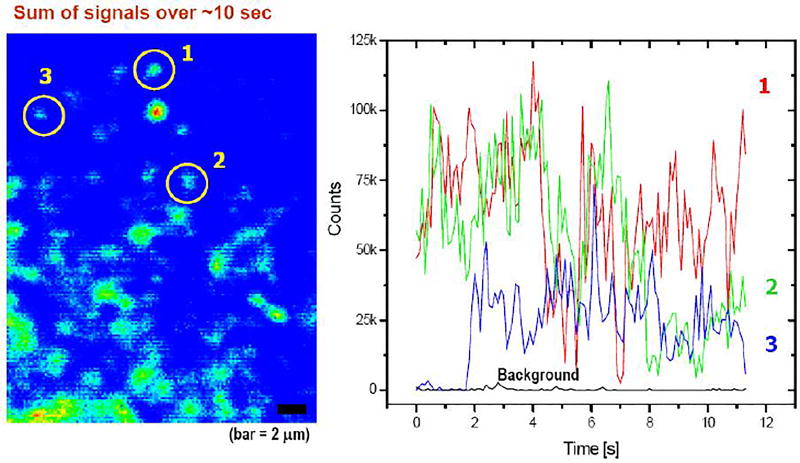
Figure 6.
left side picture is the accumulated image of quantum dots, taken by the first prototype imager shown in Figure 5 for 10 second exposure time. The sensitivity is similar to that of a typical EMCCD camera. Right side is the temporal profile of the quantum dots No.1, 2 and 3, marked on the left picture.
6. CONCLUSIONS
In this paper, we have described an innovative concept of ultra-fast single-photon-counting Mega-pixel imager. The fully developed detector is expected to have single-photon sensitivity with 100 psec time resolution and an operational global counting rate above 1 MHz. Such a device can be realized by the combination of three key components:
Image Intensifier with GaAsP photocathode, three layer of MCP, and P-47 phosphor
Mega-pixel CMOS camera together with FPGA and Optical Data Link
> 1 GHz sampling FADC followed by FPGA and Optical Data Link
Since we are to our knowledge combining the state-of-the-art technology in three areas for the first time, such a device has not been materialized before. Therefore it is expected to revolutionize time-resolved fluorescence imaging and spectroscopy from the single-molecule to whole animal level in the near future.
The first prototype has been assembled and tested with an existing Image Intensifier and preliminary results show promising results and high sensitivity. The final system is expected to be complete in 2006 and hopefully it will be applied to the real bio/medical imaging for scientific measurements.
Acknowledgments
We would like to thank Junichi Takeuchi, Toshikatsu Hakamata, Itaru Mizuno and many others at Hamamatsu Photonics Co., Japan for their continuous supports and developments of custom-made Image Intensifiers. This work was supported by discretionary funds provided by Vice Chancellor of Resource at UCLA, Roberto Peccei. This work was also supported in part by NIH grant 5 R21 RR017474 (XM & SW) and DOE grant ER63421 0008273 (XM & SW).
References
- 1.Weiss S. Fluorescence Spectroscopy of Single Biomolecules. Science. 1999;283:1676–1683. doi: 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S. Measuring Conformational Dynamics of Biomolecules by Single Molecule Fluorescence Spectroscopy. Nature Structural Biology. 2000;7:724–729. doi: 10.1038/78941. [DOI] [PubMed] [Google Scholar]
- 3.Dahan M, Laurence T, Pinaud F, Chemla DS, Alivisatos AP, Sauer M, Weiss S. Time-gated biological imaging by use of colloidal quantum dots. Optics Letters. 2001;26(11):825–827. doi: 10.1364/ol.26.000825. [DOI] [PubMed] [Google Scholar]
- 4.Michalet X, Lacoste TD, Weiss S. Ultrahigh-Resolution Colocalization of Spectrally Separable Point-like Fluorescent Probes. Methods. 2001;25:87–102. doi: 10.1006/meth.2001.1218. [DOI] [PubMed] [Google Scholar]
- 5.Michalet X, Weiss S. Single-molecule spectroscopy and microscopy. Comptes rendus Physique. 2002;2(4):619–644. [Google Scholar]
- 6.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arisaka K. New Trends in Vacuum-Based Photon Detectors. Nucl. Instrum. Meth; 2nd International Conference on New Developments in Photodetection (Beaune 99), Beaune; France. 21–25 Jun 1999.2000. [Google Scholar]
- 8.Arisaka K. Summary Talk. at 4th International Conference on New Developments in Photodetection (Beaune 05), Beaune; France. 19–24 Jun 2005; unpublished. [Google Scholar]
- 9.Hübner CG, Krylov V, Renn A, Nyffeler P, Wild UP. In: Single Molecule Spectroscopy. Rigler R, Orrit M, Basche T, editors. Springer Verlag; Stockholm: 2001. [Google Scholar]
- 10.Kelly LA, Trunk JG, Polewski K, Sutherland JC. Rev. Sci. Instr. 1995;66:1496–1498. [Google Scholar]
- 11.Kelly LA, Trunk JG, Sutherland JC. Rev. Sci. Instr. 1997;68:2279–2286. [Google Scholar]
- 12.Emiliani V, Sanvitto D, Tramier M, Piolot T, Petrasek Z, Kemnitz K, Durieux C, Coppey-Moisan M. Appl. Phys. Lett. 2003;83:2471–2473. [Google Scholar]
- 13.Lampton M, Paresce F. Rev. Sci. Instr. 1974;45:1098–1105. [Google Scholar]
- 14.Lampton M, Malina RF. Rev. Sci. Instr. 1976;47:1360–1362. [Google Scholar]
- 15.Siegmund OHW, Gummin MA, Stock J, Marsh D, Raffanti R, Sasseen T, Tom J, Welsh B, Gaines G, Jelinsky P, Hull J. Proc. SPIE. 1994;2280:89–100. [Google Scholar]
- 16.Michalet X, Siegmund OHW, Vallerga JV, Jelinsky PN, Millaud JE, Weiss S. Nucl. Instrum., Meth. A. 2006 submitted. [Google Scholar]