Abstract
Crohn's disease and ulcerative colitis are chronic inflammatory disorders affecting the gastrointestinal tract. Faecal calprotectin is a protein complex of the S-100 family of calcium-binding proteins present in inflammatory cells that can be measured in stool samples, which act as a biomarker for bowel inflammation. Elevated faecal calprotectin has been shown to reflect the presence of ongoing mucosal inflammation, which improves with mucosal healing. The aim of this review was to evaluate the available evidence on the ability of faecal calprotectin to predict a relapse in inflammatory bowel disease. Multiple retrospective studies have shown that patients who relapse have significantly higher levels of calprotectin in their stool compared with non-relapsers, especially in ulcerative colitis. Elevated faecal calprotectin postoperatively in Crohn's disease was also shown to be indicative of a relapse. However, the association of a raised faecal calprotectin and relapse is not universal and may be explained by the different patterns of mucosal inflammatory activity that exist. In conclusion, we put forward our hypothesis that changes such as a rise in faecal calprotectin levels may be more predictive of a relapse than absolute values.
Keywords: CROHN'S DISEASE, INFLAMMATORY BOWEL DISEASE, ULCERATIVE COLITIS
Introduction
Inflammatory bowel diseases (IBD) comprising Crohn's disease (CD) and ulcerative colitis (UC) are chronic inflammatory disorders primarily affecting the gastrointestinal tract (GI).1 Both CD and UC are distinguished by their different phenotypic expression of inflammation in the GI tract but share the pattern of activity common to many inflammatory disorders of a chronic-progressive or relapsing-remitting inflammation.
Faecal calprotectin (FC) is a protein complex of the S-100 family of calcium-binding proteins present in neutrophils, monocytes and macrophages. It is stable for measurement by quantitative assays in stool, which act as a biomarker for bowel inflammation that is non-invasive and cost-effective.2 There is good evidence of its use to distinguish functional bowel symptoms from those with an inflammatory origin3 4 as well as act as a surrogate biomarker of mucosal healing.5 While FC has an established role in identifying the presence of bowel inflammation at the time of testing, its ability to predict future relapse in IBD is less clear. This review aims to look at the evidence of FC's role in predicting future relapse and challenge our perceived ideas about the pattern of inflammatory activity in the natural history of IBD.
A patient's bowel symptoms are often subjective and poorly reflect disease activity, which requires validation with objective tests such as serum inflammatory markers, faecal antigens, endoscopy and radiological imaging. However, even colonoscopic and histological evaluation of inflammation can be subjective and can be made more objective by using scoring systems such as the CD endoscopic index of severity6 or one of the 22 known histological scoring systems.7 These different markers of disease activity, that is, clinical, biochemical, endoscopic, histological and radiological, allow assessment of response and need for escalation of treatment, since individually each modality is susceptible to variability but collectively they present a more robust and objective assessment. There continues to be a drive to identify non-invasive, cost-effective and reliable biomarkers to assess ongoing bowel inflammation and FC has been seen by many to fulfil this role.
The concepts of relapse and remission in IBD are difficult to define strictly. There is a move away from using clinical symptoms to define remission to the use of endoscopic mucosal healing. However, some argue that mucosal healing should be assessed histologically, while others have even suggested the use of mucosal cytokine gene expression to define treatment success.8 For now, the gold standard remains endoscopic mucosal healing, with good evidence that FC correlates well with endoscopic mucosal healing9 10 as well as histological scoring of inflammation.10 11 As such, since FC correlates with mucosal inflammation, does an elevated FC merely indicate the lack of complete mucosal healing, which would be associated with a clinical relapse in a model where inflammation is chronic and progressive? However, if mucosal inflammation is variable in a model of relapsing and remitting activity, then an elevated FC indicative of ongoing inflammation may not predict or progress to a clinical relapse in a particular individual. Conversely, a normal FC may not be protective by predicting a lower rate of clinical relapse.
Using FC to predict relapse in CD and UC
Many different studies (table 1) have looked at the use of FC in predicting clinical relapse. The majority of studies investigated patients with CD and patients with UC in clinical remission, measuring their baseline FC and following them up for at least 12 months to identify patients who had a clinical relapse. Median FC levels were compared between relapsers and non-relapsers. The majority of studies found a statistically higher baseline FC level in patients with CD and patients with UC who subsequently relapsed compared with those who did not. The exceptions were the cohort of patients with CD studied by Costa et al 12 and D'Inca et al,13 Sipponen's and Kolho's14 paediatric patients with IBD and Laharie et al's15 patients with CD who newly achieved clinical remission with infliximab (IFX). These findings suggest that FC may be a better marker of colonic inflammation since all studies in adult patients with UC were significant in contrast to variably significant results in patients with CD. The findings by Laharie et al 15 is in contrast to a later study by Ferreiro-Iglesias et al of patients in remission on adalimumab (ADA)16 and IFX17 where a difference in FC levels was found between relapsers and non-relapsers. A crucial difference may be that the FC levels were done at week 14 of IFX treatment15 in contrast to the patients who were in remission for at least 6 months on ADA16 and IFX.17
Table 1.
Summary of studies looking retrospectively at baseline FC levels in patients who relapse or stay in remission
| Authors | Disease type | Patients (n) | Definition of relapse | Median FC levels |
p Value | FC cut-off | Sensitivity (%) | Specificity (%) | Relapse risk | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Relapse/active disease group | Non-relapse/remission group | |||||||||
| 2000 | Tibble et al 24 | CD | 43 | CDAI>150 and rise>100 | 122 mg/L | 42 mg/L | <0.0001 | 50 mg/L* | 90 | 83 | |
| UC | 37 | HBI>4 and rise>2 | 123 mg/L | 29 mg/L | <0.0001 | ||||||
| 2005 | Costa et al 12 | CD | 38 | CDAI>150 and new Rx | 220.1 μg/g | 220.5 μg/g | 0.395 | 150 μg/g | 87 | 43 | 2 |
| UC | 41 | UCAI>4 and new Rx | 220.6 μg/g | 67 μg/g | <0.0001 | 150 μg/g | 89 | 82 | 14 | ||
| 2008 | D'Inca et al 13 | CD | 65 | CDAI>150 and rise>50 | 207 mg/kg | 88 mg/kg | 0.055 | 130 mg/kg | 65 | 62 | 1.7 |
| UC | 97 | ET>4 and new Rx | 190 mg/kg | 49 mg/kg | 0.02 | 130 mg/kg | 70 | 70 | 2.4 | ||
| 2009 | Gisbert et al 25 | CD | 89 | CDAI>150 | 266 μg/g | 145 μg/g | 0.002 | 150 μg/g | 28 | 93 | |
| UC | 74 | Modified TWI>11 | 213 μg/g | 126 μg/g | 0.03 | 150 μg/g | 31 | 91 | |||
| 2010 | Garcia-Sanchez et al 26 | CD | 66 | CDAI>150 | 524 μg/g | 123 μg/g | <0.01 | 200 μg/g | 80 | 65 | 4 |
| UC | 69 | Modified TWI≥11 | 298 μg/g | 105 μg/g | <0.01 | 120 μg/g | 81 | 63 | 6 | ||
| 2010 | Kallel et al 27 | CD | 53 | CDAI>150 or rise>100 | 380.5 μg/g | 155 μg/g | <0.001 | 340 μg/g | 80 | 90.7 | 18.8 |
| 2010 | Sipponen and Kolho14 | IBD | 72 | PGA | 409 μg/g | 282 μg/g | 0.44 | 108.5 μg/g | 38 | 72 | |
| 2011 | Laharie et al 15 | CD | 50 | CDAI>150 and rise>70 | 200 μg/g | 150 μg/g | ns | 130 μg/g | 61 | 48 | |
| 2013 | Lasson et al 28 | UC | 69 | New treatment | 263 μg/g | 102 μg/g† | 0.009 | 262 μg/g | 64.4 | 70.8 | |
| 2013 | De Vos et al 29 | UC | 87 | New treatment, Mayo≥2 | 125 mg/kg‡ | 27 mg/kg‡ | <0.001 | 300 mg/kg | 58.3 | 93.3 | |
| 2014 | Naismith et al 30 | CD | 92 | New treatment, surgery | 414 μg/g | 96 μg/g | 0.005 | 240 μg/g | 80 | 74.4 | 12.2 |
| 2015 | Ferreiro-Iglesias et al 16 | CD | 37 | HBI>4 | 625 μg/g | 45 μg/g | <0.005 | 204 μg/g | 100 | 85.7 | |
| 2015 | Ferreiro-Iglesias et al 17 | CD | 33 | HBI>4 | 287 μg/g | 94 μg/g | <0.005 | 160 μg/g | 87.5 | 84 | |
| UC | 20 | PMI>2 | 420 μg/g | 136 μg/g | <0.005 | 198 μg/g | 100 | 81.3 | |||
| 2015 | Scaioli et al 31 | UC | 74 | SCCAI>3 | 218 μg/g | 48 μg/g | <0.01 | 193 μg/g | 65 | 98 | |
| 2015 | Mooiweer et al 32 | IBD | 72 | New Rx, admission and endoscopic activity | 284 mg/kg | 37 mg/kg | <0.01 | 56 mg/kg§ | 64 | 100 | |
| 2016 | Delefortrie et al 33 | CD | 29 | Not stated | 261.5 μg/g | 37.6 μg/g | <0.05 | 106.5 μg/g | 87.5 | 95.2 | |
| 2016 | Zittan et al 34 | IBD | 58 | SES-CD≥3 and MES=0 | 1180 μg/g | 100 μg/g | <0.0001 | 100 μg/g¶ | 71 | 91 | |
*Combined CD and UC.
†Mild disease activity.
‡Mean values.
§Predict absence of relapse.
¶Predict endoscopic remission.
CD, Crohn's disease; CDAI, Crohns Disease Activity Index; ET, Edwards and Truelove score; FC, faecal calprotectin; HBI, Harvey–Bradshaw Index; IBD, inflammatory bowel disease; MES, Mayo endoscopic score; ns, non-significant; PGA, Physician's Global Assessment; PMI, partial Mayo index; Rx, treatment; SCCAI, Simple Clinical Colitis Activity Index; SES, simple endoscopic score; TWI, Truelove and Witt Index; UC, ulcerative colitis.
The available studies (table 1) suggest a FC cut-off for UC ranging from 120 to 300 μg/g yielding a wide sensitivity of 31% to 100% and specificity of 63% to 98%. FC cut-offs for CD range from 130 to 340 μg/g with a sensitivity of 28% to 100% and specificity of 43% to 95%.
An ongoing study, Fecal marker of Intestinal inflammation for RElapse prediction in routine monitoring of patients with CD (FIRE) is prospectively trying to answer this particular question.18 FIRE is a prospective, multicentre study in Germany that follows patients with CD in remission (Harvey–Bradshaw Index (HBI)<5) with 3-monthly FC and HBI for up to 2 years or when clinical relapse (HBI≥5) occurs. From the initial results presented in abstract so far, FIRE did not find a statistically significant difference in FC levels relative to HBI, treatment with immunosuppressants, anti-tumour necrosis factor and combination treatment or whether mucosal ulceration was present.19 Further analysis found that relapsers had significantly higher median HBI, C reactive protein and FC levels at baseline compared with non-relapsers although on multivariate regression analyses only female gender and HBI≥1 but not FC were prognostic factors for a mild-to-severe relapse (HBI≥5).18 These findings underpin the complexity of using FC to predict relapse in a multifaceted disorder such as CD.
Using FC to detect postoperative recurrence in CD
In 2006, Orlando et al 20 investigated 39 patients with CD postoperatively with an FC at 3 months and colonoscopy at 1 year. A total of 19 patients had endoscopic recurrence at 1 year and with FC>200 mg/L, giving a sensitivity of 63% and specificity 75% for endoscopic recurrence. Of the 19 patients with endoscopic recurrence, 12 had FC>200 (true-positive), while 7 had FC<200 (false-negative). Of the 20 patients without endoscopic recurrence, 5 had FC>200 (false-positive), while 15 had FC<200 (true-negative). While these findings are useful, the interval time between FC collection at 3 months and colonoscopy at 1 year limits the applicability of these results.
Lamb et al,21 in 2009, looked at CD recurrence in patients treated with an ileocaecal resection. It prospectively showed that in asymptomatic patients, FC levels resolved by 2 months and stayed low. However, in symptomatic patients, an early rise in FC at 1 month was related to postoperative complications while a late rise at 9 months was due to CD relapse. Additionally, this study showed that FC correlated significantly with HBI. Long-term follow-up of these patients showed that an elevated FC correlated significantly with escalation of treatment or further surgery over 5 years.22
More recently, Wright et al 23 also found that FC levels in patients with CD dropped postoperatively at 6 months, and that FC levels were higher in patients with endoscopic disease recurrence and correlated with severity of recurrence. Step-up treatment of those with endoscopic recurrence resulted in reduction of FC levels at 12 and 18 months.
Can FC predict relapse in IBD?
The answer to this question is not clear. While FC has been shown to correlate with CD Activity Index (CDAI) and HBI scores suggestive of clinical relapse as shown above, this is not universal. FC also correlates with endoscopic and histological scores showing mucosal inflammation although again this is not universal. Additionally, there is no universal agreement or ‘gold standard’ of what constitutes a relapse, is it clinical, endoscopic or histological? We postulate that FC can identify but not necessarily accurately predict a relapse.
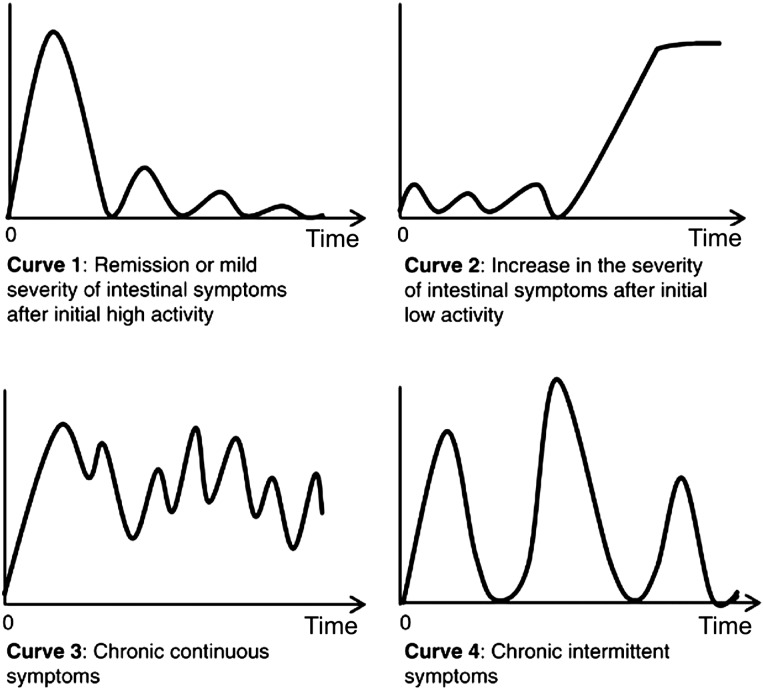
Part of the complexity in answering this question is the variable disease states that patients are in. These disease states include being in remission, subclinical or clinical relapse. Remission can be defined as clinical, endoscopic or histological remission, while relapse can be subclinical, with only histological changes or a ‘full house’ clinical relapse with clinical, endoscopic, histological and imaging changes. This complexity is further confounded by variability in the pattern of inflammatory activity, that is, whether it is chronic and progressive as is often the case in colonic inflammation in contrast to relapsing and remitting inflammation that is a feature of some of our patients with CD (figure 1).35 In light of this, absolute FC values may be less predictive than ΔFC that is, change in FC levels. Deep remission with neither symptoms nor active inflammation is strongly associated with normal FC,32 while active inflammation in a symptomatic individual is equally associated with very elevated FC levels. A moderately elevated FC level may be seen in patients with resolving inflammation (going into remission) or in patients heading for a flare or in patients who have ongoing elevated levels of inflammation. The direction and magnitude of changes in FC may be able to add better prediction to the use of FC, for example, a rise in FC may herald a pending relapse in contrast to a drop in FC that may be protective.
Figure 1.
Graphical representation of possible patterns of mucosal inflammatory activity in inflammatory bowel disease; adapted from Solberg et al.
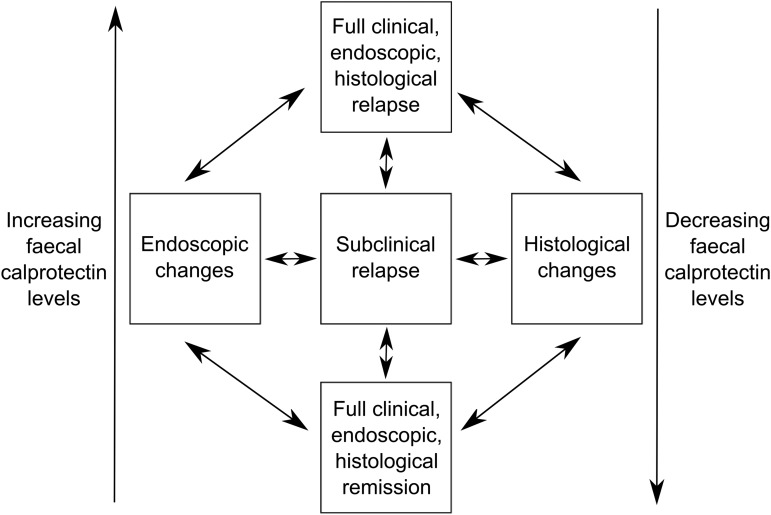
We suggest a schematic representation to understand this complexity (figure 2). In conclusion, the answer to the question of whether FC can predict a relapse in IBD is that it identifies inflammatory activity but is not able to accurately predict all individuals who will relapse. It remains to be seen whether identification of a rising FC (ΔFC) in patients who do not have symptoms of active disease is sufficiently predictive to use it clinically to direct pre-emptive treatment. Further research using the non-invasive property of serial FC may also allow identification of factors that provoke subsequent inflammatory relapses in patients in clinical remission.
Figure 2.
Schematic representation of pathways between full remission and complete relapse that faecal calprotectin may be able to predict.
Footnotes
Contributors: TSC and JCM jointly reviewed the literature available on this topic and wrote this article.
Competing interests: TechLab has previously supplied JCM with IBD-SCAN ELISA testing kits for research purposes.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380:1590–605. doi:10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- 2. Lamb CA, Mansfield JC. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol 2011;2:13–18. doi:10.1136/fg.2010.001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waugh N, Cummins E, Royle P, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013;17:xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caviglia GP, Pantaleoni S, Touscoz GA, et al. Fecal calprotectin is an effective diagnostic tool that differentiates inflammatory from functional intestinal disorders. Scand J Gastroenterol 2014;49:1419–24. doi:10.3109/00365521.2014.934913 [DOI] [PubMed] [Google Scholar]
- 5. Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004;39:1017–20. doi:10.1080/00365520410007971 [DOI] [PubMed] [Google Scholar]
- 6. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989;30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014;8:1582–97. doi:10.1016/j.crohns.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 8. Zahn A, Giese T, Karner M, et al. Transcript levels of different cytokines and chemokines correlate with clinical and endoscopic activity in ulcerative colitis. BMC Gastroenterol 2009;9:13 doi:10.1186/1471-230X-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Theede K, Holck S, Ibsen P, et al. Level of Fecal Calprotectin Correlates With Endoscopic and Histologic Inflammation and Identifies Patients With Mucosal Healing in Ulcerative Colitis. Clin Gastroenterol Hepatol 2015;13:1929–36.e1. doi:10.1016/j.cgh.2015.05.038 [DOI] [PubMed] [Google Scholar]
- 10. Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–9. doi:10.1111/j.1365-2036.2008.03835.x [DOI] [PubMed] [Google Scholar]
- 11. Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2001;33:14–22. doi:10.1097/00005176-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 12. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut 2005;54:364–8. doi:10.1136/gut.2004.043406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Inca R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol 2008;103:2007–14. doi:10.1111/j.1572-0241.2008.01870.x [DOI] [PubMed] [Google Scholar]
- 14. Sipponen T, Kolho KL. Faecal calprotectin in children with clinically quiescent inflammatory bowel disease. Scand J Gastroenterol 2010;45:872–7. doi:10.3109/00365521003782389 [DOI] [PubMed] [Google Scholar]
- 15. Laharie D, Mesli S, El Hajbi F, et al. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther 2011;34:462–9. doi:10.1111/j.1365-2036.2011.04743.x [DOI] [PubMed] [Google Scholar]
- 16. Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, et al. Usefulness of a rapid faecal calprotectin test to predict relapse in Crohn's disease patients on maintenance treatment with adalimumab. Scand J Gastroenterol 2016;51:442–7. doi:10.3109/00365521.2015.1115546 [DOI] [PubMed] [Google Scholar]
- 17. Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, et al. Fecal calprotectin as predictor of relapse in patients with inflammatory bowel disease under maintenance infliximab therapy. J Clin Gastroenterol 2016;50:147–51. doi:10.1097/MCG.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 18. Nikolaus S, Lang D, Wittig BM, et al. Faecal calprotectin as a tool for relapse prediction in clinical routine in Crohn's Disease patients—preliminary Results from the prospective, population-based FIRE study. J Crohns Colitis 2015;9:S47–8. [Google Scholar]
- 19. Nikolaus S, Schreiber S, Nurwakagari P, et al. Clinical epidemiology of fecal calprotectin: population data from the FIRE study, a prospective, longitudinal study in Germany to evaluate fecal calprotectin in routine monitoring of Crohn's disease. Gastroenterology 2014;146 (Suppl 1):S-423. [Google Scholar]
- 20. Orlando A, Modesto I, Castiglione F, et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn's disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci 2006;10:17–22. [PubMed] [Google Scholar]
- 21. Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn's disease. Br J Surg 2009;96:663–74. doi:10.1002/bjs.6593 [DOI] [PubMed] [Google Scholar]
- 22. Perowne R, Lamb C, Speight R, et al. PWE-095 faecal calprotectin is useful in predicting long term disease recurrence in post-operative Crohn's. Gut 2014;63 (Suppl 1):A165–6. [Google Scholar]
- 23. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease after surgery. Gastroenterology 2015;148:938–47.e1. doi:10.1053/j.gastro.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 24. Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15–22. doi:10.1053/gast.2000.8523 [DOI] [PubMed] [Google Scholar]
- 25. Gisbert JP, Bermejo F, Perez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis 2009;15:1190–8. doi:10.1002/ibd.20933 [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Sanchez V, Iglesias-Flores E, Gonzalez R, et al. Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J Crohns Colitis 2010;4:144–52. doi:10.1016/j.crohns.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 27. Kallel L, Ayadi I, Matri S, et al. Fecal calprotectin is a predictive marker of relapse in Crohn's disease involving the colon: a prospective study. Eur J Gastroenterol Hepatol 2010;22:340–5. doi:10.1097/MEG.0b013e32832bab49 [DOI] [PubMed] [Google Scholar]
- 28. Lasson A, Simren M, Stotzer PO, et al. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis 2013;19:576–81. doi:10.1097/MIB.0b013e31827e78be [DOI] [PubMed] [Google Scholar]
- 29. De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 2013;19:2111–17. doi:10.1097/MIB.0b013e31829b2a37 [DOI] [PubMed] [Google Scholar]
- 30. Naismith GD, Smith LA, Barry SJ, et al. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn's disease. J Crohns Colitis 2014;8:1022–9. doi:10.1016/j.crohns.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 31. Scaioli E, Scagliarini M, Cardamone C, et al. Clinical application of faecal calprotectin in ulcerative colitis patients. Eur J Gastroenterol Hepatol 2015;27:1418–24. doi:10.1097/MEG.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 32. Mooiweer E, Severs M, Schipper ME, et al. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. J Crohns Colitis 2015;9:50–5. doi:10.1093/ecco-jcc/jju003 [DOI] [PubMed] [Google Scholar]
- 33. Delefortrie Q, Schatt P, Grimmelprez A, et al. Comparison of the Liaison(R) Calprotectin kit with a well established point of care test (Quantum Blue—Buhlmann-Alere(R)) in terms of analytical performances and ability to detect relapses amongst a Crohn population in follow-up. Clin Biochem 2016;49:268–73. doi:10.1016/j.clinbiochem.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 34. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis 2016;22:623–30. doi:10.1097/MIB.0000000000000652 [DOI] [PubMed] [Google Scholar]
- 35. Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44:431–40. doi:10.1080/00365520802600961 [DOI] [PubMed] [Google Scholar]