Introduction
On 7 December 2015, the British Society of Gastroenterology and the Royal College of Physicians held a joint conference: GI cancer in the UK: can we do better? The meeting was timely as, although outcomes for patients with most gastrointestinal cancers in the UK have steadily improved in the past 10 years, survival figures remain substantially worse than in many other comparable nations.
After defining the scale of the problem, the issues around early diagnosis were discussed. Screening as prevention has huge potential where there are defined premalignant conditions. Uptake into the Bowel Cancer Screening Programme (BCSP) is variable but in some areas remains low. It is hoped that with the National Screening Committee recommendation to replace the guaiac faecal occult blood test (gFOBT) with the faecal immunochemical test (FIT), the planned age extension and the continued roll-out of bowel scope screening by the National Health Service (NHS) will extend the value of the programme further.
The view from primary care suggested that many factors affect the decision to make a referral for suspected cancer. Lack of direct access to testing was highlighted as a concern, as was the compounding issue of the many patients who delay seeking care. The Independent Cancer Force is the latest of several bodies calling for general practitioners (GPs) to be able to refer ‘direct to test’. However, a particular concern from secondary care relates to further stretching of diagnostic resources already under pressure—and how that can be addressed in times of austerity. Waiting times for endoscopy have begun to rise, yet capacity to expand is limited. Training, recruitment and retention of clinical staff were all highlighted as key issues limiting the availability of these procedures. Various practical ways to improve detection with endoscopy were proposed, as were possible ways to support and retain staff.
While much discussion related to colorectal cancer (CRC), the conference also heard about innovative methods of addressing the substantial problem of preventing oesophageal and pancreatic cancer, or at least making the diagnosis early enough to influence survival. As well as describing the presentations, this report attempts to capture the key points that emerged from the audience discussions.
Cancer survival
Professor Michel Coleman of the London School of Hygiene and Tropical Medicine discussed learning from the vast amount of data on cancer survival. Epidemiological studies and cancer registries provide a wide range of information on incidence, survival, prevalence, quality of life and mortality at the population level. Such data can help inform national cancer strategy and health service planning, but they provide few answers to doctors assessing individual patients. Clinical trials obtain evidence from highly selected patient groups who do not reflect the broader population and frequently present only the highest achievable survival. In the real world, cancer survival is unequal across populations.
A clear downward gradient is seen in cancer survival from the most to the least affluent socio-economic groups. Overall survival has improved in the past 20–30 years, but that has mainly been driven by improvements in the more affluent groups, meaning the gradient has grown steeper.1 Additionally, in the 1990s, 5-year relative survival in England, Scotland and Wales for colon cancer was well below the average for Europe.2 This situation led to the launch of the NHS Cancer Plan in England in 2000, which seemed to improve 1-year survival from 2003 onwards for various cancers.3
For CRC, postoperative outcomes in England vary substantially between hospitals and NHS trusts.4 In 1998–2006, across 150 NHS trusts, 30-day postoperative mortality was 6.7% overall. Leading prognostic factors were age, comorbidity, Dukes stage at diagnosis, deprived socio-economic status and operative urgency. Five-year survival is positively affected by survival in the first year.5 Avoidable premature deaths have declined to some degree, but if survival for CRC in Britain had been as high as the highest in Europe from 1995 to 1999, 8429 fewer premature deaths would have occurred.6 For rectal cancer, if the 3-year survival in the least affluent group had been as high as that in the most affluent group, around 600 premature deaths per year would have been avoided during 1996–2000.7
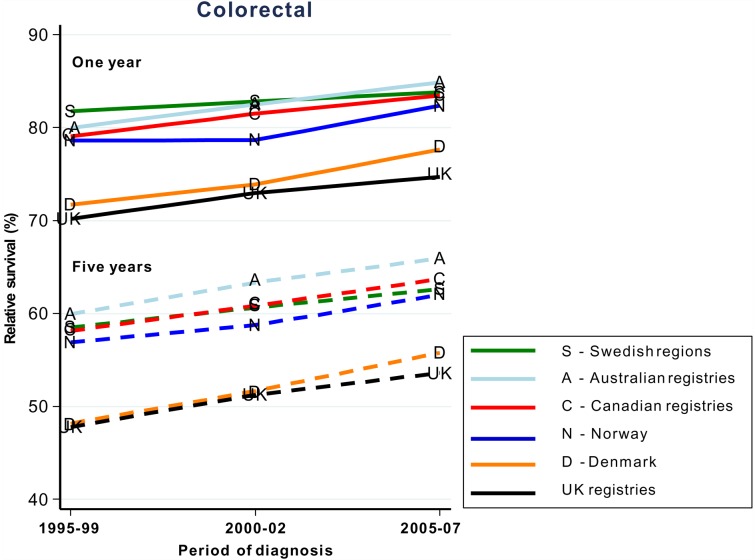
In countries where patients have universal access to healthcare, trends for 1-year and 5-year survival are roughly parallel and have changed little over time (figure 1).8 Some differences are seen for stage at diagnosis and excess deaths.9 These values, however, are difficult to compare against countries with other types of healthcare systems because of incomplete data. Staging is also classified with multiple different systems, none of which allows complete mapping from the others. Routine recording of disease stage at diagnosis (eg, in registries) and standardisation of the methods or data, which might allow mapping by algorithms, would be extremely useful.10
Figure 1.
Age-standardised 1-year and 5-year relative survival trends 1995–2007 in countries with universal healthcare systems.8
The CONCORD-2 study11 has initiated global surveillance of cancer survival based on individual data for 25.7 million patients with cancer diagnosed as having one of 10 common cancers between 1995 and 2009, provided by 279 cancer registries in 67 countries. For CRC, age-standardised net survival was lower in the UK than in many other countries in Europe, although the overall differences between countries were small. Rankings in Asia improved vastly, mainly due to aggressive strategies for early-stage identification and treatment. Compared with other cancers such as breast cancer in countries with universal access to healthcare, survival trends for CRC were much worse.
Irrespective of how large or small the numbers of potentially avoidable deaths, what remains important is that they are not being avoided. Stage at diagnosis is recognised as a crucial factor for survival, but full understanding of its contribution is hindered by differences in coding between registries and incomplete data. Wider consensus is needed on recording of data on stage at diagnosis and on the methods by which cancer stage is determined.10
Screening for gastrointestinal cancers
Screening for gastrointestinal cancer was addressed by Professor Robert Steele, University of Dundee. Bowel, stomach, liver and oesophageal cancers are among the 10 most commonly diagnosed cancers worldwide. Early detection of these malignancies or precursor conditions could improve outcomes. Screening is an option, although its benefits are debated.
Programmatic screening aims to detect disease or disease precursors in asymptomatic individuals and, therefore, is only suitable if the disease is common and treatable and early diagnosis will improve the effectiveness of treatment.12 Without improved survival, screening merely leads to patients knowing about their disease for longer. The method of screening must be acceptable to society. A drawback of programmatic screening is that it is subject to various biases, such as self-selection (volunteers being disposed to seek healthcare or being health conscious), lead-time (erroneous extension of survival due to early detection) and length bias (increased survival within a given time frame because of a long latency or preclinical period).13 To prove whether screening works, individuals who do and do not participate must be followed up.
Programme validity is determined by outcomes and costs, including whether screening does any harm (eg, overdiagnosis leading to unnecessary tests or treatments in people with low-risk cancers), which should be established with randomised controlled trials.14 The diagnostic tests for most gastrointestinal cancers are not supported by extensive data from randomised controlled trials and, therefore, do not meet all the criteria for screening. Some approaches do show promise: direct endoscopy, Helicobacter pylori status and measurement of pepsinogen and gastrin 17 for stomach cancer; direct endoscopy and cytology for oesophageal cancer; and radiological imaging of patients with cirrhosis, hepatitis B virus infection or primary biliary cirrhosis for liver cancer. By contrast, the α-fetoprotein blood test shows poor sensitivity for liver cancer and no tests are yet suggested for pancreatic cancer.
Screening for large bowel cancer with the gFOBT meets all the criteria,15–18 which has led to the BCSP being introduced across the UK. In a pilot study from 2000 to 2006 in Scotland, CRC mortality was compared in people aged 50–69 years offered screening and those not offered screening. The groups were matched for sex and socio-economic status. A 10% relative reduction in CRC mortality was seen (0.90, 95% CI 0.83 to 0.99) in the population as a whole, and among study participants alone the relative reduction was 27% (0.73, 95% CI 0.65 to 0.82).19 Screening by colonoscopy has a positive predictive value of 15% for carcinoma, 36% for adenoma and 49% for no neoplasia. With this method, though, interval cancers are a problem, especially in women in whom fewer cancers but more interval cancers are detected than in men.20
The FIT is proposed to replace gFOBT to improve specificity for human haemoglobin and because it is easier to perform and yields quantitative results. In 40 125 participants, FIT screening led to 909 (2.26%) referrals for colonoscopy and detection of 30 cancers and 31 interval cancers. Uptake of FIT is higher than that of gFOBT in men and by people in lower socio-economic groups.21 The cut-off for positivity is currently 2.0%, but reducing this threshold would improve detection of interval cancers. The number of colonoscopy referrals would increase, but would remain lower than with no screening.
Sigmoidoscopy improves detection of high-risk adenomas in people aged 55–64 years, has reduced CRC mortality and incidence by 23% (HR 0.77, 95% CI 0.70 to 0.84) and 31% (0.69, 95% CI 0.59 to 0.82), respectively, and has good uptake.22 Of note, though, when assessed by cancer site, incidence was reduced by 50% for distal cancers, but by only 3% for proximal cancers. In England, one-off flexible sigmoidoscopy is available to the public at age 55 years, but only by request or offer. In Scotland, a randomised controlled trial of flexible sigmoidoscopy is underway in people aged around 60 years. Flexible sigmoidoscopy should be rolled out across the UK soon to increase screening options, although it is unlikely to alter the socio-economic uptake gradient.23 Colonoscopy has excellent sensitivity and specificity for cancer and adenoma,24 but also has little effect on proximal cancers.25
Several new screening approaches are on the horizon but require much more assessment: multitarget faecal DNA testing, which is more sensitive but less specific than FIT (although that might improve if the cut-off were altered); peripheral blood tests for methylated DNA, tumour-associated proteins, miRNA and autoantibodies; detection of volatile organic compounds (dogs can detect these with 91% sensitivity and 99% specificity in breath and with 97% and 99% in stools); CT/MRI; and capsule endoscopy. Whether these will lessen the effects of socio-economic status remains to be seen.
Early diagnosis of gastrointestinal cancers
Early diagnosis is important for all gastrointestinal cancers, although the best data are currently available for CRC. The 10-year survival has improved significantly since the 1970s, being 90% in people with stage I bowel cancer at diagnosis, compared with <10% in people who present with stage IV disease.26 Yet only 45% of bowel cancer is diagnosed at an early stage. Emergency presentation of many types of gastrointestinal cancer remains all too common, leading to negative patient experience and poor outcomes.26
Dr Sara Hiom of Cancer Research UK highlighted some of the issues working against the ability to pick up a cancer diagnosis sooner. Barriers to early diagnosis in the UK include low public awareness of signs and symptoms, practical difficulties with making appointments and poor uptake of screening. Patients might delay visiting their GP because of embarrassment, attribution of symptoms to age or comorbidities and not wanting to ‘bother the doctor’ or be a burden to the NHS.27 The national series of ‘Be Clear on Cancer’ campaigns is aiming to raise awareness of cancer symptoms among the general public and encourage early presentation to primary care.
Barriers to patients accessing care are lack of direct access to diagnostic investigations for GPs, poor communication between primary and secondary care services, and pressure on GPs to avoid inappropriate referral. The latest National Institute for Health and Care Excellence (NICE) guidelines for symptomatic referral have lowered the threshold to a positive predictive value of 3%28 to capture more patients and cut time spent on testing, waiting for results and reconsultation. However, rates of investigation in the UK remain lower than in many other countries.29 Waiting times for colonoscopy in England have increased because diagnostic capacity is so stretched.30 A new standard of 28 days from referral to diagnosis and communication of results for 95% of patients has been set for 2020. Thus, methods to improve diagnosis and exclusion must be considered now.
The Accelerate, Coordinate, Evaluate programme (ACE) was introduced to improve and test new diagnostic pathways in the UK. Wave 1 supported uniform implementation of best practice, which, in some areas, has reduced endoscopy waiting times by at least 10 days so far. Wave 2 will involve piloting new diagnostic pathways for patients with non-specific but concerning symptoms through multidisciplinary diagnostic clinics. ACE has also encouraged thinking on issues such as the potential for expert diagnosticians (for example gastroenterologists or specialised GPs) and clearer guidelines on when referrals are inappropriate; GPs see many patients with non-specific gastrointestinal symptoms, making those at risk of cancer hard to identify.
The view from primary care
Earlier access to definitive tests
Dr Terry Bowley, Macmillan GP advisor, opened the discussion on the interface between primary and secondary care.
Despite an 18% increase in the NHS budget since 2005/2006, funding for GP care has dropped by 8% in real terms. This drop has had an impact on the size of the workforce, which is delaying access to healthcare professionals, with obvious implications for early diagnosis.
A GP might see eight or nine new cases of cancer per year and 30–40 patients (based on an average list size of 2000 patients per GP) living with cancer or the consequences of treatment at any given time. While GPs see relatively few new cancer diagnoses per year, they see a huge number of patients where malignancy is a possibility. GPs, therefore, prefer to refer patients via the 2-week wait pathway to rule out cancer rather than wait for red-flag symptoms to develop. Patients with vague symptoms, however, might need to be reviewed several times by the GP before referral,31 32 and waiting times for investigations, such as ultrasonography, can cause further delay. NICE guidance from 2015 has been based on positive predictive values. Reference values are available for many common cancers and their presenting symptoms. Macmillan, in collaboration with BMJ Informatica, has devised a support tool that is easily incorporated into general practice software and which is aimed at helping GPs in clinical decision-making and referring patients with suspected cancer.33 The tool has a symptom checker that enables GPs to calculate percentage risk values for specific cancers. It is also a ‘prompt’ functionality that picks up symptoms coded by GPs in previous consultations and highlights when patients are at increased risk of certain cancers. These prompts are especially helpful if patients do not always see the same GP. Assessment of the use of this tool showed that it changed decision-making in 50% of cases and that 20% of referrals would not have been made if the tool had not been used.33 Performance in relation to outcomes, such as early diagnosis, is being assessed in ACE.
Living with cancer
In her presentation, Professor Jane Maher, Macmillan Cancer Support, noted that improving outcomes in cancer is not just about early diagnosis, but must also take into account people living with cancer. There are 200 different cancers that can roughly be separated into three broad categories: good outcomes (the largest category, with life expectancy of at least 10 years), intermediate outcomes (life expectancy 1–5 years) and poor outcomes (life expectancy up to 2 years).34 Patients in the intermediate group are generally the least visible but often undergo many lines of cancer treatments.35
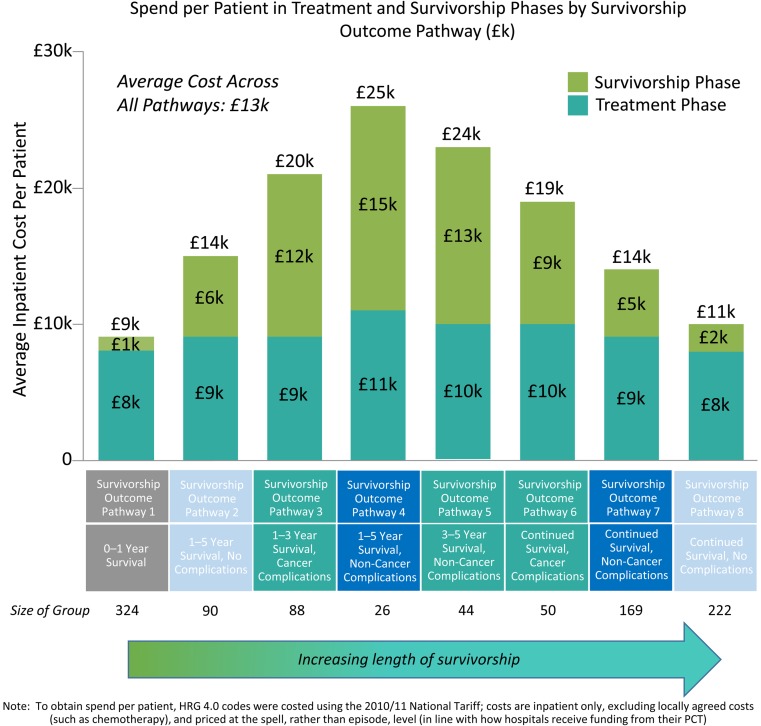
Better quality of life in the good group and survival in the poor group are needed to improve cancer outcomes. Costs are highest in survivors who live longest after cancer treatment is completed and accumulate after cancer treatment has ended (figure 2).36 They might also be compounded by coexisting disorders, such as hypertension, obesity, mental health issues and chronic heart and kidney disease. Currently, 2.5 million people in the UK are living with cancer, which is 400 000 more than in 2010, and the number is increasing by around 3% per year.37 Although an extremely positive step, more and older people are living with consequences of cancer and treatment for longer and, therefore, are not necessarily living well.38 39
Figure 2.
Costs of cancer survival.36
The Independent Cancer Taskforce40 has published recommendations to improve cancer outcomes, including reducing and managing the long-term consequences of treatment. Cancer can be separated into three main categories: rare and complex (several hundred patients requiring highly specialist care), intermediate (tens of thousands of people who need proactive care) and common (hundreds of thousands of people with a broad range of risks and requirements). Guidelines are available from Macmillan for GPs on the management of treatment consequences, including when to refer and where to find educational resources for patients. The Royal College of General Practitioners has implemented a 1-year project that aims to raise awareness and assess outcomes for consequences of treatment. It will use surveys of GPs' awareness of consequences of treatment to create a GP ‘toolkit’ for identification and management of patients at risk.
The view from secondary care
Endoscopy in gastrointestinal cancer
Dr Andrew Veitch, New Cross Hospital, Wolverhampton, and immediate past vice president (endoscopy) of the British Society of Gastroenterology, noted that endoscopy is integral to the diagnosis, prevention and treatment of gastrointestinal cancers, especially upper gastrointestinal cancers. To improve outcomes, the time to diagnosis needs to be shortened and the detection rate for early neoplasia increased.
The incidence and mortality for oesophageal cancer in the UK are the highest in Europe and are increasing, although for stomach cancer they are reducing.41 The average rate for missed upper gastrointestinal cancers within 3 years of gastroscopy worldwide is roughly 8% (range 2–40%). Many of these cancers may have been treatable endoscopically if detected earlier.
Various standards have been developed for upper gastrointestinal endoscopy, but these focus mainly on mechanistic and safety factors. For example, of 22 key performance indicators in the American Society for Gastrointestinal Endoscopy quality standards,42 only two relate to mucosal visualisation. In the UK, diagnostic gastroscopy is allocated 20 min, but the procedure takes <5 min. Other positive changes to improve detection that could also be made through training are dedicated surveillance lists, sedation of patients, cleaning of the gastrointestinal tract (a common practice in the caecum/rectum for colonoscopy) with mucolytics or washing and photo-documentation to reveal and record subtle lesions.
Another priority for gastrointestinal cancers is to improve the evidence base. Important research directions include confirmation of whether the incidence of early gastric neoplasms in Western populations is sufficient to warrant a change of practice and whether early endoscopic intervention in high-grade dysplasia or intra-mucosal carcinoma will influence the natural history of the disease.
Endoscopy capacity
Hilary Brown and Steve Wyatt presented data from their study undertaken at the University of Birmingham to assess endoscopy capacity across England and which was commissioned by Cancer Research UK.43 Surveys and interviews identified several key workforce issues, including staff recruitment (of all staff groups, but particularly nurses) and retention, which were exacerbated by staff shortages, and the training of inexperienced staff to take on scoping work. Units were finding it challenging to continue to improve efficiency, although at the time of the study the Productive Endoscopy Toolkit had only just been introduced. Several factors, including public campaigns to raise awareness of symptom, were increasing demand and thereby the pressure on units, which in some instances was adversely affecting surveillance cases and non-urgent assessments. New technology and tests, such as CT colonography, have increased the complexity of procedures and, hence, time pressures. Nevertheless, some units were making improvements in productivity by paying closer attention to the teams and having well-trained administration staff.
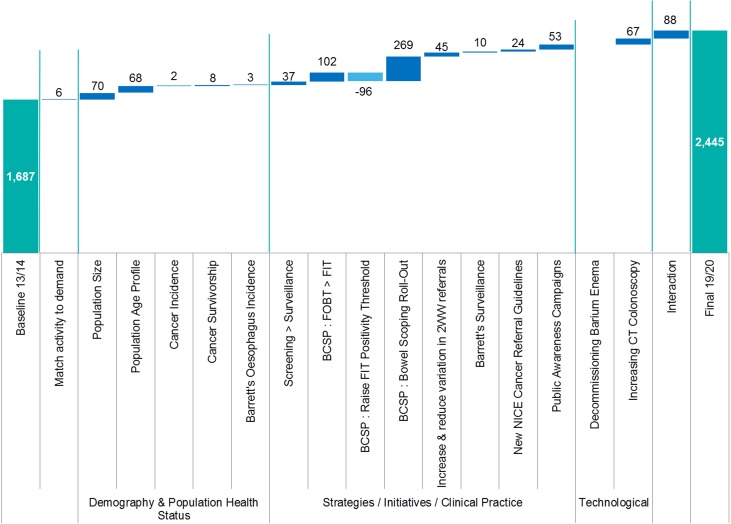
Modelling of the potential effects of changes in gastrointestinal endoscopy activity estimated that demand would reach 2.4 million endoscopies in 2019/2020, an increase of roughly 44% (6.5% per year). Around a quarter of growth was estimated to be driven by demography and population health status factors and the remainder was driven by NHS factors and new technology. The biggest single contributor was the roll-out of the BCSP (figure 3). Use of endoscopy and flexible sigmoidoscopy was predicted roughly to double, and identification of cancer through symptoms and screening to increase substantially. Reductions in non-attendance by patients offer only a limited opportunity to free up endoscopy capacity.
Figure 3.
Modelled changes in gastrointestinal endoscopy activity 2013/2014 to 2019/2020.43 2WW, 2-week wait; BCSP, Bowel Cancer Screening Programme; FIT, faecal immunochemical test; FOBT, faecal occult blood test; NICE, National Institute for Health and Care Excellence.
Investment in the workforce through careful job planning and training was recommended to address the projected increase in demand. Joint Advisory Group (JAG) accreditation and use of the Productive Endoscopy Toolkit were encouraged to further improve efficiency, as were engagement with commissioners and increased collaboration between primary and secondary care. Finally, better reporting for non-screening and screening activities could improve strategic planning.
Since the study, the government has introduced a training scheme for 200 further non-medical endoscopists in England and pledged up to £300 million more investment in diagnostics per year by 2020. The system, however, is likely to remain under pressure, and in a time of austerity, the interplay between different initiatives and interventions, such as the full roll-out of the bowel scope screening programme and the introduction of FIT, must be considered in terms of maximising impact and value for money.
Oesophageal cancer
Professor Rebecca Fitzgerald, University of Cambridge, discussed early detection of oesophageal cancer. In Western countries, the incidence of oesophageal adenocarcinoma has been increasing. Outlook is poor, with overall 5-year survival of 13%, but can be improved with early diagnosis by up to 80–90% in patients with high-grade dysplasia or stage T1a disease at presentation and 60% for stage T1b submucosal disease. Unfortunately, around 70% of patients present with stage 3 disease or worse. Barrett's oesophagus is a pre-cancerous stage at which it might be useful to act to detect or prevent possible cancer. Although most people who progress to Barrett's oesophagus do not develop cancer, 0.3% per year do. Ablation therapy can be preventive.44 45
The NICE guideline for Barrett's oesophagus recommends against offering endoscopy routinely for diagnosis, but suggest considering it for patients with gastro-oesophageal reflux disease, after discussion about risk factors and preferences.46 The previous age limit for referral has been removed in recognition of patients age 35–44 years being more likely than older patients to present as emergency cases.47
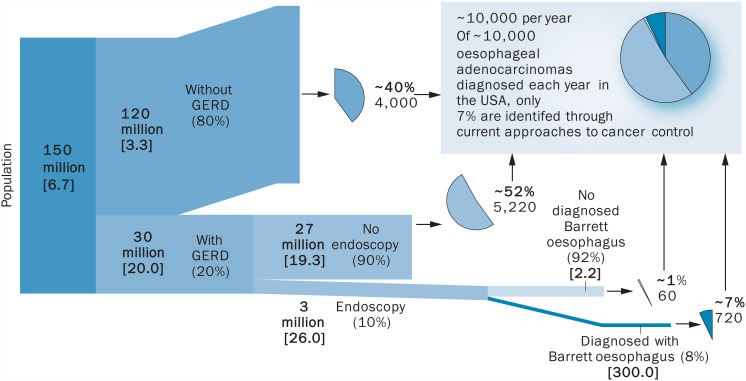
Endoscopic assessment of all patients with reflux is not feasible and, therefore, prioritisation is essential. In the USA, around 10 000 people per year have Barrett's oesophagus, of whom only about 7% develop oesophageal adenocarcinoma. Among 30 million individuals who have reflux disease, 90% do not undergo endoscopy yet account for around 52% of people who develop oesophageal adenocarcinoma and, therefore, this could be a pool worth investigating (figure 4).48 Patients with reflux or dyspepsia could be identified and investigated. However, owing to the high number of investigations that would be required, an alternative to endoscopy would be helpful.
Figure 4.
Identifying possible populations at risk of oesophageal cancer.48 GERD, gastroesophageal reflux disease.
Cytosponge is one such alternative. This device is swallowed in a capsule that dissolves in 5 min, after which the sponge is pulled out through the mouth by the attached string. It collects cell samples from the length of the oesophagus that can be assayed for disease biomarkers.21 44 49 Sensitivity for Barrett's oesophagus is 73–90% and improves with increasing length of Barrett's segments.50 51 Furthermore, costs are lower than those for endoscopy plus endotherapy in early cancers.52 For low-risk patients, testing with Cytosponge every 3 years would be appropriate, with endoscopy referral if dysplasia develops.53 Gene mutations, such as in TP53, which are associated with high-grade dysplasia Barrett's oesophagus, might help to stratify patients further and prompt immediate endoscopy.54–56
Pancreatic cancer
Professor Stephen Pereira of University College London introduced his presentation by saying that “pancreatic cancer is in such a bad state that things can only get better”. There have been no improvements in survival in the past 20–30 years. More than half of patients are seen as emergencies, and 5-year survival is only around 4%,57 yet pancreatic cancer is allocated only 1% of cancer research funding.
The UK government has highlighted the need for screening, access to care, earlier diagnosis and improved patient experience for people with pancreatic cancer. Small T2 lesions are associated with much better survival than tumours >3 cm. Importantly, there are windows of opportunity for diagnosis and detection of at-risk patients before symptoms develop.
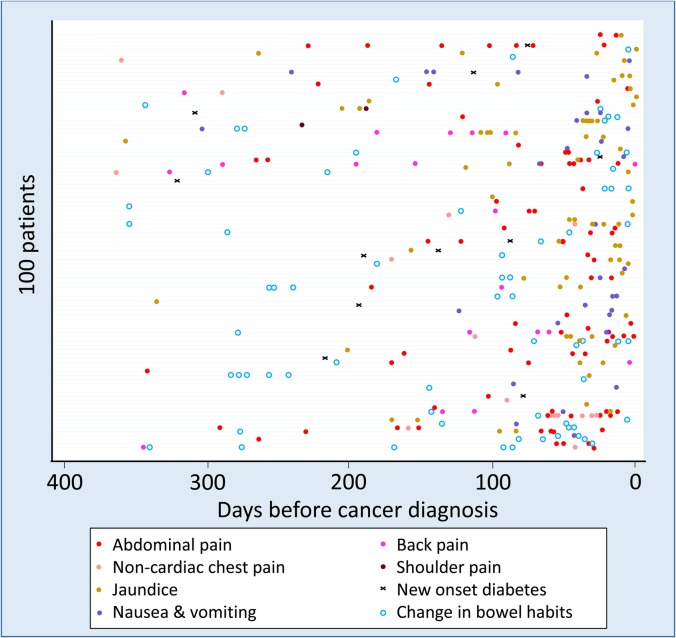
In an at-risk cohort, 91% of patients had relevant symptoms in the 2 years before diagnosis, such as abdominal pain, indigestion, change in bowel habits, back pain and nausea, but saw GPs a median of three times (range 0–22) before referral.58 However, individual symptoms are often vague, although they increase in frequency strikingly in the year before diagnosis (figure 5) and the visits tended to be clustered in that period. Creating an algorithm to combine and score specific alarm symptoms would be helpful.
Figure 5.
Incidence and timing of common symptoms in pancreatic cancer before diagnosis.58
To encourage early diagnosis, one programme is the Early Diagnosis of Abdominal Symptoms project, which uses cross-sectional information to calculate risk in multidisciplinary diagnostic centres to which GPs have direct access. There are four pilot pathways to referral: painless jaundice, >3 kg weight loss, non-specific abdominal pain and two visits to accident and emergency or the GP with severe unexplained abdominal pain. Several centres in the UK offer screening of people with family history of pancreatic cancer with or without relevant gene mutations. Opportunistic identification of cystic lesions in the pancreas should prompt further investigation. Patients with serous or mucinous or indeterminate cysts might require intervention or ongoing surveillance.
Several imaging modalities, such as CT MRI, endoscopy, ultrasonography and endoscopic retrograde cholangiopancreatography sampling, are emerging as diagnostic approaches for pancreatic cancer. Good progress in biomarker discovery has been made in the past 24 months. Use of 200 000 serum samples gathered for the United Kingdom Collaborative Trial of Ovarian Cancer Screening as a resource has enabled proteomics analysis. In the pre-diagnosis setting, CA19-9 and CA125 have shown good performance as pre-clinical biomarkers at least 1 year before diagnosis,59 and further studies are underway. Various proteins in urine, which can be collected non-invasively and is simpler to analyse than blood, correlate strongly with precursor lesions.60 Assessment of biomarkers might, therefore, extend the window of opportunity for diagnosis of pancreatic cancer by years,61 although whether they would improve detection in at-risk groups needs to be assessed.
Endoscopy of the pancreas is difficult and carries the risk of pancreatitis. Confocal laser endomicroscopy and real-time molecular imaging with fluorescing proteins offer potential new applications in addition to non-invasive imaging.
“Be Clear on Cancer” campaign
Public Health England, the Department of Public Health and the NHS are taking a collaborative approach to improve awareness of cancer with the “Be Clear on Cancer” campaign, which was discussed by Mr William Allum of the Royal Marsden NHS Foundation Trust. Launched in 2010/2011, the campaign was developed to contribute to the government's commitment of saving 5000 additional lives per year by 2014/2015.28 “Be Clear on Cancer” continues to be an integral part of work to improve early diagnosis. The campaigns are targeted at people older than 50 years and aim to raise awareness of symptoms of various cancers, including upper gastrointestinal cancer, and encourage people to see their GP without delay.
To design campaigns, public understanding of the relevance of symptoms to cancer was surveyed. For upper gastrointestinal cancer, heartburn, dyspepsia and dysphagia were selected as symptoms to promote. Dysphagia needed clarification, but heartburn was well understood. Seven local pilots were done first, followed by regional and national campaigns. Campaign activity included advertising in national media (television, press and radio), digital adverts targeted at people shopping for heartburn medication, face-to-face events and a supporting programme of public relations. Leaflets and posters were distributed to GP surgeries and these advertisements also featured on pharmacy bags.
Pharmacists repeatedly selling over-the-counter or prescription preparations to an individual were encouraged to mention the campaign. Several national pharmacy chains also carried advertising to encourage these people to see their GPs.
For the national campaign, a module on gastrointestinal cancer on Doctors.net.uk was made available, which had reasonable uptake. For the regional campaign, the number of urgent GP referrals (2-week wait) increased, as did the number of cancers diagnosed after these referrals in the target age group.62 Full analysis of the data from the national campaign is pending and will include how endoscopy services have been affected and other potential knock-on effects.
Gastrointestinal cancer: doing better in austere times
Referral
There is currently roughly a 10% survival deficit for gastrointestinal cancer in the UK compared with similar countries, began Michael Machesney from Barts Health NHS Trust. Cancers with lower prevalence have their own clinical reference groups and budgets, whereas common cancers have many clinical commissioning groups (CCGs). The interface for GP referral to secondary care needs to be developed. Enabling GPs to commission services that would produce good data and from groups that will make those data available for interpretation would be useful. Services should also address variation, develop strategies for early diagnosis and improve treatment safety and follow-up. It is hoped that the shift to early diagnosis will also improve the safety of treatment.
Patients with CRC are mainly referred through GPs, but around a quarter still initially present as emergency cases.63 The speed of GP referral can vary substantially64 and the cycle of tests and reassessment can be costly and time-consuming. Direct access to services on the request of GPs or after triage of patients by the hospital could send the most urgent cases straight to testing. Finally, the recurring limitations on endoscopy capacity must be resolved.
Screening uptake can be improved if patients are contacted directly.65 GPs are frequently unaware of whether their patients have participated in screening. In some cases, GPs receive formal notification, but in others non-participation might be marked merely by a non-flagged read code. The BCSP does not include people aged 50–54 years, despite good preventive value with bowel endoscopy in these patients. Including younger age groups in screening programmes might be a cost-effective way to increase detection in low-resource regions.
Commissioning
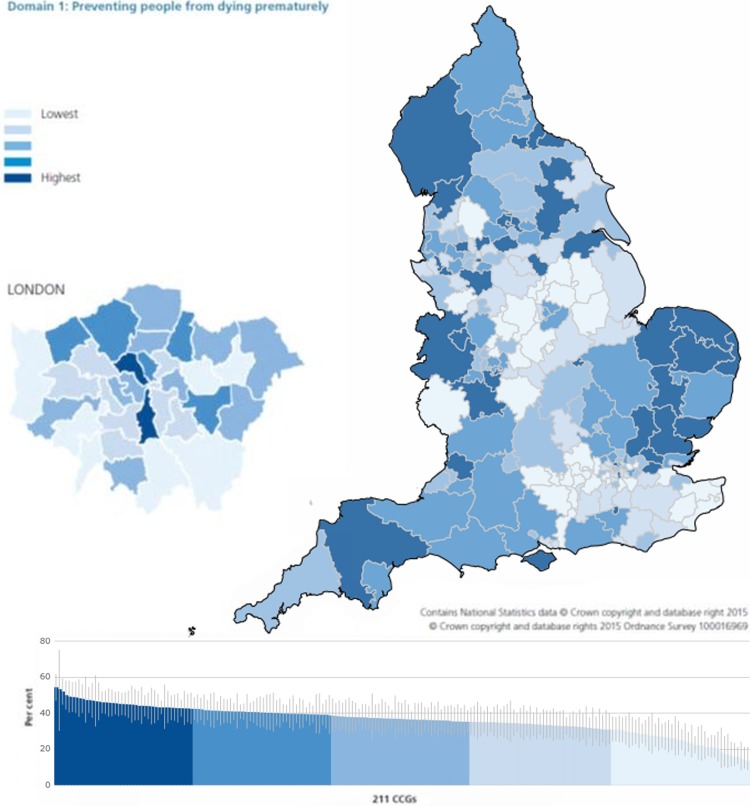
Dr Michael Glynn, National Clinical Director for GI and Liver Services, NHS England, discussed commissioning in austere times and emphasised that commissioning for value is particularly important and needs to be information based. The national data released by Public Health England show that diagnosis of stage 1 and 2 CRC varies by around 40–50% across CCGs.66
Rather than trying to explain away these variations, they should be seen as opportunities to do better. The Right Care Approach (http://www.rightcare.nhs.uk) has been set up to maximise value at the individual and population levels. CCGs are invited to use The NHS Atlas of Variation in Healthcare (figure 6)67 to investigate differences, such as burden of disease and treatment, and to address these in five key strands: clinical leadership, indicative data, clinical engagement, evidential data and effective process. For example, if all providers in England were to match the day-case surgery rates for cholecystectomy in the upper quartile, the estimated annual saving could release more than £64 million.
Figure 6.
Percentage of new cancers that were diagnosed at stage 1 or stage 2 by clinical commissioning groups (CCGs), 2013.67
For CRC, several aspects would need to be addressed, including local awareness (eg, GPs introducing campaigns), primary care systems (eg, flagging symptoms of concern to patients and making referrals), commissioning of services and implementing appropriate service models. Careful attention is needed to ensure adequate provision of pathology services. These services also need to be improved, but have not traditionally been at the forefront of CCG attention, and even seem to have fallen off the CCG radar. Changes leading to early diagnosis should in turn produce substantial savings through less treatment of stage 3 and 4 disease, as well as saving lives.
Research in primary care
Professor Roger Jones, King's College London, and Editor, British Journal of General Practice, began his presentation by stating that cancer has become a headline problem in general practice. To augment the changes that are required, general practices and GPs must drive towards definitive diagnosis earlier. How patients respond to information, campaigns and symptoms needs to be more clearly understood, and GPs need to be better engaged. The propensity of GPs to explore and act on groups of symptoms varies substantially, which might also need to be addressed.
The training of GPs over the past 20 years has often emphasised a watch and wait approach to diagnosis, but must now move towards making more timely and accurate diagnoses. Thresholds for investigation also need to be lowered to improve access to care for patients. Computer-assisted tools to help with assessment of symptoms are very useful, but many include mainly late-stage symptoms (eg, weight loss), which will not improve early diagnosis. Safety-net systems (eg, proactive follow-up) need to be put in place. For the many cancers with ‘whispering’ symptoms, continued assessment and possible retesting if results do not indicate cancer will be important.
Overall, however, the key to earlier diagnosis will be collaborative research between primary and secondary care.
Discussion
The audience was invited to raise questions and discuss any of the issues raised throughout the conference. Here were present a selection of questions and answers provided by the speakers and other audience members.
- The JAG data website says that 93% of key trusts are participating in the JAG standards accreditation programme and that 66% are currently accredited. As timing targets are part of this scheme, why are they not being met? One centre lost accreditation because of this issue, and it could lead to a domino effect.
- Good progress has been achieved, but all the quick wins have been used up and now services are experiencing diminishing returns from changes. The biggest challenge is still the workforce gap (including gastroenterologists), with 30% of roles unfilled. All units need to improve effectiveness of staff.
- The workforce is grappling with the logistics of early diagnosis. In the meantime, many patients, including a lot without cancer, remain in the system. Is the system becoming anti-patient?
- Public expectation has been set up for early diagnosis, but this increases a risk of damage to patients who do not have cancer, which needs to be limited. Multidisciplinary approaches might serve these patients better. For example, in Denmark, the health service does not just rule out cancer, it also asks what else might be wrong with those patients.
- Targets can lead to patients being ‘bounced’ between primary and secondary care. The multidisciplinary approach in ACE might improve this effect.
- Data are crucial. Many are available for people with cancer but few for the people who are referred and do not have cancer, and better tracking of these patients is needed.
- 25% of referred patients are older than 80 years, but they are not being caught by screening. Even the BCSP has not increased screening uptake to more than 60%.
- The ambition for 2020 is 75% screening uptake. Macmillan are working with GPs to try to improve the role they can play in raising awareness of screening. An ambition is to have a Macmillan GP in all CCGs.
- Data are being gathered by Macmillan on the fitness of older people to withstand various procedures.
- In terms of changing from gFOBT to FIT, now could be a good time. Also, starting screening from younger ages could mean that it becomes the norm and is accepted sooner. The introduction of FIT is expected to increase uptake by around 10%. Direct contact with patients is effective, and the UK has better uptake than in countries that do not employ direct contact, but having GPs endorse (but not asking them to carry out) testing would probably improve uptake further. Roll-out of new methods must not impinge on the public making informed choices, including refusal. Targeted approaches might also help. For example, the gFOBT test was found to be unpopular among the Asian community in Leicester because Muhammad is a common name, but as it is also the Islamic prophet's name, writing it on a card next to faeces is particularly distasteful.
- The Danish health system differs from those in other Nordic countries and is more similar to the UK primary care system. Therefore, are GPs the problem?
- Problems with late diagnosis are not all the fault of GPs, but anything that can help GPs refer these people should be approved. Survival in Denmark seems to have improved more quickly than in the other Nordic countries because of changes in referral patterns, which are now being copied.
- How can interaction between primary and secondary care be improved (eg, by access to more information, etc)?
- Direct access to all investigations might not be possible depending on the setup.
- General practice is on its knees, but by the time it gets off its knees things will be very different (eg, technology and methods of communication). Perhaps it should be left alone to recover and adapt by itself.
- Electronic communication has helped the system enormously.
- If GPs see so few patients with cancer per year, how easy will it be to educate them and other primary care staff to improve detection?
- Improved relationships between healthcare providers and knowledge of practitioners at all levels can make a difference.
- Telephone triage by nurses who can do colonoscopy can help to ensure that patients are suitable for the procedure before referral and that the preparation requirements are met.
Summary
Substantial steps are being made towards early diagnosis. A range of tools are available to help GPs appropriately categorise early symptoms during routine consultations. Various promising new tests and devices are being explored, especially for cancers that frequently present at late stages. The continuing increase in demand on endoscopy services is a major concern, not least because of the shortage of trained practitioners and other healthcare staff. However, screening and collaborative streamlining initiatives might help to improve the relevance of referrals. The question posed in the title of the conference was rhetorical, but a positive answer seems potentially achievable, even in austere times, through facilitating uptake of screening, working to develop the primary–secondary care interface, educating the public and by protecting funds for research.
Footnotes
Contributors: IF conceived, planned and chaired the meeting. WA, TB, HB, MPC, RF, MG, SH, RJ, MM, JM, SPP, RS, AV and SW were speakers at the conference. RA wrote the drafts. All authors reviewed and corrected the content.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Coleman MP, Rachet B, Woods LM, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer 2004;90:1367–73. doi:10.1038/sj.bjc.6601696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coleman MP, Gatta G, Verdecchia A, et al. , EUROCARE Working Group. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol 2003;14(Suppl 5):v128–49. doi:10.1093/annonc/mdg756 [DOI] [PubMed] [Google Scholar]
- 3. Rachet B, Maringe C, Nur U, et al. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol 2009;10:351–69. doi:10.1016/S1470-2045(09)70028-2 [DOI] [PubMed] [Google Scholar]
- 4. Morris EJ, Taylor EF, Thomas JD, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806–13. doi:10.1136/gut.2010.232181 [DOI] [PubMed] [Google Scholar]
- 5. Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol 2007;8:773–83. doi:10.1016/S1470-2045(07)70245-0 [DOI] [PubMed] [Google Scholar]
- 6. Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer 2009;101 2):S115–24. doi:10.1038/sj.bjc.6605401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis L, Coleman MP, Rachet B. How many deaths would be avoidable if socioeconomic inequalities in cancer survival in England were eliminated? A national population-based study, 1996–2006. Eur J Cancer 2012;48:270–8. doi:10.1016/j.ejca.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 8. Coleman MP, Forman D, Bryant H, et al. , ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127–38. doi:10.1016/S0140-6736(10)62231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maringe C, Walters S, Rachet B, et al. , ICBP Module 1 Working Group. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol 2013;52:919–32. doi:10.3109/0284186X.2013.764008 [DOI] [PubMed] [Google Scholar]
- 10. Walters S, Maringe C, Butler J, et al. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer 2013;132:676–85. doi:10.1002/ijc.27651 [DOI] [PubMed] [Google Scholar]
- 11. Allemani C, Weir HK, Carreira H, et al. , CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977–1010. doi:10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Public Health England. Guidance: criteria for appraising the viability, effectiveness and appropriateness of a screening programme. 2015. https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme (accessed 26 Jan 2016).
- 13. Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol 2010;37:202–15. doi:10.1053/j.seminoncol.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:762–71. doi:10.7326/0003-4819-155-11-201112060-00375 [DOI] [PubMed] [Google Scholar]
- 15. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7. doi:10.1016/S0140-6736(96)03386-7 [DOI] [PubMed] [Google Scholar]
- 16. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467–71. doi:10.1016/S0140-6736(96)03430-7 [DOI] [PubMed] [Google Scholar]
- 17. Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008;95:1029–36. doi:10.1002/bjs.6136 [DOI] [PubMed] [Google Scholar]
- 18. Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999;91:434–7. doi:10.1093/jnci/91.5.434 [DOI] [PubMed] [Google Scholar]
- 19. Libby G, Brewster DH, McClements PL, et al. The impact of population-based faecal occult blood test screening on colorectal cancer mortality: a matched cohort study. Br J Cancer 2012;107:255–9. doi:10.1038/bjc.2012.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansouri D, McMillan DC, Grant Y, et al. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS ONE 2013;8:e66063 doi:10.1371/journal.pone.0066063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steele RJ, McDonald PJ, Digby J, et al. Clinical outcomes using a faecal immunochemical test for haemoglobin as a first-line test in a national programme constrained by colonoscopy capacity. United European Gastroenterol J 2013;1:198–205. doi:10.1177/2050640613489281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkin WS, Edwards R, Kralj-Hans I, et al., UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. doi:10.1016/S0140-6736(10)60551-X [DOI] [PubMed] [Google Scholar]
- 23. McGregor LM, Bonello B, Kerrison RS, et al. Uptake of Bowel Scope (Flexible Sigmoidoscopy) Screening in the English National Programme: the first 14 months. J Med Screen 2016. 23:77–82. doi:10.1177/0969141315604659 [DOI] [PubMed] [Google Scholar]
- 24. Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58:241–8. doi:10.1136/gut.2008.156448 [DOI] [PubMed] [Google Scholar]
- 25. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. doi:10.1056/NEJMoa1301969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cancer Research UK. Bowel cancer statistics. 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer#heading-Zero (accessed 26 Jan 2016).
- 27. Hall N, Birt L, Banks J, et al. Symptom appraisal and healthcare-seeking for symptoms suggestive of colorectal cancer: a qualitative study. BMJ Open 2015;5:e008448 doi:10.1136/bmjopen-2015-008448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NICE. Suspected cancer: recognition and referral. 2015. http://www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-1837268071621 (accessed 26 Jan 2016).
- 29. Department of Health. Improving outcomes: a strategy for cancer. First annual report 2011 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213715/dh_131787.pdf (accessed 26 Jan 2016).
- 30. NHS England. Monthly diagnostics data 2014-15 https://www.england.nhs.uk/statistics/statistical-work-areas/diagnostics-waiting-times-and-activity/monthly-diagnostics-waiting-times-and-activity/monthly-diagnostics-data-2014-15/ (accessed 26 Jan 2016).
- 31. Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353–65. doi:10.1016/S1470-2045(12)70041-4 [DOI] [PubMed] [Google Scholar]
- 32. RCGP. National audit of cancer diagnosis in primary care. 2011. http://www.rcgp.org.uk/policy/rcgp-policy-areas/~/media/Files/Policy/National%20Audit%20of%20Cancer%20Diagnosis%20in%20Primary%20Care%20Document%20FINAL%20with%20amends%201Dec11%20RW.ashx (accessed 26 Jan 2016).
- 33. Macmillan Cancer Support. The Macmillan cancer decision support tool: project summary update. 2015. http://www.macmillan.org.uk/Documents/AboutUs/Health_professionals/EarlyDiagnosis/CDSSummaryUpdateAprilMay2015.pdf (accessed 26 Jan 2016). [Google Scholar]
- 34. McConnell H, White R, Maher J. Three cancer groups: explaining the different complexity intensity and longevity of broad clinical needs. 2015. a. http://www.macmillan.org.uk/Documents/AboutUs/Research/Academicposters/Threecancergroups.pdf (accessed 26 Jan 2016).
- 35. McConnell H, White R, Maher J. Understanding variations: outcomes for people diagnosed with cancer and implications for service provision. 2015. b. https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwib2v3_vs_KAhWG7xQKHSoXBDAQFgglMAA&url=https%3A%2F%2Fwww.fmlm.ac.uk%2Fsites%2Fdefault%2Ffiles%2FAppendix%2520B%2520-%2520Understanding%2520variations%2520across%2520cancer%2520types_0.pdf&usg=AFQjCNEg7mr4Zow7rFt4O5GzRk82VyFySA&sig2=yli95PxOjS9fjISHGAcFQw (accessed 26 Jan 2016).
- 36. Macmillan Cancer Support. Living with and beyond cancer: taking action to improve outcomes. 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/181054/9333-TSO-2900664-NCSI_Report_FINAL.pdf (accessed 26 Jan 2016).
- 37. Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br J Cancer 2012;107:1195–202. doi:10.1038/bjc.2012.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armes J, Crowe M, Colbourne L, et al. Patients’ supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol 2009;27:6172–9. doi:10.1200/JCO.2009.22.5151 [DOI] [PubMed] [Google Scholar]
- 39. Maher J and McConnell H. New pathways of care for cancer survivors: adding the numbers. Br J Cancer 2011;105:S5–S10. doi:10.1038/bjc.2011.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Independent Cancer Taskforce. Achieving world-class cancer outcomes: a strategy for England 2015-2020. http://www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf (accessed 26 Jan 2016).
- 41. IARC and WHO. Globocan 2012: estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 26 Jan 2016).
- 42. Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI Endoscopic procedures. Gastrointest Endosc 2015;81:1–80. doi:10.1016/j.gie.2014.07.052 [DOI] [PubMed] [Google Scholar]
- 43. Brown H, Wyatt S, Croft S, et al. Scoping the future: an evaluation of endoscopy capacity across the NHS in England. http://www.cancerresearchuk.org/sites/default/files/scoping_the_future_-_final.pdf (accessed 26 Jan 2016).
- 44. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209–17. doi:10.1001/jama.2014.2511 [DOI] [PubMed] [Google Scholar]
- 45. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277–88. doi:10.1056/NEJMoa0808145 [DOI] [PubMed] [Google Scholar]
- 46. NICE. Oesophageal cancer. 2015. https://www.nice.org.uk/guidance/conditions-and-diseases/cancer/oesophageal-cancer (accessed 26 Jan 2016).
- 47. Abel GA, Shelton J, Johnson S, et al. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br J Cancer 2015;112(Suppl 1):S129–36. doi:10.1038/bjc.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaughan TL and Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015;12:243–8. doi:10.1038/nrgastro.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lao-Sirieix P, Boussioutas A, Kadri SR, et al. Non-endoscopic screening biomarkers for Barrett's oesophagus: from microarray analysis to the clinic. Gut 2009;58:1451–9. doi:10.1136/gut.2009.180281 [DOI] [PubMed] [Google Scholar]
- 50. Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 2010;341:c4372 doi:10.1136/bmj.c4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. , BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780 doi:10.1371/journal.pmed.1001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett's esophagus. Gastroenterology 2013;144:62–73.e6. doi:10.1053/j.gastro.2012.09.060 [DOI] [PubMed] [Google Scholar]
- 53. Liu X, Wong A, Kadri SR, et al. Gastro-esophageal reflux disease symptoms and demographic factors as a pre-screening tool for Barrett's esophagus. PLoS ONE 2014;9:e94163 doi:10.1371/journal.pone.0094163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478–86. doi:10.1038/ng.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ross-Innes CS, Becq J, Warren A, et al. , Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Study Group; Oesophageal Cancer Clinical and Molecular Stratification OCCAMS Study Group. Whole-genome sequencing provides new insights into the clonal architecture of Barrett's esophagus and esophageal adenocarcinoma. Nat Genet 2015;47:1038–46. doi:10.1038/ng.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weaver JM, Ross-Innes CS, Shannon N, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 2014;46:837–43. doi:10.1038/ng.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Office for National Statistics. Cancer survival rates, cancer survival in England: patients diagnosed 2007-2011 and followed up to 2012. 2013. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-320365 (accessed 26 Jan 2016).
- 58. Keane MG, Horsfall L, Rait G, et al. A case–control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 2014;4:e005720 doi:10.1136/bmjopen-2014-005720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Brien DP, Sandanayake NS, Jenkinson C, et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res 2015;21:622–31. doi:10.1158/1078-0432.CCR-14-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Radon TP, Massat NJ, Jones R, et al. Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma. Clin Cancer Res 2015;21:3512–21. doi:10.1158/1078-0432.CCR-14-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumar V, Abbash AK, Fausto N. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia, PA: Saunders Elsevier, 2004. [Google Scholar]
- 62. National Cancer Intelligence Network, Public Health England. Be Clear on Cancer: Oesophago-gastric cancer awareness regional pilot campaign: Interim evaluation report. 2015. http://www.ncin.org.uk/publications/ (accessed 26 Jan 2016).
- 63. Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer—determining the patient journey using multiple routine data sets. Br J Cancer 2012;107:1220–6. doi:10.1038/bjc.2012.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Siminoff LA, Rogers HL, Thomson MD, et al. Doctor, what's wrong with me? Factors that delay the diagnosis of colorectal cancer. Patient Educ Couns 2011;84:352–8. doi:10.1016/j.pec.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wardle J, von Wagner C, Kralj-Hans I, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet 2016;387:751–9 doi:10.1016/S0140-6736(15)01154-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Public Health England. The NHS atlas of variation in healthcare. 2015. http://www.rightcare.nhs.uk/atlas/RC_nhsAtlas3_HIGH_150915.pdf (accessed 26 Jan 2016).
- 67. Right Care. NHS atlas of variation in healthcare 2015. 2015. http://www.rightcare.nhs.uk/atlas/2015_IAb/atlas.html (accessed 26 Jan 2016).