Abstract
The agricultural crops are often affected by the scarcity of fresh water. Seasonal drought is a major constraint on Northeast Indian agriculture. Almost 80% of the agricultural land in this region is acidic and facing severe drought during the winter period. Apart from classical breeding and transgenic approaches, the application of plant-growth-promoting bacteria (PGPB) is an alternative strategy for improving plant fitness under stressful conditions. The 1-aminocyclopropane-1-carboxylate (ACC) deaminase-producing PGPB offer drought stress tolerance by regulating plant ethylene levels. The aim of the present study was to evaluate the consortium effect of three ACC-deaminase producing rhizobacteria – Ochrobactrum pseudogrignonenseRJ12, Pseudomonas sp.RJ15 and Bacillus subtilisRJ46 on drought stress alleviation in Vigna mungo L. and Pisum sativum L. Consortium treatment significantly increase seed germination percentage, root length, shoot length, and dry weight of treated plants. An elevated production of reactive oxygen species scavenging enzymes and cellular osmolytes; higher leaf chlorophyll content; increase in relative water content and root recovery intension were observed after consortium treatment in comparison with the uninoculated plants under drought conditions. The consortium treatment decreased the ACC accumulation and down-regulated ACC-oxidase gene expression. This consortium could be an effective bio-formulator for crop health improvement in drought-affected acidic agricultural fields.
Introduction
Climate change is the greatest threat to world’s agricultural sustainability in the 21st century1. Drastic changes in various climatic factors (e.g., precipitation, heat, light, etc.) can tremendously influence the global reduction in crop yields2. The improvement in crop yields under unfavourable conditions by classical breeding or gene transfer techniques pose certain limitations in terms of ethical issues and time requirements3. Again, drought stress tolerance is often a complex phenomenon involving clusters of gene networks4–6. Although many of the networks are resolved, a large gap still remains7. The inadequate resolution of the diverse gene networks among large numbers of cultivars of a single crop is another serious problem in developing stress-resistant varieties by utilizing the gene technology approach8. Therefore, alternative eco-friendly approaches are much more appreciable at this time. One such strategy could be the use of stress-resistant plant growth promoting bacteria (PGPB) with critical roles in enhancing plant growth performance under stressed environments. PGPB either thrive freely in the soil or colonize the rhizosphere, phyllosphere, or plant tissue interior (endophytes). They are already being used as an efficient candidate to improve plant growth and development during normal as well as during stressful environmental conditions4. PGPB are capable of producing different plant growth hormones (auxin, gibberellins, cytokinins, and ethylene) and other growth-enhancing molecules (siderophore, hydrogen cyanide, phosphatase, nitrogenise, etc.) which have potential impacts on plant growth and development under abiotic stresses9. Under ambient conditions, ethylene confers a beneficial effect on plant health; however, an abrupt increase in ethylene production during biotic and abiotic stresses has negative effects, too, which leads to senescence10,11. Interestingly, some PGPB strains with ACC deaminase activity can lessen the inhibitory effects of ethylene in stress-inflicted plants by cleaving the ethylene ACC into α-ketobutyrate and ammonia10. Under abiotic stress, rhizobacteria with ACC deaminase activity can improve plant growth and development by regulating ethylene synthesis10,12,13. There are many other reports on plant health improvement through inoculating ACC deaminase positive bacterial strains during droughts14,15, flooding stress16, excessive salinity17,18, heavy metal stress19,20 etc. However, some of the investigations did not directly describe the causality of the bacterial ACC deaminase enzyme in stress resistance. There might exist some other bacterial determinants for stress alleviation in affected plants. Plant-hormone-related bacterial traits, such as the regulation of indole acetic acid (IAA) levels, also have a distinct role in eliciting stress tolerance in host plants21. Recently, Ledger et al. reported the role of volatile compounds of ACC deaminase mutant P. phytofirmans PsJN strains in inducing salinity resistance in Arabidopsis thaliana22. Thus, screening ACC deaminase producing strains, as well as different plant-growth-promoting (PGP) traits, to ameliorate abiotic stresses seems to be of greater importance for stressed agricultural systems.
In tropical countries, drought has been identified the main constraint leading to the reductions in crop yields8. During stress conditions, the plant-water relation at the cellular level gets destabilized, thus affecting the whole plant23. However, plants respond to water shortages with several morphological and physiological modifications24. Stress-tolerant PGPB can play a crucial role in these modifications and helps the plants survive. Changes in root architecture (i.e., root system topology and spatial distribution of primary and lateral roots) are the most important adaptive measures in plants during a drought. However, PGPB treatments promote root growth and alter root architecture, leading to an increase in root surface area and improved water and nutrient uptake25. Similarly, PGPB inoculation could maintain shoot growth at near-normal levels, resulting in improvements in crop health and productivity15. Again, there is a direct correlation between increases in plant relative water content and PGPB treatment. Casanovas et al. (2002) reported a positive correlation between bacterialabscisic acid (ABA) production and RWC content in maize plants, which thereby induces stomata closure when inoculated with Azospirillum brasilense BR11005spp26. Cellular osmotic adjustment by increased content of cellular osmotica is another key adaptation in plants during a drought. PGPB treatments lead to increases in plant cellular osmolytes and help plants to withstand stress15,27,28. In severely drought-stressed plants, free radical accumulation leads to the damage of cell membranes and other cellular machinery29. Antioxidant enzymes, like catalase (CAT) and peroxidase (POD), have the ability to eliminate free radicals and prevent cell membranes and DNA content from further damage30. Certain PGPB can raise the levels of reactive oxygen species (ROS) scavenging enzymes in plants. For instance, Kohler et al. reported high antioxidant enzyme activity in lettuce plants (Lactuca sativa L.) inoculated with Pseudomonas mendocina and Glomus intraradices, which contribute to enhancing tolerance against drought28 Figueiredo et al. detected enhanced antioxidant enzymatic activity in common bean plants (Phaseolus vulgaris L.) coinoculated with Rhizobium tropici and Paenibacillus polymyxa under drought stress conditions31. Furthermore, ROS scavenging enzymatic activity was increased in green gram plants (Vigna radiata L.) that were inoculated with Pseudomonas fluorescens, Bacillus subtilis and Pseudomonas aeruginosa15,27. In another study, the Pseudomonas putida strain GAP-P45, which has the ability to produce exopolyschharides (EPS), alleviated drought stress in sunflower (Helianthus annuus L.) seedlings by activating the host plant’s antioxidant enzyme machinery32.
Henceforth, inoculation of bacterial isolates that are able to alleviate drought stress could be preferable in the context of environmentally sustainable agriculture. Considerable progress in this context has been made worldwide. However, very little has been done in Northeast India, despite its rich biodiversity in the Indo-Burma Mega hotspot zone33. Nearly, 80% of agricultural lands in Northeast India are acidic due to Al3+ toxicity34,35. Also, although it is known as high rainfall area, these lands experience severe water scarcity during the winter season34,35. Increases in Al3+ toxicity and subsequent drought stress are resulting in root growth retardation, leading to fewer uptakes of water and nutrients and, thereby, lower crop productivity35. However, the alkaline/acidic environments sustain a diverse microbial community with PGP attributes36. Previously, few acidotolerant bacterial genera have been found in acidic environments and have sorted out their PGP attributes in low pH conditions37,38. Similarly, the acidic soil of north-east India may harbour rich microbial communities that might be useful in agriculture. Previously, our group did substantial work on fluorescent pseudomonads mediated drought stress tolerance in mung beans (Vigna radiata L.)15. A comprehensive work based on physiological and molecular approaches have established Pseudomonas aeruginosa GGRJ21 as a very efficient osmotic stress tolerant strain, having the attributes to enhance drought stress tolerance in host plants. Thus, in a continuation of the previous work, the present investigation focused on screening potent ACC deaminase producers from drought-prone agricultural fields of this region and their effect on drought stress alleviation. Black gram (Vigna mungo L.) and the garden pea (Pisum sativum L.) were used as model plants. The experimental plants were selected based on their wide cultivation in the sampling sites. The seasonal drought in the winter season has a tremendous negative effect on their growth and production39. The rhizosphere bacterial strains were screened for osmotic stress tolerance and ACC deaminase activity for plant growth promotion. Possible inherent mechanisms of drought tolerance were investigated by determining the accumulation of ROS scavenging enzymes (e.g., CAT, POD) and osmolytes (as proline and total phenolics) in the bacterial consortium inoculated plants under water stress conditions. Furthermore, the accumulation of ACC in tested plants and a preliminary investigation on the possible molecular mechanism of bacterial ACC deaminase action on inoculated plants was conducted by examining the expression level of ACC synthase (ACS) and ACC oxidase (ACO), coding mRNA transcripts by real-time qPCR.
Results
Identification, characterization and in vitro plant growth promoting traits of selected bacterial isolates
The selected isolates were identified based on their morphological, biochemical, and molecular characteristics. The cells of RJ12 were gram-negative and non-spore forming. Gram-negative rod-shaped cells of RJ15 did not produce spores. However, the cells of RJ46 were found to be gram-positive, spore-forming and rod-shaped. RJ12 showed positive activity for catalase, oxidase, and methyl red test but showed negativity in starch hydrolysis and nitrate reduction. RJ15 was positive for oxidase, catalase, and nitrate reductase but was negative for starch hydrolysis and methyl red test. RJ46 was found to be positive for catalase, oxidase, nitrate reductase and starch hydrolysis but had negative activity in methyl red. Further, the isolates RJ12, RJ15 and RJ46 were identified as Ochrobactrum pseudogrignonense (KM271984), Pseudomonas sp. (KJ801950) and Bacillus subtilis (KM083797), respectively, based on their 16S rRNA gene sequence homology.
The in vitro multiple PGP traits of the selected osmotic stress resistant strains, viz RJ12, RJ15 and RJ46 were detected in normal and osmotic stress conditions (Table 1). A comparison of all the three strains with the osmotic stress susceptible strain Serratia nematodiphila RJ10 revealed a significant effect of osmotic stress on PGP traits. The quantitative estimation showed a significant reduction (p = 0.05) in PGP traits, except for ACC deaminase, in all the bacterial strains in osmotic stress condition. However, the reduction rate was lower in RJ12, RJ15 and RJ46 than in RJ10. The isolate RJ12 was recorded as the most efficient ACC deaminase producer, even under osmotic stress conditions (114 nmol mg−1 h−1), followed by RJ46 (110 nmol mg−1h−1) and RJ15 (46 nmol mg−1h−1). Also, RJ12 showed superior activity in other PGPR traits during normal and osmotic stress conditions. All the strains could produce IAA (68–85 µg ml−1), phosphatase (37.3–42.6 µg ml−1), siderophore (6.2–11.32 μmol benzoic acid ml−1) and HCN (9.4–14.3 nmol mg cellular protein−1) during osmotic stress conditions. Compatibility assay among the three strains did not show any antagonistic effects against one another (Supplementary Material Figure S1).
Table 1.
Plant growth promoting traits of bacterial strains, Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp RJ15 and Bacillus subtilis RJ46 under normal and osmotic stress condition (−0.73 MPa). An osmotic stress sensitive PGP strain Serratia nematodiphila RJ10 was taken for comparison throughout the experiment.
| Bacteria strain | ACC deaminase (n mol mg−1 h−1) | IAA (µg ml−1)at 100 µg ml−1 tryptophan | Phosphate Solubilisation index (µg ml−1) | Siderophore production μmol benzoic acid ml−1 | HCN production (nmole mg cellular protein−1) | Nitrogen fixation |
|---|---|---|---|---|---|---|
| Bacterial growth under normal condition | ||||||
| Ochrobactrum pseudogrignonense J12 | 125 ± 2.14a | 120.9 ± 1.4a | 85.4 ± 0.93a | 21.76 ± 1.4a | 36.2 ± 1.2a | + |
| Pseudomonas sp. RJ15 | 57 ± 1.06b | 100.7 ± 1.5b | 69.2 ± 0.88b | 14 ± 0.35b | 26.7 ± 0.9b | + |
| Bacillus subtilis RJ46 | 116 ± 1.21c | 75.1 ± 0.86c | 82.1 ± 1.12a | 18.3 ± 0.92a | 30.3 ± 0.9c | + |
| Serratia nematodiphila RJ10 | 132 ± 1.24a | 117.3 ± 1.3a | 86 ± 0.24a | 26 ± 1.1c | 38.4 ± 1.2a | + |
| Bacterial growth under osmotic stress condition (−0.73 MPa) | ||||||
| Ochrobactrum pseudogrignonense RJ12 | 110 ± 1.87a | 85 ± 1a | 42.6 ± 0.73a | 11.32 ± 0.2a | 14.3 ± 0.7a | + |
| Pseudomonas sp. RJ15 | 46 ± 0.87b | 72 ± 1.12b | 37.3 ± 0.54b | 6.2 ± 0.11a | 9.4 ± 0.2a | + |
| Bacillus subtilis RJ46 | 114 ± 1.54a | 68 ± 0.91c | 39.2 ± 0.42b | 9.43 ± 0.4a | 11.5 ± 0.3a | + |
| Serratia nematodiphila RJ10 | 4.1 ± 0.41c | 10.3 ± 0.51d | 3.2 ± 0.01c | 1.4 ± 0.14b | 2.2 ± 0.14b | − |
One-way ANOVA was performed in both the table considering the activity of Serratia nematodiphila strain RJ10 as independent variable, followed by Tukey’s test. Means within a column sharing same lowercase letter are not significantly different at p = 0.05; figures are means ± standard deviation (n = 5). Table key: “+” positive for the test; “−” negative for the test.
Bacterial effect on seed germination and plant growth promotion
Consortium treatment had a significant effect on seed germination and vigor index compared to individual bacterial treatment or a random combination of any of the two bacterial strains in stress condition (Table 2). Seed treatment with consortium resulted in 100% germination in both test plants. A higher vigor index in both black gram (vigor indexcontrol = 1325, vigor indexconsortium = 3100) and garden pea (vigor indexcontrol = 1328, vigor indexcontortium = 2870) plants clearly showed a significant (p = 0.05) increase in root length and shoot length in germinated seedlings treated with bacterial consortium compared to a combination of any two isolates or individual bacterial treatments (Table 2).
Table 2.
Effect of bacterial inoculation on growth attributes of black gram and garden pea. (RJ12 - Ochrobactrum pseudogrignonense, RJ15 - Pseudomonas sp, RJ46 - Bacillus subtilis).
| Treatments | Germination (%) | Root length (cm) | Shoot length (cm) | Vigor index | ||||
|---|---|---|---|---|---|---|---|---|
| Black gram | Garden pea | Black gram | Garden pea | Black gram | Garden pea | Black gram | Garden pea | |
| Un-inoculated seeds | 87 ± 1b | 83 ± 4.7c | 4.1 ± 0.76a | 3 ± 0.34e | 11 ± 1.3b | 13 ± 1.1b | 1325 f | 1328 f |
| RJ12 inoculated seeds | 98 ± 2.3a | 97 ± 4.7a | 7.5 ± 1b | 6 ± 0.82d | 17.1 ± 2.1a | 15.7 ± 1.5a | 2410b | 2104c |
| RJ15 inoculated seeds | 97 ± 4.7a | 90 ± 8b | 5.2 ± 0.87a | 5.8 ± 0.9d | 16.7 ± 1.7a | 16 ± 1.7a | 2215d | 1962e |
| RJ46 inoculated seeds | 90 ± 1.7b | 93 ± 4.7b | 6 ± 0.45b | 7 ± 0.23c | 16 ± 1.8a | 16.2 ± 1.6a | 1980e | 2157c |
| RJ12 + RJ15 inoculated seeds | 96 ± 1.6a | 94 ± 5b | 8.3 ± 1.2b | 7 ± 0.64c | 15.8 ± 2.01a | 15 ± 1.0a | 2313c | 2068d |
| RJ12 + RJ46 inoculated seeds | 98 ± 2.5a | 97 ± 3a | 9.1 ± 1b | 8.5 ± 1b | 16.2 ± 1.3a | 16.4 ± 2.0a | 2479b | 2415b |
| RJ15 + RJ46 inoculated seeds | 94 ± 1.9b | 96 ± 4.3a | 8 ± 1.5b | 7.3 ± 0.56c | 15 ± 2.0a | 15.7 ± 1.45a | 2162d | 2208c |
| Consortium treated seeds | 100 ± 0a | 100 ± 0a | 14 ± 1a | 12 ± 1a | 17 ± 1.5a | 16.7 ± 1.0a | 3100a | 2870a |
Means within a column sharing same lowercase letter are not significantly different according to Student’s t-test at p = 0.05; figures are means ± standard deviation (n = 5).
Pot study under water stress
The negative effect of water stress was noticed in uninoculated water stress (drought) imposed pulse crops with stunted growth, reduced vigor, and less chlorophyll content (Figs 1 and 2, Table 3). However, significant (p = 0.05) plant growth and development was noticed in bacteria-inoculated plants under drought stress (Figs 1 and 2, Table 3, Supplementary Material Table S3). The combined action of the three bacterial strains was found to be more promising than the action of any two combined strains or of each strain used individually (Table 3, Supplementary Material Table S3). The consortium helped the plants to thrive best at the soil moisture content, even at 9.5% (data not shown). At 20% soil moisture content, the consortium treatment increased the root length of black gram and garden pea plants significantly (p = 0.05) by 287% and 269%, respectively when compared to the control plants; however, these values in uninoculated stressed plants were 41% and 39% for black gram and garden pea, respectively. Treatment with individual bacterium showed a similar trend, but one that was not superior to their combined action. Consortium treatment did not show any notable difference in shoot length elongation as compared to treatment with individual isolates or a combination of any two of them under stressed conditions. Compared to control plants, 4.2% (black gram) and 17.3% (garden pea) decreased in dry weight in consortium-treated stressed plants. However; this rate for the uninoculated stressed plants was 63.8% and 61.7%, respectively (Table 3). Similarly, remarkable increases of 85% and 88% in RWC (relative water content) was observed in consortium-inoculated stress-imposed black gram and garden pea plants, respectively, as compared to the uninoculated stressed plants. Root recovery intension is one of the most reliable and sensitive indicators of drought tolerance in plants. Consortium treatment was also found to be effective in root recovery intension. Furthermore, the isolates enhanced plant growth promotion in normal irrigated conditions, too (Table 3, Supplementary Material Table S3).
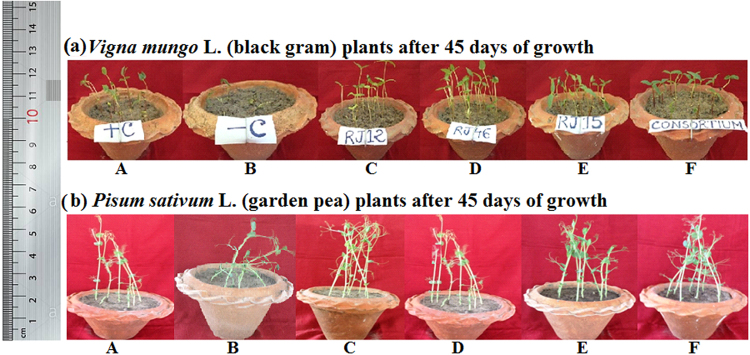
Figure 1.
Effect of bacteria inoculation on plant growth promotion under water stress (drought). Bacterial individual and combined effect (consortium) increases survival rate of (a) black gram and (b) garden pea plants after 45th days of growth stress induction as compared to the negative control (stressed plants without inoculation). A - uninoculated watered plants as positive control, B- uninoculated plants under drought stress as negative control, C – inoculated with Ochrobactrum pseudogrignonense RJ12 under water stress, D – inoculated with Bacillus subtilis RJ46 under water stress, E – inoculated with Pseudomonas sp. RJ15 under drought stress, F – consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp. RJ15) inoculated plants with drought stress.
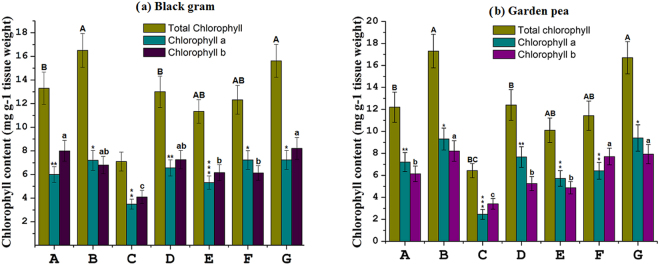
Figure 2.
Quantitative estimation of chlorophyll a, chlorophyll b, and total chlorophyll content of (a) black gram and (b) garden pea leaves growing under different treatment condition. The quantification was carried out after 20 days of stress induction. Treatment conditions, A- positive control, B- consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated with sufficient water supply, C- negative control (stressed plants without inoculation), D-inoculated with Ochrobactrum pseudogrignonense RJ12 under stress, E-inoculated with Pseudomonas sp.RJ15 under stress, F- inoculated with Bacillus subtilis RJ46 under stress, G- consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated under stress. Two-way ANOVA was performed considering water supply and bacteria inoculation as two independent variables followed by Tukey’s post-test for each treatment. Error bars show the standard deviation of the mean values of five replicates. Different symbols on bars indicates a significant difference at p = 0.05 in chlorophyll content under different treatment conditions.
Table 3.
Effect of bacteria inoculation on plant growth promotion in black gram and garden pea plants (RJ12 - Ochrobactrum pseudogrignonense, RJ15 - Pseudomonas sp, RJ46 - Bacillus subtilis. Consortium is the mixture of all the three strains in equal ratio (1:1:1).
| Treatments | Root length (cm) | Shoot length (cm) | Dry weight (g) | Relative water content (%) | Root recovery intension (mg g−1 hr−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BG | GP | BG | GP | BG | GP | BG | GP | BG | GP | |
| Uninoculated plants with sufficient water supply | 3.12 ± 0.3 f | 2.76 ± 0.21 g | 5.43 ± 0.42 h | 7.54 ± 0.26 g | 47 ± 1.9c | 65.3 ± 0.76b | 62 ± 1 f | 73 ± 2.6e | 0.76 ± 0.1b | 0.79 ± 0.21b |
| Uninoculated plants under stress | 4.4 ± 0.33e | 3.86 ± 0.25e | 2.52 ± 0.12i | 4.0 ± 0.5 h | 17 ± 0.23 h | 25 ± 0.43i | 40 ± 0.65 h | 42 ± 0.38i | 0.36 ± 0.02e | 0.34 ± 0.01 f |
| Inoculated with RJ12 and sufficient water supply | 3.87 ± 0.53 f | 3.3 ± 0.13 f | 6.3 ± 1.5 g | 12.5 ± 1.1e | 53 ± 0.54b | 67.43 ± 0.4b | 78 ± 0.54b | 88 ± 0.21b | ND | ND |
| Inoculated with RJ12 under stress | 8.2 ± 0.46b | 7.14 ± 0.13b | 14.3 ± 0.41b | 15.2 ± 0.65c | 43 ± 1.7fd | 48 ± 0.24 f | 68 ± 0.17e | 70 ± 0.64 f | 0.62 ± 0.14c | 0.67 ± 0.27c |
| Inoculated with RJ15 and sufficient water supply | 2.76 ± 0.13 g | 2.96 ± 0.76 g | 7 ± 1 f | 10.7 ± 1.4 f | 49 ± 2c | 52 ± 1e | 68 ± 1.7e | 76 ± 1.6d | ND | ND |
| Inoculated with RJ15 under stress | 6.11 ± 0.38d | 6.51 ± 0.31d | 11.2 ± 0.27d | 12.45 ± 0.34e | 28 ± 0.27 g | 36 ± 0.65 h | 61 ± 0.14 f | 58 ± 0.61 h | 0.51 ± 0.18d | 0.64 ± 0.22d |
| Inoculated with RJ46 and sufficient water supply | 3.43 ± 0.23 f | 2.8 ± 0.15 g | 6.86 ± 0.89 f | 11.1 ± 1 f | 38 ± 0.54e | 57 ± 1.8c | 72 ± 2.1d | 75 ± 2d | ND | ND |
| Inoculated with RJ46 under stress | 7.1 ± 0.43c | 6.82 ± 0.65c | 12.67 ± 0.31c | 14.3 ± 0.52d | 32 ± 0.26 f | 41 ± 0.27 g | 58 ± 0.32 g | 66 ± 0.46 g | 0.52 ± 0.16d | 0.65 ± 0.13d |
| Consortium inoculated with sufficient water supply | 4.15 ± 0.48e | 3.45 ± 0.5 f | 16.2 ± 0.28a | 17.35 ± 0.43a | 62.65 ± 0.65a | 87.43 ± 0.32a | 87 ± 0.32a | 91 ± 0.43a | 0.78 ± 0.13a | 0.81 ± 0.15a |
| Inoculated with Consortium under stress | 12.1 ± 0.86a | 10.2 ± 0.32a | 10.73 ± 0.36e | 16.71 ± 0.54b | 45 ± 0.23d | 54 ± 0.32d | 74 ± 0.25c | 79 ± 0.26c | 0.63 ± 0.21c | 0.56 ± 3.16e |
Two-way ANOVA was performed considering water supply and bacteria inoculation as two independent variables, followed by Tukey’s post-test for each treatment. For each figure in a column, values represented by the same lowercase letters are not significantly different at p = 0.05.; figures are means ± standard deviation (n = 5). Table key: BG - black gram, GP - garden pea, ND – not determined.
Leaf chlorophyll content was determined to examine the impact of the rhizobacterial strains as well as their consortium on the photosynthetic efficiency of host plants (Fig. 2). Total leaf chlorophyll content did not bear any significant difference in consortium-treated stressed plants in comparison to uninoculated watered plants (positive control). However, the contents of leaf chlorophylls a, b, and a + b in consortium-treated stressed plants increased by 106%, 100%, 120% (in black gram plants) and 283%, 132%, and 159% (in garden pea plants) in comparison with the uninoculated stressed plants (negative control). This indicates the efficiency of PGPB strains on the maintenance of chlorophyll content under drought conditions.
Effects on antioxidant enzymes and osmolyte accumulation in plants
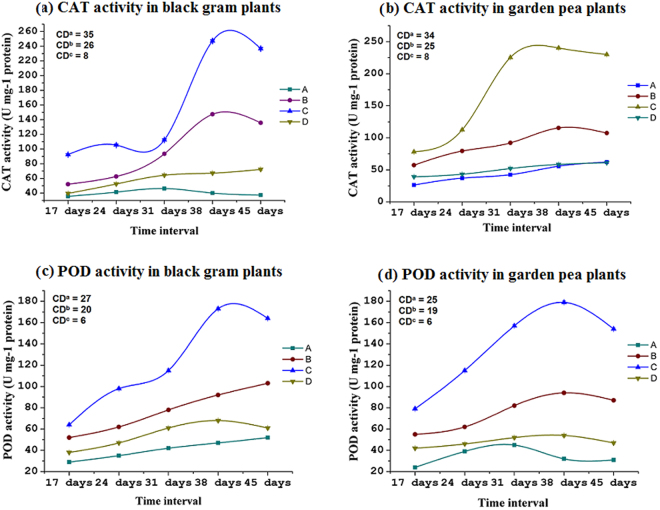
The activation of a plant’s inherent enzymatic and non-enzymatic systems is always crucial for the detoxification of the ROS under stress conditions. The results of this study clearly indicated that consortium significantly (p = 0.05) stimulates the CAT activity in both black gram and garden pea plants (Fig. 3a,b). CAT is a major enzyme for hydrogen peroxide detoxification in stressed plants. In consortium-treated stressed plants, CAT activity was increased rapidly from the seventeenth day to the thirty-eighth day, after which it declined gradually. Moreover, an increasing trend was also noticed in stress-induced plants without inoculation, but this trend was not as prominent as it was in the consortium-inoculated plants. The control plants and consortium-treated normally-watered plants did not show any significant changes in CAT activity during the experiments. POD was located in every ROS-producing compartment and functions as a fine regulator of intracellular ROS level. As with CAT, a similar increasing trend was also observed in POD activity in consortium-inoculated stress-induced plants as a function of time (Fig. 3c,d). POD activity was gradually increased in the negative control and consortium-treated drought-stressed plants. Furthermore, enzyme activity increased significantly (p = 0.05) in consortium-treated plants compared to plants under negative control.
Figure 3.
Activity of catalase and peroxidase in stress experienced black gram and garden pea plants in different time interval. Ten samples were analyzed for each replication, and each treatment consisted of five replications. (a) catalase activity in black gram, (b) catalase activity in garden pea, (c) peroxidase activity in black gram, d) peroxidase activity in garden pea. Treatments: A- uninoculated watered plants as positive control, B – consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated plants with drought stress induction, C- uninoculated plants under drought stress as negative control, D – consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated with sufficient water supply. Critical difference (CD) was computed at p = 0.05. CDa, CDb, and CDc represent critical differences for treatments, time intervals, and treatment × time intervals, respectively.
Proline and phenolics are other biomarkers of plants under stress. The increases in proline and phenolic accumulation are very much essential for maintaining the osmotic potential of plant tissues and, thereby, protect plants from over dehydration during droughts. Bacterial inoculation had a direct effect on plant osmolytes (Table 4). Proline content was increased significantly (p = 0.05) in consortium-inoculated plants when compared to both positive and negative control plants. However, in consortium-inoculated regularly-watered plants, a marked difference was not observed in comparison to control plants. The leaf phenolics content of bacteria-treated stress-exposed plants was measured from the thirty-eighth day of treatment. Bacterial-combined action in the form of consortium increased the phenolics content by 196% and 216% in black gram and garden pea plants, respectively when compared to the positive control plants. However, these values for uninoculated stress-imposed plants were 97% and 68% in black gram and garden pea, respectively.
Table 4.
Activity of proline and phenolics in black gram and garden pea plants under water stress condition. Proline activity was measured at different interval of time, i.e. 17th to 45th days of sowing with seven days of interval. Phenolics activity measured on the 38th day of sowing. Consortium is the mixture of all the three strains in equal ratio (1:1:1).
| Proline activity (µmoles g−1 fresh weight) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Time Interval | |||||||||||
| 17th day | 24th day | 31st day | 38th day | 45th day | ||||||||
| BG | GP | BG | GP | BG | GP | BG | GP | BG | GP | |||
| Uninoculated plants with sufficient water supply | 2.86 ± 0.34c | 1.96 ± 0.05c | 3.32 ± 0.72c | 2.85 ± 0.21c | 3.57 ± 0.23c | 2.97 ± 0.61c | 3.69 ± 0.45c | 3.12 ± 0.3b | 3.74 ± 0.21c | 3.31 ± 0.41c | ||
| Uninoculated plants under stress | 3.96 ± 0.14b | 3.20 ± 4.05b | 4.30 ± 1.77b | 5.34 ± 0.43a | 5.87 ± 0.77b | 6.21 ± 0.35b | 7.32 ± 0.32b | 7.80 ± 0.34a | 9.76 ± 0.42b | 7.91 ± 0.32b | ||
| Consortium inoculated with sufficient water supply | 3.65 ± 0.25b | 3.12 ± 0.18b | 3.98 ± 0.22b | 3.45 ± 0.3b | 4.12 ± 0.28c | 3.56 ± 0.11c | 4.19 ± 0.23c | 3.76 ± 0.12b | 4.31 ± 0.33c | 3.89 ± 0.6c | ||
| Inoculated with Consortium under stress | 4.71 ± 0.26a | 4.26 ± 0.54a | 5.82 ± 0.81a | 5.37 ± 0.31a | 8.25 ± 0.12a | 7.62 ± 0.18a | 9.78 ± 0.54a | 8.43 ± 0.42a | 12.71 ± 0.34a | 10.67 ± 0.55a | ||
| Total phenolic (mg g −1 fresh weight) | ||||||||||||
| Treatments | BG | GP | ||||||||||
| Uninoculated plants with sufficient water supply | 7.2 ± 1.14c | 4.87 ± 0.21c | ||||||||||
| Uninoculated plants under stress | 14.24 ± 2.21b | 8.21 ± 0.15b | ||||||||||
| Consortium inoculated with sufficient water supply | 8.32 ± 1.1c | 5.65 ± 0.14c | ||||||||||
| Inoculated with Consortium under stress | 21.34 ± 2.12a | 15.43 ± 0.52a | ||||||||||
Two-way ANOVA was performed considering water supply and bacteria inoculation as two independent variables, followed by Tukeys post-test for each treatment. For each figure in a column, values represented by the same lowercase letters are not significantly different at p = 0.05; figures are means ± standard deviation (n = 5). Table key: BG - black gram, GP - garden pea.
ACC accumulation in plants
Changes in ACC levels in positive control plants, consortium-inoculated stress-induced plants, uninoculated stress-induced plants, and consortium-inoculated regularly-watered plants were further estimated to confirm the positive interaction of ACC deaminase producers with the experimental black gram and garden pea plants. There was significant (p = 0.05) reduction in ACC accumulation in consortium-treated stress-induced plants as compared to the uninoculated stress-induced plants (Fig. 4).
Figure 4.
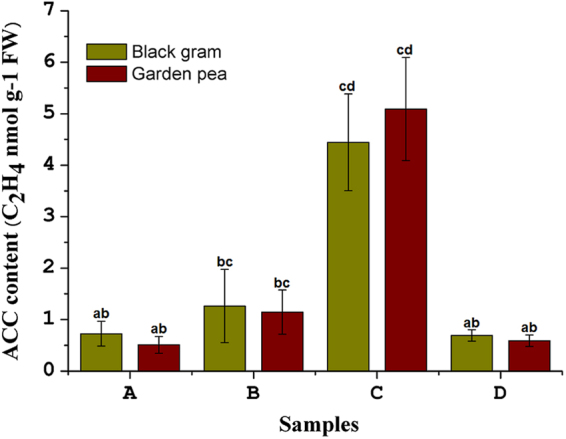
1-aminocyclopropane-1-carboxylic acid (ACC) content in the root tips of black gram and garden pea plants growing under different treatment condition. The quantification was carried out on 45th day of stress induction. Treatments: A- uninoculated watered plants as positive control, B-consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated plants with drought stress induction, C- uninoculated plants under drought stress as negative control, D – consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated with sufficient water supply. Two- way ANOVA was performed considering water supply and bacteria inoculation as two independent variables followed by Tukey’s post-test for each treatment. Different symbols on bars indicates a significant difference at p = 0.05 in ACC accumulation under different treatment condition.
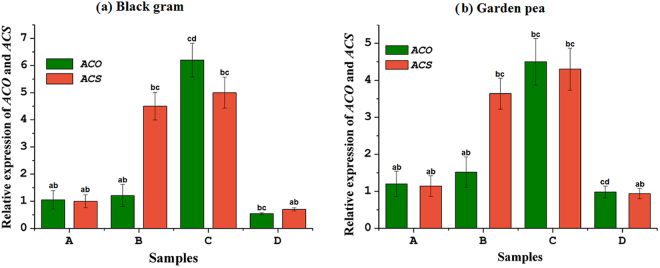
Relative quantification of ethylene synthesis regulatory genes
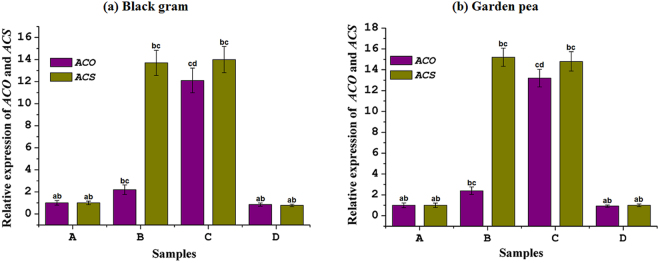
Among the four reference genes, ACT11, Ubq, β-Tub9, and 18S rRNA, ACT11 was verified to have the lowest average expression stability (M) when samples experiencing osmotic stress were analyzed (data not shown). Therefore, ACT11 was selected as the housekeeping gene for overall expression analysis. The relative expression level of ACS and ACO genes in consortium-treated black gram and garden pea plants were studied over four experimental conditions: plants with a normal water supply, consortium-inoculated plants with normal water supply, consortium-inoculated plants with induced drought stress, and uninoculated plants under drought stress. The plants with a normal water supply were considered a calibrator for the experiment. Similar to the negative control plants, the transcript copy number of ACS was significantly increased in leaf (Fig. 5a,b) and root (Fig. 6a,b) tissues of consortium-treated plants, indicating no significant role of consortium action on the expression of the ACS gene transcript. The expression of ACS was 8 to 9 times greater in root tissues than in leaf tissues. However, consortium treatment regulated 3 to 5 and 10 times greater down-regulation of ACO in leaf and root tissues, respectively, as compared to the uninoculated stress-induced plants (Figs 5a,b and 6a,b).
Figure 5.
Relative gene expression level of ACO and ACS in leaf tissue of (a) black gram and (b) garden pea plants in different treatment conditions. Treatments: A- uninoculated watered plants as positive control, B-consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated plants with drought stress induction, C- uninoculated plants under drought stress as negative control, D-consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated with sufficient water supply. Two-way ANOVA was performed considering water supply and bacteria inoculation as two independent variables followed by Tukey’s post-test for each treatment. Different symbols on bars indicates a significant difference at p = 0.05 in gene expression under different treatment condition.
Figure 6.
Relative gene expression level of ACO and ACS in root tissue of (a) black gram and (b) garden pea plants in different treatment conditions. A- uninoculated watered plants as positive control, B-consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated plants with drought stress induction, C- uninoculated plants under drought stress as negative control, D – consortium (Ochrobactrum pseudogrignonense RJ12 + Bacillus subtilis RJ46 + Pseudomonas sp.RJ15) inoculated with sufficient water supply. Two-way ANOVA was performed considering water supply and bacteria inoculation as two independent variables followed by Tukey’s post-test for each treatment. Different symbols on bars indicates a significant difference at p = 0.05 in gene expression under different treatment condition.
Toxicity test
An acute oral toxicity/pathogenicity test was carried out by APT Testing and Research Private Limited (Pune, India) for all three bacterial strains, which were found to be non-toxic according to the EPA 712-C-96–322, OPPTS 8853550 Guidelines (adopted February 1996).
Discussion
In recent days, water shortage is one of the main challenges faced by worldwide agricultural practices. This issue has been limiting crop yields of arable land40. Thus, achieving better crop health and production under water shortage conditions is the biggest challenge for sustainable global agriculture. Again, there is an indirect relationship between soil acidity and reduction in crop yields during drought stress. In general, soil acidity is toxic to plant roots and leads to poor and abnormal root development35. This, in turn, leads to reduced water and nutrient uptake. Even a restriction of root penetration into lower soil layers may occur due to increases in acidity of subsoils41. Thus, high soil acidity magnifies the negative effects of drought during average rainfall levels, too. In this study, the aim was to identify efficient indigenous osmotic stress tolerant bacterial strains from drought affected acidic agricultural soils of Northeast India for their ability to confer drought resistance in crop plants. Three osmotic stress-tolerant ACC deaminase-producing bacterial strains, (i.e. Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15, and B. subtilis RJ46) were screened, and their PGP activities were evaluated in black gram and garden pea plants under water deficit conditions. Huang et al. reported that the direct interactions between different microbial members often result in the promotion of key PGP processes and of plant growth and development in a typical rhizosphere ecosystem42. Again, the syntrophic relationship between different organisms is quite common in microbial ecosystems43. Thus, the use of mixed microbial inoculants (consortium) acting synergistically is more beneficial for better yields and quick/improved results. Moreover, higher concentrations of bacterial cells in a consortium have the potential to supply more nutrients to host plants, thus exerting greater PGP effects44. The bacterial strains selected for the present study were compatible with each other. The results of in-vivo experiments revealed tremendous improvements in plant growth promotion of test plants under both normal and water deficit conditions when the three strains were applied in consortium compared to individual treatments or mixtures of any two strains.
To validate the effect of ACC deaminase on stress-induced experimental plants, we measured the level of ACC accumulation, as well as mRNA expressions of ACS (responsible for ACC production) and ACO (responsible for stress ethylene production) gene transcripts in black gram and garden pea plants. Consortium inoculation significantly reduced ACC accumulation in stress-induced black gram and garden pea plant roots when compared to the negative control plants (uninoculated plants under stress). The active ACC deaminase enzyme of bacterial origin might have played a significant role in the reduction of ACC levels in roots, as reported in previous research45. Moreover, an up-regulation of ACS was recorded in the tested plants in the stress condition. The higher expression of ACS may trigger ACC accumulation in plant tissues during the initial phase of stress induction. However, root-or-seed-surface-anchored PGPB with ACC deaminase activity may act as a sink for ACC that lowers their levels in the inoculated plants, as mentioned in previous reports45,46. Furthermore, the down-regulation of ACO in leaf and root tissues of consortium-inoculated stress-induced plants and the up-regulation of ACO mRNA transcripts in negative control plants had strengthened the correlation between bacteria inoculation and reduction in deleterious stress ethylene accumulation in drought-stress-experienced plants. The consortium inoculation might have some effect on ACO down-regulation and, thereby, the prevention of deleterious ethylene accumulation in stressed plants. The results are corroboratory with previous findings. Camilios-Neto et al. reported the impact of bacterial colonization in ACO transcript down-regulation, which leads to a lowering of ethylene accumulation in wheat seedlings during nutrient limitation47. Similarly, an excessive down-regulation of ACO was noticed in stress-imposed pepper plants (Capsicum annuum L.) upon inoculation with PGPB (Bacillus sp. and Arthrobacter sp.) strains48. The lower expression pattern of ACO in consortium-treated stressed plants might be due to the substrate-based competition between ACC deaminase and ACO for binding with ACC46. The reduction in ACC levels would lead to a reduced ACO expression and subsequent declines in stress ethylene production. Hence, the bacterial consortium is capable of regulating stress ethylene levels, thereby conferring drought stress tolerance in the tested host plants.
Microorganisms with multi-faceted mechanisms of action are beneficial for plant growth promotion during abiotic stress condition49. Therefore, different inherent PGP attributes were also screened and quantified in the selected ACC deaminase positive bacterial strains. The bacterial strains were potent for many PGP traits, such as IAA production, siderophore production, HCN production, phosphate solubilisation, and nitrogen fixation even under high osmotic stress conditions. According to Glick et al. (2007), bacterial PGP traits have a positive influence on plant growth and development by increasing nutrient availability during stressful conditions50. It is well-known that IAA stimulates the transcription of the plant enzyme ACC synthase51, which catalyzes the formation of ACC. In this case, IAA induces ethylene production in the plant. However, increases in ethylene levels have feedback inhibitory effects on IAA signal transduction, which thereby limits the activity of ACC synthase51. Thus, the association of IAA-producing bacteria in plants will ultimately trigger the production of relatively high concentrations of ACC and, subsequently, the feedback inhibition of IAA synthesis. However, PGPB with both IAA- and ACC-deaminase-producing activity will control the excess ethylene production level and thereby lessen the ethylene feedback inhibition of IAA biosynthesis. This is because a large portion of the additional ACC produced due to a cumulative effect of plant and bacterial IAA is cleaved by bacterial ACC deaminase. Therefore, the overall result of this cross-talk well defines the role of IAA to enhance plant growth promotion under stressful conditions in the presence of ACC deaminase activity. Previous studies showed the effect of bacterial IAA on root length elongation under a drought stress regime52,53. Recently, Sorty et al. have reported the influence of bacterial IAA in seed germination and seedling growth in wheat under saline stress54. Similar predispositions of bacterial IAA production in seed germination and root length elongation were noticed in our experimental plants. Further, the phosphate-solubilizing bacterial isolates of drought agrosystems are likely to be more useful for plant health improvement under water deficit conditions. Again, the three osmotic stress tolerant strains were not only restricted to the inorganic phosphate bound to calcium ions (i.e. Ca3(PO)4) but can also act upon aluminium phosphates, which seem to be more effective in acidic soil of Assam15. Again, plants are more vulnerable to pathogens during abiotic stress. Moreover, a solution to provide cross-protection against phytopathogens during abiotic stress is always appreciable. The members of the consortium were also efficient producers of bacterial siderophores. The siderophores play a significant role in the biological availability of iron to plant roots55. Low molecular mass siderophores can bind to most of the iron available in the rhizosphere with very high avidity, which inhibits the proliferation of fungal pathogens in host plant roots due to lack of available iron56,57. Earlier report has demonstrated the activity of siderophores overproducing mutants in protecting the plants against heavy metal stress58. Further, the bacterial strains were efficient producers of HCN. HCN-producing bacteria are antagonistically active against different microorganisms. The HCN may provide protection against pathogenic entry in plants during stressful conditions. Similarly, the bacterial HCN can induce systemic resistance in plants by acting as extracellular signals, subsequently triggering a series of internal processes. Ultimately, the signal translocated is perceived by plant cells which activate cellular defense mechanisms. Hence, the consortium has additional advantages for protecting plants against phytopathogen attacks during abiotic stress. Thus, apart from ACC deaminase activity, multiple PGP traits of the selected strains also may have a cumulative effect in plant growth promotion under drought stressed environment.
Exposure to ACC deaminase positive bacteria has successfully benefitted the host plants by various biochemical and physiological modifications as well. Firstly, successful plant seedling establishment is one of the major concerns for the survival of crop plants during stress conditions. Our tested bacterial consortium showed 100% germination of the tested plant seeds, demonstrating their high performance in plant growth and development during the presence of environmental stressors. Additionally, bacterial priming enhanced the ROS scavenging enzyme activity in stress-induced plants in comparison to the uninoculated stressed plants, showing consistent coalition with previous findings27,28,59,60. In principle, the increase of ROS scavengers in host plants is recognized as an important parameter for drought stress alleviation by the microorganism. The level of CAT and POD increased significantly (p = 0.05) in consortium-treated stress-affected plants in comparison to the negative controls. The increase in activity of ROS scavenging enzymes (i.e., CAT and POD) provided the protective mechanism in stress-exposed experimental plants by detoxifying the reactive hydrogen peroxide (H2O2), hydrogen radical (•OH) and singlet oxygen (1O2), as stated by Kohler et al.28. One of the major early responses to water stress in plants is a decrease in photosynthetic efficiency. In general, a significant reduction of photosynthesis is observed in plants during drought stress, which thereby decreases energy production and metabolite accumulation. Inoculation of garden pea and black gram plants with ACC deaminase positive consortium partially eliminated the deleterious water stress effects on growth by maintaining the chlorophyll content, which was also noted in previous reports61,62. This revealed the higher photosynthetic activity of the consortium-treated plants compared to the negative controls. Furthermore, bacterial inoculation showed a direct effect on proline and phenolic compounds accumulation. Plant cellular osmolytes, like proline and phenolics, are important determinants of plant response to environmental stresses63. There is a correlation between increases in osmolyte accumulation and decreases in cellular osmotic potential. The decrease in cellular osmotic potential helps maintain adequate water absorption from drying soil, which thereby increases cell turgor pressure, improving the physiological activity of plants, even during prolonged water deficit64–66. Later on, the osmolytes can also act as molecular chaperones, stabilizing the cellular structure of proteins, and they can defend host cell walls by strengthening the exodermis and several cortical cell layers67. Our experimental results showed a direct correlation between bacterial inoculation and osmolyte accumulation in treated plants, which could also be an important bacterial determinant for drought stress alleviation in the tested host plants.
Conclusion
The isolation and characterization of stress-tolerant rhizobacteria are not only essential for understanding their ecological role in the rhizosphere but also their utilization in eco-friendly and sustainable agro-technologies. The overall study has established the combined action of O. pseudogrignonense RJ12, Pseudomonas sp. RJ15, and B. subtilis RJ46 towards the alleviation of water stress in black gram and garden pea plants. The inoculation of black gram and garden pea plants with consortium resulted in higher seed germination rates, enhanced root length elongation, increased synthesis of total leaf chlorophyll, and accelerated production of antioxidant enzymes and cellular osmolytes. Also, down-regulation of the ACO gene transcript was observed upon consortium inoculation in the stressed plants. Besides these mechanisms, the inherent PGP traits of individual bacteria may provide an indirect mechanism for water stress alleviation in the tested plants by providing sufficient phosphate, iron, available nitrogen, and cross-protection against pathogen entry. Thus, the use of such microbial consortium/consortia, which can induce drought stress tolerance and also enhance plant growth and development during normal condition, might be very much beneficial for sustainable agriculture68. The integrative application of such consortium having the characteristics of a potential biotic and abiotic stress suppressor might appear to be a very effective strategy for drought stress alleviation in other crops as well. Moreover, the bacterial strain and their consortium formulation require further field evaluation and validation before being confirmed as bio-inoculants to combat various abiotic stresses in the acidic soil based agro-ecosystems of Northeast India.
Methods
Soil sampling, isolation of ACC deaminase producing rhizobacteria and screening for osmotic stress tolerance
Rhizosphere soil samples were collected from drought-affected and normally-irrigated agricultural plots of Jorhat district, Assam (26° 45’ 0” North and 94° 13’ 0” East of Northeast India with an average altitude of 116 ma. s. l. and warm-to-temperate climatic conditions). The monsoon months (June to October) receive heavy rainfall, with an average of 412 mm; however, rainfall is scanty during winter (November to February), with an average of 15 mm (Metallurgical Department, Govt. Assam, India). The soils of the selected agricultural field were vertisol type69 and clay loam in texture with pH 3 to 5.5. The samplings were carried out during the month of November 2013–2014 and 2014–2015. A total of Fifty-two soil samples were collected aseptically from roots of five different vegetable crops (Brassica juncea L., Phaseolus vulgaris L., Pisum sativum L., Brassica oleracea L. and Vigna mungo L.). For each crop, ten to twelve plants were randomly selected for rhizosphere soil sampling. The root-associated soil samples were collected during plant growing season as a 15 cm2 by 30 cm depth lump. After collection, the roots were shaken vigorously by hand for 10 minutes to remove the loosely-adhering soil particles. The soil particles that were tightly adhered to the roots were then scraped with a brush and tweezers, transferred into separate sterile Hi-dispo Bags (HiMedia, Mumbai, India) and immediately transported to the laboratory by an air-conditioned sampling van at room temperature. Soil suspensions in phosphate saline were spread on a Dworkin and Foster (DF) minimal salt medium with 3 mM ACC as the sole source of nitrogen for selective isolation of ACC-deaminase-producing-rhizobacteria70. The pH of the medium was adjusted at the range of 3 to 5.5. Colony PCR with the degenerate primers DegACC5′ (5′-GGBGGVAAYAARMYVMGSAAGCTYGA) and DegACC3′ (5′-TTDCCHKYRTANACBGGRTC) was carried out to amplify partial acdS gene for better detection of ACC deaminase positive strain, as mentioned earlier71. Bacterial osmotic stress tolerance was checked by monitoring their growth curve under different water potentials (−0.05, −0.15, −0.30, −0.49, and −0.73 MPa). The osmotic stress condition was developed in the Nutrient broth (NB) growth medium by adding the required amount of polyethylene glycol (PEG 6000) as described by Michel and Kaufmann 197372. One millilitre of overnight grown bacterial culture (1 × 108 CFU ml−1) was inoculated to the PEG supplemented NB and incubated at 30 ± 2 °C for 24 hours with a continuous agitation of 120 rpm. Bacterial growth was monitored colourimetrically by measuring the absorption spectra at 600 nm as a function of time using Specord 200 (Analytik Zena, Germany). Out of 70 ACC deaminase producers, only three isolates – i.e., RJ12, RJ15 (inhabitants of Vigna mungo L. rhizosphere), and RJ46 (inhabitant of Pisum sativum L. Rhizosphere) showed vigorous growth at high osmotic stress conditions (−0.30, −0.49, and −0.73 MPa) (Supplementary Material Table S1). Therefore, these three isolates were further considered in the rest of the study. Furthermore, the growth of the isolates was checked in a varied acidic pH range (3 to 5.5) and observed vigorous growth in the pH ranges with the maximum at 4.5 (Supplementary Material Table S2).
Phenotypic and biochemical characterization of bacterial isolates
The morphology of the three isolates was examined using gram staining and light microscopy. Further biochemical characterization was carried out according to Bergey’s Manual of Determinative Bacteriology73.
Identification of bacterial isolates
The three selected osmotic stress-resistant bacteria strains were identified up to the genus/species level by 16S rRNA signature sequencing. Purified bacterial genomic DNA was taken as the template to amplify 16S rRNA signature sequence with bacterial universal primer 27 F and 1492 R. The amplicons (approx. 1450 bp) were purified using GeNeiPure™ Quick PCR purification kit and sequence were determined by fluorescent terminators (Big Dye, Applied Biosystems) run in an Applied Biosystems ABI prism-automated DNA sequencer (3130 × l). The partial 16S rRNA sequences were compared with NCBI GenBank database using the online software BLASTN. The trimmed 16S rRNA sequences were submitted further in NCBI gene bank and sequence IDs retrieved.
Quantification of ACC deaminase and other PGP traits
The screening and quantification of in vitro PGP traits of the selected bacterial strains were performed in bacterial strains under both normal and osmotic stress conditions. The required amount of PEG was added to develop osmotic stress (−0.73 MPa) in the growth mediums for quantification of the PGP traits under stress. Moreover, the same standard protocols were used for the quantification of PGP traits of the bacterial strains growing under normal and osmotic stress conditions. The quantitative estimation of ACC deaminase was carried out as mentioned by Honma and Shimomura74. The three bacterial isolates were grown in a DF minimal broth (pH 4.5) supplemented with 10 µg of ACC (Sigma-Aldrich). After 48 hours of incubation, colourimetric estimations for enzyme activity were carried out and expressed in micromoles of α-ketobutyrate produced per milligram of cellular protein per hour (µmol mg−1 h−1). The production of an IAA-like molecule was carried out as described by Gordon and Weber75. The bacterial strains were inoculated in a DF salts minimal medium with L-tryptophan of different concentrations (0, 50, 100, 200 and 500 µg ml−1). The 48-hours-old bacterial cultures were harvested by centrifugation (4000 × g for 20 minutes at 4 °C). A preliminary screening of indole production was performed by mixing the supernatant with Salkowski’s reagent (50 ml, 35% perchloric acid and 1 ml 0.5 M FeCl3) in a ratio of 1:4 (supernatant: reagent) at room temperature (28 °C) for 20 minutes. The development of a pink colour indicated the production of indoles. Indole production was quantified by spectrophotometric absorption (Specord 200, Analytik Jena, Germany) at 535 nm with three replications. A standard curve was prepared by using pure IAA (Sigma Aldrich, USA). The phosphate solubilisation efficiency was monitored by aluminium-phosphate-supplemented modified Pikovskaya agar, as tricalcium phosphate (TCP) has been reported to be an unreliable and relatively weak factor in determining the solubilization of inorganic phosphate in the acidic soil of Assam15,76. Further, the quantitative estimation of phosphatase was carried out77. Nitrogen fixation, HCN production, and siderophore production were monitored by previous standard protocols78–80. Moreover, the compatibility of the three rhizobacterial strains with each other was tested by dual culture plate assay on nutrient agar and agar well diffusion method81.
Bacterial inoculum preparation
The cells of overnight-grown bacteria (1 × 108 CFU ml−1) were harvested by centrifugation (4500 rpm for 20 minutes). The harvested cells were washed twice with 60 mM phosphate saline buffer and resuspended thereof. An optical density of 0.5 at 535 nm was achieved to maintain the uniform cell density of 1 × 108 CFU ml−1. For consortium (either any two bacteria or mixed suspension of all three), the cell suspensions were mixed at 1:1 or 1:1:1 ratio.
Effect of selected isolates on plant growth promotion
The PGP efficiency of the bacterial strains was performed using standard roll towel method82. The individual effect of the selected isolates (RJ12, RJ15, and RJ46), a mixture of any two isolates (RJ12 + RJ15, RJ12 + RJ46, RJ15 + RJ46), and the combination of all isolates (consortium) on seed germination and seedling vigor were determined. The black gram (var PU 40) and garden pea (var Goldie) seeds (50 of each) were surface-sterilized with 70% alcohol and 1% sodium hypochlorite and inoculated with 10 ml bacterial inoculum (1 × 108 CFU ml−1) containing 0.1% of carboxymethyl cellulose (CMC) as an adhesive agent. After incubation at room temperature for 2–3 hours, the seeds were dried with sterile blotting paper. Surface sterilized uninoculated seeds were considered as the control group. Both inoculated and control seeds were seeded in Hoagland solutions with polyethylene glycol (PEG-6000) and incubated at 25 ± 2 °C in a plant growth chamber (Fitotron, Weiss Technik, UK). The temperature was maintained at 35 °C and 25 °C (day and night) with a relative humidity of 60%. The PEG-6000 was added to develop artificial stress in the Hoagland solution. After 7 days of incubation, seed germination percentage, root length, shoots length, and vigor indexes (VI) were calculated. The VIs was calculated by the formula, VI = % of seed germination × (root length + shoot length)83. The whole experiment was repeated five times and carried out with five replications individually for each treatment.
Growth promotion under osmotic stress (drought stress) condition
The surface sterilized black gram and garden pea plants were treated with bacterial inoculums as mentioned in the earlier section. Averages of 10 seeds/pot were sown in earthen pots containing a sterile soil mixture (clay loam/sand/cow dung at 1:1:1 w/w/w ratio). Seedlings were grown in a greenhouse with 28/20 °C day/night temperatures and ~70% relative humidity under conditions of a 16/8 hours light/dark cycle (approx.). After 10 days of seedling growth, plants were divided into the following categories with five replications of each, viz. (1) uninoculated watered plants as positive control; (2) individual bacteria-inoculated plants under water stress; (3) individual bacteria-inoculated under normal watered condition; (4) combined inoculation of RJ12 and RJ15 under water stress and normal watered conditions, RJ12 + RJ15; (5) combined inoculation of RJ12 and RJ46 under water stress and normal watered conditions, RJ12 + RJ46; (6) combined inoculation of RJ15 and RJ46 under water stress and normal watered condition, RJ15 + RJ46; (7) combined inoculation of all three isolates (RJ12 + RJ15 + RJ46) under water stress; (8) combined inoculation of all three isolates (RJ12 + RJ15 + RJ46) with a normal water supply, and (9) uninoculated plants under drought stress as negative control. The osmotic stress in the pots was artificially induced by irrigating the pot with a PEG-6000 nutrient solution. The concentration of PEG-6000 (g/L of water) was determined using the equation of Michel and Kaufmann72.
The osmotic potential of the stress-induced pots was gradually decreased at a rate of -0.04 MPa/day. On the twenty-fifth day of sowing (15 days after the stress induction), the osmotic pressure reached 0.51 MPa. The soil moisture content on the twenty-fifth day was 20% in the negative control and bacteria-inoculated stress-induced plants, and the same condition was maintained up to the forty-fifth day of plant growth. The soil moisture content was determined using 5TE soil moisture sensors (Decagon Devices, Inc., Pullman, WA, USA). The pH of the sterile soil mixture was maintained at 4.5 by watering the plants with leftover (cold) coffee, diluted 50–50 with water. The pH of the soil mixture was measured by a pre-calibrated pH electrode (Mettler Toledo, USA) in 1:5 suspensions of soil and water84.
Morphological and physiological characterization of the experimental plants
The plants were harvested randomly on 45th day (5 plantlets/replicate/treatment, i.e., a total of 25 plantlets per treatment). The harvested plants were further studied for any changes in morphological parameters, such as shoot length, root length, and dry weight. The root water content (RWC) of leaves was determined from the 25 randomly collected plantlets of each treatment using the formula RWC (%) = (Fresh weight − Dry weight/completely turgid weight − Dry weight) × 10085. Total chlorophyll content was estimated from two grams of randomly collected leaf samples of each treatment via the standard protocol86. Root vigor (expressed in terms of root recovery intension), was also measured according to the triphenyltetrazolium chloride (TTC) method87.
Biochemical characterization
Biochemical characterizations of the experimental plants (bacteria treated, positive control, and negative control) were started on the 17th day after sowing with 7-day intervals up to the 45th day of plant growth. The leaf samples were collected randomly from twenty plantlets of each treatment for further biochemical characterization.
Two grams of fresh leaves were homogenized in 2 ml of a 50 mM ice-cold phosphate buffer (pH 6.0) with pre-chilled mortar and pestle. The homogenate was centrifuged at 15000 × g for 15 minutes at 4 °C. The supernatant was used for enzyme assays. The protein concentration was determined according to the Bradford method using bovine serum albumin (BSA) as standard88. The reaction mixture for the POD assay contained a potassium phosphate buffer (160 μl, 100 mM, pH 6.0), an H2O2 solution (80 μl, 0.5% w/w) and a pyrogallol solution (160 μl, 5% w/v) making the final volume 1.5 ml. Fifty microlitre enzyme extracts were added to the assay solution. The reaction was monitored at 420 nm after 3 minutes of reaction, and the activity was expressed in terms of U mg−1 protein with five replicates89. CAT activity was measured according to Beer and Sizer (1952), with minor modifications90. The reaction mixture consisted of a 100 mmol l−1 phosphate buffer (pH 7.0), 0.1 mmol l−1 EDTA, 20 mmol l−1 H2O2, and 20 μl enzyme extract. The reaction was started by the addition of 20 µl enzyme extract. After 3 minutes of enzymatic reaction, the decrease of H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient (36 M−1cm−1), and the results were expressed as units mg−1 protein (U = 1 mM of H2O2 reduction min−1 mg−1 protein) with 5 replicates. The total phenolics and proline content were measured with standard protocols and expressed in mg g−1, fresh weight, and µmoles g−1 fresh weight, respectively, with 5 replicates91,92.
Extraction and measurement of ACC
The ACC contents in the roots of 25 randomly collected plantlets on the forty-fifth day of plant growth were extracted and analyzed from different treatment conditions (uninoculated watered plants as a positive control, consortium-inoculated plants with drought stress induction, uninoculated plants under drought stress as a negative control, and consortium-inoculated with sufficient water supply)93. Root apexes were crushed in liquid nitrogen, followed by homogenization in 80% ethanol at 55 °C for 10 to 15 minutes. After centrifugation (10000 × g for 10 minutes), supernatants of same samples were evaporated to dryness under vacuum at 55 °C. The final products were suspended in distilled water. Further, the amounts of extracted ACC were quantified indirectly by converting ACC to ethylene. The evolved ethylene was measured by gas chromatography. The whole experiment was repeated three times with five replications for each treatment.
RNA isolation and two steps real-time PCR
The total RNA extraction from the leaf and root samples of normal plants, as well as of plants that experienced stress for 45 days, were extracted by the RNeasy plant mini kit (Qiagen, Leusden, The Netherlands) and immediately reverse-transcribed to cDNA with a 2 × Verso cDNA synthesis kit (Thermo Scientific, USA) using random hexamers as per the manufacturer’s instruction. A quantitative amplification reaction for reference and target genes were carried out in a 96-well StepOnePlusTM Real-Time PCR System (Applied Biosystems, USA) using a Thermo DYNAMOTM 4 C SYBR Green qPCR master mix (Thermo Scientific, USA). cDNAs were replaced by sterile water for no-template control reaction. Fifteen nanograms (15ng) of cDNAs were used for the relative expression analysis. The reaction conditions were set as follows: 10 min. at 42 °C; 10 min. at 95 °C; 40 cycles of cDNA amplification for 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C with fluorescent signal recording. At the end, a final step of 15 s at 95 °C, and of 1 min. at 60 °C and fluorescence measured at each 0.7 °C variation (from 60 °C to 95 °C) was included to obtain the melting curve. Four reference genes, i.e. Act11, the Ubiquitin-conjugating enzyme (Ubq), Tubulin β-9 (β-Tub9), and 18S rRNA were used for the proper normalization of real-time qPCR reaction. Two important stress ethylene regulatory genes ACC oxidase (ACO) and ACC synthase (ACS) were selected as target genes for the experiment. The sequences of the genes studied were obtained from NCBI GenBank and the primers were designed with the aid of the OLIGO software (version 5.0; Molecular Biology Insights). The sequences, Genebank accession ID, and other parameters of primers have been listed in Table 5.
Table 5.
Primers sequences and other properties used in real time PCR expression analysis.
| Target genes | Genebank ID | Target plants | Forward sequence (5′-3′) | Reverse sequence (5′-3′) | Tm (°C) | Product size (bp) | PCR efficiency value (E ± SD) |
|---|---|---|---|---|---|---|---|
| ACO | AB128037.1 | GP | CTTGTCCTAAACCGGCACTC | CACTTCGAGTGGATCACCAA | 59 | 179 | 1.854 ± 0.052 |
| AM180696.1 | BG | TGCTGTGATTTCTCCAGCAC | ACGGCCTTCATAGCTTCAAA | 59 | 150 | 1.932 ± 0.031 | |
| ACS | AF016458.1 | GP | GGAGGATTCAAACGTGATGG | GGAGGATTCAAACGTGATGG | 60 | 234 | 2.012 ± 0.061 |
| M94863.1 | BG | TCTGCTGCAGGTTTCATTTG | TGCTCTCCCACCTCTCACTT | 60 | 181 | 1.883 ± 0.048 | |
| ACT11 | U76192.1 | GP | TGAAGCTCCGCTTAACCCTA | ATCGCATGTGGAAGTGCATA | 60 | 207 | 2.121 ± 0.056 |
| NM001278957.1 | BG | TCCTCTCACCTTGCCTCTGT | TCCAGCCTTAACCATTCCAG | 60 | 151 | 1.922 ± 0.024 | |
| Ubq | L29077.1 | GP | CCGTATGCTGGAGGTGTTTT | GGATCAGTCAGCAATGAGCA | 59 | 209 | 1.787 ± 0.047 |
| CM003604.1 | BG | GCTCAAGGATTTGCAGAAGG | TTGGTGGCTTGAAGGGATAG | 60 | 170 | 1.865 ± 0.032 | |
| β- Tub9 | FE676365.1 | GP | GGATCTCGAACCTGGAACAA | CCAAGACGGAATCGATGAGT | 60 | 155 | 1.951 ± 0.027 |
| X60216.1 | BG | CCGTTGTGGAGCCTTACAAT | TCCACTCATGGTGGCAGATA | 60 | 170 | 1.843 ± 0.043 | |
| 18S rRNA | AH001723.2 | GP | CATGATAACTCGTCGGATCG | CGTTTCTCAGGCTCCATCTC | 59 | 220 | 2.011 ± 0.027 |
| AH001765.2 | BG | AGCGGATGTTGCTTTTAGGA | GCACCACCACCCATAGAATC | 59 | 225 | 1.943 ± 0.048 |
Table key: BG - black gram, GP - garden pea, Tm- melting temperature.
Toxicity test
All three bacterial strains underwent toxicity tests at APT Testing and Research Private Ltd., Pune, India, to investigate acute oral toxicity/pathogenicity.
Data analyses
A one-way ANOVA, followed by Tukey’s test, was conducted to analyze the data sets obtained from the quantitative estimation of PGP traits (Table 1). Student’s t-test was used to analyze the data of seed germination/vigor index experiments (Table 2). However, the rest of the greenhouse experiments and real-time PCR generated results were analyzed by a two-way ANOVA, considering water supply and bacteria inoculation as two independent variables, followed by Tukey’s post-test for each treatment using SPSS software (ver. 10.1, SPSS Inc., www.spss.com). The significance level for all analyses was p = 0.05. In real-time qPCR experiments, ten-fold serial dilution of cDNA curves was used to calculate the amplification efficiency for all genes using the formula E = 10(−1/slope)94. The threshold cycle (CT) was compared with the log10 relative copy number of the sample from a dilution series. The CT values and log copy numbers of cDNAs for all the genes maintained a linear relationship having a range of correlation coefficient (R2) from 0.95 to 0.99, indicating a proportionate change in CT values related to the serial dilution of the samples. The E-value ranged from 1.787 to 2.121, indicating the efficient amplification near the theoretical optimum level of 295. The relative expression levels obtained for target genes were compared when the candidate normalizer genes were used individually. Then, the best combination was obtained by geNorm software96. The expression level calculated by the formula 2−ΔΔCt represents the x-fold difference from the calibrator.
Electronic supplementary material
Acknowledgements
The work is supported by a Network Project (PMSI, BSC-0117), sponsored by the Council of Scientific and Industrial Research (Ministry of Science and Technology), Government of India, New Delhi. The authors are thankful to the funding agency. The authors are also thankful to the Director, CSIR-NEIST, Jorhat, Assam for providing necessary facilities to carry out the work and BIF Centre, CSIR-NEIST, Jorhat for providing the computational facilities.
Author Contributions
J.S., R.D., R.K.S., R.S. conceived and designed the experiments. J.S., R.D., R.K.S. performed the experiments. J.S., R.K.S., R.S.,V.K.G., R.B. analyzed the data. A.Y., R.S. contributed reagents/materials/computational resources. R.K.S., R.S. wrote the paper. All authors have reviewed the manuscript and have given approval to the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Juthika Saikia, Rupak K. Sarma and Rajashree Dhandia contributed equally to this work.
A correction to this article is available online at https://doi.org/10.1038/s41598-018-25174-5.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21921-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/30/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Papworth A, Maslin M, Randalls S. Is climate change the greatest threat to global health? The Geograph. J. 2015;181:413–422. doi: 10.1111/geoj.12127. [DOI] [Google Scholar]
- 2.FAO. The State of Food and Agriculture 2016. Available online: http://www.fao.org/3/a-i6030e.pdf (2016).
- 3.Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009;27:744–752. doi: 10.1016/j.biotechadv.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Zhu JK. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001;4(5):401–406. doi: 10.1016/S1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhu JK. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabbani MA, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003;133:1755–1767. doi: 10.1104/pp.103.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleury D, Jefferies S, Kuchel H, Langridge P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010;61:3211–3222. doi: 10.1093/jxb/erq152. [DOI] [PubMed] [Google Scholar]
- 8.Kim, Y. C., Glick, B. R., Bashan, Y. & Ryu, C. M. Enhancement of plant drought tolerance by microbes (ed Aroca, R.) 383-413 (Springer Verlag, 2012).
- 9.Bashan, Y. & de-Bashan, L. E. Bacteria/plant growth-promotion (ed Hillel, D.) 103–115 (Elsevier Oxford, 2005).
- 10.Glick BR, Penrose DM, Li J. A model for lowering plant ethylene concentrations by plant growth promoting rhizobacteria. J. Theorit. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 11.Czarny JC, Grichko VP, Glick BR. Genetic modulation of ethylene biosynthesis and signalling in plants. Biotechnol. Adv. 2006;24:410–419. doi: 10.1016/j.biotechadv.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ovakim DH, Charles TC, Glick BR. An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Cur. Microbiol. 2000;41:101–105. doi: 10.1007/s002840010101. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Z, Park E, Glick BR. 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007;53:912–918. doi: 10.1139/W07-050. [DOI] [PubMed] [Google Scholar]
- 14.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [Google Scholar]
- 15.Sarma RK, Saikia R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014;377:111–126. doi: 10.1007/s11104-013-1981-9. [DOI] [Google Scholar]
- 16.Grichko VP, Glick BR. Amelioration of flooding stress by ACC-deaminase containing plant growth promoting bacteria. Plant Physiol. Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- 17.Kausar R, Shahzad SM. Effect of ACC-deaminase containing rhizobacteria on growth promotion of maize under salinity stress. J. Agri. Soc. Sci. 2006;2:216–218. [Google Scholar]
- 18.Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 2007;53:1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- 19.Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR. Tolerance of transgenic canola expressing 1-aminocyclopropane-1- carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol. Biochem. 2005;43:701–708. doi: 10.1016/j.plaphy.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Belimov AA, et al. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L Czern.) Soil Biol. Biochem. 2005;37:241–250. doi: 10.1016/j.soilbio.2004.07.033. [DOI] [Google Scholar]
- 21.Egamberdieva D, Jabborova D, Hashem A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi. J. Biol. Sci. 2015;522:773–779. doi: 10.1016/j.sjbs.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledger T, et al. Volatile-Mediated Effects Predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016;7:1838. doi: 10.3389/fmicb.2016.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagarajan, S. & Nagarajan, S. Abiotic stress adaptation in plants (ed. Pareek, A. et al.) 1–11 (Springer, 2009).
- 24.Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 25.Vacheron J, et al. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanovas EM, Barassi CA, Sueldo RJ. Azospirillum inoculation mitigates water stress effects in maize seedlings. Cereal Res. Commun. 2002;30:343–350. [Google Scholar]
- 27.Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol. Plant. 2011;33:203–209. doi: 10.1007/s11738-010-0539-1. [DOI] [Google Scholar]
- 28.Kohler J, Hernandez JA, Caravaca F, Roldan A. Plant growth-promoting rhizobacteria and abuscularmycorrhizal fungi modify alleviation biochemical mechanisms in water stressed plants. Funct. Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- 29.Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 30.Scandalios, J. G. Regulation and properties of plant catalases (ed. Foyer, C.H. & Mullineaux, P.M.) 275–315 (CRC Press, 1994).
- 31.Figueiredo MVB, Burity HA, Martinez CR, Chanway CP. Alleviation of drought stress in common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 32.Sandhya V, Ali SKZ, Grover M, Reddy G, Venkateswarlu B. Alleviation of drought tress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soil. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- 33.Myers N, Mittermeir RA, Mittermeir CG, da Fonseca GAB, Kents J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 34.Saha, R., Chaudhary, R. S. & Somasundaram, J. Soil Health Management under Hill Agroecosystem of North East India. Appl. Env. Soil Sci. 10.1155/2012/696174 (2012).
- 35.Awasthi JP, et al. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS One. 2017;27(12(4)):e0176357. doi: 10.1371/journal.pone.0176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma, P., Yadav, A. N., Kumar, V., Singh, D. P. & Saxena, A. K. Beneficial Plant-Microbes Interactions: Biodiversity of Microbes from Diverse Extreme Environments and Its Impact for Crop Improvement (ed. Singh, D.P. et al.) 543–580 (Springer Nature, 2017).
- 37.Yadav S, Kaushik R, Saxena AK, Arora DK. Diversity and phylogeny of plant growth promoting bacilli from moderately acidic soil. J Basic Microbiol. 2011;51:98–106. doi: 10.1002/jobm.201000098. [DOI] [PubMed] [Google Scholar]
- 38.Yadav S, Yadav S, Kaushik R, Saxena AK, Arora DK. Genetic and functional diversity of fluorescent Pseudomonas from rhizospheric soils of wheat crop. J Basic Microbiol. 2013;54:425–437. doi: 10.1002/jobm.201200384. [DOI] [PubMed] [Google Scholar]
- 39.Parida BR, Oinam B. Unprecedented drought in North East India compared to Western India. Cur. Sci. 2015;109:2121–2126. doi: 10.18520/cs/v109/i11/2121-2126. [DOI] [Google Scholar]
- 40.Kang Y, Khan S, Ma X. Climate change impacts on crop yield, crop water productivity and food security – A review. Prog. Nat. Sci. 2009;19:1665–1674. doi: 10.1016/j.pnsc.2009.08.001. [DOI] [Google Scholar]
- 41.Haling RE, Simpson RJ, Culvenor RA, Lambers H, Richardson AE. Effect of soil acidity, soil strength and macropores on root growth and morphology of perennial grass species differing in acid-soil resistance. Plant Cell Env. 2011;34:444–456. doi: 10.1111/j.1365-3040.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- 42.Huang XF, et al. Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany. 2014;92:267–275. doi: 10.1139/cjb-2013-0225. [DOI] [Google Scholar]
- 43.Morris BE, Henneberger R, Huber H, Moissl-Eichinger C. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 2013;37(3):384–406. doi: 10.1111/1574-6976.12019. [DOI] [PubMed] [Google Scholar]
- 44.Bashan Y. Inoculants of plant growth‐promoting bacteria for use in agriculture. Biotechnol. Adv. 1998;16:729–770. doi: 10.1016/S0734-9750(98)00003-2. [DOI] [Google Scholar]
- 45.Penrose DM, Moffatt BA, Glick BR. Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can. J. Microbiol. 2001;47:77–80. doi: 10.1139/w00-128. [DOI] [PubMed] [Google Scholar]
- 46.Madhaiyan M, Poonguzhali S, Ryu J, Sa T. Regulation of ethylene levels in canola (Brassica campestris) by 1 aminocyclopropane- 1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta. 2006;224:268–278. doi: 10.1007/s00425-005-0211-y. [DOI] [PubMed] [Google Scholar]
- 47.Camilios-Neto D, et al. Dual RNAseq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genom. 2014;15:378. doi: 10.1186/1471-2164-15-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.) Can. J. Microbiol. 2007;53:1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- 49.Ruzzi M, Aroca R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015;196:124–134. doi: 10.1016/j.scienta.2015.08.042. [DOI] [Google Scholar]
- 50.Glick BR, et al. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007;26:227–242. doi: 10.1080/07352680701572966. [DOI] [Google Scholar]
- 51.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimkpa C, Weinand T, Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32(12):1682–94. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 53.Vurukonda SS, Vardharajula S, Shrivastava M, Skz A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Sorty AM, et al. Effect of Plant Growth Promoting Bacteria Associated with Halophytic Weed (Psoralea corylifolia L) on Germination and Seedling Growth of Wheat Under Saline Conditions. Appl. Biochem. Biotechnol. 2016;180(5):872–882. doi: 10.1007/s12010-016-2139-z. [DOI] [PubMed] [Google Scholar]
- 55.Sorty AM, Shaikh NR. Novel co-enrichment method for isolation of magnetotactic bacteria. J. Basic Microbiol. 2015;55:520–526. doi: 10.1002/jobm.201400457. [DOI] [PubMed] [Google Scholar]
- 56.Castignetti D, Smarrelli J. Siderophores, the iron nutrition of plants, and nitrate reductase. FEBS Lett. 1986;209:147–151. doi: 10.1016/0014-5793(86)81100-0. [DOI] [Google Scholar]
- 57.O’Sullivan DJ, O’Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 1992;56:662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burd GI, Dixon DG, Glick BR. Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- 59.Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Habib SH, Kausar H, Saud HM. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. Biomed. Res. Int. 2016;2016:6284547. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang CJ, et al. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One. 2012;7(12):e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastouri F, Bjorkman T, Harman GE. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interac. 2012;25:1264–1271. doi: 10.1094/MPMI-09-11-0240. [DOI] [PubMed] [Google Scholar]
- 63.Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- 64.Harb A, Krishnan A, Ambavaram MMR, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010;154:1254–1271. doi: 10.1104/pp.110.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson AD, Hits ED. Metabolic responses of mesophytes to plant water deficits. Annu. Rev. Plant Physiol. 1982;33:163–203. doi: 10.1146/annurev.pp.33.060182.001115. [DOI] [Google Scholar]
- 66.Rhodes, D. & Samaras, Y. Genetic control of osmoregulation in plants (ed. Stange, K.) 347–361 (CRC Press, 1994).
- 67.Hayat S, et al. Role of Proline under ChangingEnvironments: A Review. Plant Signal Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakkeeran, S., Fernando, W. G. D. M. & Siddiqui, Z. A. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases (ed. Siddiqui, Z.A.) 257–296 (Springer, 2005).
- 69.USDA. Soil taxonomy. A basic system of soil classification for making and interpreting soil surveys. United States Department of Agriculture, Washington, DC (2010).
- 70.Dworkin M, Foster JW. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958;75:592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hontzeas N, et al. Evidence for horizontal gene transfer (HGT) of ACC deaminase genes. Appl. Environ. Microbiol. 2005;71:7556–7558. doi: 10.1128/AEM.71.11.7556-7558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;5:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergey, D. H. et al. Bergey’s manual of determinative bacteriology (ed. Williams & Wilkins) 1860–1937 (Baltimore, 1994).
- 74.Honma M, Shimomura T. Metabolism of 1- aminocyclopropane-1-carboxylic acid. Agri. Biol. Chem. 1978;42:1825–1831. [Google Scholar]
- 75.Gordon SA, Weber RP. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bashan Y, Kamnev AK, de-Bashan LE. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol. Fertil. Soils. 2013;49:465–479. doi: 10.1007/s00374-012-0737-7. [DOI] [Google Scholar]
- 77.Fiske CH, Subbarow Y. A colorimetric determination of phosphorus. J. Biol. Chem. 1925;66:375–400. [Google Scholar]
- 78.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 79.Cappuccino, J. G. & Sherman, N. Biochemical activities of microorganisms. Microbiology, A Laboratory Manual (The Benjamin / Cummings, 1992).
- 80.Kremer RJ, Souissi T. Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Cur. Microbiol. 2001;43:182–186. doi: 10.1007/s002840010284. [DOI] [PubMed] [Google Scholar]
- 81.Schillinger U, Lucke FK. Antibacterial activity of Lactobacillussake isolated from meat. Appl. Env. Microbiol. 1989;55:1901–1906. doi: 10.1128/aem.55.8.1901-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ISTA. Proceedings of the International Seed Testing Association, international rules for seed testing. Seed Sci. Technol. 21, 25–30 (1993).
- 83.Baki AAA, Anderson JD. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. doi: 10.2135/cropsci1973.0011183X001300060013x. [DOI] [Google Scholar]
- 84.Rayment, G, E. & Higginson, F. R. Australian Laboratory Handbook of Soil and Water Chemical Methods (Inkata Press Melbourne, 1992).
- 85.Teulat B, et al. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003;108:181–188. doi: 10.1007/s00122-003-1417-7. [DOI] [PubMed] [Google Scholar]
- 86.Lorenzen CJ. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Ass. Sci. Limnol. Oceano. 1967;12:343–346. doi: 10.4319/lo.1967.12.2.0343. [DOI] [Google Scholar]
- 87.Liu RX, Zhou ZG, Gu WQ, Chen BL. Oosterhuis DM. Effects of N fertilization on root development and activity of water-stressed cotton (Gossypium hirsutum L.) plants. Agri. Water Manag. 2008;9:1261–1270. doi: 10.1016/j.agwat.2008.05.002. [DOI] [Google Scholar]
- 88.Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 89.Chance, B. & Maehly, A. C. Assay of catalases and peroxidases. Methods in Enzymology Vol-II, 764–775 (Academic Press New York, 1995).
- 90.Beer RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 91.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 92.Malik, C. P. & Singh, M. B. Plant enzymology and histo enzymology (Kalyani Publishers New Delhi, 1980).
- 93.Wang H, Woodson WR. Reversible inhibition of ethylene action and interruption of petal senescence in carnation flowers by norbornadiene. Plant Physiol. 1989;89:434–438. doi: 10.1104/pp.89.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkening S, Bader A. Quantitative real-time polymerase chain reaction: methodical analysis and mathematical model. J. Biomol. Tech. 2004;15:107–111. [PMC free article] [PubMed] [Google Scholar]
- 96.Vandesompele J, et al. Accurate normalization of real-timequantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.