Abstract
Among traditional Chinese medicine injections, intravenous Shuang-Huang-Lian (IV-SHL) has the highest incidence of injection-induced immediate hypersensitivity reactions (IHRs). The precise mechanisms of IV-SHL-induced IHRs remain ambiguous. In this study, we investigated the mechanisms of SHL injection (SHLI)-induced IHRs. Our data showed that serum total IgE and mouse mast cell protease 1 (MMCP1) levels were higher in the SHLI antiserum; however, these effects of SHLI disappeared in the antibiotic-treated mice. SHLI caused intraplantar vasopermeability and shock during the first local or systemic injection. SHLI-induced nonallergic IHRs were attributed to its intermediate fraction F2 (the extract of Lonicerae Japonicae Flos and Fructus forsythiae), and could be blocked by antagonists for histamine or C5a, rather than PAF or C3a. Eight constituents of F2 were able to directly activate C5 to promote local vasopermeability at the mg/mL level. In conclusion, SHLI-induced IHRs are not mediated by IgE. SHLI or its F2 can directly activate blood C5. Subsequently, C5a is likely to provoke histamine release from its effector cells (e.g., mast cells and basophils), indicating that histamine is a principal effector of IHRs induced by SHLI.
Introduction
Shuang-Huang-Lian (SHL), a modern antimicrobial formulation comprising alcohol-water extracts of three herbs (Lonicerae Japonicae Flos, Scutellariae Radix, and Fructus Forsythiae), is officially recorded in the Chinese Pharmacopoeia and is approved for production by the Chinese Food and Drug Administration (CFDA)1. Since 1973, intravenous SHL (IV-SHL) has been widely used in China to treat respiratory infection when an oral preparation is ineffective2. However, despite its benefits3, IV-SHL has been recorded to induce severe immediate hypersensitivity reactions (IHRs), namely anaphylaxis4, at a very low frequency5. Therefore, the CFDA has raised an alarm regarding this potentially life-threatening outcome6.
In China, about four hundred million patients are treated with traditional Chinese medicine injections (TCMI) per year. Adverse drug reactions (ADRs) have been reported with an incidence of approximately 1.51%7,8, representing around 67.3% of ADRs occurred within 1 h which belonged to IHRs9. Of these, IV-SHL has the highest incidence of ADRs10,11. Of the 11001 patients administered with IV-SHL, 182 (1.65%) had cutaneous and mucosal allergies (e.g., urticaria and rashes), while 14 (0.127%) suffered anaphylaxis12. Consequently, many researchers have focused on evaluating IHRs caused by IV-SHL. Some reports suggested that SHL injection (SHLI) significantly increased rat serum total IgE (tIgE) level13; whereas, others reported that SHLI could cause nonallergic IHRs (NA-IHRs)14. However, to design new strategies to prevent or treat SHLI-induced IHRs, the precise mechanism should be determined. To achieve this, we carried out a systemic study of the mechanisms of SHLI-induced IHRs. The two main possible mechanisms are IgE-mediated responses and complement activation-related pseudo-allergy (CARPA)15. Our results showed that the mechanism of SHLI-induced IHRs is CARPA rather than an IgE-mediated response. Our data indicated that SHLI can directly activate blood C5, which might subsequently stimulate its effector cells (e.g., mast cells and basophils) to release histamine16, which is the principal effector of IHRs induced by SHLI.
Methods
Materials and reagents
SHLI and its two intermediate fractions (F1, the extract of Scutellariae Radix; F2, the extract of Lonicerae Japonicae Flos and Fructus Forsythiae) were prepared by Duoduo Pharmaceutical Co., Ltd. (Jiamusi, Heilongjiang, China) according to the Chinese Pharmacopoeia1. Compound 48/80 (C48/80), propranolol, CV3988, triprolidine, globulins, cimetidine, and SB290157 were purchased from Sigma-Aldrich (St Louis, MO, USA). PMX53 was from GL Biochem Ltd. (Shanghai, China). Mouse tIgE ELISA kit was from Biolegend Co. (San Diego, CA, USA). Rehydragel® aluminum adjuvant was from General Chemical (Parsippany, NJ, USA). Anti-human CD54-allophycocyanin (APC) antibody and its REA control-APC were from Miltenyi Biotec. (Bergisch Gladbach, German). Human C5a ELISA kit, fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD86 antibody and its isotype control were from BD Biosciences (San Diego, CA, USA). Gentamicin, ampicillin, 2-methyl-5-nitroimidazole-1-ethanol, and fradiomycin were from TCI Chemicals (Tokyo, Japan). Vancomycin was obtained from BBI Life Sciences Corporation (Shanghai, China). The mouse mast cell protease 1 (MMCP1) ELISA kit was from Invitrogen (San Diego, CA, USA). Protein G PLUS-Agarose was from Santa Cruz Biotechnology, Inc. (SantaCruz, CA, USA).
Cells and animals
The human mast cell line LAD2 (from Michael D. Gershon, MD, Columbia University, USA) was a gift from Prof. Renshan Sun (the Third Military Medical University, Chongqing, China). The human THP-1 cell line was purchased from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). Balb/c mice (female, 18–20 g) and SD rats (female, 160–180 g) were from Vital River Experimental Animal Services (Beijing, China). All the experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Animals Ethics Committee of the IMPLAD of Chinese Academy of Medical Sciences.
Preparation of shrimp tropomyosin (ST)
ST from Metapenaeus ensis was extracted and purified using an isoelectric precipitation method as previously described17.
Preparation of ST or SHLI antisera
Balb/c mice were injected (i.p.) weekly with aluminum adjuvant (100 μL/mouse) containing SHLI (200 μL/mouse) or ST (60 μg/mouse). ST was used as a positive control allergen. The negative control (NC) mice were treated with aluminum adjuvant containing an equal volume of normal saline (NS). Five weeks later, the mice were sacrificed and the antisera were collected for subsequent assays.
Measurement of tIgE and ST specific IgE (sIgE) in the antisera
Serum tIgE level was assayed using a commercial mouse tIgE ELISA kit. The levels of sIgE were measured as previously described, with some modifications18. Briefly, IgG in the serum were removed by Protein G PLUS-Agarose, according to the manufacturer’s instructions. The 96-well microtiter plates were coated with test constituent-bovine serum albumin (BSA) conjugate or ST (10 μg/mL, 100 μL/well) in coating buffer (0.05 M carbonate buffer, pH 9.6). After overnight incubation at 4 °C, plates were washed 4 times with phosphate-buffered saline (PBS)/0.05% Tween 20 and blocked with 1% BSA-PBS at 37 °C for 1 h. After washing, the IgG-free serum samples were added to the plates and incubated overnight at 4 °C. 100 μL of the HRP-labeled rat anti-mouse IgE antibody was added 4 times washing later. The plates were incubated at 37 °C for 1 h. The reactions were developed with 3,3′,5,5′-tetramethylbenzidine for 5 min at 37 °C and stopped using 100 μL of 2 M H2SO4. The optical density (OD) was read at 450 nm.
Preparation of antibiotic-treated mice
The mice were fed autoclaved water with or without ampicillin (0.5 mg/mL), gentamicin (0.5 mg/mL), metronidazole (0.5 mg/mL), neomycin (0.5 mg/mL), and vancomycin (0.25 mg/mL) continuously via a water bottle. In the event of poor animal hydration, we supplemented control and antibiotic water with artificial sweetener19. Five weeks later, the mice were sacrificed, and the serum tIgE and MMCP1 levels were measured using ELISA.
Human cell line activation test (h-CLAT) on THP-1 cells
According to previously described method20, THP-1 cells were treated with 34 constituents (50 μg/mL) for 24 h at 37 °C and their effects on CD54 and CD86 expression on the cell surface were determined by flow cytometry. Isotype control and unstained cells were used as the negative control (NC)21.
Preparation of hapten-BSA conjugate
Forsythiaside A-BSA, arctigenin-BSA, and baicalein-BSA conjugates were prepared via a Mannich-type reaction, based on their active hydrogen, according to a previously described method22. Oleanolic acid is able to directly couple with BSA based on its free carboxyl.
Passively sensitized animals
Passive cutaneous anaphylaxis (PCA) in rats was performed according to a previously described method with some modifications23. The rats were anesthetized and received an intradermal injection of 100 μL of SHLI antiserum (or 1/200 dilution of ST antiserum). Twenty-four hours later, the rats were injected via their tail vein with 1 mL of 5 mg/mL Evans Blue containing 1 mg forsythiaside A-BSA, arctigenin-BSA, oleanolic acid-BSA, baicalein-BSA conjugate, or 500 μg ST, respectively. The resultant blue spots in the dorsal inboard skin were scored 1 h later.
In the MMCP1 assay, Balb/c mice were primed (i.v.) with NC serum (500 μL/mouse), ST antiserum (10 μL/mouse), or SHLI antiserum (500 μL/mouse). Twenty-four hours later, the mice were challenged (i.v.) with ST (200 μg/mouse) or SHLI (200 μL/mouse). The NC mice were treated with NS. Four hours later, whole blood was obtained and the MMCP1 concentration in the serum was measured using ELISA24.
Evans Blue extravasation assays
Evans Blue extravasation in mice hind paws was measured as previously described25. For the rat cutaneous Evans Blue extravasation assay, animals were subcutaneously injected with 100 μL of test substances and then immediately intravenously injected with 5 mg/mL of Evans Blue (1 mL). Fifteen minutes later, the rats were sacrificed and the resultant blue spots in the dorsal inboard skin were scored.
Anaphylactoid shock assay
Anaphylactoid shock was assessed by rectal thermometry26. To detect the effects of test substances on the temperature of normal mice, Balb/c mice were intravenously injected with the test substances, including NS, C48/80 (40 μg/mouse), SHLI (0.5 mL/mouse), F1 (4.81 mg/mouse), or F2 (16.5 mg/mouse), respectively. Thirty minutes later, the rectal temperature was measured. To increase the severity of anaphylaxis27, the mice were pretreated (i.v.) with propranolol (35 μg/mouse). Twenty minutes later, the mice were challenged (i.p.) with the test substances. After 30 min, the rectal temperature was measured. For the platelet activating factor (PAF) or histamine antagonist experiments, 1 mg/mL of antagonist (CV3988, triprolidine, and cimetidine, 200 μL/mouse) was intraperitoneally injected into the mice 10 min before the propranolol pretreatment.
Complement activation assay in vitro
Human whole blood and plasma from healthy volunteers were obtained with written informed consent. The plasma was prepared by centrifuging blood that had been collected in EDTA-coated tubes and was immediately used or frozen at −80 °C until use. Plasma (100 μL, 1:100 dilution) was mixed with 4 μL of CaCl2 (1 M), 4 μL of MgCl2 (1 M), and 10 μL of test substances, and then incubated at 37 °C. After 20 min, the reaction was stopped using 22 μL of EDTA (0.5 M, pH 8.0) and cooling to 0 °C. Tween-80 was used as a positive control28. The level of C5a in the plasma was determined using a commercial ELISA kit according to the manufacturer’s instruction.
Statistical analysis
Data were expressed as the means ± SD from at least three independent experiments and were analyzed by a one-way analysis of variance (ANOVA). A Student t-test was used when only two groups were compared. A difference with a P-value < 0.05 was considered statistically significant.
Results
SHLI-induced IHRs are not mediated by IgE
Serum tIgE and MMCP1 levels are higher in the SHLI antiserum
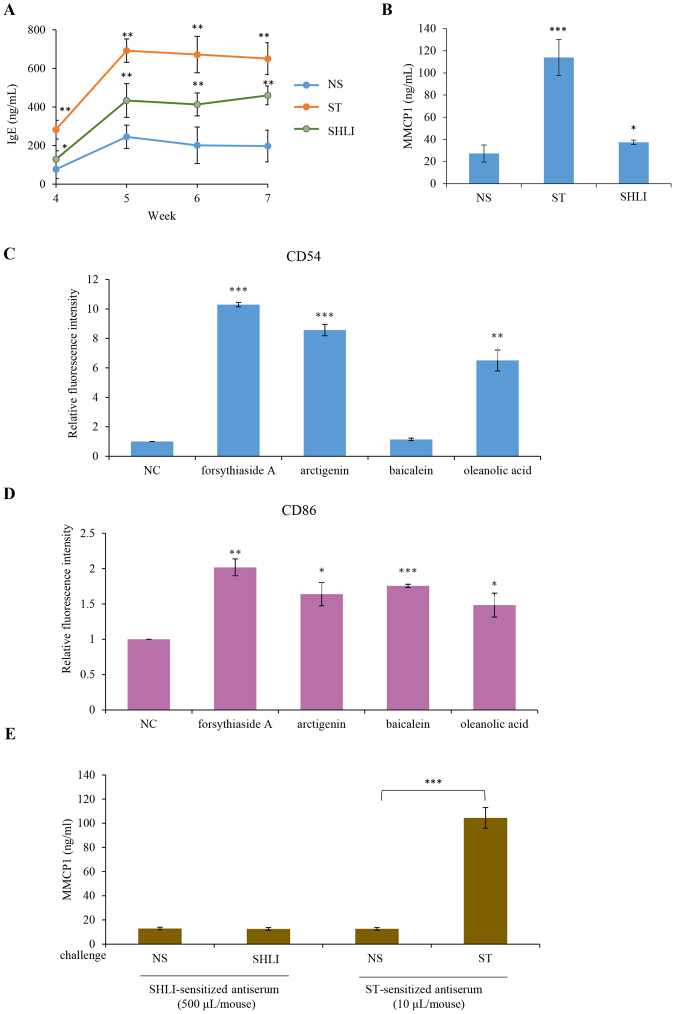
IgE-mediated IHRs are the most common allergic IHRs29. To amplify the possible Th2 response induced by SHLI, an aluminum adjuvant was used during mouse immunization. As shown in Fig. 1A, serum tIgE levels were significantly elevated by ST and SHLI, 4 weeks after intraperitoneal immunization, and peaked and stabilized between 5–7 weeks. Consistently, serum MMCP1, a specific marker for IgE-mediated allergy24, was significantly increased in the SHLI antiserum from the mice sensitized by SHLI for 5 weeks (Fig. 1B). These results seemingly suggested that SHLI might induce IgE-mediated IHRs.
Figure 1.
(A) SHLI elevates mouse serum tIgE levels 4–7 weeks after immunization (n = 8). The mice were injected weekly (i.p.) with aluminum adjuvant (100 μL/mouse) containing SHLI (200 μL/mouse) or ST (60 μg/mouse). The serum tIgE level was determined using a commercial ELISA kit. (B) SHLI causes an increase in mouse serum MMCP1 5 weeks after immunization (n = 8). The mice were injected weekly (i.p.) with aluminum adjuvant (100 μL/mouse) containing SHLI (200 μL/mouse) or ST (60 μg/mouse). The serum MMCP1 level was determined using a commercial ELISA kit. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. NS. (C,D) Four constituents in SHLI increase THP-1 cells CD54 (C) and/or CD86 (D) levels. THP-1 cells were treated with 4 constituents (50 μg/mL) for 24 h at 37 °C and then stained with anti-human CD54-APC or anti-human CD86-FITC antibodies for 15 min at 25 °C. The expression of CD54 and CD86 on THP-1 cells was assayed by flow cytometry. Isotype control and unstained cells were used as NC. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. the NC. (E) The serum MMCP1 concentration does not increased in the mice passively sensitized by SHLI antiserum. Balb/c mice were primed (i.v.) with NC serum (500 μL/mouse) or ST antiserum (10 μL/mouse) or SHLI antiserum (500 μL/mouse). Twenty-four hours later, the mice were challenged (i.v.) with ST (200 μg/mouse) or SHLI (200 μL/mouse). The NC mice were treated with an equal volume of NS. Four hours later, the whole blood was obtained and MMCP1 concentration in the serum was measured using an ELISA. ***P < 0.001.
Four suspicious allergens are not the haptens for IgE
The tIgE is the summation of sIgEs against different antigens, and MMCP1 is a specific marker for type I hypersensitivity. The fact that SHLI elevated serum tIgE and MMCP1 levels prompted us to look for the potential allergenic components from SHLI. To efficiently and quickly screen the possible haptens, the h-CLAT method was used20. Thirty-four available constituents (50 μg/mL; Table S1) in SHLI were identified based on CD54 or/and CD86 expression in THP-1 cells, of which 4 constituents (forsythiaside A, arctigenin, oleanolic acid, and baicalein) might possess sensitization potential (Fig. 1C,D). We then synthetized their respective holoantigens (hapten-BSA conjugates) to measure their respective sIgE in the SHLI antiserum using ELISA (in vitro). Unexpectedly, no sIgE was detected, while anti-ST sIgE was markedly high in the parallel experiments (data not shown). Consistently, these conjugates also could not result in blue spots in a PCA assay (data not shown), suggesting that 4 suspicious allergens are not the haptens for IgE.
There are two possibilities for the above phenomenon: 1) The haptens for IgE are not included in the 4 constituents or in the other 30 constituents; 2) SHLI does not cause IgE-mediated IHRs. To clarify this issue, serum MMCP1 of passively sensitized mice was determined. Unlike ST, SHLI could not increase the serum MMCP1 level (Fig. 1E), suggesting that SHLI-induced IHRs are not mediated by IgE.
SHLI fails to elevate serum tIgE and MMCP1 in antibiotic-treated mice
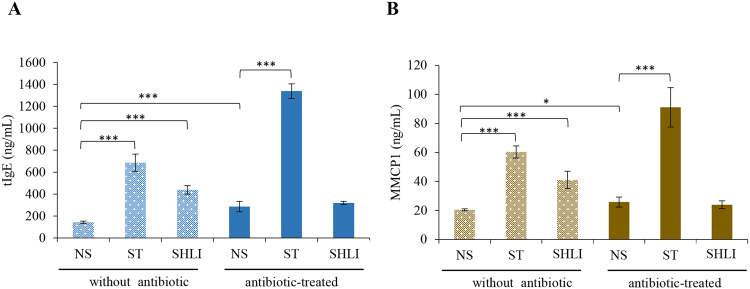
Given that commensal bacteria-derived signals can limit serum IgE levels19,30, together with the fact that SHL is an antimicrobial31, we speculated that SHLI-induced serum tIgE and MMCP1 elevation would be relevant to its suppressive effect on commensal bacteria-derived signals. To confirm this hypothesis, antibiotic-treated mice, in which the antimicrobial activity of SHLI might be completely restricted, were used19. As expected, the SHLI-mediated elevation of serum tIgE and MMCP1 (Fig. 1A,B) disappeared, while that of ST was not attenuated (Fig. 2).
Figure 2.
SHLI fails to elevate serum tIgE (A) and MMCP1 (B) levels in antibiotic-treated mice (n = 8). We fed mice autoclaved water with or without ampicillin (0.5 mg/mL), gentamicin (0.5 mg/mL), metronidazole (0.5 mg/mL), neomycin (0.5 mg/mL), and vancomycin (0.25 mg/mL) continuously via a water bottle. In the event of poor animal hydration, we supplemented control and antibiotic water with artificial sweetener. Five weeks later, the mice were sacrificed, and the serum tIgE and MMCP1 levels were measured by ELISA. *P < 0.05 and ***P < 0.001.
SHLI contributes to NA-IHRs by activating C5
IHRs can be allergic- as well as nonallergic-mediated32; therefore, we next evaluated whether SHLI could induce NA-IHRs. Our study had demonstrated that SHLI could not directly induce human mast cells LAD2 degranulation (data not shown). In fact, our previous experiment showed that SHLI could markedly dampened C48/80-induced degranulation in mast cells17. Therefore, in the present study, we evaluated whether SHLI could induce local and systemic NA-IHRs in vivo.
SHLI induces vasopermeability during the first intraplantar injection
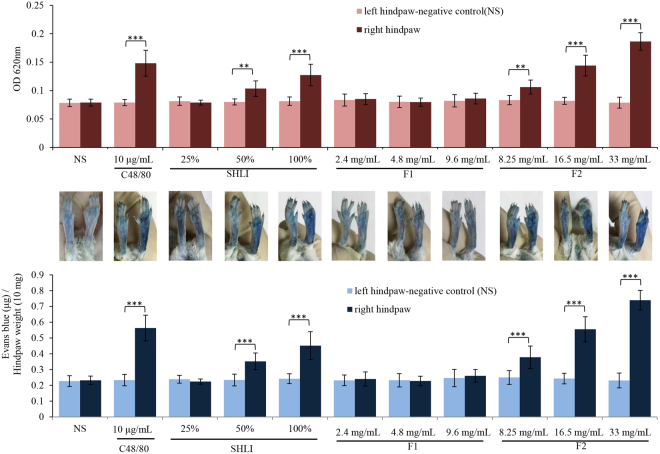
We first determined the local allergenicity of SHLI using a sensitive hindpaw Evans Blue extravasation assay. As shown in Fig. 3, the positive control C48/80 (10 μg/mL), SHLI (50% and 100%), and its intermediate fraction F2 (8.25 mg/mL–33 mg/mL; 33 mg/mL was the equivalent concentration in 100% SHLI) significantly induced vasopermeability during the first intraplantar injection, while F1 (9.6 mg/mL was the equivalent concentration in 100% SHLI) had no effect, indicating that F2 might be the major contributor in SHLI that induces local NA-IHRs.
Figure 3.
SHLI induces vasopermeability (Evans Blue extravasation) during the first intraplantar injection (n = 6). Fifteen minutes after induction of anesthesia (50 mg/kg of pentobarbital), mice were injected intravenously (i.v.) with 50 μL of 12.5 mg/mL Evans Blue. Five minutes later, 5 μL of the test substance (C48/80, SHLI, F1, or F2) was administered by intraplantar injection in the right paw and NS was administered in the left paw. Thirty minutes later, the mice were sacrificed and the paw tissue was collected, dried for 24 h at 50 °C, and weighed. Evans Blue was extracted by a 24 h incubation in formamide at 50 °C, and the OD value was read at 620 nm. **P < 0.01 and ***P < 0.001.
F2 of SHLI induces anaphylactoid shock by histamine, rather than PAF
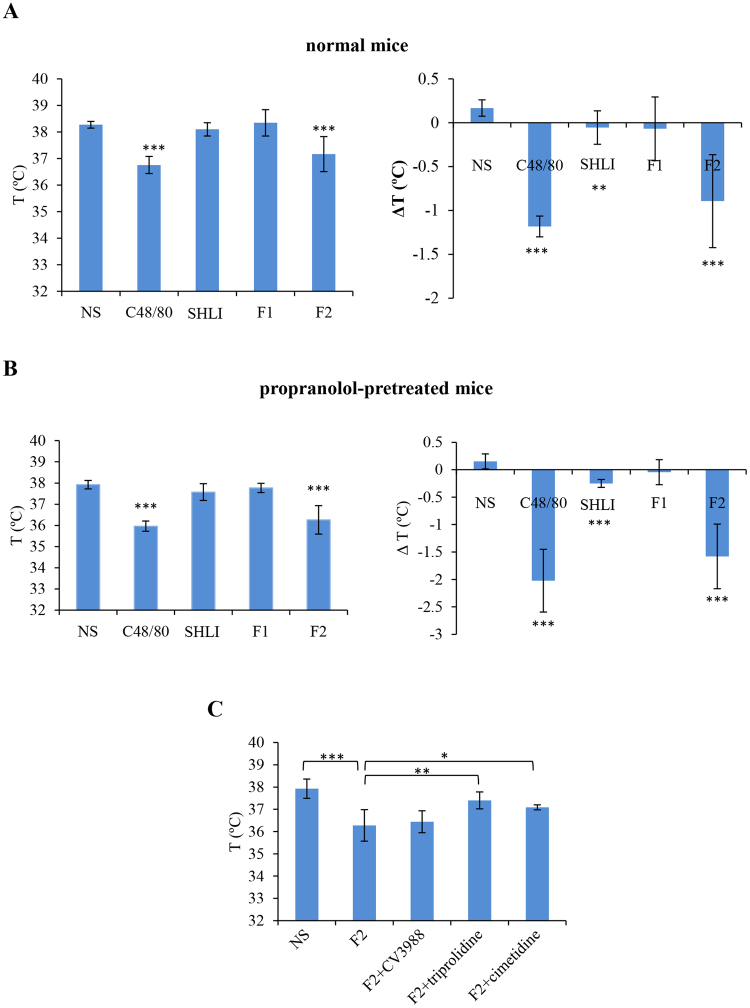
We next evaluated whether SHLI could cause anaphylactoid shock (detected as hypothermia26). Mice were made more sensitive to SHLI-induced shock by pretreatment with propranolol, which does not induce shock by itself, but can increase the severity of shock27,33. As shown in Fig. 4A,B, C48/80 and F2 contributed to obvious hypothermia in both normal and propranolol-pretreated mice, while F1 has no effect on the mice’s temperature. SHLI significantly induced hypothermia only by comparing the temperature difference (ΔT °C) before and after treatment. Given the fact that F2 showed a more obvious effect, we chose F2 instead of SHLI in the subsequent antagonist study.
Figure 4.
(A) F2 of SHLI induces anaphylactoid shock in normal mice (n = 6). The mice were intravenously injected with NS, C48/80 (40 μg/mouse), SHLI (0.5 mL/mouse), F1 (4.81 mg/mouse), or F2 (16.5 mg/mouse). Thirty minutes later, the rectal temperature was measured. **P < 0.01 and ***P < 0.001 vs. NS. (B) F2 of SHLI induces anaphylactoid shock in propranolol-pretreated mice (n = 6). The mice were pretreated intravenously (i.v.) with propranolol (35 μg/mouse). Twenty minutes later, the mice were intraperitoneally injected with NS, C48/80 (40 μg/mouse), SHLI (0.5 mL/mouse), F1 (4.81 mg/mouse), or F2 (16.5 mg/mouse). Thirty minutes later, the rectal temperature was measured. ***P < 0.001 vs. NS. (C) F2 of SHLI-induced shock is histamine dependent (n = 6). The mice were intraperitoneally treated with 0.2 mg/mouse of CV3988 (a PAF antagonist), triprolidine (an H1 antagonist), and cimetidine (an H2 antagonist), and then (10 min later) injected (i.v.) with propranolol (35 μg/mouse). Twenty minutes later, the mice were challenged (i.p.) with F2 (16.5 mg/mouse). Thirty minutes later, the rectal temperature was measured. *P < 0.05, **P < 0.01 and ***P < 0.001.
Mast cells, basophils, and macrophages contribute predominantly to the pathogenesis of anaphylaxis through their secretion of histamine or/and PAF26,34. An experiment performed using specific antagonists for PAF (CV3988), histamine H1 receptor (triprolidine) and H2 receptor (cimetidine) demonstrated that F2-induced shock was substantially ameliorated by triprolidine and cimetidine, but not CV3988 (Fig. 4C), indicating that histamine, rather than PAF, was the principal effector in F2-induced systemic anaphylaxis.
SHLI promotes local and systemic NA-IHRs by anaphylatoxin C5a
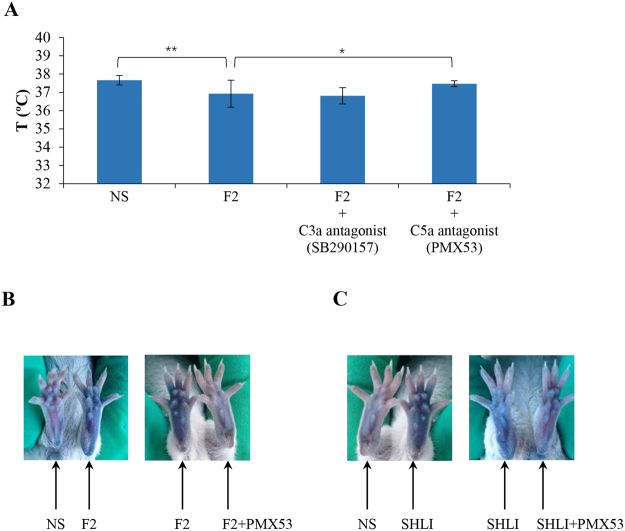
The above observations indicated that SHLI or F2 are likely to promote local and systemic NA-IHRs by inducing the release of histamine, a mast cell-derived mediator. Anaphylatoxin C3a or C5a can activate mast cells and basophils to secrete histamine35. Thus, it is possible that SHLI induces NA-IHRs via the activation of complement. Consequently, we used a C3a antagonist (SB290157) or a C5a antagonist (PMX53) to antagonize F2-induced shock. The result showed that PMX53, but not SB290157, significantly attenuated F2-induced hypothermia (Fig. 5A). Next, in a hindpaw Evans Blue extravasation assay, F2- or SHLI-induced vasopermeability could be blocked by PMX53 (Fig. 5B,C), indicating SHLI’s induction of NA-IHRs was attributed to C5a, rather than C3a.
Figure 5.
SHLI-induced NA-IHRs is mediated by C5a. (A) C5a antagonist PMX53 can block F2-induced shock (n = 10). The mice were pretreated with propranolol (i.v., 35 μg/mouse) 10 min after intraperitoneally injected with SB290157 (a C3a antagonist, 30 mg/kg) or PMX53 (a C5a antagonist, 1 mg/kg). Twenty minutes later, the mice were challenged (i.p.) with F2 (16.5 mg/mouse). Thirty minutes later, the rectal temperature was measured. *P < 0.05 and **P < 0.01. (B) Representative images of Evans Blue extravasation of mouse paw induced by F2 and blocked by PMX53 (n = 6). Fifteen minutes after induction of anesthesia (50 mg/kg of pentobarbital), mice were intraplantarly injected with 10 μL of PMX53 (1 mg/mL). Twenty minutes later, the mice were injected (i.v.) with 50 μL of 12.5 mg/mL Evans Blue. Five minutes later, 5 μL of F2 (33 mg/mL), or NS was administered by intraplantar injection in the paw. Thirty minutes later, the mice were sacrificed and the paws were photographed. (C) Representative images of Evans Blue extravasation of mouse paw induced by SHLI (10 μL/paw) and blocked by PMX53 (n = 6).
Eight constituents of F2 can induce local vasopermeability by directly activating C5
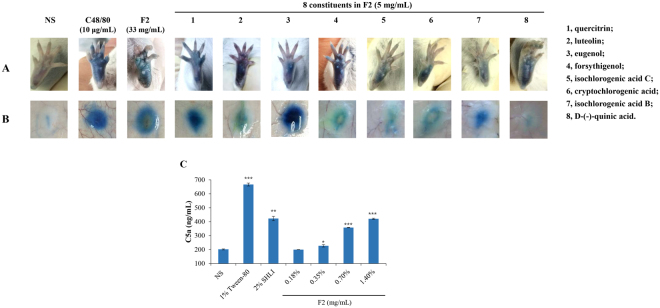
To identify the possible pseudoallergens in SHLI, we tested the effects of 29 available constituents (5 mg/mL) from F2 (Table S1) using Evans Blue extravasation assays in both mouse and rat models. Eight constituents (cryptochlorogenic acid, forsythigenol, isochlorogenic acid B and C, eugenol, quercitrin, D-(−)-quinic acid, and luteolin) significantly induced vasopermeability during the first intraplantar injection in mice (Fig. 6A); however, the effect of D-(−)-quinic acid in rats was not observed (Fig. 6B). Moreover, the effects of 8 constituents on intraplantar vasopermeability could also be blocked by the C5a antagonist PMX53 (photos not shown).
Figure 6.
(A) Representative images of Evans Blue extravasation of mouse hindpaw induced by 8 allergenic constituents in F2. Fifteen minutes after induction of anesthesia (50 mg/kg of pentobarbital), mice were injected (i.v.) with 100 μL of 6.25 mg/mL Evans Blue in saline. Five minutes later, 15 μL of the test substance was administered by intraplantar injection in one paw. Fifteen minutes later, the mice were sacrificed and the paws were observed. (B) Representative images of Evans Blue extravasation of rat dorsal skin induced by seven allergenic constituents in F2. Rats were subcutaneously injected with 100 μL of test substances and then immediately injected (i.v.) with 5 mg/mL of Evans Blue (1 mL). Fifteen minutes later, the rats were sacrificed and the resultant blue spots were scored. (C) SHLI and F2 can activate C5 in plasma in vitro. The plasma was treated with test substances at the indicated concentrations and C5a level was determined by a commercial ELISA kit. *P < 0.05, **P < 0.01 and ***P < 0.001.
Next, we determined whether the 8 constituents could directly activate C5 in vitro using a commercial C5a ELISA kit. Except for luteolin, which was likely to interfere with the ELISA system, the other 7 constituents could directly activate C5 in vitro in a concentration-dependent manner; their half effective concentration (EC50) values were shown in Table S2. We also determined the effects of SHLI and F2. As expected, SHLI or F2 increased the C5a level in plasma in a concentration-dependent manner (Fig. 6C).
Discussion
Although TCMI-induced ADRs occur at a very low frequency, anaphylaxis attracts great attention because of its life-threatening outcome. IV-SHL has the highest incidence of TCMI-induced IHRs. For this reason, many researchers have focused on evaluating IHRs induced by IV-SHL. The majority of them supported the view that IV-SHL could cause IgE-mediated IHRs13,36–39 although this was also questioned40,41, while only a few researchers reported that IV-SHL has the potential to cause an anaphylactoid reaction resulting from direct degranulation in mast cells or basophils42,43. Nevertheless, to date, the supporters of IgE-mediated IHRs have not identified the specific IgE for SHLI, and the supporters of anaphylactoid reaction cannot provide a convincing mechanism. Therefore, the mechanisms of IV-SHL-induced IHRs are a matter of debate. In the present study, we systemically researched the mechanisms of IHRs induced by IV-SHL.
IHRs, sub-divided into mild, moderate, or severe/life-threatening (namely anaphylaxis4), can be allergic- as well as nonallergic-mediated32. In mice, two immunological pathways have been defined: IgE-FcεRI mediates the classical pathway and IgG-FcγRIII mediates the alternative pathway26,29,44. Considerably more antibody and antigen are required to induce IgG- compared with IgE-mediated anaphylaxis29, which most likely reflects the much higher affinity of IgE binding to FcεRI than IgG binding to FcγRIII. By immunization with an aluminum adjuvant, SHLI significantly elevated the serum tIgE and MMCP1 levels (Fig. 1A,B), which is in agreement with a previous report13. The tIgE is the summation of sIgE against different antigens and SHLI prepared by water-extraction and alcohol-precipitation mainly comprises micromolecular compounds. Unexpectedly, screening by the h-CLAT method showed that 4 suspicious allergens were not the haptens for IgE. Moreover, after challenge by SHLI, the serum MMCP1 concentration of passively sensitized mice was not affected (Fig. 1E). These findings demonstrate that SHLI does not contribute to IgE-mediated IHRs. In fact, our data showed that the elevated effects of SHLI on serum tIgE and MMCP1 could be attributed to its antimicrobial activity (Fig. 2).
Clinically, IHRs induced by SHLI commonly occur after a first exposure45, conforming to the characteristics of NA-IHRs25. However, as a mitochondrial calcium uniporter agonist, SHLI can directly dampen mast cells or basophils degranulation17, suggesting that the IHRs induced by SHLI may occur indirectly. Thus, in this study, we evaluated SHLI-induced local and systemic NA-IHRs in vivo. Our data indicated that SHLI, predominantly its F2 fraction, contributed to local and systemic NA-IHRs (Figs 3 and 4A,B). Using specific antagonists26,34, we showed that histamine, rather than PAF, was the principal effector in SHLI-induced NA-IHRs (Fig. 4C). To our knowledge, PAF is the major mediator responsible for IgG/C3a-induced anaphylaxis in macrophages26,27,46, while histamine is a mast cell-derived mediator. Besides MrgprB2 or MrgprX2 on mast cells25, engagement of complement receptors with their ligands can also induce histamine release47. In fact, CARPA is a main class of drug-induced acute immune toxicity15. The complement-derived anaphylatoxins C3a and C5a act as cell activators by binding to their respective receptors, C3aR and C5aR. Anaphylatoxin receptors are widely expressed on the cells of myeloid origin, including mast cells, basophils and macrophages, etc. Unlike macrophages liberation PAF, histamine is released by mast cells and basophils16. Due to a very little amount of histamine released from basophils34, the major effector cells should be mast cells48. C5a has long been identified as a potent liberator of histamine49, our findings identified that SHLI promotes NA-IHRs by directly activating C5, thereby activating its effector cells to release histamine (Fig. 5). Notably, 8 constituents from F2 could directly activate C5 (Fig. 6 and Table S2) to promote local vasopermeability at the mg/mL level, which are far higher than their respective equivalent concentrations in SHLI, indicating that there might be a series of active ingredients similar to these 8 constituents that collectively activate C5 to induce NA-IHRs.
In summary, this is the first study to show that SHLI-induced IHRs is a CARPA, rather than an IgE-mediated allergy. SHLI-induces C5 activation and then provokes histamine release, which might be a principal effector of its IHRs. The results of the present study might lead to the design of new strategies to prevent or treat IHRs induced by IV-SHL.
Electronic supplementary material
Acknowledgements
This work was supported by the CAMS Innovation Fund for Medical Sciences (2016-I2M-3-015); the National S&T Major Project and Scientific Researchers Aiding Enterprise Item from the Ministry of Science and Technology of the People’s Republic of China (Nos 2014ZX09201022-006 and 2015ZX09501004-001-003); and the National Natural Science Foundation of China (Nos 81601385 and 81274163).
Author Contributions
G.Y. and Q.Y. designed and wrote the study; G.Y., H.R. and H.Y. performed the main experiments; F.Q. performed some biological experiments; C.R. analyzed the data. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21843-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.State Pharmacopoeia Committee. Chinese Pharmacopoeia, Vol. 1, 846–848 (Chemical Industry Press, 2010).
- 2.Kong XT, et al. Treatment of acute bronchiolitis with Chinese herbs. Arch. Dis. Child. 1993;68:468–471. doi: 10.1136/adc.68.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JR, Zhang D, Zhang XM, Zhang B. Systematic review of Shuanghuanglian jnjection in the treatment of acute upper respiratoryInfection. Chin. J. Pharmacoepidemiol. 2016;25:269–274. [Google Scholar]
- 4.Johansson SG, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 5.Long XW, Ma JX. Clinical analysis of Shuanghuanglian injection-induced allergic reaction (64 cases) Chinese Journal of Information on Traditional Chinese Medicine. 2008;15:112. [Google Scholar]
- 6.National drug adverse reaction monitoring center. Information bulletin of adverse drug reaction (No. 22)–warning the severe adverse reactions induced by levofloxacin and Shuanghuanglian injection. China Food and Drug Administration Web. http://www.sda.gov.cn/WS01/CL0078/38014_1.html (2009).
- 7.Wu, Z. Talk about revaluation of the safety of traditional Chinese medicine injection. China Food and Drug Administration Web. http://www.sda.gov.cn/WS01/CL0049/39953.html (2009).
- 8.Wen ZY, et al. Preliminary report of the monitoring of traditional Chinese medicine injection-induced adverse reaction. Traditional Chinese Drug Research & Clinical Pharmacology. 2003;17:278–281. [Google Scholar]
- 9.Liang JQ, et al. Literature research for the characteristic and rule of traditional Chinese medicine injection-induced adverse reaction. Chinese Journal of Drug Application and Monitoring. 2010;7:158–161. [Google Scholar]
- 10.Huang XP. Analysis of the adverse drug reactions induced by traditional Chinese medicine injection (3640 cases) Modern Medicine & Health. 2007;23:3461–3462. [Google Scholar]
- 11.Ding JW. Comprehensive analysis of traditional Chinese medicine injection-induced allergic shock (481 cases) Chinese Journal of Clinical Pharmacy. 2009;18:293–296. [Google Scholar]
- 12.Tang W, et al. Meta-analysis on incidence of adverse drug reaction induced by Shuanhguanglian injection. China Journal of Chinese Materia Medica. 2016;41:2732–2742. doi: 10.4268/cjcmm20161427. [DOI] [PubMed] [Google Scholar]
- 13.Li ZG, Gao Y, Wang HS, Liu Z. A rat model of ShuangHuang Lian injection-inducedanaphylaxis. Asian Pac. J. Allergy Immunol. 2010;28:185–191. [PubMed] [Google Scholar]
- 14.Wang HS, et al. Hypotensive response in rats and toxicological mechanisms induced by shuanghuanglian, an herbal extract mixture. Drug Discov. Ther. 2010;4:13–18. [PubMed] [Google Scholar]
- 15.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128:36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, et al. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci. Rep. 2017;7:38736. doi: 10.1038/srep38736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999;103:206–214. doi: 10.1016/S0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 19.Hill DA, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi H, Miyazawa M, Yoshida Y, Ito Y, Suzuki H. Prediction of preservative sensitization potential using surface marker CD86 and/or CD54 expression on human cell line, THP-1. Arch. Dermatol. Res. 2007;298:427–437. doi: 10.1007/s00403-006-0714-9. [DOI] [PubMed] [Google Scholar]
- 21.Ashikaga T, et al. Development of an in vitro skin sensitization test using human cell lines: the human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicol. In Vitro. 2006;20:767–773. doi: 10.1016/j.tiv.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, et al. Preparation of highly specific anti-zearalenone antibodies by using the cationic protein conjugate and development of an indirect competitive enzyme-linked immunosorbent assay. Analyst. 2012;137:229–236. doi: 10.1039/C1AN15487G. [DOI] [PubMed] [Google Scholar]
- 23.Church MK, Miller P. Time courses of the anti-anaphylactic and anti-inflammatory effects of dexamethasone in the rat and mouse. Br. J. Pharmacol. 1978;62:481–486. doi: 10.1111/j.1476-5381.1978.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc. Natl. Acad. Sci. USA. 2011;108:12413–12418. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil BD, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J. Allergy Clin. Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 27.Khodoun M, et al. Peanuts can contribute to anaphylactic shock by activating complement. J. Allergy Clin. Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiszhár Z, et al. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012;45:492–498. doi: 10.1016/j.ejps.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J. Clin. Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill DA, Artis D. The influence of commensal bacteria-derived signals on basophil-associated allergic inflammation. Gut Microbes. 2013;4:76–83. doi: 10.4161/gmic.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, et al. Analysis of multiple constituents in a Chinese herbal preparation Shuang-Huang-Lian oral liquid by HPLC-DAD-ESI-MSn. J. Pharm. Biomed. Anal. 2007;44:430–438. doi: 10.1016/j.jpba.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Johansson SG, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 33.TenBrook JA, et al. Should beta-blockers be given to patients with heart disease and peanut-induced anaphylaxis? A decision analysis. J. Allergy Clin. Immunol. 2004;113:977–982. doi: 10.1016/j.jaci.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimura Y, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AR, Hugli TE, Müller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067–1080. [PMC free article] [PubMed] [Google Scholar]
- 36.Guo SS, et al. Establishment and applicability evaluation of the BN rat model for assaying the immediate hypersensitivity reaction induced by traditional Chinese medicines injections. China Journal of Chinese Materia Medica. 2011;36:1845–1849. [PubMed] [Google Scholar]
- 37.Zhang RX, et al. Comparison of the anaphylaxis reactions induced by chlorogenic acid and Shuang Huang Lian power-injeciton. World Science and Technology (Modernization of Traditional Chinese Medicine and Materia Medica) 2010;12:1005–1008. [Google Scholar]
- 38.Li JC, Li Y, Chen Q, Cheng ZH, Tan S. Immunotoxicity research on intravenous Shuang Huang Lian. Traditional Chinese drug Research & Clinical Pharmacology. 2008;5:172–174. [Google Scholar]
- 39.Wang ZX, et al. Anaphylaxis study on three traditional Chinese medicines injection. Journal of Shanxi College of Traditional Chinese Medicine. 2011;12:5–7. [Google Scholar]
- 40.Huang CG, et al. Effect of traditional Chinese medicine injections on I-type allergy. China Journal of Chinese Materia Medica. 2011;36:801–805. [PubMed] [Google Scholar]
- 41.Lu, Z. H. Study on immunosensitizing potential of Shuanghuanglian injection using popliteal lymph node assay. Doctoral Dissertation, Shandong University (2010).
- 42.Luo X, Wang Q, Zhou L, Dong Y, Jiang YP. Effects of some traditional Chinese medicine injections on the degranulation in RBL-2H3 cell. Traditional Chinese drug Research & Clinical Pharmacology. 2009;20:506–510. [Google Scholar]
- 43.Han S, et al. New method of screening allergenic components from Shuanghuanglian injection: with RBL-2H3/CMC model online HPLC/MS system. J. Pharm. Biomed. Anal. 2014;88:602–608. doi: 10.1016/j.jpba.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Oettgen HC, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 45.Yan G, et al. Investigation of toxicological effects of Shuanghuanglian injection in Beagle dogs by metabonomic and traditional approaches. Exp. Biol. Med. (Maywood) 2010;235:1356–1364. doi: 10.1258/ebm.2010.009390. [DOI] [PubMed] [Google Scholar]
- 46.Jiao D, et al. Macrophages are the dominant effector cells responsible for IgG-mediated passive systemic anaphylaxis challenged by natural protein antigen in BALB/c and C57BL/6 mice. Cell Immunol. 2014;289:97–105. doi: 10.1016/j.cellimm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol. Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- 48.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J. Allergy Clin. Immunol. 2016;137:1674–1680. doi: 10.1016/j.jaci.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollmann TJ, Mueller-Ortiz SL, Braun MC, Wetsel RA. Disruption of the C5a receptor gene increases resistance to acute Gram-negative bacteremia and endotoxic shock: opposing roles of C3a and C5a. Mol. Immunol. 2008;45:1907–1915. doi: 10.1016/j.molimm.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.