Abstract
Acute graft-versus-host disease (GVHD) in the gut is common following hematopoetic cell transplantation (HCT) and is associated with high mortality. However, it remains unclear whether Th1 or Th17 CD4+ T cells can initiate acute gut GVHD. In this issue of the JCI, Ullrich and colleagues identified a subset of CD4+ T cells that express high levels of IL-7Rα and granulocyte-macrophage CSF (IL-7RαhiGM-CSF+) cells that are involved in the induction of acute gut GVHD in murine models. The IL-7RαhiGM-CSF+ effector memory cells were BATF dependent, RORγt independent, produced large amounts of GM-CSF and IFN-γ, and released little IL-17. CD4+IL-7RαhiGM-CSF+ cells were not classical Th17 cells but had more of a Th1-like phenotype, despite their dependence on BATF. This work suggests that targeting the IL-7R/BATF/GM-CSF axis has therapeutic potential for treating acute gut GVHD.
Organ and stage specificity of GVHD-mediating T cells
Graft-versus-host disease (GVHD) is an exaggerated systemic immune response initiated by alloreactive T cells that primarily target the gut, liver, lung, and skin (1). The progression of GVHD can be divided into acute and chronic phases. While acute GVHD is characterized by T cell infiltration and tissue cell apoptosis (2), chronic GVHD is often a continuation of acute GVHD that is perpetuated by graft-derived memory T cells along with de novo thymus-generated T cells and characterized by subsiding T cell infiltration and increasing collagen production (3, 4).
When activated, naive CD4+ T cells can differentiate into Th1, Th2, or Th17 or Tregs cells, depending on the cytokine milieu, and are controlled by specific transcription factors . Th1 differentiation is controlled by the transcription factors T-bet and STAT1/STAT4; Th2 by Gata3 and STAT6; Th17 by RORγt and Stat3/BATF; and Treg cells by FOXP3 and STAT5 (5–7). After hematopoietic cell transplantation (HCT), alloreactive T cells also differentiate into Th1, Th2, or Th17 or Treg cells and reciprocally regulate each other (8). For example, IFN-γ from Th1 cells can suppress Th2 and Th17 cell differentiation, and IL-17 from Th17 cells can inhibit Th1 cell differentiation (8, 9). The organ-specific tissue damage mediated by subsets of Th cells is associated with tissue-specific tropism that is mediated by differential expression of homing and chemokine receptors (8). For example, Th1 cells express high levels of integrin α4β7 and the chemokine receptors CCR9 and CXCR3, while gut tissue expresses the α4β7 ligand MAdCAM-1, the CCR9 ligand CCL25, and the CXCR3 ligand CCL9-11 (8). Early after HCT, the acute inflammatory environment caused by the conditioning regimen favors differentiation of Th1 cells and cytotoxic T lymphocytes (Tc1), the predominant infiltrating T cells in the gut (8, 10). Th17 cells express CCR4 and CCR6, and lung and skin tissues express the CCR4 ligands CCL17 and CCL22, as well as the CCR6 ligand CCL20 (8). Thus, Th2 and Th17 cells preferentially infiltrate lung and skin tissues (8). Skin-infiltrating T cells are observed mostly as Th1 cells early after HCT, while Th17 cell infiltration occurs gradually and becomes obvious during chronic GVHD (11). Th17 cells mediate cutaneous chronic GVHD via IL-22 production (12); therefore, tissue infiltration of Th subsets is target-organ specific and inflammation-phase dependent. Gut tissue–infiltrating Th cells during the early acute phase of GVHD are predominantly IFN-γ–producing Th1 and Tc1 cells.
IFN-γ- but not IL-17–producing CD4+ T cells ignite acute gut GVHD
Alloreactive T cell activation has been proposed to begin in the mesenteric lymph nodes (MLNs) and Peyer’s patches (13, 14). Acute GVHD in the gut, the most common site of acute GVHD, results in a high mortality rate (15) and is primarily mediated by alloreactive CD4+ T cells. While transient in vivo depletion of donor CD4+ T cells effectively prevents the induction of acute gut GVHD (16), the subset of CD4+ T cells that initiates gut GVHD remains unclear. In this issue of the JCI, Ullrich et al. have identified a subset of CD4+ T cells that initiates acute gut GVHD (17). These CD4+ T cells exhibited an effector memory (Tem) phenotype, with high levels of IL-7 receptor α (IL-7Rα) expression and production of granulocyte-macrophage CSF (GM-CSF). These IL-7RαhiGM-CSF+CD4+ Tem cells produced large amounts of IFN-γ, TNF-α, and GM-CSF and little IL-17. Expression of IL-7Rα was required for expansion of this subset, and production of GM-CSF was required for optimal pathogenicity. The generation of these CD4+ Tem cells was BATF dependent but RORγt independent. The CD4+ Tem cells were augmented with IL-23 to produce GM-CSF, without production of IL-17.
Another subset of donor CD4+ T cells with a central memory (Tcm) phenotype and expression of surface CD11c and IL-23R (CD11c+IL-23R+CD4+ Tcm cells) was recently identified by the Drobyski group as colitogenic CD4+ Tcm cells in acute GVHD recipients (18). CD11c+IL-23R+ CD4+ Tcm cells also produced large amounts of IFN-γ and TNF-α, with little IL-17. Although expression of IL-23R was required for CD11c+IL-23R+CD4+ Tcm cell pathogenicity, IL-23 did not induce IL-17 production. This is consistent with the observation that IL-23 augmented IL-7RαhiGM-CSF+CD4+ Tem cell production of GM-CSF in the absence of IL-17 (17), although it is not yet clear whether the CD11c+IL-23R+CD4+ Tcm cells are also BATF dependent and RORγt independent. Together, these observations indicate that the CD4+ T cells that initiate acute gut GVHD are IFN-γ–producing, Th1-like cells but not IL-17–producing Th17 cells. Interestingly, Fu et al. have reported that the prevention of acute gut GVHD requires T-bet deficiency in donor CD4+ T cells (19).
Acute gut GVHD pathogenesis differs from chronic GVHD and autoimmune colitis
BATF was found to be required for the differentiation of RORγt-dependent Th17 cells that produced IL-17 and GM-CSF in a mouse model of experimental autoimmune encephalomyelitis (EAE), as well as in colitis-associated colon cancer (6, 20); however, the BATF-dependent IL-7RαhiGM-CSF+ CD4+ Tem cells in acute GVHD gut tissue were RORγt independent and did not produce IL-17, but instead produced IFN-γ, which is usually produced by T-bet–dependent Th1 cells (17). The mechanisms that regulate the differential generation of BATF-dependent, RORγt-independent CD4+ Tem cells in acute gut GVHD as compared with BATF-dependent, RORγt-dependent Th17 cells in autoimmune EAE and colitis remain unclear, and these differences are likely conferred by the specific cytokine environment encountered. Acute GVHD is characterized by a cytokine storm with high concentrations of IFN-γ, TNF-α, IL-1, and IL-6 and low levels of TGF-β, a cytokine environment that favors Th1, but not Th17, differentiation (10). Moreover, IFN-γ from Th1 cells can further inhibit this Th17 differentiation (8). As acute inflammation subsides and transitions into chronic inflammation, concentrations of IFN-γ decrease, those of TGF-β increase, and the cytokine environment gradually begins to favor Th17 differentiation (10). Thus, Th17 may participate in the pathogenesis at the early chronic GVHD phase. Although studies indicate that Th17 cells contribute to the pathogenesis of acute GVHD in mouse models and in humans (21, 22), kinetic analysis of gut tissue–infiltrating Th1 and Th17 cells has not been reported. Additionally, transplants from mice lacking RORγt (Rorγt–/–) do not prevent the induction of acute gut GVHD, despite a reduction in the severity of gut GVHD (23). Together, these observations indicate that Th17 cells are unlikely to initiate or play a critical role in acute gut GVHD.
Perspectives
Chung et al. recently reported that delta-like 1/4 (DLL1/4) ligand–mediated notch signaling is involved in the interaction between alloreactive CD4+ T cells and host fibroblast stromal cells (FSCs) and/or fibroblast reticular cells (FRCs) in the lymph nodes and plays a critical role in priming the alloreactive CD4+ T cells within 48 hours of HCT (24). Thus, it is likely that interaction of the Notch receptor with its DLL1/4 ligands plays an important role in activating the memory CD4+ T cells that initiate gut GVHD.
Although both CD11c+CD23R+CD4+ Tcm and IL-7RαhiGM-CSF+CD4+ Tem cells initiate acute gut GVHD, the relationship between these subsets remains unclear. While depletion of naive T cells reduces chronic GVHD in both mouse models and humans, memory T cells are still able to induce acute gut GVHD (25). Therefore, it is possible that a subset of memory CD4+ T cells can initiate gut GVHD, followed by amplification of the disease by activation and expansion of naive alloreactive T cells. Here, we propose a hypothesis that the memory CD4+ T cells ignite and promote acute gut GVHD development via interaction with host FSCs and FRCs, naive alloreactive T cells, and other innate immune cells.
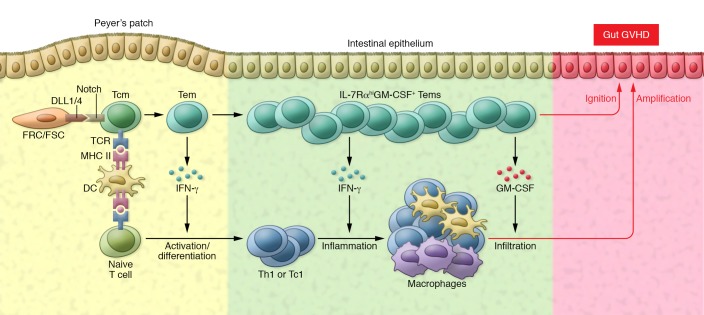
Shortly after HCT, the CD11c+IL-23R+CD4+ Tcm cells and naive alloreactive T cells migrate into MLNs and Peyer’s patches (Figure 1). Here, the Tcm CD4+ T cells immediately interact with host DCs via their T cell receptor–MHC II binding and interact with host FSCs and FRCs via Notch receptors and DLL1/4 ligands. The reactivated Tcm cells rapidly develop into Tem cells that produce large amounts of IFN-γ and other cytokines to augment naive alloreactive T cell activation and expansion. At the same time, Tem cells that express the gut-homing molecules α4β7 and CCR9 migrate into gut tissues and mediate local inflammation via production of GM-CSF and IFN-γ. The high-level of IL-7Rα expression and production of IL-2 make these Tem cells resistant to tissue programmed death ligand 1–mediated (PDL1-mediated) tolerance, as previously demonstrated (16). The production of GM-CSF and other cytokines and chemokines by Tem cells helps augment infiltration of innate immune cells and expansion of the alloreactive T cells derived from naive T cells, leading to full-blown gut GVHD. High concentrations of IFN-γ and low concentrations of TGF-β in the tissues during the early phase of acute gut GVHD can inhibit Th17 cell differentiation (8, 10). However, as acute inflammation transitions into the chronic phase, and as tissue concentrations of IFN-γ decrease and those of TGF-β increase, the effect of TGF-β, along with IL-6 and IL-1, leads to Th17 cell differentiation. Therefore, BATF-dependent, RORγt-independent Th1-like CD4+ T cells initiate acute gut GVHD development, and RORt-dependent Th17 cells may participate in the early stage of chronic GVHD.
Figure 1. Diagram depicting the interactions of BATF-dependent, RORγt-independent Tcm cells and Tem cells with host DCs, FSC/FRC, and donor naive T cells during development of acute gut GVHD.
IFN-γ from Tem cells augments naive T cell activation and differentiation into Th or Tc1 cells in the Peyer’s patch. Tem cells produce GM-CSF to augment the infiltration of innate immune cells such as macrophages, while producing IFN-γ to augment local inflammation. Tem cells ignite gut GVHD, and other cells amplify this GVHD. TCR, T cell receptor.
Future studies are warranted to test this hypothesis. In particular, several questions need to be answered. First, can CD11c+IL-23R+CD4+ Tcm cells give rise to the BATF-dependent, RORγt-independent IL-7RαhiGM-CSF+ Tem cells, and are these cells T-bet dependent? Second, how do Tcm cells interact with FSCs and FRCs in MLNs and Peyer’s patches? Finally, as dysbiosis plays an important role in augmenting acute gut GVHD (26), how does the intestinal microbiota influence the activation and expansion of Tcm cells, or vice versa?
In summary, BATF-dependent, RORγt-independent IL-7RαhiGM-CSF+ Tem cells play a critical role in initiating acute gut GVHD pathogenesis, and targeting the IL-7R/BATF/GM-CSF axis has therapeutic potential for treating acute gut GVHD.
Acknowledgments
The author’s research is supported by a grant from the National Institute of Allergy and Infectious Diseases (NIAID), NIH (R01 AI 066008). The author thanks his lab members Qingxiao Song and Kaniel Cassady for their help: Qingxiao for making the artful diagram of Figure 1, and Qingxiao and Kaniel Cassady for their critical review of this Commentary.
Version 1. 01/29/2018
Electronic publication
Version 2. 03/01/2018
Print issue publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Reference information: J Clin Invest. 2018;128(3):897–899. https://doi.org/10.1172/JCI98685.
See the related article at BATF-dependent IL-7RhiGM-CSF+ T cells control intestinal graft-versus-host disease.
References
- 1. Cutler C, Antin JH. Manifestations and treatment of acute graft-versus-host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, eds. Thomas’ Hematopoietic Cell Transplantation. Oxford, United Kingdom: Wiley-Blackwell; 2009:1287–1303. [Google Scholar]
- 2.Chakraverty R, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203(8):2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu T, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191(1):488–499. doi: 10.4049/jimmunol.1300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179(5):3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16(3):149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 8.Yi T, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114(14):3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi T, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112(5):2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henden AS, Hill GR. Cytokines in graft-versus-host disease. J Immunol. 2015;194(10):4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, et al. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016;127(18):2249–2260. doi: 10.1182/blood-2015-09-668145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gartlan KH, et al. A critical role for donor-derived IL-22 in cutaneous chronic GVHD. Am J Transplant. doi: 10.1111/ajt. [published online ahead of print September 23, 2017]. https://doi.org/10.1111/ajt.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murai M, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4(2):154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 14.Beilhack A, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111(5):2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naymagon S, et al. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist. Nat Rev Gastroenterol Hepatol. 2017;14(12):711–726. doi: 10.1038/nrgastro.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni X, et al. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest. 2017;127(5):1960–1977. doi: 10.1172/JCI91138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ullrich E, et al. BATF-dependent IL-7RhiGM-CSF+ T cells control intestinal graft-versus-host disease. J Clin Invest. 2018;128(3):916–930. doi: 10.1172/JCI89242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou V, et al. A colitogenic memory CD4+ T cell population mediates gastrointestinal graft-versus-host disease. J Clin Invest. 2016;126(9):3541–3555. doi: 10.1172/JCI80874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu J, et al. T-bet is critical for the development of acute graft-versus-host disease through controlling T cell differentiation and function. J Immunol. 2015;194(1):388–397. doi: 10.4049/jimmunol.1401618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punkenburg E, et al. Batf-dependent Th17 cells critically regulate IL-23 driven colitis-associated colon cancer. Gut. 2016;65(7):1139–1150. doi: 10.1136/gutjnl-2014-308227. [DOI] [PubMed] [Google Scholar]
- 21.Kappel LW, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113(4):945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossard C, et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft-versus-host disease. Leukemia. 2012;26(7):1471–1474. doi: 10.1038/leu.2012.41. [DOI] [PubMed] [Google Scholar]
- 23.Fulton LM, et al. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORγt. J Immunol. 2012;189(4):1765–1772. doi: 10.4049/jimmunol.1200858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung J, et al. Fibroblastic niches prime T cell alloimmunity through Δ-like Notch ligands. J Clin Invest. 2017;127(4):1574–1588. doi: 10.1172/JCI89535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleakley M, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125(7):2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shono Y, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]