Abstract
Uveal melanoma, a rare subset of melanoma, is the most common primary intraocular malignancy in adults. Despite effective primary therapy, nearly 50% of patients will develop metastatic disease. Outcomes for those with metastatic disease remain dismal due to a lack of effective therapies. The unique biology and immunology of uveal melanoma necessitates the development of dedicated management and treatment approaches. Ongoing efforts seek to optimize the efficacy of targeted therapy and immunotherapy in both the adjuvant and metastatic setting. This review provides a comprehensive, updated overview of disease biology and risk stratification, the management of primary disease, options for adjuvant therapy, and the current status of treatment strategies for metastatic disease.
Keywords: adjuvant therapy, immunotherapy, liver-directed therapy, targeted therapy, uveal melanoma
Epidemiology and biology of uveal melanoma
Uveal melanoma represents less than 5% of all melanoma cases in the United States, but it is the most common primary intraocular malignancy in adults, accounting for 85–95% of all ocular melanoma cases.1 Uveal melanoma arises from melanocytes along the uveal tract, including the iris, ciliary body and choroid. The majority of cases originate in the choroid (~85%), with remaining cases arising from the ciliary body (5–8%) and the iris (3–5%).1–3
Although the rate of cutaneous melanoma continues to rise, the incidence of uveal melanoma in the United States has remained stable at approximately 5.1 per million since the 1970s.4 The median age at diagnosis is about 62 years. Risk factors for the development of uveal melanoma include fair skin, light eye color (green or blue), welding, ocular melanocytosis, dysplastic nevus syndrome and the presence of a germline BRCA1-associated protein 1 (BAP1) mutation.5–9 Patients harboring BAP1 mutations typically present at a younger age, between 30 and 59 years.
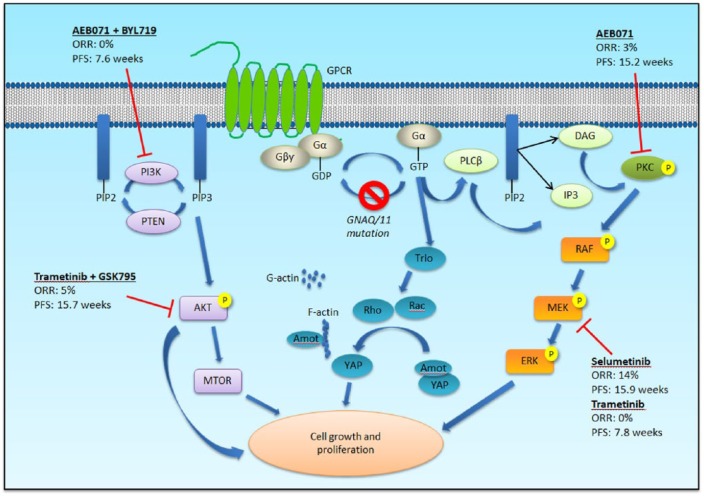
The biology of uveal melanoma differs from that of cutaneous melanoma. The vast majority (85–95%) of uveal melanoma is characterized by activating mutations in genes encoding the G-protein-alpha subunits GNAQ or GNA11, which lead to stimulation of the MAPK and phosphatidylinositol 3-kinase (PI3K)/Akt pathways,10–13 as well as the transcriptional co-activator Yes-associated protein 1 (YAP1) through the Trio-Rho/Rac signaling circuit (Figure 1).14 Additional mutations mutually exclusive to those in GNAQ/11 have been identified in phospholipase C β4 (PLCB4) and the G-protein coupled receptor cysteinyl leukotriene receptor 2 (CYSLTR2), affirming the importance of the G-alpha signaling pathway in uveal melanoma.15,16
Figure 1.
G-alpha signaling pathway in uveal melanoma (adapted from Patel M et al. Clin Cancer Res 2011; 17(8): 2087–2100). G-protein coupled receptors (GPCR) signal through the heterotrimeric proteins, Gα and Gβγ. Mutations in GNAQ or GNA11 lead to constitutive activation of Gα and downstream stimulation of the mitogen-activated protein kinase (MAPK) pathway via phospholipase C (PLCβ) and protein kinase C (PKC). The phosphotidylinositol-3 kinase (PI3K)/Akt/mTOR and the Yes-activated protein (YAP) pathways are also activated. All three signaling pathways contribute to tumor growth and proliferation. Targeted therapy against various downstream effectors have had limited clinical efficacy. ORR - overall response rate; PFS - progression-free survival.
Several other genetic alterations have been implicated in the development of uveal melanoma. Inactivating mutations in BAP1, a tumor suppressor gene located on chromosome 3p, are found in approximately 47% of primary uveal melanoma and 84% of metastatic uveal melanoma cases, consistent with the association between BAP1 mutations and poor prognosis.9 Mutations in splicing factor 3B subunit 1 (SF3B1), involved in pre-messenger RNA splicing, while associated with more favorable prognostic features than BAP1 mutations, are also found in cases of delayed metastasis, with a median of 8.2 years.17,18 EIF1AX encodes for eukaryotic translation initiation factor 1A. These mutations are mutually exclusive from BAP1 and SF3B1 and are associated with a longer disease-free survival and a more favorable prognosis.17–20
Prognosis and risk stratification
Despite excellent rates of local disease control, nearly 50% of patients will ultimately succumb to metastatic disease, with the most common initial site being the liver. Outcomes are exceedingly poor following the development of distant disease. Approximately 20–30% of patients diagnosed with primary uveal melanoma die of systemic metastases within 5 years of diagnosis, and 45% die within 15 years.4,21
Various clinical, pathological and genetic features have been shown to predict metastatic disease and survival. Clinical stage using the American Joint Committee on Cancer (AJCC) staging system, based on tumor size, the degree of extraocular extension and ciliary body involvement, is a validated risk stratification method.22 Findings from a recent meta-analysis of phase II trials in metastatic uveal melanoma reported elevated lactate dehydrogenase (LDH) and increasing diameter of the largest liver lesion to be associated with inferior progression-free survival (PFS).23 Prognostic factors for inferior overall survival (OS) include ECOG performance status ⩾1, increasing age, male sex, elevated LDH, elevated alkaline phosphatase and increasing diameter of the largest liver lesion. Recurrent cytogenetic abnormalities also hold prognostic significance. Monosomy of chromosome 3 and amplification of 8q are associated with increased metastatic risk and worse survival. The concurrent presence of these two alterations portends a particularly poor prognosis.24 Other alterations associated with increased metastatic risk include loss of 8p, 6q and 1p.25
Gene expression profiling has emerged as an important prognostic tool that predicts metastatic risk with greater accuracy than clinical stage or chromosome 3 status. A prospectively validated, commercially available 15-gene expression panel developed by Castle Biosciences categorizes patients as Class 1 (low metastatic risk) or Class 2 (high metastatic risk).26 Class 1 disease is further subdivided into Class 1a and Class 1b, with a superior prognosis for Class 1a disease. The 5-year metastatic risk for patients with Class 1a, 1b and 2 uveal melanomas are 2%, 21% and 72%, respectively.27 More recently, the preferentially expressed antigen in melanoma (PRAME) has been reported to be an independent prognostic biomarker. In one analysis of 389 uveal melanoma patients, PRAME mRNA expression was the most significant predictor of metastatic risk in patients with Class 1 or disomy 3 tumors.28 The 5-year rate of metastasis was 0% for PRAME–/Class 1, 38% for PRAME+/Class 1 and 71% for Class 2 disease. A second study demonstrated that aberrant hypomethylation and activation of PRAME was associated with increased metastatic risk in both Class 1 and 2 tumors.29 Notably, PRAME expression was directly associated with SF3B1 mutations in Class 1 uveal melanomas, consistent with the development of late metastases in patients with SF3B1-mutated tumors.
The Rare Tumor Project of The Cancer Genome Atlas (TCGA) recently performed a comprehensive multiplatform analysis of 80 primary uveal melanoma tumors.30 Four molecularly distinct subtypes with varying clinical outcomes were identified: two associated with poor-prognosis monosomy 3 (M3) and two associated with better-prognosis disomy 3 (D3). Similar to prior reports, BAP1 alterations were detected in 83.3% of M3 tumors and are associated with a unique global DNA methylation profile distinct from the pattern seen in D3 tumors. Despite the shared methylation profile, M3 tumors can be further divided into two subgroups characterized by different biological pathways and clinical outcomes. For example, DNA damage repair, MYC, and hypoxia-inducible factor 1 alpha (HIF1A) signaling are more active in one M3 subgroup, whereas elevated levels of MAPK and AKT are more prominent in the other subgroup, suggesting these uveal melanoma subsets may benefit from tailored treatment strategies that target these differentially upregulated cellular pathways. Notably, M3 tumors also harbor higher levels of immunological genes associated with the presence of a CD8+ T cell infiltrate, interferon-γ signaling, T cell invasion, and immunosuppressive factors such as IDO1 and TIGIT. Agents targeting these suppressive factors, under investigation in ongoing trials, may therefore have clinical activity and help overcome the resistance to immune checkpoint inhibitors. Within the two D3 subtypes, EIF1AX- and SRSF2 (serine and arginine rich splicing factor 2)/SF3B1-mutant tumors harbor distinct genomic and DNA methylation profiles. However, the previously observed association between EIF1AX versus SF3B1 mutations and low versus intermediate metastatic risk was not seen, possibly due to relatively short follow-up times.
Management of primary uveal melanoma
Local treatment for uveal melanoma consists of either globe-preserving therapies (radiation, laser therapy, surgical resection) or enucleation. Based on a 2006 Collaborative Ocular Melanoma Study Group (COMS) study that demonstrated equivalent survival outcomes for patients with medium-sized choroidal melanomas randomized to iodine-125 brachytherapy versus enucleation, the majority of primary uveal melanomas in the United States are treated with first-line plaque brachytherapy.
Surgery
Enucleation was the historical approach to definitive, local treatment and is still appropriate in the presence of large tumor size, extensive extraocular growth, and a low probability of retaining vision. However, there has generally been a movement toward vision- and eye-preserving modalities after the 2006 COMS study failed to show a survival benefit with enucleation compared to brachytherapy.31 Of note, the follow-up COMS quality-of-life assessment did find that patients who underwent enucleation experienced less anxiety during subsequent clinic visits than patients who received brachytherapy.32
Transretinal endoresection and transscleral resection offer eye-preserving surgical approaches. Although transscleral resection is associated with improved retention of visual acuity compared to plaque brachytherapy, likely due to a reduction in late radiation adverse effects such as radiation retinopathy and neovascular glaucoma, it is a complicated procedure with more immediate complications including retinal detachment, ocular hypertension and submacular hemorrhage.33 Moreover, local recurrence rates are higher with transscleral resection than with brachytherapy or enucleation. In one matched case–control study, the rate of tumor recurrence was 6.1% after brachytherapy and 32.6% after transscleral resection.34 Several other studies have found similarly high rates of local recurrence.35,36
Radiation therapy
Brachytherapy, which involves suturing a radioactive plaque to the sclera to deliver focal radiation to the tumor, is the most frequently employed modality in the US. The most commonly employed radioisotopes are iodine-125 (125I) and ruthenium-106 (106Ru). Palladium 103 (103Pd) is rarely used, and cobalt 60 (60Co) was used in the past. 125I is the preferred isotope in the US and emits gamma radiation, which penetrates more deeply into tumors than the beta-emitting 106Ru, but is associated with increased toxicity to surrounding tissue.37 Following treatment, regular ophthalmologic exams should be performed to monitor for complications including radiation-induced retinopathy, cataracts, neovascular glaucoma and macular edema, which can develop up to 5 years after therapy.38 The use of intravitreal anti-vascular endothelial growth factor (VEGF) after brachytherapy has been shown to reduce or delay the rate of macular edema, moderate vision loss and poor visual acuity.39
For medium to large tumors or those in a location that may not be amenable to plaque brachytherapy, charged-particle radiotherapy can be used. This technique is preferred for tumors surrounding the optic disk and fovea, where plaques cannot be placed directly. Due to the physical properties of charged particles, specifically the sharp decline in radiation dose beyond the targeted area, collateral damage to normal ocular tissue is reduced. As a result, a high rate of local tumor control (>95% at 15 years)40 can be achieved without significantly worse complications than plaque brachytherapy. One prospective, randomized study comparing helium-ion therapy and brachytherapy for medium-sized choroidal and ciliary body melanomas found improved local control, eye preservation and disease-free survival with charged-particle therapy.41 The study did include patients with tumors close to the optic disk, but improved outcomes were still observed with charged-particle therapy after excluding these patients. That being said, plaque brachytherapy can achieve similar outcomes with careful patient and tumor selection.42
Stereotactic proton-beam therapy is another option for large tumors and may help spare the need for enucleation and vision loss. A large retrospective study of 492 patients with T3 and T4 choroidal melanomas demonstrated a 5-year local control rate of 94% and a 19.5% enucleation rate that decreased over time.43
Laser and photodynamic therapy
Photocoagulation or transpupillary thermotherapy (TTT) utilize focused thermal energy to destroy tumor cells. The techniques may be used as a primary therapy for small choroidal lesions,44,45 but given the variable efficacy in the primary setting, with a possible risk of extrascleral extension,46 and associated adverse effects including retinal vascular occlusions, vitreous hemorrhage, and retinopathy, patient selection is critical for successful outcomes. Rather, photocoagulation or TTT can be more widely used for residual disease or adjunctive therapy.47,48 One study found higher rates of local control, eye-globe preservation and recurrence-free survival with simultaneous ruthenium brachytherapy and TTT,49 whereas a separate analysis found no clinical benefit and only worse visual outcomes when TTT was administered either before or after brachytherapy.50 Photodynamic therapy, which involves the injection of a light-sensitive compound such as verteporfin followed by exposure to light in order to generate damaging free oxygen radicals, is also sometimes used for the treatment of small melanomas, but long-term disease control and recurrence-free survival remain unclear.51
Novel approaches
Tissue factor is a transmembrane cytokine receptor constitutively expressed in sub-endothelial tissues that, upon binding to its ligand factor VII, initiates the extrinsic coagulation cascade. Additional downstream signaling effects include activation of various growth and angiogenic pathways.52 Tissue factor is expressed at elevated levels in uveal melanoma cells compared to normal uveal melanocytes.53 Expression levels also correlate with the number of blood vessels in primary uveal melanoma tumors, suggesting its potential role in tumor angiogenesis, growth and metastasis. ICON-1 is an immunoconjugate protein in development by Iconic Therapeutics that consists of a structural variant of human factor VII. Binding of ICON-1 to cells that overexpress tissue factor eliminates pathologic neovascularization and targets tumor cells for removal by the immune system. An ongoing phase I study is evaluating the safety and activity of single and repeated escalating intravitreal doses of ICON-1 in patients with primary uveal melanoma prior to enucleation or brachytherapy [ClinicalTrials.gov identifier: NCT02771340].
Another novel therapy in development by Aura Biosciences is AU-011, which consists of viral nanoparticles, modeled on the human papillomavirus (HPV), conjugated to infrared-activated photodynamic dye. Following intravitreal injection, the viral conjugates selectively bind to cancer cells due to overexpression and modification of heparan sulfate proteoglycans on tumor cells, and destroy the cell membrane upon activation with an ophthalmic laser. AU-011 is currently being tested in a phase Ib/II trial in patients with small primary choroidal melanoma [ClinicalTrials.gov identifier: NCT03052127].
Adjuvant therapy and surveillance
Given the high metastatic risk associated with Class 2 tumors and the poor long-term prognosis of metastatic disease, an improved understanding of the biological mechanisms underlying disease dissemination and the development of effective adjuvant therapies are critical. Thus far, no systemic adjuvant therapy has been shown to reduce the risk of metastasis or improve OS. Dacarbazine, an alkylating agent that prevailed as the standard of care for metastatic cutaneous melanoma prior to the development of immunotherapy, offered no survival advantage compared to observation in a randomized adjuvant trial.54 Although adjuvant interferon (IFN) is approved by the FDA for use in resected cutaneous melanoma, two non-randomized studies failed to show any survival benefit with IFN therapy compared to matched historical controls following primary tumor treatment.55,56 A phase II study evaluating the combination of dacarbazine and IFN [ClinicalTrials.gov identifier: NCT01100528] has completed accrual, with results anticipated in the near future.
A number of novel therapies based on purported biological mechanisms are being investigated in the adjuvant setting. The growth factor receptors, c-Met and c-Kit, are highly expressed in uveal melanoma and may play a role in metastatic progression.57,58 Crizotinib is a tyrosine kinase inhibitor (TKI) that has been shown to inhibit phosphorylation of c-Met and in vitro migration of uveal melanoma cells. In a murine model of metastatic uveal melanoma, crizotinib significantly reduced the development of distant metastasis as compared to the untreated control arm.59 Interestingly, at doses that selectively inhibit c-Met, crizotinib only marginally reduced the growth of established tumors, suggesting that other tyrosine kinase receptors such as epidermal growth factor receptor (EGFR) and insulin growth factor receptor 1 (IGFR1) are critical for uveal melanoma cell proliferation and survival.60,61 Sunitinib, another TKI that inhibits c-Kit, vascular endothelial growth factor receptor (VEGFR) and other receptors, yielded a 5-year survival benefit (75% versus 55%) compared to matched controls in a retrospective study.62 Both crizotinib and sunitinib are being assessed in ongoing adjuvant trials [ClinicalTrials.gov identifiers: NCT02223819, NCT02068586].
Loss of BAP1 is associated with loss of melanocytic differentiation and increased metastatic potential in uveal melanoma. Histone deacetylase (HDAC) inhibitors have been shown to reverse the phenotypic effects of BAP1 loss by inducing morphologic differentiation and transition from a high-risk to a low-risk gene expression profile in uveal melanoma cells.63 Based on the potential role for HDAC inhibition in the adjuvant setting, valproic acid and vorinostat are being evaluated in ongoing trials [ClinicalTrials.gov identifiers: NCT02068586, NCT01587352]. Various other strategies are also being investigated (Table 1), including immune-based therapies such as immune checkpoint inhibition and an autologous dendritic cell vaccine.
Table 1.
Current adjuvant clinical trials in uveal melanoma.
| Mechanism | Trial | Phase | Identifier | Status |
|---|---|---|---|---|
| Chemotherapy | Dacarbazine + interferon-alfa | II | NCT01100528 | Accrual complete |
| Cisplatin, tamoxifen + sunitinib | II | NCT00489944 | Unknown | |
| Fotemustine versus observation | III | EudraCT Number 2008-005691-27 | Accrual complete | |
| Targeted therapy | Crizotinib | II | NCT02223819 | Recruiting |
| Sunitinib versus valproic acid | II | NCT02068586 | Recruiting | |
| Immunotherapy | Ipilimumab + nivolumab | II | NCT01585194 | Recruiting |
| Dendritic cell vaccine | I/II | NCT00929019 | Recruiting | |
| Liver-directed therapy | Prophylactic liver RT | II | NCT02336763 | Terminated* |
Study terminated due to lack of accrual.
There are no consensus guidelines regarding the optimal surveillance strategy following primary treatment. Various imaging modalities have been evaluated.64 Of these, magnetic resonance imaging (MRI) appears to have the greatest sensitivity in detecting small liver lesions that may not be seen on ultrasound, computed tomography (CT) or positron-emission tomography.65,66 For patients with low-risk disease based on cytogenetics or gene expression profiling, we generally recommend consideration of routine imaging with a CT scan of the chest and an MRI of the abdomen and pelvis every 6 to 12 months. Patients with a high risk of metastatic recurrence warrant closer observation, with imaging obtained every 3–6 months.
Treatment of metastatic uveal melanoma
Outcomes for patients with advanced disease are dismal, with a median OS ranging from 4 to 15 months.21,67,68 There is no FDA-approved standard of care for metastatic uveal melanoma. Various treatments have been evaluated, including systemic chemotherapy, immunotherapy, targeted agents against the MAPK pathway, and liver-directed therapies, but response rates are generally less than 10%, and no therapy has been shown to improve OS.69–71 A recent meta-analysis of 29 phase II trials in metastatic uveal melanoma conducted between 1988 and 2015 sought to define historical benchmarks of PFS and OS, and found disappointing outcomes across all the treatment groups.23 The median PFS was 3.29 months (6-month PFS 27%), and the median OS was 10.2 months (1-year OS 43%).
Liver-directed therapies
Uveal melanoma most commonly metastasizes to the liver. An analysis of patients enrolled in the COMS study found that 93% of patients had liver metastases at the time of death.72 Of those who had only one site of metastasis, the liver was involved in 95% of cases. Resection of hepatic lesions in highly select cases may offer long-term survival and cure, but the survival advantage may partly reflect patient selection.73 Radiofrequency ablation, stereotactic radiotherapy, regional chemotherapy such as hepatic intra-arterial infusion and isolated hepatic perfusion (IHP), and various embolization techniques are other liver-directed approaches. There is limited prospective data regarding the efficacy of liver-directed therapies, but available evidence suggests some clinical benefit. Interestingly, in the previously discussed meta-analysis, 6-month PFS was significantly higher with liver-directed therapy compared to chemotherapy, immunotherapy and targeted therapy, even after adjusting for prognostic factors.23
A phase III European Organization for the Research and Treatment of Cancer (EORTC) study randomized 171 patients with uveal melanoma and hepatic metastases to intra-arterial or intravenous fotemustine.74 Although there was no difference in OS (14.6 versus 13.8 months) between the two arms, there was a significant improvement in ORR (10.5 versus 2.5%) and PFS (4.5 versus 3.5 months) with the intra-arterial versus intravenous approach. IHP is a form of intra-arterial chemotherapy in which the liver is completely isolated from systemic circulation, allowing delivery of a high concentration of chemotherapy with minimal systemic exposure. A second phase III trial compared percutaneous isolated hepatic perfusion (PHP) with melphalan and best alternative care in 93 patients with melanoma metastatic to the liver (88% ocular, 12% cutaneous).75 Again, an improvement in ORR and PFS was seen with hepatic infusion. There was no improvement in OS, but this may have been confounded by crossover at the time of hepatic progression. There are two ongoing, randomized controlled, phase III trials further evaluating the efficacy of IHP. The SCANDIUM study seeks to evaluate whether IHP improves OS compared to best alternative care in patients with isolated liver metastases from uveal melanoma [ClinicalTrials.gov identifier: NCT01785316], and the FOCUS study by Delcath Systems Inc. is randomizing patients with liver-dominant disease to either PHP with melphalan or one of four options under best alternative care: transarterial chemoembolization, dacarbazine, ipilimumab or pembrolizumab [ClinicalTrials.gov identifier: NCT02678572].
Embolization techniques include bland embolization, hepatic arterial chemoembolization using a variety of chemotherapy agents (fotemustine, BCNU, cisplatin) followed by administration of an embolic agent, radioembolization using yttrium-90 (90Y)-labeled microspheres, and immunoembolization with granulocyte-macrophage colony-stimulating factor (GM-CSF). A randomized phase II study comparing immunoembolization (IE) and bland embolization (BE) in 52 patients with metastatic uveal melanoma found a numerically higher response rate (21.2% versus 16.7%) and OS (21.5 versus 17.2 months) in the IE group; the survival advantage was statistically significant in patients with at least 20% liver involvement.76 Given tumor destruction via embolization may lead to increased release of tumor antigens to the immune system, concurrent use of systemic immune checkpoint inhibitors is an area of interest and active investigation.
Chemotherapy
Chemotherapy regimens adopted from cutaneous melanoma, for example, dacarbazine, temozolomide, cisplatin, treosulfan, fotemustine, and various combinations, have been used in uveal melanoma with disappointing results. Response rates range from 0% to 15%, and no agent has been shown to prolong survival.77–81
Immunotherapy
Advances in immunotherapy, in particular the development of immune checkpoint inhibitors targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1), have dramatically improved outcomes for patients with advanced cutaneous melanoma. Unfortunately, a similar clinical benefit has not been borne out in metastatic uveal melanoma. The low mutational burden observed in uveal melanoma may partly account for the limited success of immune checkpoint blockade. Moreover, as identified in the TCGA analysis, upregulation of immunosuppressive factors such as IDO1 and TIGIT may contribute to treatment resistance and suggests a role for combination immune therapies targeting these additional factors.30
Anti-CTLA-4 therapy
CTLA-4 delivers a negative modulatory signal to T cells upon binding to CD80 or CD86 on antigen-presenting cells. Ipilimumab, a monoclonal antibody that blocks the CTLA-4 receptor, was the first agent to demonstrate an improvement in OS for patients with advanced cutaneous melanoma. The randomized phase III studies that led to its approval by the FDA in 2011 did not include patients with uveal melanoma, but smaller prospective and retrospective studies have found limited clinical activity.
One of the larger retrospective, multicenter analyses included 39 patients with metastatic uveal melanoma treated with ipilimumab, the majority of whom received the 3 mg/kg dose. An immune-related response rate of 5.1% was observed (one complete response, one late partial response), and the median OS from the first dose of ipilimumab was 9.6 months. A number of other series have demonstrated similarly low response rates ranging from 0% to 5%, a PFS of ~3 months, and an OS of less than ~10 months.
There have been two single-arm phase II studies evaluating the efficacy of ipilimumab. In the Spanish Melanoma Group (GEM) study, 32 treatment-naïve patients with progressive metastatic disease received ipilimumab 10 mg/kg every 3 weeks for 4 doses followed by a maintenance dose every 12 weeks. Interim findings presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in 2014 included 13 evaluable patients.82 After a median follow up of 5.5 months, one patient (7.7%) achieved a partial response and six patients (46.2%) had stable disease. The median OS was 9.8 months. The Dermatologic Cooperative Oncology Group (DeCOG) subsequently conducted a phase II study evaluating the efficacy of ipilimumab 3 mg/kg in patients with different subtypes of metastatic melanoma.83 Of the 53 patients with uveal melanoma (45 pre-treated and 8 treatment-naïve), 16 patients (47%) experienced stable disease at 12 weeks (21% at 24 weeks). There were no partial or complete responses. The median PFS and OS were 2.8 months and 6.8 months, respectively. Of note, 19 patients (36%) experienced grade 3–4 treatment-related adverse events, a higher number than has been reported in prior cutaneous melanoma studies.
Tremelimumab, another anti-CTLA-4 antibody, was also evaluated in a prospective phase II study in patients with untreated metastatic uveal melanoma.84 There were no responses among the 11 enrolled patients. The median PFS was 2.9 months, and the median OS was 12.8 months. Due to a lack of efficacy, the study was terminated after the interim analysis.
Anti-PD-1 therapy
The PD-1 pathway is responsible for inhibiting T cell proliferation and activity in peripheral tissues. Nivolumab and pembrolizumab are two anti-PD-1 receptor antibodies that are approved for the treatment of advanced cutaneous melanoma. Studies demonstrate superior outcomes and a more favorable toxicity profile with anti-PD-1 therapy compared to ipilimumab. Given that CTLA-4 and PD-1 downregulate different phases of T cell activation, combination therapy has been shown to be more effective than single-agent therapy in cutaneous melanoma.85 However, whether this benefit holds true in uveal melanoma remains unclear, given the phase III CheckMate-067 trial excluded uveal melanoma patients. The largest series to date evaluated outcomes for 58 patients with metastatic uveal melanoma treated with pembrolizumab (n = 38), nivolumab (n = 16) or atezolizumab (n = 2) at nine different academic centers.71 Of these, 36 patients (62%) had received prior ipilimumab. Of the 56 evaluable patients, 2 attained partial responses (3.6%) and 5 had stable disease (8.9%). Median PFS and OS were 2.8 months and 7.6 months, respectively. Several other smaller case series have reported variably low response rates and limited clinical benefit.86–88 Prospective trials of anti-PD-1 therapy, alone or in combination with other agents, are ongoing (Table 2).
Table 2.
Current clinical trials in metastatic uveal melanoma.
| Mechanism | Trial | Phase | Identifier | Status |
|---|---|---|---|---|
| Targeted therapy | ||||
| PKC/MEK | AEB071 + BYL719 | I | NCT02273219 | Accrual complete |
| Intermittent selumetinib | I | NCT02768766 | Recruiting | |
| Selumetinib +/– paclitaxel | II | ISRCTN29621851 | Recruiting | |
| Binimetinib + AEB071 * | I/II | NCT01801358 | Terminated | |
| LXS196 | I | NCT02601378 | Recruiting | |
| Multikinase inhibition | Sorafenib (STREAM) | II | NCT01377025 | Accrual complete |
| Cabozantinib versus temozolomide/dacarbazine | II | NCT01835145 | Accrual complete | |
| Immunotherapy | ||||
| Checkpoint blockade | Pembrolizumab | II | NCT02359851 | Accrual complete |
| Ipilimumab + nivolumab | II | NCT01585194 | Recruiting | |
| Ipilimumab + nivolumab | II | NCT02626962 | Accrual complete | |
| Ipilimumab + radioembolization * | I | NCT01730157 | Terminated | |
| Ipilimumab + nivolumab + radioembolization | 0 | NCT02913417 | Recruiting | |
| TILs | Tumor infiltrating lymphocytes | II | NCT01814046 | Accrual complete |
| Cellular adoptive immunotherapy + ipilimumab | I | NCT03068624 | Recruiting | |
| T cell redirection | IMCgp100 (second-line) | I | NCT02570308 | Recruiting |
| IMCgp100 (first-line) | II | NCT03070392 | Recruiting | |
| Antibody–drug conjugate | Glembatumumab vedotin | II | NCT02363283 | Accrual complete |
| Epigenetic therapy | ||||
| HDAC inhibition | Vorinostat | II | NCT01587352 | Accrual on hold |
| Pembrolizumab + entinostat (PEMDAC) | II | NCT02697630 | Recruiting | |
| BET inhibition | PLX51107 | I | NCT02683395 | Recruiting |
| Liver-directed therapy | ||||
| IHP | IHP versus best alternative care (SCANDIUM) | III | NCT01785316 | Recruiting |
| PHP | PHP versus TACE, dacarbazine, ipilimumab, or pembrolizumab (FOCUS) | III | NCT02678572 | Recruiting |
Study terminated for scientific or other reasons. BET, bromodomain and extra-terminal; HDAC, histone deacetylase; IHP, isolated hepatic perfusion; PHP, percutaneous hepatic perfusion; TIL, tumor infiltrating lymphocyte.
Novel immune-based approaches
Despite the disappointing results with immune checkpoint inhibition, a number of novel immune-based therapies have shown promising signs of clinical activity. IMCgp100 is a bispecific biologic in development by Immunocore, comprising targeting and effector moieties. The targeting end constitutes a soluble T cell receptor (TCR) that recognized the melanocyte-associated antigen glycoprotein 100 (gp100) presented in the context of HLA-A2, which is expressed in approximately 50% of patients with uveal melanoma, and the effector end includes an anti-CD3 single-chain variable fragment (scFv). In vitro, IMCgp100 redirects a potent T cell-mediated immune response toward gp100 positive melanoma cells.
The first-in-human (FIH) phase I study of IMCgp100 enrolled 84 patients with advanced melanoma, including 16 patients with uveal melanoma.89 In general, toxicities other than infusion-related reactions were grade 1 and 2 in severity. The most frequent treatment-related adverse effects included rash (100%), pruritus (64%), pyrexia (50%) and periorbital edema (46%). More severe infusion-related reactions involving grade 3 or higher hypotension typically occurred during the first 2 weeks of treatment. The observed side effects were largely attributed to chemokine release, movement of CD3+ T lymphocytes into tumor and normal tissues, and rarely cytokine release syndrome. Of the 15 patients with uveal melanoma evaluable for response, 3 (20%) achieved a partial response (2 confirmed, 1 unconfirmed), and 7 (47%) had stable disease. Six patients (40%) experienced disease control for ⩾24 weeks. The 1-year OS was 73%, a figure much higher than the previously reported OS benchmark of 43%.23
Based on the promising activity observed in uveal melanoma, a subsequent phase I study was initiated in patients with metastatic uveal melanoma [ClinicalTrials.gov identifier: NCT02570308]. To maximize response and minimize the risk of severe hypotension, an intra-patient dose-escalation design was used. In a retrospective review of response data from the FIH study, greater responses were generally noted at higher dose levels (65–85 mcg weekly). However, such high initial doses could not be achieved due to dose-limiting toxicities seen with the first and second weekly doses of IMCgp100. To address this issue, patients receive 20 mcg and 30 mcg on days 1 and 8, respectively, followed by a higher fixed-dose level in subsequent weeks. Preliminary findings were recently presented at the 2017 ASCO Annual Meeting.90 A total of 19 heavily pre-treated patients with metastatic uveal melanoma (median of four prior lines of therapy) received weekly IMCgp100 across four target dose cohorts (60, 70, 75 and 80 mcg). The 75 mcg dose was identified to be the maximum tolerated dose (MTD) and recommended phase II dose. The toxicity profile was similar to what was previously observed; the most common drug-related adverse effects were pruritus (84%), pyrexia (84%), fatigue (74%), hypotension (74%) and peripheral edema (63%). Grade 3–4 adverse effects included AST elevation (15%), erythema (15%) and hypotension (15%). Of the 16 evaluable patients, 2 (11%) achieved partial responses, and 5 (26%) had a minor response as defined by a ⩾10% reduction in the size of target lesions. Notably, the median PFS of 5.6 months is nearly double the PFS of 2.6 to ~3 months seen in prior immune checkpoint inhibition studies. The estimated 1-year OS of 79.5% again far exceeds the previously reported OS benchmark. The pivotal phase II study of IMCgp100 versus investigator’s choice of dacarbazine, pembrolizumab or ipilimumab in treatment-naïve patients with metastatic uveal melanoma was recently initiated [ClinicalTrials.gov identifier: NCT03070392].
The relatively long latency period between initial diagnosis and metastatic recurrence in uveal melanoma suggests some degree of immune surveillance, which may be augmented for therapeutic purposes. A recent study comparing the attributes of tumor infiltrating lymphocytes (TILs) in liver metastases from uveal melanoma and cutaneous melanoma found significantly greater anti-tumor reactivity in TILs from cutaneous melanoma versus uveal melanoma samples.91 However, a subset of uveal melanoma TILs displayed a comparable, robust level of reactivity. In an effort to determine whether adoptive transfer of such reactive TILs could induce tumor regression, a single-arm, phase II study was conducted [ClinicalTrials.gov identifier: NCT01814046]. At the time of interim analysis, a total of 21 patients with metastatic uveal melanoma were treated with lympho-depleting conditioning chemotherapy (cyclophosphamide and fludarabine) followed by a single infusion of autologous TILs and high-dose interleukin-2.92 Of the 20 evaluable patients, 7 (35%) had objective tumor regression (6 partial responses, 1 complete response), with 3 patients experiencing ongoing response at the time of report. Grade 3 or higher adverse effects were largely due to induction chemotherapy; all patients experienced lymphopenia, neutropenia and thrombocytopenia. Infection occurred in six patients (29%), and there was one treatment-related death due to sepsis and multi-organ failure.
Glembatumumab vedotin is a monoclonal antibody–drug conjugate directed against glycoprotein NMB, a transmembrane protein highly expressed in multiple tumor types, including uveal melanoma, and linked to the microtubule inhibitor monomethyl auristatin E (MMAE). Results from a single-arm, phase II study of glembatumumab in metastatic uveal melanoma [ClinicalTrials.gov identifier: NCT02363283] will be presented at the 2017 International Congress of the Society for Melanoma Research.93 A total of 35 patients were enrolled. Of the 31 evaluable patients, the ORR was 6%. An additional 17 patients (55%) had stable disease. The most frequent treatment-related adverse effects included alopecia, transaminitis and rash.
The cancer-testis antigen, preferentially expressed antigen in melanoma (PRAME), is a known prognostic biomarker of metastatic risk in patients with uveal melanoma29 and has also been proposed as a therapeutic target, given its lack of expression on normal cells. In fact, 69% of metastatic uveal melanoma tumors from a retrospective study expressed PRAME.94 Additionally, the investigators demonstrated that HLA-A2 restricted, PRAME-specific T cells were able to recognize and react against PRAME-positive uveal melanoma cell lines, suggesting a potential role for PRAME-directed immunotherapy. Vaccines targeting PRAME are being evaluated in PRAME-positive cutaneous melanoma [ClinicalTrials.gov identifier: NCT01149343] and non-small cell lung cancer [ClinicalTrials.gov identifier: NCT01853878], and may be a potential strategy in uveal melanoma as well.
Targeted therapy
Since uveal melanoma is characterized by mutations in GNAQ or GNA11, which lead to constitutive activation of the MAPK and PI3K/Akt pathways, therapies that target downstream effectors of these pathways such as MEK, Akt and protein kinase C (PKC) are being investigated. Unfortunately, results have been disappointing so far, with response rates generally less than 10% (Figure 1).
Selumetinib is a potent and highly selective inhibitor of MEK. A randomized, phase II study in 101 pre-treated or treatment-naïve patients with metastatic uveal melanoma demonstrated improved clinical outcomes with selumetinib compared to chemotherapy (temozolomide or dacarbazine).95 The primary endpoint of PFS was significantly longer among patients who received selumetinib compared to those who received chemotherapy (15.9 versus 7 weeks, p < 0.001). While no responses were seen in the chemotherapy arm, 7 patients (14%) in the selumetinib arm had a partial response. There was no significant difference in OS (11.8 versus 9 months, p = 0.09), but 86% of patients randomized to chemotherapy crossed over to receive selumetinib at the time of disease progression. These promising results prompted the subsequent phase III SUMIT trial that randomized 129 patients with treatment-naïve metastatic uveal melanoma to selumetinib plus dacarbazine versus dacarbazine alone. The SUMIT trial unfortunately did not meet its primary endpoint; median PFS was not significantly improved in the selumetinib plus dacarbazine arm compared to the dacarbazine alone arm based on blinded independent central review (2.8 versus 1.8 months, p = 0.32). Similarly, there was no significant difference in ORR by central review (3.1 versus 0%, p = 0.36).
Efforts to optimize the efficacy of MEK inhibition are ongoing. Constant drug exposure may lead to feedback pathway reactivation, as supported by the development of resistance in BRAF-mutant melanoma cells exposed continuously to vemurafenib.96 Moreover, cumulative drug exposure and toxicity may limit the doses achieved with continual dosing. In an effort to maximize target inhibition with higher doses, while minimizing toxicity, and reduce the effects of paradoxical feedback activation, an intermittent dosing schedule of selumetinib (three days on, four days off) is being evaluated in a phase Ib trial in patients with metastatic uveal melanoma [ClinicalTrials.gov identifier: NCT0276766]. The SelPac trial in the UK is a randomized, phase II study evaluating three treatment arms: continuous selumetinib, continuous selumetinib plus paclitaxel, and intermittent selumetinib plus paclitaxel [ISRCTN29621851].
Trametinib is another potent MEK inhibitor that was evaluated in a phase I study in patients with advanced melanoma, 16 of whom had primary uveal melanoma. Among these patients, there were no objective responses; two patients (13%) achieved a 24% tumor reduction and four patients (25%) had stable disease for ⩾16 weeks. The median PFS was 1.8 months.97 Given that oncogenic GNAQ and GNA11 mutations activate both MEK and Akt, simultaneous inhibition of these two pathways may represent a promising approach. Selumetinib combined with the Akt inhibitor MK2206 induced a synergistic decrease in the viability of GNAQ-mutant uveal melanoma cells and inhibited tumor growth in xenograft mouse models.98 Unfortunately, a randomized, phase II trial evaluating the efficacy of trametinib with or without the Akt inhibitor GSK2141795 (GSK795) in 40 patients with metastatic uveal melanoma failed to detect any survival benefit with the addition of Akt inhibition.99 Median PFS was 15.6 weeks in the GSK795 arm and 15.7 weeks in the trametinib alone arm. Only one partial response was observed in each arm (ORR ~5%), so accrual was held.
Stimulation of the MAPK pathway in uveal melanoma occurs via activation of phospholipase C β (PLCβ) and PKC. In a phase I study of the PKC inhibitor AEB071 (sotrastaurin), partial responses were observed in only 4 of 153 patients (3%). In total, 76 patients (50%) had stable disease, and the median PFS was 3.5 months.100 LXS196 is another oral PKC inhibitor that is being evaluated in a phase I study [ClinicalTrials.gov identifier: NCT02601378]. Preliminary findings from 50 evaluable patients in the dose-escalation cohort demonstrated an ORR of 8% (two confirmed and two unconfirmed partial responses).101 An additional 33 patients (66%) had stable disease. The most common adverse effects included GI toxicities, hypotension and fatigue. The expansion cohort at the recommended dose for expansion (RDE) of 300 mg BID is ongoing. Concurrent inhibition of PKC and PI3K may offer another approach to targeting the dual signaling pathways. The combination of AEB071 and the PI3Kα inhibitor BYL719 synergistically inhibited proliferation and induced apoptosis in GNAQ- and GNA11-mutant cell lines102 and is being investigated in a phase Ib trial in metastatic uveal melanoma [ClinicalTrials.gov identifier: NCT02273219]. Results from 21 enrolled patients will be presented at the 2017 International Congress of the Society for Melanoma Research.103 Although the combination regimen was tolerable, no objective responses were observed. 67% of patients achieved stable disease, with a median PFS of 7.6 weeks.
Additional therapeutic targets include various growth factor receptors that are overexpressed in uveal melanoma, such as c-Kit and c-Met, the receptor for hepatocyte growth factor. Sunitinib is a nonselective c-Kit inhibitor currently being investigated in the adjuvant setting [ClinicalTrials.gov identifier: NCT02068586]. However, the phase II SUAVE trial failed to show any survival benefit with sunitinib compared to dacarbazine in patients with untreated metastatic uveal melanoma.104 Cabozantinib, a multi-kinase inhibitor of c-Met, Axl and VEGF, has shown anti-tumor activity in a xenograft mouse model of metastatic uveal melanoma.105 Subset analysis of 23 uveal melanoma patients treated with cabozantinib in a discontinued phase II trial demonstrated encouraging clinical activity with a median PFS and OS of 4.8 months and 12.6 months, respectively.106 Based on these findings, a phase II trial comparing cabozantinib with chemotherapy (temozolomide or dacarbazine) in patients with metastatic disease was initiated [ClinicalTrials.gov identifier: NCT01835145]. Enrollment has been completed, and results are anticipated later this year. Sorafenib is another oral multikinase inhibitor that was evaluated in a phase II randomized discontinuation study (STREAM) in 152 uveal melanoma patients.107 Two patients (1.3%) achieved a partial response, and 37 (24%) had disease progression. The 78 patients (51%) with stable disease were then randomized to continuation or discontinuation of sorafenib. Continuation of treatment was associated with a significant improvement in PFS compared to placebo (5.5 versus 1.9 months, p = 0.0079), suggesting some degree of clinical activity despite the overall low ORR. The combination of sorafenib with carboplatin and paclitaxel yielded an ORR of 0%, but the 45% minor response rate (tumor regression <30%) and the 4-month PFS again support some level of anti-tumor activity.108
Epigenetic approaches
Given the genetic simplicity of uveal melanoma, epigenetic dysregulation plays a critical role in its pathogenesis. Genes encoding epigenetic regulatory enzymes are downregulated in high-risk disease.109 Recent integrative analysis of 80 uveal melanoma tumors found a distinct global DNA methylation state associated with the poor-prognosis subtype characterized by monosomy 3 and BAP1 loss.30 As previously discussed, HDAC inhibitors induce cell-cycle arrest as well as morphologic and transcriptomic changes associated with lower metastatic risk in preclinical models.63 Valproic acid and vorinostat are currently being evaluated in the adjuvant and metastatic setting, respectively [ClinicalTrials.gov identifiers: NCT02068586, NCT01587352].
Bromodomain and Extra-Terminal (BET) protein inhibition offers a novel therapeutic approach in uveal melanoma. The BET family of proteins, including BRD2, BRD3, BRD4 and BRDT, are epigenetic regulators that bind to acetylated lysine residues on histone tails in order to direct the assembly of nuclear complexes that regulate DNA replication, chromatin remodeling and transcription.110,111 More specifically, BRD4 may regulate oncogenic drivers including MYC by binding to super-enhancers, noncoding DNA regions densely occupied by master transcription factors responsible for cell identity.112,113 Since MYC amplification plays an important role in uveal melanoma, BET inhibition was hypothesized to have therapeutic activity via downregulation of MYC and MYC-dependent genes. JQ1, a first-generation BET inhibitor that competitively displaces BRD4 from acetylated histones, has potent cytotoxic activity in GNAQ/11-mutant cell lines and in a uveal melanoma mouse model.114 Interestingly, the anti-tumor effects of BET inhibition appear to be mediated by suppression of Bcl-xL and Rad51, which regulate apoptosis and DNA damage response, respectively. Concomitant silencing of Bcl-xL and Rad51 was sufficient to induce apoptosis in uveal melanoma cells independently of c-Myc.114 PLX51107 is an oral small-molecule BET inhibitor currently being assessed in a multicenter phase Ib dose-escalation study in various solid and hematologic malignancies [ClinicalTrials.gov identifier: NCT02683395]. Based on promising preclinical data and early signs of clinical activity in uveal melanoma, a phase II study in patients with metastatic uveal melanoma is planned.
Conclusion
Uveal melanoma is a rare form of melanoma that is biologically and clinically distinct from cutaneous melanoma. Despite usual success in achieving local control, nearly 50% of patients will eventually develop metastatic recurrence. Gene expression profiling has improved our ability to risk-stratify patients, and the recent TCGA analysis has uncovered new molecularly distinct, clinically relevant subsets that may guide future efforts to devise more individualized treatment strategies. Outcomes for patients with metastatic disease remain incredibly poor. The therapeutic advances that have translated to improved patient survival in cutaneous melanoma have unfortunately not yielded similar benefits in advanced uveal melanoma. However, our expanding knowledge of disease biology and immunology and the encouraging results seen with new agents such as IMCgp100 offer promise for future effective therapies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: RDC serves as a consultant and/or advisory board member for AstraZeneca, Aura Biosciences, Iconic Therapeutics, Janssen, Merck, Novartis, Rgenix, and Thomson Reuters. BPM serves as a consultant for Aura Biosciences. The other authors report no conflicts of interest in this work.
Contributor Information
Jessica Yang, Division of Hematology/Oncology, Columbia University Medical Center, New York, NY, USA.
Daniel K. Manson, Division of Hematology/Oncology, Columbia University Medical Center, New York, NY, USA
Brian P. Marr, Department of Ophthalmology, Columbia University Medical Center, New York, NY, USA
Richard D. Carvajal, Assistant Professor of Medicine, Director of Experimental Therapeutics and Melanoma Services, Division of Hematology/Oncology, Columbia University Medical Center, 177 Fort Washington Avenue, MHB 6GN-435, New York, NY 10032, USA; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY, USA.
References
- 1. McLaughlin CC, Wu XC, Jemal A, et al. Incidence of noncutaneous melanomas in the U.S. Cancer 2005; 103: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 2. Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res 2010; 16: 6083–6092. [DOI] [PubMed] [Google Scholar]
- 3. Komatsubara KM, Carvajal RD. Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep 2017; 19: 45. [DOI] [PubMed] [Google Scholar]
- 4. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 2011; 118: 1881–1885. [DOI] [PubMed] [Google Scholar]
- 5. Hammer H, Olah J, Toth-Molnar E. Dysplastic nevi are a risk factor for uveal melanoma. Eur J Ophthalmol 1996; 6: 472–474. [DOI] [PubMed] [Google Scholar]
- 6. Shah CP, Weis E, Lajous M, et al. Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis. Ophthalmology 2005; 112: 1599–1607. [DOI] [PubMed] [Google Scholar]
- 7. Shields CL, Kaliki S, Livesey M, et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol 2013; 131: 993–1003. [DOI] [PubMed] [Google Scholar]
- 8. Weis E, Shah CP, Lajous M, et al. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol 2006; 124: 54–60. [DOI] [PubMed] [Google Scholar]
- 9. Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010; 330: 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009; 457: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010; 363: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piperno-Neumann S, Desjardins L. Advances in uveal melanoma. Rev Prat 2014; 64: 83–84. [PubMed] [Google Scholar]
- 13. Griewank KG, van de Nes J, Schilling B, et al. Genetic and clinico-pathologic analysis of metastatic uveal melanoma. Mod Pathol 2014; 27: 175–183. [DOI] [PubMed] [Google Scholar]
- 14. Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014; 25: 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson P, Aoude LG, Wadt K, et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget 2016; 7: 4624–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore AR, Ceraudo E, Sher JJ, et al. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat Genet 2016; 48: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harbour JW, Roberson ED, Anbunathan H, et al. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet 2013; 45: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yavuzyigitoglu S, Koopmans AE, Verdijk RM, et al. Uveal melanomas with SF3B1 mutations: a distinct subclass associated with late-onset metastases. Ophthalmology 2016; 123: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 19. Luke JJ, Triozzi PL, McKenna KC, et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res 2015; 28: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin M, Masshofer L, Temming P, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet 2013; 45: 933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003; 44: 4651–4659. [DOI] [PubMed] [Google Scholar]
- 22. Shields CL, Kaliki S, Furuta M, et al. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013; 120: 2066–2071. [DOI] [PubMed] [Google Scholar]
- 23. Khoja L, Atenafu EG, Joshua AM. Meta-analysis of phase II trials in metastatic uveal melanoma (MUM) to determine progression-free (PFS) and overall survival (OS) benchmarks for future phase II trials: an irci-ocular melanoma initiative. J Clin Oncol 2016; 34(15 Suppl.): 9567–9567. [Google Scholar]
- 24. Versluis M, de Lange MJ, van Pelt SI, et al. Digital PCR validates 8q dosage as prognostic tool in uveal melanoma. PLoS One 2015; 10: e0116371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ewens KG, Kanetsky PA, Richards-Yutz J, et al. Genomic profile of 320 uveal melanoma cases: chromosome 8p-loss and metastatic outcome. Invest Ophthalmol Vis Sci 2013; 54: 5721–5729. [DOI] [PubMed] [Google Scholar]
- 26. Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012; 119: 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol 2014; 25: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res 2016; 22: 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Field MG, Durante MA, Decatur CL, et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget 2016; 7: 59209–59219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson AG, Shih J, Yau C, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017; 32: 204–220.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch Ophthalmol 2006; 124: 1684–1693. [DOI] [PubMed] [Google Scholar]
- 32. Melia M, Moy CS, Reynolds SM, et al. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS report no. 3. Arch Ophthalmol 2006; 124: 226–238. [DOI] [PubMed] [Google Scholar]
- 33. Caminal JM, Padron-Perez N, Arias L, et al. Transscleral resection without hypotensive anaesthesia vs iodine-125 plaque brachytherapy in the treatment of choroidal melanoma. Eye (Lond) 2016; 30: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kivela T, Puusaari I, Damato B. Transscleral resection versus iodine brachytherapy for choroidal malignant melanomas 6 millimeters or more in thickness: a matched case–control study. Ophthalmology 2003; 110: 2235–2244. [DOI] [PubMed] [Google Scholar]
- 35. Puusaari I, Damato B, Kivela T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefes Arch Clin Exp Ophthalmol 2007; 245: 522–533. [DOI] [PubMed] [Google Scholar]
- 36. Bechrakis NE, Petousis V, Willerding G, et al. Ten-year results of transscleral resection of large uveal melanomas: local tumour control and metastatic rate. Br J Ophthalmol 2010; 94: 460–466. [DOI] [PubMed] [Google Scholar]
- 37. Damato B. Progress in the management of patients with uveal melanoma: the 2012 Ashton Lecture. Eye (Lond) 2012; 26: 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology 2011; 118: 402–407. [DOI] [PubMed] [Google Scholar]
- 39. Shah SU, Shields CL, Bianciotto CG, et al. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology 2014; 121: 269–275. [DOI] [PubMed] [Google Scholar]
- 40. Gragoudas E, Li W, Goitein M, et al. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol 2002; 120: 1665–1671. [DOI] [PubMed] [Google Scholar]
- 41. Mishra KK, Quivey JM, Daftari IK, et al. Long-term results of the UCSF-LBNL randomized trial: charged particle with helium ion versus iodine-125 plaque therapy for choroidal and ciliary body melanoma. Int J Radiat Oncol Biol Phys 2015; 92: 376–383. [DOI] [PubMed] [Google Scholar]
- 42. Damato B, Patel I, Campbell IR, et al. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys 2005; 63: 385–391. [DOI] [PubMed] [Google Scholar]
- 43. Bensoussan E, Thariat J, Maschi C, et al. Outcomes after proton beam therapy for large choroidal melanomas in 492 patients. Am J Ophthalmol 2016; 165: 78–87. [DOI] [PubMed] [Google Scholar]
- 44. Turcotte S, Bergeron D, Rousseau AP, et al. Primary transpupillary thermotherapy for choroidal indeterminate melanocytic lesions. Can J Ophthalmol 2014; 49: 464–467. [DOI] [PubMed] [Google Scholar]
- 45. Mashayekhi A, Shields CL, Rishi P, et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: importance of risk factors in tumor control. Ophthalmology 2015; 122: 600–609. [DOI] [PubMed] [Google Scholar]
- 46. Singh AD, Eagle RC, Jr, Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma – clinicopathologic correlations. Arch Ophthalmol 2003; 121: 397–400. [DOI] [PubMed] [Google Scholar]
- 47. Badiyan SN, Rao RC, Apicelli AJ, et al. Outcomes of iodine-125 plaque brachytherapy for uveal melanoma with intraoperative ultrasonography and supplemental transpupillary thermotherapy. Int J Radiat Oncol Biol Phys 2014; 88: 801–805. [DOI] [PubMed] [Google Scholar]
- 48. Saakian SV, Val’skii VV, Semenova EA, et al. Transpupillary thermotherapy in the treatment of recurrent and residual choroidal melanomas: preliminary results. Vestn Oftalmol 2009; 125: 11–15. [PubMed] [Google Scholar]
- 49. Yarovoy AA, Magaramov DA, Bulgakova ES. The comparison of ruthenium brachytherapy and simultaneous transpupillary thermotherapy of choroidal melanoma with brachytherapy alone. Brachytherapy 2012; 11: 224–229. [DOI] [PubMed] [Google Scholar]
- 50. Tarmann L, Wackernagel W, Avian A, et al. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br J Ophthalmol 2015; 99: 1644–1649. [DOI] [PubMed] [Google Scholar]
- 51. Fabian ID, Stacey AW, Papastefanou V, et al. Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma. Eye (Lond) 2017; 31: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108: 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walker TM, Van Ginkel PR, Gee RL, et al. Expression of angiogenic factors Cyr61 and tissue factor in uveal melanoma. Arch Ophthalmol 2002; 120: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 54. Desjardins L, Dorval T, Lévy C, et al. Randomised study on adjuvant therapy by DTIC in choroidal melanoma. Ophtalmogie 1998; 12: 168–173. [Google Scholar]
- 55. Lane AM, Egan KM, Harmon D, et al. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology 2009; 116: 2206–2212. [DOI] [PubMed] [Google Scholar]
- 56. Richtig E, Langmann G, Schlemmer G, et al. Safety and efficacy of interferon alfa-2b in the adjuvant treatment of uveal melanoma. Ophthalmologe 2006; 103: 506–511. [DOI] [PubMed] [Google Scholar]
- 57. Mallikarjuna K, Pushparaj V, Biswas J, et al. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: an immunohistochemical study. Curr Eye Res 2007; 32: 281–290. [DOI] [PubMed] [Google Scholar]
- 58. All-Ericsson C, Girnita L, Muller-Brunotte A, et al. c-Kit-dependent growth of uveal melanoma cells: a potential therapeutic target? Invest Ophthalmol Vis Sci 2004; 45: 2075–2082. [DOI] [PubMed] [Google Scholar]
- 59. Surriga O, Rajasekhar VK, Ambrosini G, et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol Cancer Ther 2013; 12: 2817–2826. [DOI] [PubMed] [Google Scholar]
- 60. Xu H, Stabile LP, Gubish CT, et al. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res 2011; 17: 4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu X, Zhou J, Rogers AM, et al. c-Met, epidermal growth factor receptor, and insulin-like growth factor-1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res 2012; 22: 123–132. [DOI] [PubMed] [Google Scholar]
- 62. Valsecchi ME, Orloff M, Sato R, et al. Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology 2017. pii: S0161-6420(17)30329-9. [DOI] [PubMed] [Google Scholar]
- 63. Landreville S, Agapova OA, Matatall KA, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012; 18: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Francis JH, Patel SP, Gombos DS, et al. Surveillance options for patients with uveal melanoma following definitive management. Am Soc Clin Oncol Educ Book 2013: 382–387. [DOI] [PubMed] [Google Scholar]
- 65. Piperno-Neumann S, Servois V, Mariani P, et al. Prospective study of surveillance testing for metastasis in 100 high-risk uveal melanoma patients. J Fr Ophtalmol 2015; 38: 526–534. [DOI] [PubMed] [Google Scholar]
- 66. Marshall E, Romaniuk C, Ghaneh P, et al. MRI in the detection of hepatic metastases from high-risk uveal melanoma: a prospective study in 188 patients. Br J Ophthalmol 2013; 97: 159–163. [DOI] [PubMed] [Google Scholar]
- 67. Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group report no. 26. Arch Ophthalmol 2005; 123: 1639–1643. [DOI] [PubMed] [Google Scholar]
- 68. Kuk D, Shoushtari AN, Barker CA, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016; 21: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carvajal RD, Schwartz GK, Tezel T, et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017; 101: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blum ES, Yang J, Komatsubara KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park) 2016; 30: 29–32, 34,–43, 48. [PubMed] [Google Scholar]
- 71. Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016; 122: 3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol 2001; 119: 670–676. [DOI] [PubMed] [Google Scholar]
- 73. Agarwala SS, Eggermont AM, O’Day S, et al. Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 2014; 120: 781–789. [DOI] [PubMed] [Google Scholar]
- 74. Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol 2014; 25: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pinqpank JF, Hughes M, Alexander HR, et al. 9304 ORAL percutaneous hepatic perfusion (PHP) vs. best alternative care (BAC) for patients (pts) with melanoma liver metastases: efficacy update of the phase 3 trial (NCT00324727). Eur J Cancer 2011; 47(Suppl. 1): S653. [Google Scholar]
- 76. Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol 2015; 26: 523–532.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev 2012; 38: 549–553. [DOI] [PubMed] [Google Scholar]
- 78. Spagnolo F, Grosso M, Picasso V, et al. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res 2013; 23: 196–198. [DOI] [PubMed] [Google Scholar]
- 79. Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol 2009; 148: 119–127. [DOI] [PubMed] [Google Scholar]
- 80. Schmittel A, Schmidt-Hieber M, Martus P, et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann Oncol 2006; 17: 1826–1829. [DOI] [PubMed] [Google Scholar]
- 81. Homsi J, Bedikian AY, Papadopoulos NE, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in patients with metastatic uveal melanoma. Melanoma Res 2010; 20: 507–510. [DOI] [PubMed] [Google Scholar]
- 82. Piulats Rodriguez JM, Ochoa de Olza M, Codes M, et al. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): the GEM-1 trial. ASCO Meeting Abstracts 2014; 32: 9033. [Google Scholar]
- 83. Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One 2015; 10: e0118564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Joshua AM, Monzon JG, Mihalcioiu C, et al. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res 2015; 25: 342–347. [DOI] [PubMed] [Google Scholar]
- 85. Wolchok JD, Chiarion-Sileni V, Gonzalez R. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kottschade LA, McWilliams RR, Markovic S, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. ASCO Meeting Abstracts 2015; 33: 9010. [Google Scholar]
- 87. Piperno-Neumann S, Servois V, Mariani P. Activity of anti-PD1 drugs in uveal melanoma patients. J Clin Oncol 2016; 34(Suppl.): 9588. [Google Scholar]
- 88. Karydis I, Chan PY, Wheater M, et al. Clinical activity and safety of pembrolizumab in ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 2016; 5: e1143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Middleton MR, Steven NM, Evans TJ. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific T cell redirector with solid tumour activity: results from the FIH study in melanoma. J Clin Oncol 2016; 34(Suppl.): 3016. [Google Scholar]
- 90. Sato T, Nathan PD, Hernandez-Aya LF. Intra-patient escalation dosing strategy with IMCgp100 results in mitigation of T-cell based toxicity and preliminary efficacy in advanced uveal melanoma. J Clin Oncol 2017; 35(Suppl.): 9531. [Google Scholar]
- 91. Rothermel LD, Sabesan AC, Stephens DJ, et al. Identification of an immunogenic subset of metastatic uveal melanoma. Clin Cancer Res 2016; 22: 2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chandran SS, Somerville RPT, Yang JC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 2017; 18: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Patel S, Lewis KD, Olencki T. A phase II study of glembatumumab vedotin for metastatic uveal melanoma. In: 14th international congress of the Society for Melanoma Research, Brisbane, Australia, 18–21 October 2017 Brisbane: Brisbane Convention and Exhibition Centre. [Google Scholar]
- 94. Gezgin G, Luk SJ, Cao J, et al. PRAME as a potential target for immunotherapy in metastatic uveal melanoma. JAMA Ophthalmol 2017; 135: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014; 311: 2397–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013; 494: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ambrosini G, Musi E, Ho AL, et al. Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death. Mol Cancer Ther 2013; 12: 768–776. [DOI] [PubMed] [Google Scholar]
- 99. Shoushtari AN, Kudchadkar RR, Panageas KS. A randomized phase 2 study of trametinib with or without GSK2141795 in patients with advanced uveal melanoma. J Clin Oncol 2016; 35(Suppl.): 9511. [Google Scholar]
- 100. Piperno-Neumann S, Kapiteijn E, Larkin JMG. Phase I dose-escalation study of the protein kinase C (PKC) inhibitor AEB071 in patients with metastatic uveal melanoma. J Clin Oncol 2014; 32(5 Suppl.): 9030. [Google Scholar]
- 101. Piperno-Neumann S, Carlino MS, Boni V. A phase I trial of LXS196, a PKC inhibitor for uveal melanoma. In: 14th international congress of the Society for Melanoma Research, Brisbane, Australia, 18–21 October 2017 Brisbane: Society for Melanoma Research. [Google Scholar]
- 102. Musi E, Ambrosini G, de Stanchina E, et al. The phosphoinositide 3-kinase alpha selective inhibitor BYL719 enhances the effect of the protein kinase C inhibitor AEB071 in GNAQ/GNA11-mutant uveal melanoma cells. Mol Cancer Ther 2014; 13: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Komatsubara KM, Shoushtari AN, Feun L. A phase Ib study of AEB071, a PKC inhibitor, and BYL719, a PI3Ka inhibitor, in patients with metastatic uveal melanoma (UM). In: 14th international congress of the Society for Melanoma Research, Brisbane, Australia, 18–21 October 2017 Brisbane: Society for Melanoma Research. [Google Scholar]
- 104. Sacco JJ, Nathan PD, Danson S, et al. Sunitinib versus dacarbazine as first-line treatment in patients with metastatic uveal melanoma. ASCO Meeting Abstracts 2013; 31: 9031. [Google Scholar]
- 105. Yeh I, Griewank KG, Ding VW. c-Met inhibition is effective in a mouse xenograft model of metastatic uveal melanoma. In: 102nd annual meeting of the American Association for Cancer Research, Orlando, FL, 2–6 April 2011, 3587. Orlando, FL: American Association for Cancer Research. [Google Scholar]
- 106. Daud A, Kluger HM, Kurzrock R, et al. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br J Cancer 2017; 116: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Scheulen ME, Kaempgen E, Kielholz U. STREAM: a randomized discontinuation, blinded, placebo-controlled phase II study of sorafenib (S) treatment of chemonaive patients (pts) with metastatic uveal melanoma (MUM). J Clin Oncol 2017; 35(Suppl.): 9511. [Google Scholar]
- 108. Bhatia S, Moon J, Margolin KA, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One 2012; 7: e48787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Herlihy N, Dogrusoz M, van Essen TH, et al. Skewed expression of the genes encoding epigenetic modifiers in high-risk uveal melanoma. Invest Ophthalmol Vis Sci 2015; 56: 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fu LL, Tian M, Li X, et al. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget 2015; 6: 5501–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature 2010; 468: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Loven J, Hoke HA, Lin CY, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013; 153: 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ambrosini G, Sawle AD, Musi E, et al. BRD4-targeted therapy induces Myc-independent cytotoxicity in Gnaq/11-mutatant uveal melanoma cells. Oncotarget 2015; 6: 33397–33409. [DOI] [PMC free article] [PubMed] [Google Scholar]