Short abstract
Background
Lymphopenia is a well-known adverse event of fingolimod, a disease-modifying drug for multiple sclerosis (MS).
Objectives
The objective of this paper is to investigate risk factors for predicting fingolimod-induced lymphopenia in MS by frequent hematological monitoring.
Methods
We retrospectively reviewed data of fingolimod-treated MS patients. Data assessed were sex, age, disease duration, medication history, body mass index, all attacks, Kurtzke’s Expanded Disability Status Scale score, and absolute lymphocyte count (ALC) within two days before initiating fingolimod (baseline), on the day after first administration (day 2), and at least every other month after initiating fingolimod therapy.
Results
Of 41 MS patients, marked lymphopenia (ALC <200/µl) was confirmed in 12 patients (lymphopenia group) within one year. A significantly more frequent history of treatment with any interferon-beta and lower median baseline ALC was observed in the lymphopenia group than in the non-lymphopenia group (n = 29) (91.7% vs. 44.8%; p = 0.006 and 1469/µl vs. 1879/µl; p = 0.005). An ALC of ≤952/μl on day 2 was the most responsible risk factor for predicting marked lymphopenia (sensitivity, 92%; specificity, 76%; area under the curve, 0.823; p < 0.001).
Conclusions
Low baseline ALC and treatment history with any interferon-beta were risk factors for fingolimod-induced lymphopenia, possibly predicted from ALC on day 2.
Keywords: Fingolimod, multiple sclerosis, lymphopenia, risk factors, prediction
Introduction
Fingolimod, a sphingosine-1-phosphate receptor (S1PR) modulator, reduces the recirculation of autoaggressive lymphocytes and is approved as a once-daily oral therapy (dose, 0.5 mg) for multiple sclerosis (MS) in many countries.1,2 Lymphopenia is a well-known adverse event that affects continuous drug administration because the Summary of Product Characteristics (SPC) in Europe states that if the absolute lymphocyte count (ALC) is confirmed to be <200/µl, treatment should be interrupted until recovery.3–6 Patients with a low baseline lymphocyte count and women with a low body mass index (BMI) were suggested to have a risk of lymphopenia in a German and Swedish cohort.7 However, no studies were based on the data of frequent laboratory tests. Here we aimed to investigate the risk factors for predicting fingolimod-induced lymphopenia in patients with MS by frequent hematological monitoring.
Patients and methods
Patients
First, 70 patients with MS who were treated with fingolimod at Chiba University Hospital until May 2017 were retrospectively recruited. They fulfilled the McDonald criteria proposed in 2010.8
Patients who were treated with other S1PR regulators before initiating fingolimod therapy (n = 3) and those who were followed for <1 year after initiating fingolimod therapy (n = 18) were excluded. Eight patients who did not undergo hematological monitoring at any of the following time points were also excluded: within two days before initiating fingolimod therapy (baseline), on the day after the first administration (day 2) of fingolimod therapy, and at least every other month after initiating fingolimod therapy.
Grades of lymphopenia were defined according to the Common Terminology Criteria for Adverse Events (CTCAE v4.0): grade 1 (ALC of 800/μl to the lower limit of normal), grade 2 (ALC 500–799/μl), grade 3 (ALC 200–499/μl) and grade 4 (ALC <200/μl).9 Patients who exhibited grade 4 lymphopenia within one year after initiating fingolimod were categorized as the lymphopenia group, whereas the remaining patients were categorized as the non-lymphopenia group.
Abnormal liver function tests were defined as any liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase) were ≥3-fold of the upper limit of normal at two different laboratory examinations.
All patients were originally treated with fingolimod at a dose of 0.5 mg daily; however, 18 patients were switched to intermittent drug holiday therapies such as one drug holiday per week, two holidays per week, or every other day administration.10 The reasons for drug holiday were the presence of lymphopenia only (grade 4, n = 9; grade 3, n = 3), abnormal liver function test results only (n = 3), and both grade 4 lymphopenia and abnormal liver function test results (n = 3).
Informed consent was provided by all patients for participation in this study.
Evaluation points and definitions
We reviewed the patients’ data such as sex, age, disease duration, clinical course, medication history, current smoking habits, history of all attacks, weight, BMI, body surface area (BSA) using the Du Bois method, and laboratory tests, including creatinine clearance (CrCl), using the Cockcroft–Gault equation at the initiation of fingolimod therapy.
Moreover, Kurtzke’s Expanded Disability Status Scale (EDSS)11 score and hematological monitoring data, including ALC and liver function test results, were assessed at the following time points: within two days before initiating fingolimod therapy (baseline), on the day after the first administration of fingolimod therapy (day 2), and at least every other month after initiating fingolimod therapy.
Attacks were defined as new or reactivated neurological symptoms caused by an acute inflammatory demyelinating event in the central nervous system, with a duration of at least 24 hours in the absence of fever or infection, and they occurred at least 30 days after the preceding episode.8
Statistical analysis
Statistical comparisons between the two groups were performed using the Mann–Whitney U test or Wilcoxon signed-rank test. Fisher’s exact test was performed to compare dichotomous variables. Univariable and multivariable logistic regression analysis models were used to estimate the risk factors associated with grade 4 lymphopenia. The receiver operating characteristic curve was used to identify the selection of cut-off points for regression analysis, and area under the curve (AUC) was used for model comparison to identify the responsible predictor for grade 4 lymphopenia; Kaplan–Meier survival plot was used to test the efficacy of early predictors.
Differences of p values of <0.05 were considered to be statistically significant. All statistical analyses were performed using the JMP® 12 software (SAS Institute Inc, Cary, NC, USA).
Results
In total, 41 patients fulfilled the inclusion criteria. The mean number of hematological monitoring within one year after initiating fingolimod therapy was 14.2 (range, 11–30). The baseline characteristics of the patients in the lymphopenia group (n = 12) and non-lymphopenia group (n = 29) are summarized in Table 1. The distributions of sex, age, disease duration, clinical patterns, smoking habits, annualized relapse rates (ARRs), EDSS scores, weight, BMI, BSA, and CrCl were not significantly different between the two groups.
Table 1.
Comparison of baselinea characteristics in the lymphopenia and non-lymphopenia group.
| Characteristic | Lymphopenia group (n = 12) | Non-lymphopenia group (n = 29) | p value |
|---|---|---|---|
| Clinical pattern of MS, RR:SP | 11:1 | 27:2 | 0.657 |
| Female, n (%) | 11 (91.7) | 19 (65.5) | 0.087 |
| Age, median (IQR) | 40.5 (9.3) | 35.0 (15.5) | 0.456 |
| Disease duration in years, median (IQR) | 13.2 (14.4) | 8.2 (13.9) | 0.218 |
| ARRs within previous two years, median (IQR) | 1.25 (2.75) | 1.00 (2.00) | 0.738 |
| EDSS score, median (IQR) | 3.75 (3.00) | 3.00 (4.00) | 0.394 |
| Weight, kg, median (IQR) | 54.7 (5.3) | 54.3 (16.2) | 0.785 |
| BMI, kg/m2, median (IQR) | 20.4 (3.4) | 21.3 (3.4) | 0.456 |
| BSA, m2, median (IQR) | 1.57 (0.08) | 1.56 (0.26) | 0.752 |
| CrCl, ml/min, median (IQR) | 121.6 (26.2) | 107.2 (27.8) | 0.390 |
| Current smoker, n (%) | 5 (41.7) | 10 (34.5) | 0.464 |
| Treatment history, n (%) | 11 (91.7) | 13 (44.8) | 0.006 |
| Any INF-β, n (%) | 11 (91.7) | 13 (44.8) | 0.006 |
| Natalizumab, n (%) | 1 (8.3) | 0 (0) | 0.300 |
| Absolute lymphocyte count, /µl, median (IQR) | 1469.0 (413.5) | 1879.0 (796.0) | 0.005 |
ARRs: annualized relapse rates; BMI: body mass index; BSA: body surface area; CrCl: creatinine clearance; EDSS: Expanded Disability Status Scale; IFN-β: interferon-beta; IQR: interquartile range; MS: multiple sclerosis; RR: relapsing–remitting; SP: secondary progressive.
aAt the initiation of fingolimod.
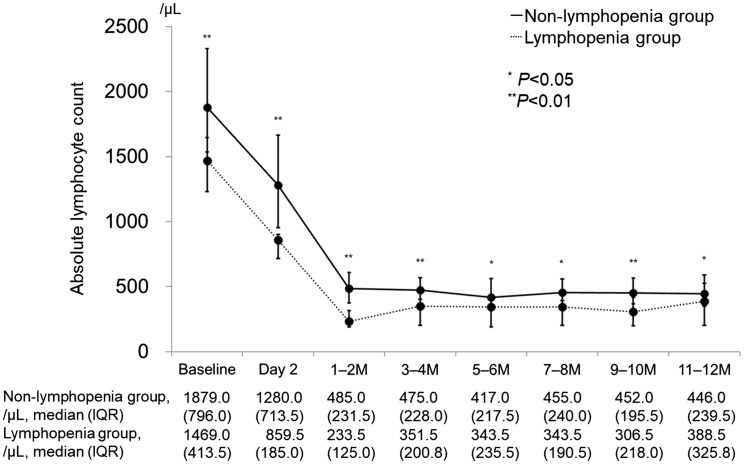
The history of treatment with any interferon-beta (IFN-β) was significantly more frequent in the lymphopenia group than in the non-lymphopenia group (91.7% vs. 44.8%; p = 0.006). The median (interquartile range; IQR) ALC at baseline was 1469.0/µl (413.5/µl) in the lymphopenia group and 1879.0/µl (796.0/µl) in the non-lymphopenia group (p = 0.005), and the time series charts of ALC in the lymphopenia group were continuously less than those in the non-lymphopenia group until one year after initiating fingolimod treatment (Figure 1).
Figure 1.
Longitudinal change of absolute lymphocyte count in lymphopenia group (n = 12) and non-lymphopenia group (n = 29). Baseline is within two days before initiating fingolimod treatment, and day 2 is the day after the first administration.
IQR: interquartile range; M: month.
The characteristics of the patients at last follow-up are summarized in Table 2. The total administration duration, ARRs, EDSS score, and proportion of patients who exhibited abnormal liver function test results were not significantly different between the two groups. In all patients, the elevations of liver enzyme levels were asymptomatic. The median (IQR) decline rate of ALC, which was obtained by dividing the lowest ALC within one year after initiating fingolimod by the baseline ALC was 11.1% (5.8%) in the lymphopenia group and 17.8% (10.9%) in the non-lymphopenia group (p < 0.001). The number of patients treated with intermittent drug holiday therapy was significantly higher in the lymphopenia group than in the non-lymphopenia group (75.0% vs. 31.0%; p = 0.012). The median (IQR) duration of intermittent drug holiday therapy was 1.9 (2.9) years in the lymphopenia group and 0.0 (0.6) years in the non-lymphopenia group (p = 0.006). Administration frequency at last follow-up was significantly lower in the lymphopenia group than in the non-lymphopenia group (4.0 days per week vs. 7.0 days per week; p = 0.001).
Table 2.
Comparison of characteristics at last follow-up after the initiation of fingolimod in lymphopenia and non-lymphopenia group.
| Characteristic | Lymphopenia group (n = 12) | Non-lymphopenia group (n = 29) | p value |
|---|---|---|---|
| Total administration duration in years, median (IQR) | 2.3 (2.0) | 3.6 (2.7) | 0.288 |
| ARRsa, median (IQR) | 0.27 (1.20) | 0 (0.26) | 0.055 |
| EDSS score, median (IQR) | 4.25 (3.75) | 3.50 (4.50) | 0.574 |
| Abnormal liver function testsb, n (%) | 3 (25.0) | 8 (27.6) | 0.595 |
| Decline rate of absolute lymphocyte countc, %, median (IQR) | 11.1 (5.8) | 17.8 (10.9) | <0.001 |
| Patients with IDH therapy, n (%) | 9 (75.0) | 9 (31.0) | 0.012 |
| Administration duration of IDH therapy in years, median (IQR) | 1.9 (2.9) | 0.0 (0.6) | 0.006 |
| Administration frequency, days per week, median (IQR) | 4.0 (3.8) | 7.0 (1.0) | 0.001 |
ARRs: annualized relapse rates; EDSS: Expanded Disability Status Scale; IDH: intermittent drug holiday; IQR: interquartile range.
aARRs were obtained by dividing the total number of relapses by total duration after the initiation of fingolimod.
bAbnormal liver function tests were defined as any liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase) were ≥3-fold of the upper limit of normal at two different laboratory tests.
cDecline rate of absolute lymphocyte count was obtained by dividing the lowest absolute lymphocyte count within one year after initiating fingolimod by the baseline absolute lymphocyte count.
The logistic regression model showed an increased risk with ALC of ≤1675/µl at baseline both in the univariable and multivariable regression analyses (Table 3). Furthermore, patients who were previously treated with any IFN-β had a higher risk of grade 4 lymphopenia. Female sex and BMI of ≤19.8 kg/m2 had no statistical effect on the risk of grade 4 lymphopenia.
Table 3.
Logistic regression model: Risk factors associated with grade 4 lymphopenia (ALC <200/µl).
| Risk factors | Odds ratio (95% confidence interval) | p value | |
|---|---|---|---|
| Sex, female vs. male | Adjusteda | 5.79 (0.92–113.52)6.60 (0.46–254.67) | 0.0640.171 |
| BMI ≤19.8kg/m2b, yes vs. no | Adjusteda | 2.63 (0.65–10.97)6.83 (0.83–181.09) | 0.1740.077 |
| History of any IFN-β treatment, yes vs. no | AdjustedaAdjustedc | 13.53 (2.20–264.18)23.50 (1.77–1250.57)10.18 (1.40–212.26) | 0.0030.0130.020 |
| ALC of ≤1675/µL at baselineb, yes vs. no | AdjustedaAdjustedc | 18.00 (2.91–352.56)38.52 (3.48–1803.71)14.15 (2.04–289.97) | <0.0010.0010.005 |
ALC: absolute lymphocyte count; BMI: body mass index; IFN-β: interferon-beta.
aAdjusted for sex, BMI ≤19.8kg/m2 or more, medication history, ALC of ≤1675/µl or more at baseline.
bThe optimal value was inferred by receiver operating characteristic curve.
cAdjusted for medication history, ALC of ≤1675/µl or more at baseline.
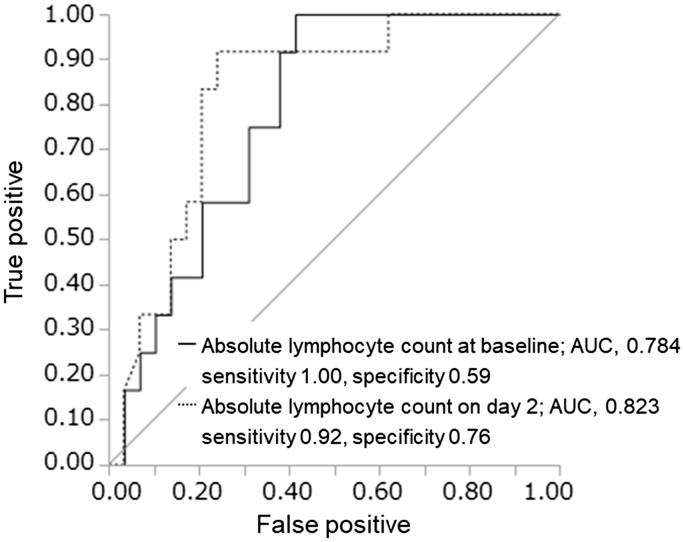
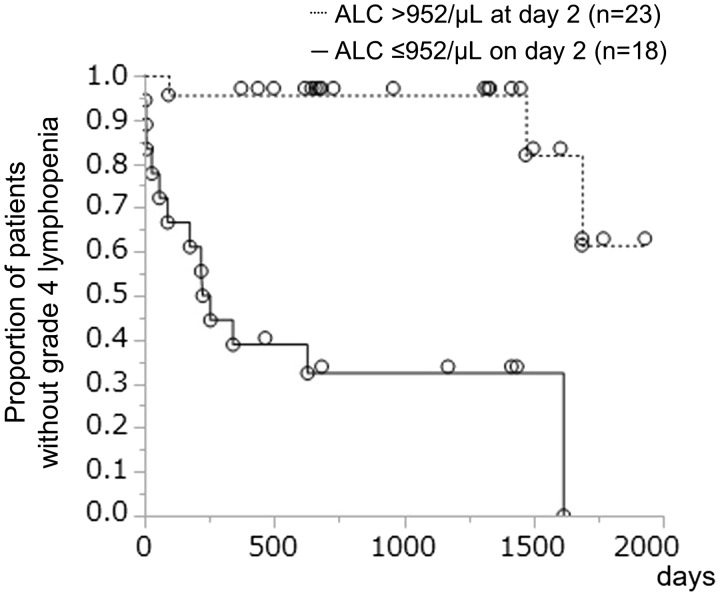
Figure 2 shows that compared with the baseline ALC, an ALC of ≤952/μl on day 2 was a more responsible risk factor for predicting grade 4 lymphopenia within one year, with a sensitivity of 92% and specificity of 76% (AUC, 0.823; p < 0.001). Furthermore, this early predictor was effective for distinguishing grade 4 lymphopenia at the last follow-up (Figure 3).
Figure 2.
Prediction of grade 4 lymphopenia (absolute lymphocyte count <200/µl) within one year by lymphocyte count at baseline (within two days before initiating fingolimod treatment) and on day 2 (the day after the first administration).
AUC: area under the curve.
Figure 3.
Days to fulfill the definition of grade 4 lymphopenia (absolute lymphocyte count <200/µl). Kaplan–Meier survival plot tested in two groups that were divided by absolute lymphocyte count on day 2 (the day after the first administration): Absolute lymphocyte count was 952/µl or less (n = 18) and more than 952/µl (n = 23). In the group with absolute lymphocyte count of 952/µl or less, the median (95% confidence interval) days to fulfill grade 4 lymphopenia were 239 days (57–1617 days, p < 0.001).
ALC: absolute lymphocyte count.
Discussion
This study showed that a low ALC at baseline and a treatment history of any IFN-β were associated with grade 4 lymphopenia, and an ALC of ≤952/µl on the day after first administration (day 2) was an early predictor.
Fingolimod has a long half-life of approximately six to nine days, and it takes >4 weeks to reach a steady-state concentration in the blood.4,12 Grade 4 lymphopenia during fingolimod therapy is a common adverse event, occurring in 15%–20% of patients.7,13 Previous studies have reported that the decrease of nadir ALC was correlated with the dosage, and patients with a low initial lymphocyte count (<1600/µl) and females with low BMI (<18.5 kg/m2) were at a higher risk of developing lymphopenia.3,7
In our study, grade 4 lymphopenia within one year was observed in 29.3% (12/41) patients, which was much higher than previously reported values. The mean BMI of the 41 patients in this study was 22.0 kg/m2 (vs. 24.3–25.4 kg/m2 in a previous study).7 Given that the lymphocyte count is affected by BMI,14 the higher proportion of patients with grade 4 lymphopenia in this study compared with those in previous studies could be attributed to lower BMI in this population. However, in this population, a low ALC at baseline was the main risk factor for grade 4 lymphopenia, and female sex and low BMI showed the same trends as those reported in a previous study.
The IFN-β family influences the production of cytokines by lymphocytes and some adverse events such as leukopenia and lymphopenia,15 which are considered to be related to myelosuppressive activities.16
Although ARRs before fingolimod administration were similar between the lymphopenia and non-lymphopenia groups, ARRs after fingolimod therapy tended to be slightly higher in the lymphopenia group. This finding might be because of the higher rates of patients treated with intermittent drug holiday therapy in the lymphopenia group. There is a possibility that the change from conventional therapy to intermittent drug holiday has a risk of rebound effects,17 and it was reported that the efficacy of an alternate-day fingolimod administration was not effective enough to inhibit disease activities, though dose-reduction therapy was safe overall and well tolerated with some efficacy.12,18,19 Our study was a retrospective one, and we had not defined the number of lymphocyte counts to start intermittent drug holiday therapy. However, we started intermittent drug holiday therapy when an ALC was confirmed to be <200/µl or when the next ALC was supposed to be <200/µl judging from the ALC and the speed of decrease of ALCs.
In a phase III fingolimod study, the rate of infections was not correlated with the lymphocyte count.13 However, whether fingolimod-induced lymphopenia affects progressive multifocal leukoencephalopathy and other opportunistic infections remains unclear.20 As described in the SPC, fingolimod-induced lymphopenia should be detected as soon as possible.6 The predictor identified in this study may aid in the early detection of lymphopenia, resulting in the prevention of adverse events, including infections.
The limitations of this study include its retrospective design and small sample size; furthermore, frequent hematological monitoring was performed only for one year. The different percentages of patients who were treated with intermittent drug holiday therapy, and different drug administration durations of intermittent drug holiday therapy and frequencies between lymphopenia and non-lymphopenia groups, might have affected the comparisons of the last follow-up characteristics. Moreover, the lack of uniformity in the lymphopenia group due to the retrospective design of our study including concomitant disorder such as liver dysfunction might also have affected the results. Accordingly, a large randomized controlled trial should be performed to confirm our findings.
In conclusion, a low baseline ALC and a treatment history with any IFN-β were risk factors for fingolimod-induced lymphopenia, and patients with an ALC of ≤952/µl on the day after first administration were more likely to develop lymphopenia after fingolimod treatment; such patients may need to be carefully monitored and prepared for the appearance of adverse infectious events.
Acknowledgments
Authorship: RO drafted the first manuscript and contributed to acquiring the data. MM revised the manuscript, leading to the final approval of the current submission. MM, TU, AU, HM and JL contributed to data collection. SK supervised the study and revised the manuscript, leading to the final approval of the current submission. All authors read and approved the final manuscript.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Pelletier D andHafler DA.. Fingolimod for multiple sclerosis. N Engl J Med 2012; 366: 339–347. [DOI] [PubMed] [Google Scholar]
- 2.Saida T, Kikuchi S, Itoyama Y, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult Scler 2012; 18: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 3.Budde K, Schmouder RL, Brunkhorst R, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol 2002; 13: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 4.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355: 1124–1140. [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 6.Gilenya 0.5 mg hard capsules. Last Updated on eMC 30-Jun-2017. Novartis Pharmaceuticals UK Ltd, http://www.medicines.org.uk/emc/medicine/24443 (accessed 18 October 2017).
- 7.Warnke C, Dehmel T, Ramanujam R, et al. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology 2014; 83: 2153–2157. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Bethesda, MD, USA. 2010, http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. (accessed 5 December 2017).
- 10.Tanaka M, Kinoshita M, Tanaka K. Intermittent drug holidays in fingolimod therapy for multiple sclerosis. Mult Scler. Epub ahead of print 1 July 2017. DOI: 10.1177/1352458517722647. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M andTanaka K.. Dose reduction therapy of fingolimod for Japanese patients with multiple sclerosis: 24-month experience. Clin Exp Neuroimmunol 2014; 5: 383–384. [Google Scholar]
- 13.Francis G, Kappos L, O’Connor P, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler 2014; 20: 471–480. [DOI] [PubMed] [Google Scholar]
- 14.Furuncuoğlu Y, Tulgar S, Dogan AN, et al. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur Rev Med Pharmacol Sci 2016; 20: 1300–1306. [PubMed] [Google Scholar]
- 15.Nikfar S, Rahimi R, Abdollahi M, et al. A meta-analysis of the efficacy and tolerability of interferon-beta in multiple sclerosis, overall and by drug and disease type. Clin Ther 2010; 32: 1871–1888. [DOI] [PubMed] [Google Scholar]
- 16.Naldini A andFleischmann WR Jr.. In vivo myelosuppression by combination interferon treatment: Antagonism of MuIFN-gamma and MuIFN-beta myelosuppressive effects. J Biol Response Modif 1987; 6: 546–555. [PubMed] [Google Scholar]
- 17.Hatcher SE, Waubant E, Nourbakhsh B, et al. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 2016; 73: 790–794. [DOI] [PubMed] [Google Scholar]
- 18.Zecca C, Merlini A, Disanto G, et al. Half-dose fingolimod for treating relapsing–remitting multiple sclerosis: Observational study. Mult Scler. Epub ahead of print 1 February 2017. DOI: 10.1177/1352458517694089. [DOI] [PubMed] [Google Scholar]
- 19.Yamout BI, Zeineddine MM, Sawaya RA, et al. Safety and efficacy of reduced fingolimod dosage treatment. J Neuroimmunol 2015; 285: 13–15. [DOI] [PubMed] [Google Scholar]
- 20.Killestein J, Vennegoor A, van Golde AE, et al. PML-IRIS during fingolimod diagnosed after natalizumab discontinuation. Case Rep Neurol Med 2014; 2014: 307872. [DOI] [PMC free article] [PubMed] [Google Scholar]