Abstract
Atherosclerosis is a chronic inflammatory disease of the vasculature that is initiated by cholesterol deposition into the arterial wall, which triggers the infiltration of immune and inflammatory cells, including monocytes and macrophages. As atherosclerotic plaques progress, localized hypoxia promotes compensatory angiogenesis from the vasa vasorum. Immature neovessels are prone to leakage, thus destabilizing the plaque and leading to intraplaque hemorrhage. Macrophages with different phenotypes, ranging from classical inflammatory subtypes to alternatively activated antiinflammatory macrophages, have been identified in atherosclerotic lesions. Antiinflammatory hemoglobin-scavenging CD163+ macrophages are present in neovessel- and hemorrhage-rich areas; however, the role of these macrophages in atherogenesis has been unclear. In this issue of the JCI, Guo, Akahori, and colleagues show that CD163+ macrophages promote angiogenesis, vessel permeability, and leucocyte infiltration in human and mouse atherosclerotic lesions through a mechanism involving hemoglobin:haptoglobin/CD163/HIF1α-mediated VEGF induction. This study thus identifies proatherogenic properties of CD163+ macrophages, which previously were thought to be beneficial.
Macrophages contribute to intraplaque neovascularization
Atherosclerosis is a chronic, progressive disease of the large arteries that over decades culminates in acute cardiovascular events, such as myocardial infarction and stroke. Early lipid deposition, endothelial cell activation, and leucocyte recruitment in the vascular intima result in progression to complex atherosclerotic lesions, the stability of which determines the incidence of cardiovascular events due to plaque rupture or erosion and thrombus formation. Current therapeutic strategies aim to stabilize existing plaques, for instance, by decreasing inflammation (1). Plaque angiogenesis and intraplaque hemorrhage, which occur when plaques increase in size, are important contributors to plaque progression and instability (Figure 1 and ref. 2). Plaque neovascularization is thought to be mainly driven by local hypoxia (3) that is provoked by progressive thickening of the neointimal layer and overconsumption of O2 by plaque macrophages (4). The hypoxia stabilizes HIF1α protein, which subsequently induces the expression and secretion of proangiogenic factors such as VEGF (3). Neovessel formation includes a construction phase (tubulogenesis), which consists of proteolytic degradation of the extracellular matrix (ECM) and proliferation and migration of endothelial cells, and a stabilization phase (maturation), during which the integrity of the vessel is established by reconstruction of ECM around the neovessel (5). In advancing atherosclerosis, angiogenesis is frequently incomplete, resulting in vascular leakage and hemorrhage and subsequent uptake of RBC, hemoglobin (Hb), and iron by surrounding macrophages (6–8), ultimately leading to destabilization (2). Therefore, apart from being involved in cholesterol uptake and foam cell formation, macrophages are involved in numerous processes during atherogenesis.
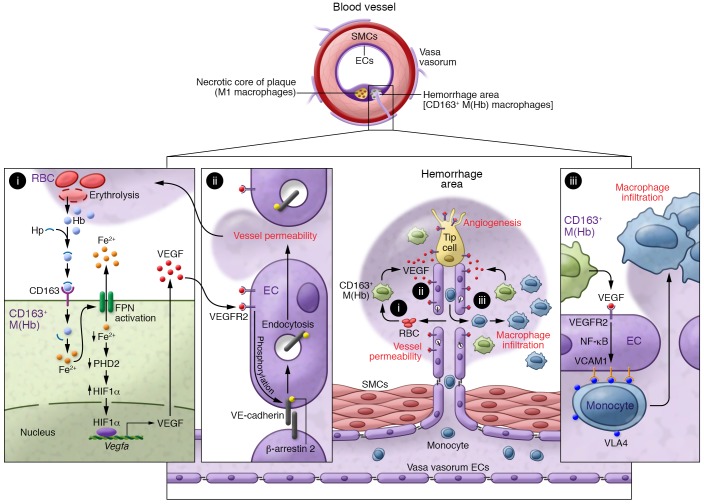
Figure 1. Alternative CD163+ macrophages associate with atherosclerotic plaque hemorrhage and leakage.
CD163+ M(Hb) macrophages are abundant in hemorrhage areas, in which erythrolysis provides high amounts of Hb (i). In complex with Hp, Hb is scavenged by CD163 and induces the expression of FPN, which promotes intracellular Fe2+ depletion (i). Ferrous iron efflux leads to decreased prolyl hydroxylase 2 (PHD2) activity and subsequent stabilization of HIF1α, which enhances VEGF expression (i) and intraplaque neovascularization. VEGF promotes VEGFR2-dependent VE-cadherin internalization (ii) and neovessel leakage (ii). VEGF also enhances VCAM expression and macrophage infiltration (iii), therby increasing plaque progression and erosion. ECs, endothelial cells; SMCs, smooth muscle cells.
While the Manichean concept that macrophages can be divided into proinflammatory M1 and antiinflammatory M2 subsets has mainly been described in vitro (9), this framework has allowed the identification of heterogeneous macrophages in atherosclerosis. Subsequent studies have identified a wide spectrum of intermediary macrophage phenotypes within the plaque (10). Indeed, macrophage polarization depends on the microenvironment, where different pro- and antiinflammatory signals coexist with complex lipids, senescent cells, hypoxia, and accumulated Hb and iron. Depending on the stimuli, macrophages can skew toward an oxidized LDL–activated proinflammatory M1 subset, an IL-4–induced antiinflammatory M2 subset, a Hb-associated subset referred to as M(Hb) or Mhem, an oxidized phospholipid–stimulated Mox subset, or a CXCL4-activated M4 subtype (10). Macrophage behavior is therefore a highly dynamic process during which macrophage activity dynamically adapts to the microenvironment, thus allowing macrophage subsets to participate in almost every step of atherosclerosis (10). Cholesterol accumulation into the artery wall results in M1 polarization, thereby enhancing the inflammatory response. M2 macrophages are thought to contribute to tissue repair, inflammation resolution, and efficient efferocytosis by scavenging apoptotic cells. In addition, macrophages may contribute to intraplaque neoangiogenesis. Indeed, IL-4– and IL-13–activated macrophages facilitate capillary sprouting by remodeling ECM through the secretion of matrix metalloproteinases (MMP2, MMP7, MMP9, and MMP12) and tip cell guidance by secreting semaphorins (5). Moreover, these macrophages produce angiogenic factors, including TGF-β, VEGF, and EGF (5). However, incomplete neovascularization results in intraplaque hemorrhage, and erythrocyte lysis releases high amounts of Hb, which, after binding to haptoglobin (Hp), is internalized by macrophages through the scavenger receptor CD163. Exposure to Hb:Hp skews macrophages toward an alternatively activated M(Hb) phenotype (11). Intracellular accumulation of Hb and iron enhances the activity of the oxysterol-activated nuclear receptor liver X receptor α (LXRα), thereby inducing cholesterol efflux and preventing foam cell formation (6, 8). Additionally, compared with M1-polarized macrophages, CD163+ cells produce fewer proinflammatory cytokines, such as TNF-α, and more antiinflammatory cytokines, such as IL-10. Along with a noted decrease of cholesterol accumulation, CD163+ macrophages have been suggested to serve an atheroprotective role (11). However, the study from Guo, Akahori, and colleagues in this issue challenges this simple concept (Figure 1 and ref. 12).
Alternative macrophages may exert proatherogenic activities
Guo, Akahori, and coworkers show that Hb-activated CD163+ macrophages are abundantly present in atherosclerotic plaques (12). Analysis of human endarterectomy samples revealed that CD163+ macrophages are mainly present in neovascularized, VEGF-positive hemorrhagic areas and correlate with markers of plaque progression (Figure 1 and ref. 12), suggesting a role for this macrophage population in plaque angiogenesis and vascular permeability (Figure 1, i and ii). Whether these CD163+ macrophages are derived from cells newly recruited through the leaky vessel, or whether they are derived from preexisting macrophages that have been skewed toward M(Hb) is unclear. Nevertheless, these CD163+ cells were mostly present in areas of high microvessel permeability. In mice, Cd163 gene deletion in the proatherogenic Apoe–/– genetic background impaired plaque progression (12). Finally, genetic analysis of cardiovascular disease patient cohorts revealed an association between the rs7136716 GG polymorphism of CD163, which increases CD163 expression, and coronary plaque rupture and plaque angiogenesis. Moreover, the association between CD163 rs7136716 GG and adverse plaque features was independent of traditional cardiovascular risk factors. Together, these observations by Guo, Akahori, and colleagues suggest that CD163+ macrophages exert deleterious effects on atherogenesis, despite their antiinflammatory profile (12).
Furthermore, Guo, Akahori, and coworkers demonstrated that CD163+ macrophages probably contribute to plaque instability through a pathway involving CD163-mediated Hb:Hp macrophage polarization, iron-mediated HIF1α protein stabilization, and subsequently increased VEGF production (Figure 1i and ref. 12). In turn, VEGF stimulates VEGFR2-dependent VE-cadherin internalization and degradation in endothelial cells (Figure 1ii), provoking a loss of endothelial barrier function and increased vascular permeability (Figure 1ii). In addition, VEGF enhances VCAM expression through a mechanism involving NF-κB pathway activation (Figure 1iii), hence promoting inflammatory cell recruitment into the vascular wall (Figure 1iii and ref. 12). Intriguingly, even though iron-handling genes are induced in CD163+ macrophages upon Hb exposure, Guo, Akahori, and colleagues found that Hb:Hp-polarized macrophages have relatively low levels of intracellular ferrous Fe2+, an observation that may explain the stabilization of HIF1α (Figure 1i and ref. 8). These results highlight a mechanism of HIF1α-dependent angiogenesis that is dependent on iron metabolism and CD163 rather than on hypoxia. Indeed, accumulation of intracellular iron increases the expression of ferroportin (FPN), which promotes iron efflux and HIF1α-dependent activation of VEGF expression (Figure 1i). Interestingly, LXRα also induces FPN expression, inhibits expression of the FPN inhibitor hepcidin, and may therefore be an interesting target to prevent the dysfunction of these macrophages (6). Other potential mechanisms of interest include the energy sensor AMPK, which enhances VEGF, FGF2, PDGFB, and TGF-β expression and promotes intraplaque neoangiogenesis (13). In different tissues, iron depletion enhances AMPK activation (14). Whether AMPK regulates VEGF expression in Fe2+-depleted M(Hb) macrophages and/or whether changes in intracellular pO2 and a metabolic switch occur in the M(Hb) in hemorrhagic areas remains to be determined.
Conclusions
Guo, Akahori, and colleagues have shown that alternatively polarized CD163+ macrophages may contribute to plaque destabilization. Dysfunctional activity of alternative macrophages has also been observed in mannose receptor–positive macrophages, which are located adjacent to areas of calcification and display an impaired or so-called “lazy” osteoclast phenotype because of defective cathepsin K expression (15). These findings indicate that even though the pathways established upon alternative activation of macrophage polarization may appear to be mainly antiatherogenic, under specific conditions, alternatively polarized macrophages can also promote atherosclerosis progression. Strategies targeting the recruitment, differentiation, and/or activity of CD163+ macrophages may be an interesting approach to combat atherosclerosis. Guo, Akahori, and colleagues propose reducing CD163 activity and/or expression to inhibit intraplaque neovascularization and plaque progression by using CD163 antagonists or by inhibiting expression. Although data analysis from RNA sequencing and microarray experiments performed on different subsets of sorted immune cells (https://www.immgen.org/) indicates that CD163 is mainly expressed in macrophages, it is also present to a lesser extent in γδT cells. While γδT cells do not appear to be involved in early atherosclerosis (16), these cells accumulate in atheroma and may also play a role in advanced lesions. Although the overall effect of CD163 may thus involve other cell types, targeting CD163 may represent a new innovative therapeutic approach; however, additional studies are needed to ensure the feasibility and selectivity of such an approach.
Acknowledgments
Grants were provided by the European Genomic Institute for Diabetes (EGID) (ANR-10-LABX-46, to BS); ALMaVasCal (ANR-16-CE14-0001-01, to BS); and the Foundation Leducq (Leducq Epigenetics of Atherosclerosis Network [LEAN]). BS is a recipient of an Advanced European Research Council (ERC) Grant (grant number 694717).
Version 1. 02/19/2018
Electronic publication
Version 2. 03/01/2018
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2018;128(3):910–912. https://doi.org/10.1172/JCI120123.
See the related article at CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis.
Contributor Information
Benoit Pourcet, Email: benoit.pourcet@pasteur-lille.fr.
Bart Staels, Email: Bart.Staels@pasteur-lille.fr.
References
- 1.Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32(16):1977–1985, 1985a, 1985b, 1985c. doi: 10.1093/eurheartj/ehr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parma L, Baganha F, Quax PHA, de Vries MR. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol. 2017;816:107–115. doi: 10.1016/j.ejphar.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Marsch E, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34(12):2545–2553. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 5.Kalucka J, Bierhansl L, Wielockx B, Carmeliet P, Eelen G. Interaction of endothelial cells with macrophages-linking molecular and metabolic signaling. Pflugers Arch. 2017;469(3–4):473–483. doi: 10.1007/s00424-017-1946-6. [DOI] [PubMed] [Google Scholar]
- 6.Bories G, et al. Liver X receptor activation stimulates iron export in human alternative macrophages. Circ Res. 2013;113(11):1196–1205. doi: 10.1161/CIRCRESAHA.113.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle JJ, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174(3):1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn AV, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59(2):166–177. doi: 10.1016/j.jacc.2011.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 11.Guo L, Harari E, Virmani R, Finn AV. Linking hemorrhage, angiogenesis, macrophages, and iron metabolism in atherosclerotic vascular diseases. Arterioscler Thromb Vasc Biol. 2017;37(4):e33–e39. doi: 10.1161/ATVBAHA.117.309045. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128(3):1106–1124. doi: 10.1172/JCI93025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H, et al. AMP-activated protein kinase α1 in macrophages promotes collateral remodeling and arteriogenesis in mice in vivo. Arterioscler Thromb Vasc Biol. 2016;36(9):1868–1878. doi: 10.1161/ATVBAHA.116.307743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill JF, Thomson DM, Hardman SE, Hepworth SD, Willie S, Hancock CR. Iron deficiency causes a shift in AMP-activated protein kinase (AMPK) subunit composition in rat skeletal muscle. Nutr Metab (Lond) 2012;9(1):104. doi: 10.1186/1743-7075-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinetti-Gbaguidi G, et al. Human alternative macrophages populate calcified areas of atherosclerotic lesions and display impaired RANKL-induced osteoclastic bone resorption activity. Circ Res. 2017;121(1):19–30. doi: 10.1161/CIRCRESAHA.116.310262. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HY, Wu R, Hedrick CC. Gammadelta (γδ) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis. 2014;234(2):265–269. doi: 10.1016/j.atherosclerosis.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]