Abstract
Background
Mastocytoma are frequently diagnosed cutaneous neoplasms in dogs. In non-resectable mastocytoma patients, novel targeted drugs are often applied. The transcription factor STAT5 has been implicated in the survival of human neoplastic mast cells (MC). Our study evaluated the JAK2/STAT5 pathway as a novel target in canine mastocytoma.
Materials and Methods
We employed inhibitors of JAK2 (R763, TG101348, AZD1480, ruxolitinib) and STAT5 (pimozide, piceatannol) and evaluated their effects on 2 mastocytoma cell lines, C2 and NI-1.
Results
Activated JAK2 and STAT5 were detected in both cell lines. The drugs applied were found to inhibit proliferation and survival in these cells with the following rank-order of potency: R763 > TG101348 > AZD1480 > pimozide > ruxolitinib > piceatannol. Moreover, synergistic anti-neoplastic effects were obtained by combining pimozide with KIT-targeting drugs (toceranib, masitinib, nilotinib, midostaurin) in NI-1 cells.
Conclusion
The JAK2/STAT5 pathway is a novel potential target of therapy in canine mastocytoma.
Keywords: canine mastocytoma, JAK2, KIT, STAT5, targeted drugs
1. Introduction
Mast cell tumors (MCT), also referred to as mastocytoma, are the most common skin neoplasms in canines. They represent 7%-21% of all cutaneous tumors in dogs.1–3 The biological behavior of MCT is variable, ranging from solitary benign tumors to a highly invasive and metastatic disease.4 In order to classify canine MCT, a grading system was developed based on histological findings.5 Grade 1 neoplasms consist of mature, well-differentiated mast cells (MC) with a low potential to metastasize whereas in grade 3 tumors, MC are immature with a high risk of metastasis formation. Grade 2 represents an intermediate stage where MC show variable signs of differentiation. Several studies have shown that this grading system is of prognostic significance concerning survival.6–8 However, regardless of the grade all MCT are considered to be potentially malignant.
The etiology of MCT is largely unknown but probably multifactorial, as evidenced by different aberrations in neoplastic MC. One of the commonly mutated genes described in literature in neoplastic MC is KIT.4,9 The encoded protein KIT is a tyrosine kinase receptor, which activates various downstream targets including the signal transducer and activator of transcription 5 (STAT5) and plays a major role in MC growth. In many cases, mutations in KIT are associated with ligand-independent activation of the receptor and therefore with autonomous growth of MC.10–13
Standard treatment in MCT is surgery with wide excision margins for resectable tumors, chemo- or radiotherapy for non-resectable cases or a combined treatment for residual or locally recurrent MCT.6,14 Recently, 2 tyrosine kinase inhibitors (TKI) directed against KIT, namely masitinib and toceranib, have been approved for the treatment of KIT-mutated MCT.15–18 These drugs are able to suppress tumor progression in a subset of patients. However, in many cases treatment effects are only temporary.16,18 Therefore, current research attempts to identify novel targets and to develop new therapeutic approaches in MCT.
The Janus kinase 2 (JAK2) is a critical, cytoplasmic tyrosine kinase downstream of cytokine (growth factor) receptors, promoting the proliferation of normal and neoplastic cells. JAK2 activates major downstream targets, including STAT5, phosphoinositide 3-kinases (PI3K) and extracellular-signal-regulated kinases (ERK).19–21 Recently, the JAK2-targeting drug ruxolitinib has been approved by the FDA for the treatment of human primary myelofibrosis (PMF) and hydroxyurea (HU)-resistant or -intolerant polycythemia vera (PV), in which JAK2-activating mutations are prominent.22–24 In 2011, Beurlet et al. identified JAK2 mutations in canine patients suffering from PV.25 We have recently described that activated STAT5 is constitutively expressed in human neoplastic MC and triggers the proliferation and survival of these cells.26 Together, JAK2 and STAT5 are considered to be crucial mediators of growth and survival of neoplastic cells and therefore potential therapeutic targets in myeloid neoplasms.27,28
However, JAK2 and STAT5 have not been investigated in the context of canine MC neoplasms so far. The aims of this study were to examine the expression and activation of JAK2 and STAT5 in canine MCT and to explore the anti-neoplastic effects of established inhibitors of the JAK2/STAT5 pathway in these cells. For this purpose, 2 established canine MC lines, C2 and NI-1 were used both of which carry several mutations in KIT.29,30 In addition, primary neoplastic MC isolated from mastocytoma specimens of different grades according to the Patnaik classification5 were employed in order to obtain more disease-relevant information about target expression profiles. To evaluate the effects of JAK2-/STAT5 inhibition in MCT, a number of JAK2- or STAT5-targeting drugs were applied. The JAK2-blockers used in this study (R763, TG101348, AZD1480, ruxolitinib) have already been tested in clinical trials and ruxolitinib is even used in clinical practice in human patients with PMF and PV. By contrast, the STAT5-targeting drugs tested (pimozide, piceatannol) have not been applied in clinical oncology so far. We also asked whether JAK2- or STAT5 blockers and KIT-targeting drugs would exert synergistic anti-neoplastic effects in MCT. In summary, the results of our study provide evidence that targeting the JAK2/STAT5 pathway might be a potent approach to tackle otherwise untreatable mastocytoma patients.
2. Materials and Methods
2.1. Reagents
The compounds used in this study are listed in Table 1. Imatinib was dissolved in distilled water and all other drugs were dissolved in dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, Missouri). RPMI 1640 medium and antibiotics (penicillin/streptomycin) were purchased from Lonza (Basel, Switzerland), fetal bovine serum (FBS) from Gibco Life Technologies (Carlsbad, California) and 3H-thymidine from PerkinElmer (Waltham, Massachusetts). Propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich. A specification of antibodies used in this study is provided in Table 2.
Table 1.
Specification of inhibitors used in this study
| Name | Known targets | Result of target inhibition (drug action) | Clinical application | Cmax in μM (at dose/day) | Concentration range (μM)1 | Supplier2 |
|---|---|---|---|---|---|---|
| R763 | JAK2, aurora kinase A, aurora kinase B, FLT3 | G2/M phase arrest, endoreduplication | Phase 1 trial (human): 11.4-85.3 mg/day | 0.09 (85.3 mg) | 0.005-5 | Rigel Pharmaceuticals |
| TG101348 | JAK2, JAK1, JAK3, FLT3, RET, TYK2 | proliferation inhibition, apoptosis induction | Phase 1 trial (human): 100–600 mg/day | 2.8 (500 mg) | 0.005-7.5 | ChemieTek |
| AZD1480 | JAK2, JAK1, JAK3, TYK2, FGFR3, STAT3 | Proliferation inhibition, apoptosis induction | Phase 1 trial (human): 5–80 mg/day | 1.7 (30 mg) | 0.005-7.5 | ChemieTek |
| Ruxolitinib | JAK2, JAK1, JAK3 | Proliferation inhibition, apoptosis induction | Approved (human): PMF, post-PV-MF, post-ET-MF, PV3, (EU, USA) | 2.3 (50 mg) | 1-20 | ChemieTek |
| Pimozide | STAT5, D2 dopamine receptor, 5-HT7 receptor, Ca2+ channels | Unknown | Approved (human): Tourette's Syndrome (EU, USA) | 0.007 (2 mg) | 0.5-25 | Sigma-Aldrich |
| Piceatannol | STAT5, SYK, LCK | Proliferation inhibition, apoptosis induction, histamine release blockade | Animal model (rat): 20.2-80.7 mg/kg/day | 8.1 (80.7 mg/kg) | 1-75 | Sigma-Aldrich |
| Imatinib | KIT, BCR-ABL, PDGFR | Proliferation inhibition | Approved (human): CML, ALL, MDS/MPD, DFSP, HES/CEL, ASM, GIST (EU, USA) | 3.3 (400 mg) | 0.05-2.5 | ChemieTek |
| Masitinib | KIT, PDGFR, LCK, LYN, FGFR3 | Proliferation inhibition | Approved (canine): MCT (EU, USA—conditionally) | 0.9 (8.4 mg/kg) | 0.05-5 | LC Laboratories |
| Midostaurin | KIT, PKC, PKA, S6, EGFR, PDGFR | Proliferation inhibition, G2/M phase arrest | Approval pending (human): AML, mastocytosis (USA) | 7 (250 mg) | 0.05-2.5 | LC Laboratories |
| Nilotinib | KIT, BCR-ABL, PDGFR | Proliferation-/migration inhibition, apoptosis induction | Approved (human): CML (EU, USA) | 3.6 (800 mg) | 0.05-5 | ChemieTek |
| Toceranib | KIT, VEGFR, PDGFR, CSF-1, FLT3 | Proliferation inhibition, apoptosis induction | Approved (canine): MCT (EU, USA) | 0.3 (1.45 mg/kg) | 0.25-0.5 | Sigma-Aldrich |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; ASM, aggressive systemic mastocytosis; Cmax, peak plasma concentration of a drug after administration; CML, chronic myelogenous leukemia; CSF-1, colony stimulating factor 1; DFSP, dermatofibrosarcoma protuberans; EGFR, epidermal growth factor receptor; ET, essential thrombocythemia; FGFR3, fibroblast growth factor receptor 3; FLT3, fms-like tyrosine kinase 3; GIST, gastrointestinal stromal tumor; HES/CES, hypereosinophilic syndrome/chronic eosinophilic leukemia; JAK, janus kinase; LCK, leukocyte C-terminal SRC kinase; LYN, LCK/YES novel tyrosine kinase; MCT, mast cell tumor; MDS/MPD, myelodysplastic/myeloproliferative disease; PDGFR, platelet-derived growth factor receptor; PK, protein kinase; PMF, primary myelofibrosis; PV, polycythemia vera; STAT, signal transducer and activator of transcription; SYK, spleen tyrosine kinase; TYK2, tyrosine kinase 2; VEGFR, vascular endothelial growth factor receptor.
Range of concentrations used in this study.
ChemieTek, Indianapolis, Indiana; LC Laboratories, Woburn, Massachusetts; Rigel Pharmaceuticals, San Francisco, California; Sigma-Aldrich, St. Louis, Missouri.
PV patients intolerant or resistant to hydroxyurea.
Table 2.
Specification of antibodies used in this study
| Name/clone | Reactive structure | IgG subtype | Method | Dilution (diluent) | Fluorochrome | Company1 |
|---|---|---|---|---|---|---|
| 47 | pSTAT5 | mIgG1 | FC | — | Alexa Fluor 647 | BD Biosciences |
| 251610 | STAT5 | mIgG1 | FC | — | PE | R&D Systems |
| 104D2 | KIT | mIgG1 | FC | — | PE | BD Biosciences |
| MOPC-21 | mIgG1 isotype | n.a. | FC | — | Alexa Fluor 647 | BD Biosciences |
| MOPC-21 | mIgG1 isotype | n.a. | FC | — | PE | BD Biosciences |
| 11711 | mIgG1 isotype | n.a. | FC | — | PE | R&D Systems |
| Annexin V | Annexin V | n.a. | FC | — | FITC | eBioscience |
| Annexin V | Annexin V | n.a. | FC | — | APC | BioLegend |
| D2E12 | JAK2 | rIgG | ICC | 1:50 (TBS/FBS) | n.a. | Cell Signaling |
| E132 | pJAK2 | rIgG | ICC | 1:50 (Renoir red diluent) | n.a. | Millipore |
| C11C5 | pSTAT5 | rIgG | ICC/IHC | 1:20 (Renoir red diluent) | n.a. | Cell Signaling |
| C-19 | KIT | rIgG | ICC | 1:200 (TBS/FBS) | n.a. | Santa Cruz Biotechnology |
| Phospho-c-KIT (Tyr719) | pKIT | rIgG | ICC | 1:20 (TBS/FBS) | n.a. | Cell Signaling |
| Phospho-STAT5 pTyr694 | pSTAT5 | rIgG | WB | 1:1000 (TBST/BSA) | n.a. | Invitrogen |
| 89 | STAT5 | mIgG | WB | 1:1000 (TBST/BSA) | n.a. | BD Transduction Laboratories |
| AC-74 | β-Actin | mIgG | WB | 1:5000 (TBST/BSA) | n.a. | Sigma-Aldrich |
Abbreviations: APC, allophycocyanin; FC, flow cytometry; FITC, fluorescein isothiocyanate; ICC, immunocytochemistry; IHC, immunohistochemistry; m, mouse; n.a., not applicable; PE, phycoerythrin; r, rabbit; TBS/FBS, tris-buffered saline/fetal bovine serum; TBST/BSA, tris-buffered saline tween/bovine serum albumin; WB, Western blot.
BD Biosciences, San Jose, California; BD Transduction Laboratories, San Jose, California; BioLegend, San Diego, California; Cell Signaling, Massachusetts; eBioscience, San Diego, California; Invitrogen, Waltham, Massachusetts; Millipore, Billerica, Massachusetts; R&D Systems, Minneapolis, Minnesota; Santa Cruz Biotechnology, Dallas, Texas; Sigma-Aldrich, St. Louis, Missouri.
2.2. Culture of cell lines
In this study, we used 2 canine mastocytoma cell lines, C2 and NI-1. C2 cells were kindly provided by Dr. Warren Gold (Cardiovascular Research Institute, University of California, San Francisco, California).29 NI-1 cells were established in our laboratory.30 Cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin (antibiotics) at 5% CO2 and 37° C. Cells were split every 2 to 3 days and thawed from an original stock every 6 to 8 weeks. In each experiment, “control medium” (RPMI 1640 medium with 10% FBS and 1% antibiotics) was applied. In addition, vehicle control experiments (DMSO concentration corresponding to the highest drug concentration applied) were performed. In all experiments, the vehicle controls showed no effects on either pSTAT5 expression, cell proliferation, cell viability or cell cycle progression (not shown).
2.3. Isolation of primary canine neoplastic MC from mastocytoma specimens
We isolated neoplastic MC from fresh tumor samples obtained from 3 patients undergoing surgical intervention at the University of Veterinary Medicine Vienna (Vienna, Austria) (Table 3) using collagenase as described.31 In brief, tissue samples were cut into small pieces, washed thoroughly in Tyrode's buffer and were then incubated in collagenase type 2 (Worthington, Lakewood, New Jersey) at 37°C for 180 minutes. Isolated MC were recovered by filtration through nytex cloth and collected into FBS-containing tubes. After washing, cells were examined for viability (trypan blue exclusion) and MC numbers.
Table 3.
Characteristics of canine mastocytoma patients
| No | Sex | Age (years) | Breed | MCT grade 1 | pSTAT5 expression 2 |
|---|---|---|---|---|---|
| 1 | fc | 5.3 | Magyar Vizsla | 1 | n.e. |
| 2 | fc | 10.8 | Cross Breed | 2 | n.e. |
| 3 | fc | 12 | Golden Retriever | 3 | n.e. |
| 4 | fc | 10 | Pit Bull Terrier | 3 | + |
| 5 | m | 13.5 | Alpine Dachsbracke | 3 | + |
| 6 | f | 8.5 | Labrador Retriever | 3 | + |
| 7 | m | 11.5 | Golden Retriever | 2 | + |
| 8 | m | 10 | Golden Retriever | 2 | + |
| 9 | m | 7 | Golden Retriever | 2 | + |
| 10 | fc | 13.5 | German Pinscher | 1 | + |
| 11 | f | 5 | Labrador Retriever | 1 | + |
| 12 | f | 6 | Boxer | 1 | + |
Abbreviations: f, female; fc, female castrated; m, male; MCT, mast cell tumor; n.e. not examined; no, number.
According to Patnaik classification.5
Examined by immunohistochemistry.
2.4. Immunohistochemistry and immunocytochemistry
MCT sections were obtained from nine canine patients treated at the University of Veterinary Medicine Vienna (Vienna, Austria) (Table 3). Immunohistochemistry (IHC) was performed on sections prepared from paraffin-embedded, formalin-fixed mastocytoma specimens by the indirect immune-peroxidase staining technique using the anti-pSTAT5 monoclonal antibody (mAb) C11C5 (diluted 1:20 in Renoir Red Diluent, Biocare Medical, Walnut Creek, California) as described.26 Immunocytochemistry (ICC) was performed on C2 and NI-1 cells using antibodies against JAK2, pJAK2, pSTAT5, KIT and pKIT (Table 2) as reported.26 Antibody reactivity was controlled by antibody omission.
2.5. Flow cytometric evaluation of pSTAT5, STAT5 and KIT in neoplastic MC
Expression of pSTAT5 and STAT5 was examined in C2 cells and NI-1 cells using an intracellular flow cytometry staining protocol as reported.30 In brief, cells were stained with the Alexa Fluor 647-conjugated anti-pSTAT5 mAb 47, the phycoerythrin (PE)-conjugated anti-STAT5 mAb 251610, the Alexa Fluor 647-conjugated isotype-matched control antibody or the PE-conjugated isotype-matched control antibody. Phosphorylated STAT5 levels and total STAT5 levels were then analyzed on a FACS Canto 2 (Becton Dickinson, Franklin Lakes, New Jersey). To evaluate the effects of JAK2-, STAT5- and KIT inhibitors on expression of pSTAT5, C2 and NI-1 cells were incubated in control medium or in various concentrations of R763, TG101348, AZD1480, ruxolitinib, pimozide, piceatannol, imatinib, masitinib, midostaurin or nilotinib (0.05-50 μM) at 37°C for 4 hours before being analyzed using flow cytometry. To confirm surface expression of KIT on C2 and NI-1 cells, the PE-conjugated anti-KIT mAb 104D2 was applied as reported.32
2.6. Protein analysis and Western blotting
Sample preparation and Western blotting were done according to standard methods.33 Nitrocellulose membranes were incubated with antibodies directed against STAT5 or pSTAT5. β-Actin served as loading control (Table 2). To evaluate the effects of JAK2- and STAT5 blockers on expression of pSTAT5, C2 and NI-1 cells were incubated in control medium or in the presence of R763, TG101348, AZD1480, ruxolitinib, pimozide or piceatannol (1-50 μM) at 37°C for 4 hours.
2.7. Cross-validation of anti-human antibodies in the canine MC lines
The specific reactivity of the anti-JAK2 mAb D2E12 and the anti-KIT antibody C-19 for the canine species was previously confirmed by others.34,35
To validate the cross-reactivity of antibodies against human pSTAT5, pJAK2 and pKIT, we performed Western blot experiments using canine cell lines and the human MC line HMC-1.2 as positive control. In these experiments, we were able to confirm specific reactivity of NI-1 cells with antibodies against pSTAT5, pJAK2 and pKIT (not shown). In case of the fluorochrome-labeled anti-human anti-KIT mAb 104D2, no Western blot reaction was obtained neither with canine cells nor with HMC-1.2 cells. An explanation for this could be that this antibody was designed particularly for flow cytometric detection of surface KIT.
2.8. Effects of targeted drugs on proliferation of canine neoplastic mast cells
Primary neoplastic MC, C2 cells and NI-1 cells were seeded in 96-well microtiter plates and incubated in control medium or in various concentrations of JAK2 inhibitors (0.005-20 μM) or STAT5 inhibitors (0.5-75 μM) at 37°C for 48 hours. After adding 0.5 μCi 3H-thymidine (37°C, 16 hours), cells were harvested on filter membranes in a Filtermate 196 harvester (Packard Biosciences, Meriden, Connecticut). The bound radioactivity was measured in a Top-Count NXT β-counter (Packard Biosciences).
2.9. Evaluation of apoptosis and cell cycle arrest
To analyze drug effects on induction of apoptosis and cell cycle distribution, C2 and NI-1 cells were incubated in control medium or in various concentrations of JAK2-targeting drugs (0.1-7.5 μM) or STAT5-targeting drugs (5-75 μM) at 37° C for 24 or 48 hours. Apoptosis was measured by Annexin V/PI- or Annexin V/DAPI-staining and flow cytometry as described.31 For experiments with toceranib, we used Annexin V-APC combined with DAPI instead of Annexin V-FITC combined with PI due to the autofluorescence of toceranib. The total fraction of all apoptotic cells was quantified by determining the percentage of Annexin V-single positive cells plus the percentage of Annexin V- and PI/DAPI-double positive cells relative to all cells captured in the test tube. To evaluate additive or synergistic drug effects on cell survival, NI-1 cells were incubated with various drug combinations employing JAK2 inhibitors (R763, TG101348, AZD1480, ruxolitinib), the STAT5 inhibitor pimozide and KIT inhibitors (imatinib, masitinib, midostaurin, nilotinib, toceranib) for 24 hours. In these experiments, we selected suboptimal drug concentrations that showed only little or no effect on induction of apoptosis in NI-1 cells in previous experiments. As the statistical evaluation of drug combinations by CompuSyn (ComboSyn, Paramus, New Jersey) requires multiple drug concentrations, we applied an additional concentration of each single drug and each drug combination, for example pimozide (2.5 μM, 5 μM), toceranib (0.25 μM, 0.5 μM), combination (2.5 μM pimozide + 0.25 μM toceranib, 5 μM pimozide + 0.5 μM toceranib). The lower concentrations were only used for calculating statistics and are not shown. For cell cycle studies, cells were harvested after 48 hours (without prior synchronization of cells) and stained with PI as reported.31 The amount of DNA-bound PI was then determined using flow cytometry.
2.10. Statistical analysis
To determine the significance in differences between the drug-treated conditions and the untreated control condition in our experiments, the Student's t-test was applied. Results were considered statistically significant when P was < .05. Drug combination effects on apoptosis were evaluated by CompuSyn and considered to be synergistic when the combination index (CI) was < 1, additive when CI = 1 and antagonistic when CI > 1.
3. Results
3.1. Canine neoplastic MC exhibit activated JAK2, STAT5 and KIT
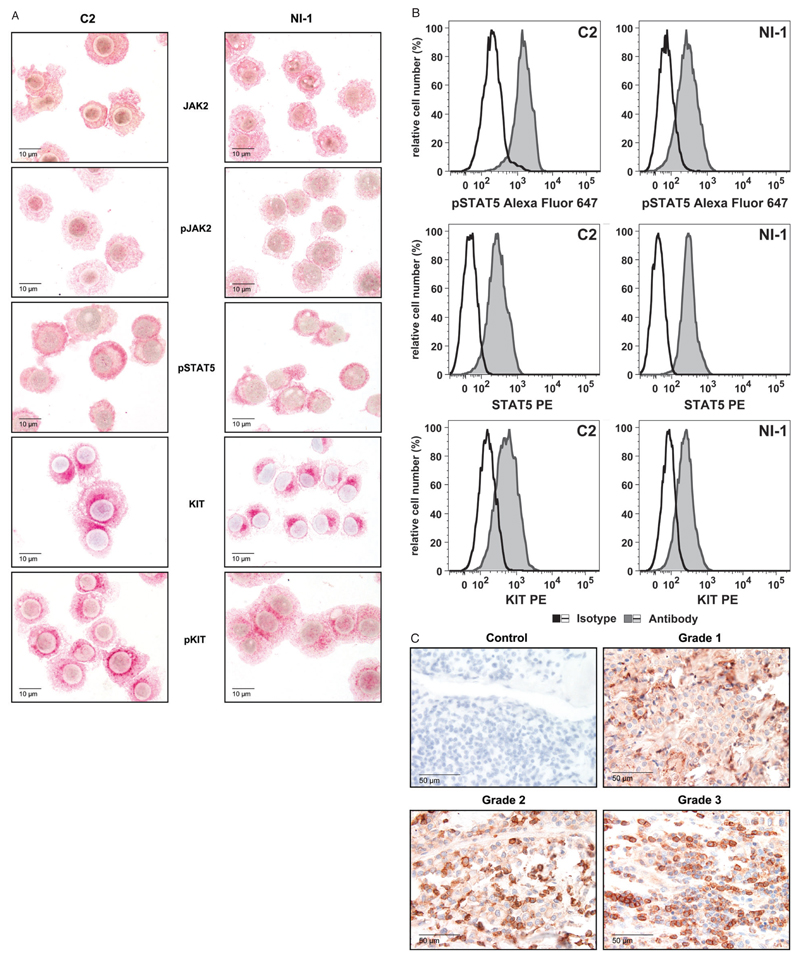
As determined by immunocytochemistry, C2 cells and NI-1 cells were found to express JAK2, pJAK2, pSTAT5, KIT and pKIT (Figure 1A). The presence of intracellular pSTAT5 and STAT5 as well as surface KIT in C2 and NI-1 cells was also demonstrable using flow cytometry (Figure 1B). In these experiments, higher levels of pSTAT5 were detected in C2 cells compared with NI-1 cells whereas STAT5- and KIT levels were comparable in the 2 cell lines. Furthermore, we were able to demonstrate the expression of pSTAT5 in primary MCT by IHC (Table 3, Figure 1C). In particular, pSTAT5 was detected in neoplastic MC in 9 of 9 canine patients examined.
Figure 1.
Expression of JAK2, STAT5 and KIT in canine neoplastic mast cells (MC). A, C2 cells (left panel) and NI-1 cells (right panel) were stained with antibodies for JAK2, pJAK2, pSTAT5, KIT or pKIT using indirect immunocytochemistry as described in the text. B, Levels of pSTAT5, STAT5 and KIT in C2 and NI-1 cells determined using flow cytometry. Cells were incubated with an Alexa Fluor 647-conjugated anti-pSTAT5 antibody (gray histograms, upper panel), a phycoerythrin (PE)-conjugated anti-STAT5 antibody (gray histograms, middle panel) or a PE-conjugated anti-KIT antibody (gray histograms, lower panel). The isotype-matched control antibodies are also shown (open histograms). MFI, mean fluorescence intensity. C, Immunohistochemical detection of pSTAT5 in neoplastic mast cells of tumor sections obtained from canine mastocytoma patients using the monoclonal anti-pSTAT5 antibody C115C. The staining technique is described in the text. Representative examples from 3 patients are provided (grades 1, 2 and 3 according to Patnaik5, as indicated). The antibody omission control is also shown (upper left panel).
3.2. JAK2-, STAT5- and KIT-targeting drugs counteract STAT5 activation in C2 and NI-1 cells
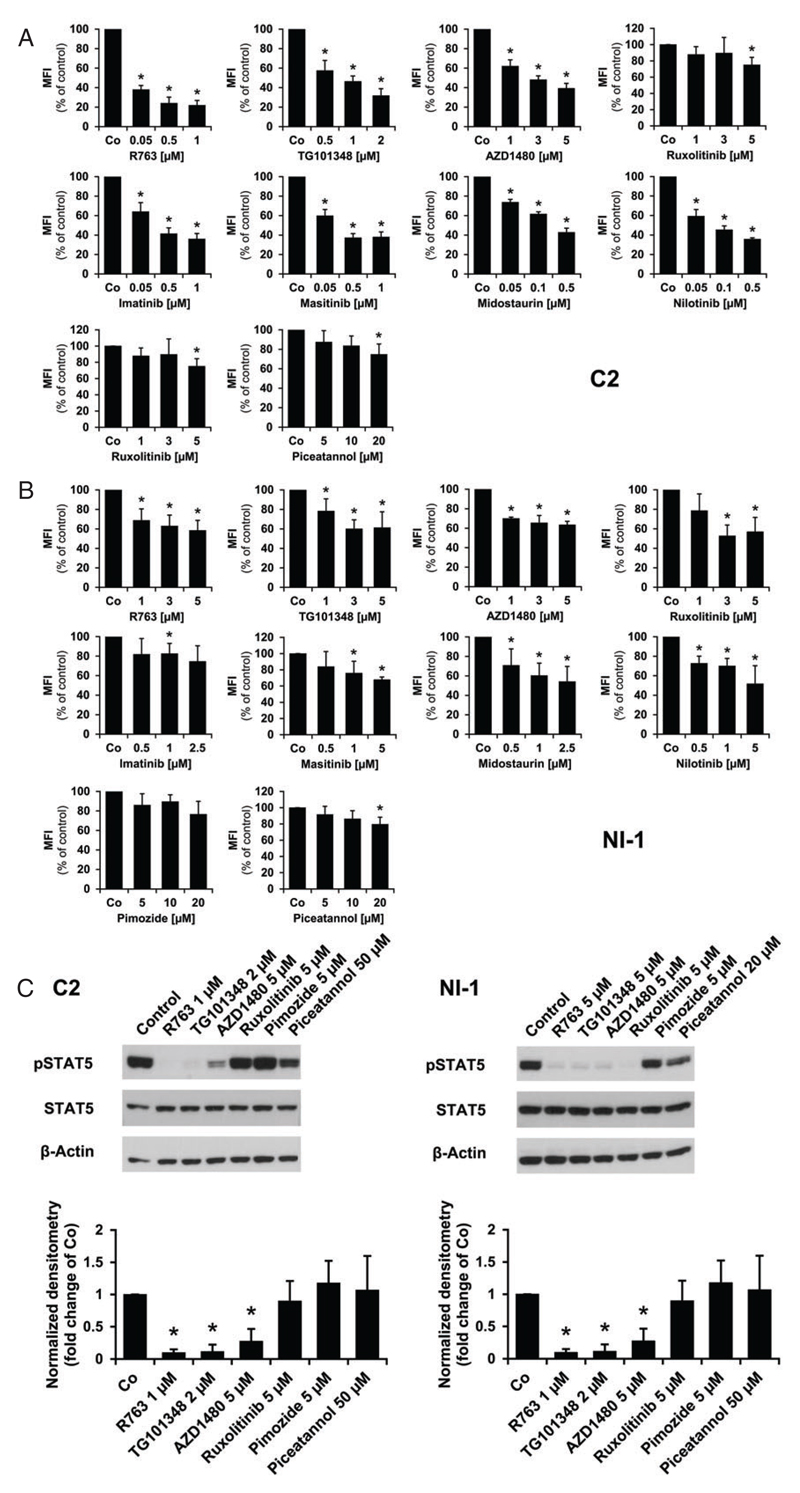
To evaluate the functional role of JAK2 and STAT5, we treated C2 and NI-1 cells with various targeted drugs. As shown in Figure 2A,B, the JAK2-targeting drugs R763, TG101348, AZD1480 and ruxolitinib (0.05-5 (μM) as well as the KIT inhibitors imatinib, masitinib, midostaurin and nilotinib (0.05-5 μM) were able to decrease the levels of pSTAT5 in C2 and NI-1 cells in a dose-dependent manner after 4 hours of treatment. In these experiments, C2 cells were more sensitive compared with NI-1 cells. The STAT5 blockers pimozide and piceatannol (5-50 μM) showed only little effects on pSTAT5 levels in both cell lines. Using Western blot, we found that most of the JAK2-targeting drugs decrease expression of pSTAT5 whereas the STAT5 blockers only showed weak effects in C2 and NI-1 cells (Figure 2C).
Figure 2.
Effects of targeted drugs on pSTAT5 expression in C2 and NI-1 cells. C2 (A) and NI-1 cells (B) were incubated in control medium (Co) or in medium containing various drug concentrations (as indicated) at 37°C for 4 hours. pSTAT5 levels were analyzed using flow cytometry and an Alexa Fluor 647-conjugated anti-pSTAT5 antibody. The staining technique is described in the text. Results show the mean fluorescence intensity (MFI) values relative to medium control (100%) and represent the mean ± SD of at least 3 independent experiments. Asterisk: P < 0.05. The medium control and dimethyl sulfoxide (DMSO) control (not shown) produced almost identical results. C, C2 (left panel) and NI-1 (right panel) cells were exposed to various inhibitors for 4 hours and levels of phosphorylated STAT5 and total STAT5 were examined using Western blotting as described in the text (upper panel). β-Actin served as loading control. The levels of pSTAT5 were quantified by densitometry and normalized to β-Actin (lower panel). Results are expressed relative to control (=1.0) and represent the mean ± SD of 3 independent experiments. *P < 0.05.
3.3. Targeting of JAK2 or STAT5 is associated with reduced proliferation of canine neoplastic MC
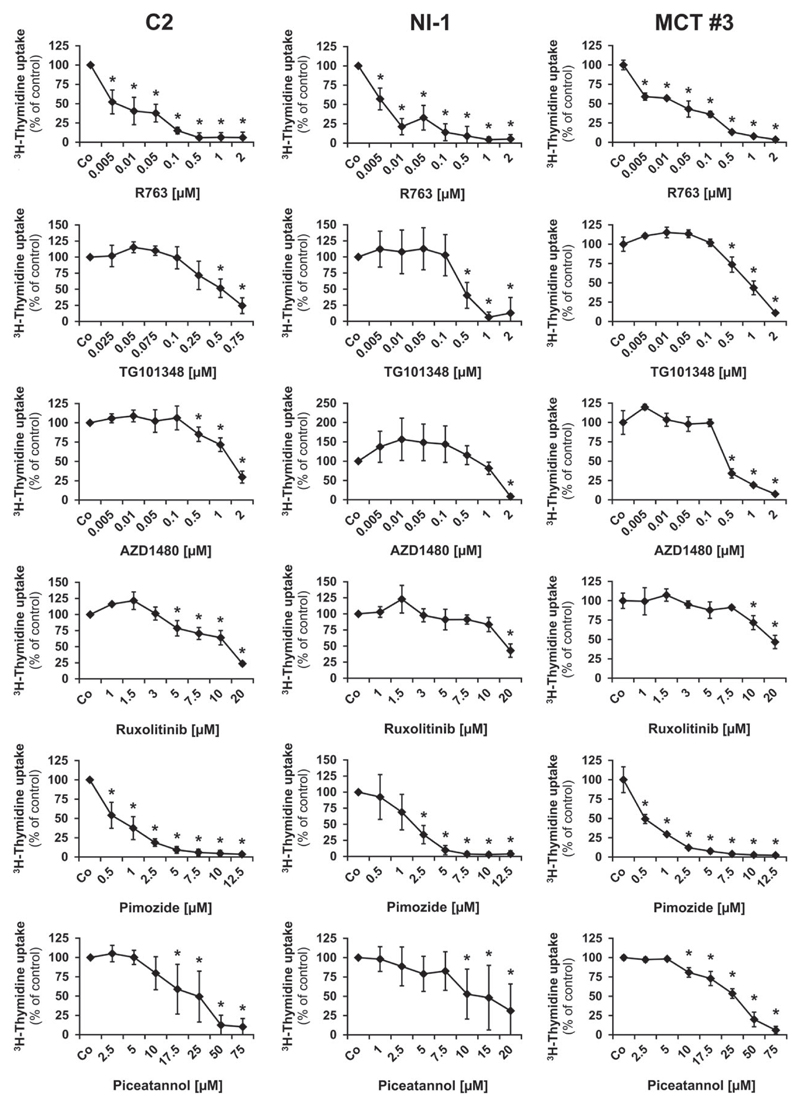
As assessed by 3H-thymidine uptake experiments, all the tested inhibitors were found to decrease the proliferation of the canine MC lines as well as proliferation of primary neoplastic MC in all 3 samples tested (patients #1, #2, #3) (Figure 3). Dose-dependent effects were seen with all drugs but the IC50 values were found to vary among the different drugs applied. The JAK2-targeting drugs R763 and TG101348 were found to block proliferation of C2 and NI-1 cells at relatively low IC50 values (<1 μM). Slightly higher IC50 values (1-2.5 μM) were obtained with AZD1480 and the STAT5-targeting drug pimozide. By contrast, no substantial effects were seen with ruxolitinib or the STAT5 blocker piceatannol (IC50: >10 μM) (Table 4). When comparing drug effects in primary neoplastic MC with the effects obtained in the 2 cell lines examined no major differences were found (Figure 3, Table 4).
Figure 3.
Growth-inhibitory effects of targeted drugs on neoplastic mast cells. C2 cells (left panel), NI-1 cells (middle panel) or cells isolated from a primary mastocytoma (patient #3, right panel) were incubated in control medium (Co), with a vehicle control, which had no effect and is not shown or with various concentrations of JAK2- or STAT5 inhibitors at 37°C for 48 hours. Thereafter, 3H-thymidine incorporation was measured. Results show 3H-thymidine uptake relative to control (=100%) and represent the mean ± SD of at least 3 different experiments (C2, NI-1) or the mean ± SD of triplicates (patient #3). *P < 0.05.
Table 4.
Effects of JAK2- and STAT5 inhibitors on proliferation of canine neoplastic mast cells
| IC50 values1 (μM) obtained with |
||||||
|---|---|---|---|---|---|---|
| Cells | R763 | TG101348 | AZD1480 | Ruxolitinib | Pimozide | Piceatannol |
| C2 | 0.005-0.01 | 0.25-0.75 | 1-2 | 10-20 | 0.5-1 | 17.5-25 |
| NI-1 | 0.005-0.01 | 0.1-0.5 | 1-2 | 10-20 | 1-2.5 | 10-15 |
| MCT #1 | 0.01-0.05 | 1-2 | 0.1-0.5 | >20 | 0.5-1 | 25-50 |
| MCT #2 | 0.005-0.01 | 1-2 | 1-2 | >20 | 2.5-5 | 25-50 |
| MCT #3 | 0.01-0.05 | 0.5-1 | 0.1-0.5 | 10-20 | 0.5-1 | 25-50 |
Abbreviations: IC, inhibitory concentration; MCT, mast cell tumor.
Assessed by 3H-thymidine uptake.
3.4. JAK2- and STAT5 inhibitors induce apoptosis in canine MC lines
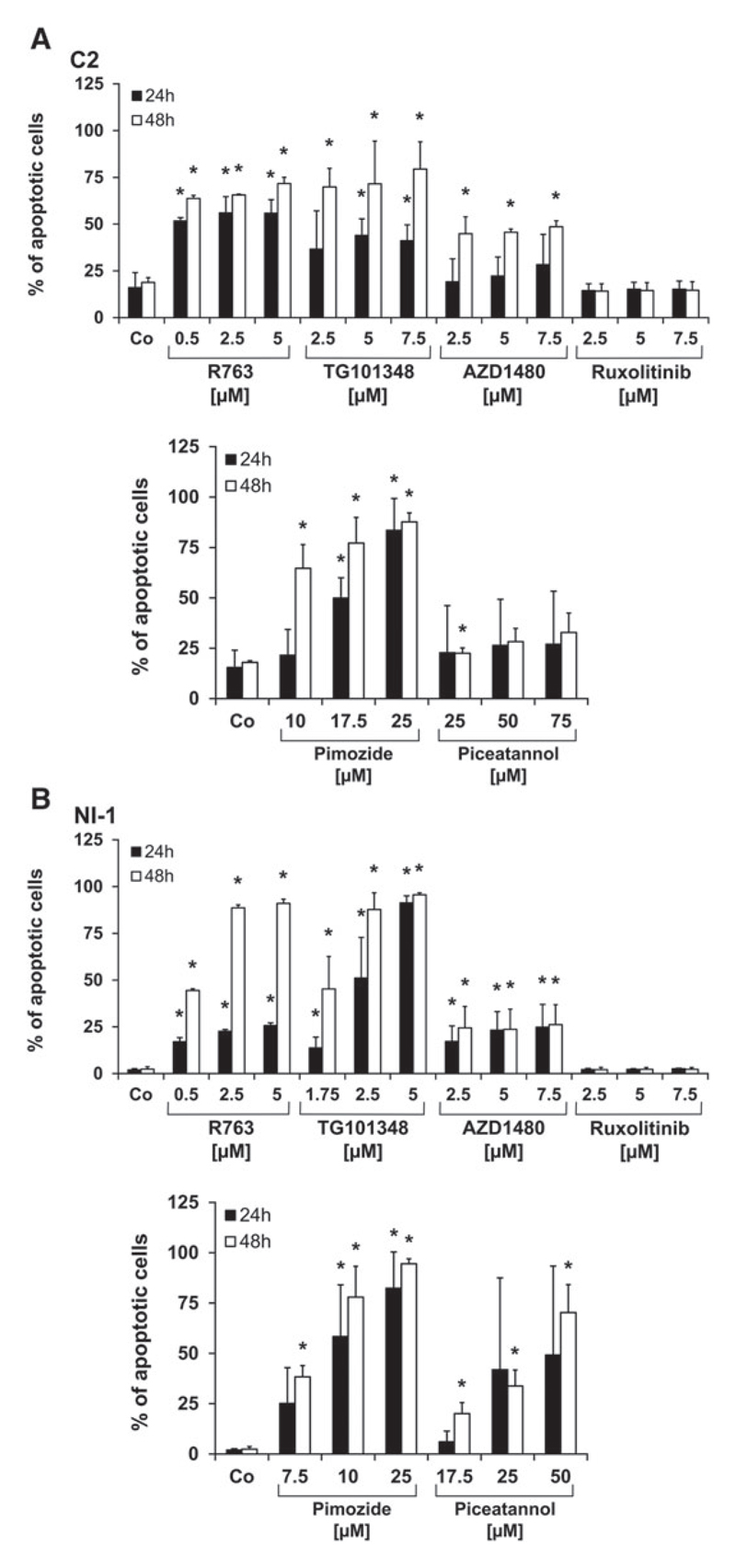
Next, we investigated the mechanism of drug action and asked whether the JAK2- and STAT5-targeting drugs applied would induce apoptosis in canine MC lines. As assessed by Annexin V/PI staining, R763, TG101348, AZD1480, pimozide and piceatannol were found to induce apoptosis in C2 cells and NI-1 cells (Figure 4). Pimozide, R763 and AZD1480 showed comparable effects in C2 and NI-1 cells whereas piceatannol and TG101348 were found to be more effective in NI-1 cells than in C2 cells. In general, higher concentrations of STAT5 blockers were necessary to induce apoptosis in canine MC lines compared with the JAK2 inhibitors applied.
Figure 4.
Induction of apoptosis in C2 and NI-1 cells by JAK2- and STAT5-targeting drugs. C2 (A) and NI-1 cells (B) were incubated in control medium (Co) or medium containing various concentrations of JAK2- and STAT5 blockers at 37°C for 24 or 48 hours (h). Thereafter, cells were examined by flow cytometry using an antibody against Annexin V and propidium iodide (PI) staining. Results show the percent of all apoptotic cells (percentage of cells stained single-positive for Annexin V plus percentage of cells stained double-positive for Annexin V and PI) and represent the mean ± SD of 3 independent experiments. *P < .05. The effects of the dimethyl sulfoxide (DMSO) control did not differ from the medium control (not shown).
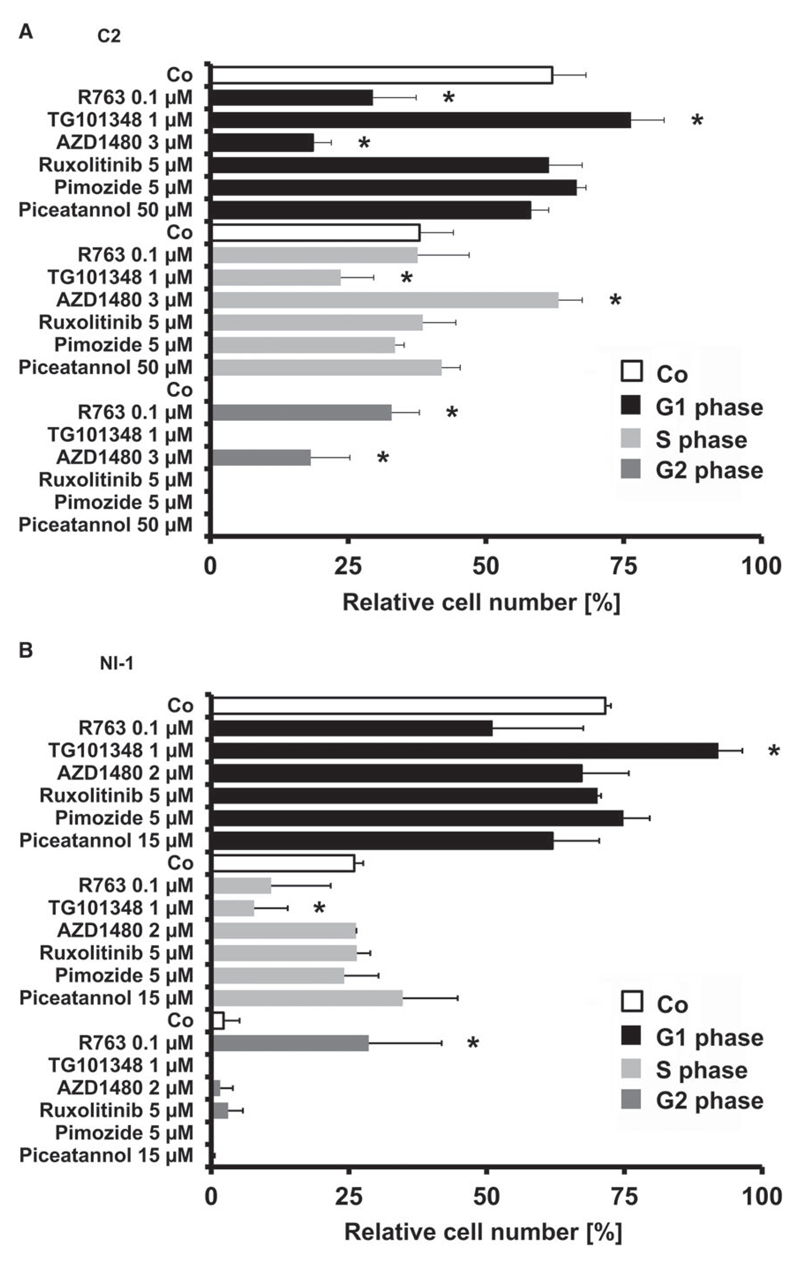
3.5. JAK2-targeting drugs induce cell cycle arrests in C2 cells and NI-1 cells
To further explore the mechanism of drug-induced inhibition of proliferation in neoplastic MC, we examined the effects of JAK2- and STAT5 inhibitors on cell cycle progression. Interestingly, these drugs were found to exert distinct and variable effects on cell cycle distribution in C2 and NI-1 cells (Figure 5). Whereas R763 induced a G2-phase arrest, TG101348 produced a G1 cell cycle arrest in both cell lines. AZD1480 was found to be effective in C2 cells in inducing an S/G2-phase arrest but did not induce an S/G2 arrest in NI-1 cells. The STAT5 inhibitors tested and the JAK2 inhibitor ruxolitinib showed no effects on cell cycle progression in C2 or NI-1 cells.
Figure 5.
Effects of JAK2- and STAT5 blockers on cell cycle progression of C2 and NI-1 cells. C2 (A) and NI-1 cells (B) were incubated in control medium (Co) or with various inhibitors at 37°C for 48 hours. After incubation, cells were harvested and examined for the amount of DNA-bound PI. Results represent the mean ± SD of 3 independent experiments. *P < .05. The effects on the cell cycle by the medium control and the vehicle control were similar (not shown).
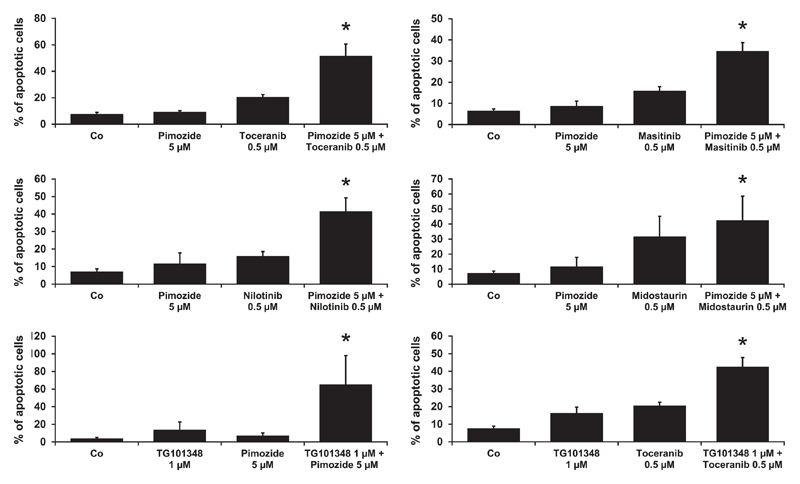
3.6. Evaluation of drug combinations in NI-1 cells
To evaluate the effects of drug combinations on apoptosis, NI-1 cells were exposed to various combinations of JAK2-, STAT5- or KIT-targeting drugs. Results were then examined by CompuSyn software to determine synergistic drug combination effects. In these experiments, we identified several drug combinations with synergistic effects regarding induction of apoptosis in NI-1 cells (CI < 1). These drug combinations included “pimozide + toceranib”, “pimozide + masitinib”, “pimozide + nilotinib”, “pimozide + midostaurin”, “TG101348 + pimozide” and “TG101348 + toceranib” (Figure 6).
Figure 6.
Effects of drug combinations on induction of apoptosis. NI-1 cells were incubated in control medium (Co), in the presence of single inhibitors or the indicated drug combinations at 37°C for 24 hours. Induction of apoptosis was determined using flow cytometry as described in the text. Results show the percent of apoptotic cells (percentage of cells stained single-positive for Annexin V plus percentage of cells stained double-positive for Annexin V and propidium iodide [PI]/4′,6-diamidino-2-phenylindole [DAPI]) and represent the mean ± SD of at least 3 independent experiments. *P < .05.
4. Discussion
MCT are frequently diagnosed skin neoplasms in canines.1–3 Although several treatment options including en-bloc resection, radiation, chemotherapy and KIT inhibitors are available, relapses are frequently seen in advanced high-grade MCT patients.6,14–18 Therefore, new treatment approaches and new targeted drugs are currently being developed.36–40
In this study, we have identified the JAK2/STAT5 pathway as a novel potential target for therapy in canine MCT. In particular, several different drugs targeting JAK2 or STAT5 produced growth inhibition and apoptosis in MCT cells. In addition, we were able to show that STAT5 inhibitors and drugs directed against JAK2 or KIT exert potent synergistic anti-neoplastic effects (CI < 1) in canine neoplastic MC.
So far, little is known about expression and activation of JAK2 and STAT5 in neoplastic MC.40 In the human system, neoplastic MC reportedly show nuclear and cytoplasmic pSTAT5.26,41 In this study, we were able to demonstrate that canine MCT cells display nuclear and cytoplasmic pSTAT5 independent of the tumor grading. Moreover, we were able to show the presence of pSTAT5 as well as pJAK2 in 2 established canine mastocytoma cell lines, namely C2 and NI-1. To the best of our knowledge, these are the first data suggesting that the JAK2/STAT5 pathway is active in canine neoplastic MC.
The JAK2/STAT5 pathway has recently been implicated in various myeloproliferative neoplasms including PV, where activating JAK2 mutations like JAK2 V617F have been detected in human patients.24 More recently, this was confirmed in the canine system.25 Moreover, it has been reported that JAK2 can be recruited and activated by KIT and indeed, SCF-induced colony growth of murine hematopoietic cell lines and human fetal liver cells was found to be reduced after treatment with JAK2 inhibitors.40,42 In addition, STAT5 has recently been described as a KIT-dependent molecular target in human neoplastic MC.11–13 In this study, we were able to show that the KIT-targeting drugs imatinib, masitinib, midostaurin and nilotinib downregulate expression of pSTAT5 in C2 and NI-1 cells, suggesting a role of KIT in STAT5 activation. However, these TKI effects were much stronger in C2 cells than in NI-1 cells, which may be explained by the fact that NI-1 cells display several different KIT mutations and are therefore more resistant against KIT-targeting TKI. C2 cells carry an internal tandem-duplication in exon 11 of KIT whereas in NI-1 cells several mutations in exon 8 and in exon 10 of KIT are present.30 Alternatively, C2 cells could be more sensitive because these cells express higher pSTAT5 levels compared with NI-1 cells.
During the past few years, a number of drugs blocking the JAK2/STAT5 pathway have been developed. In the recent past, the JAK2-targeting drug ruxolitinib has been approved for the treatment of human PMF and PV based on the clear clinical effects of this drug.22,23 Concerning human mastocytosis, ruxolitinib has recently been administered to 2 patients suffering from symptomatic systemic mastocytosis and found to reduce the symptom-burden in these patients with a substantial increase in the quality of life.43,44 However, in both disease models ruxolitinib exerts effects on the cytokine storm and inflammation rather than on cell proliferation.43–46
In this study, we applied a series of drugs known to interact with the activity of JAK2 including ruxolitinib as well as STAT5-targeting drugs. In these experiments, we were able to show that the targeted drugs applied suppress the levels of pSTAT5 in C2 cells and NI-1 cells. The effects of the JAK2 blockers on pSTAT5 levels are in line with the assumption that STAT5 is a JAK2-downstream target. We also examined the effects of the JAK2- and STAT5-targeting drugs on growth of canine neoplastic MC. These inhibitors were indeed found to block the proliferation of C2 and NI-1 cells in a dose-dependent manner with the following rank-order of potency: R763 > TG101348 > AZD1480 > pimozide > ruxolitinib > piceatannol. Furthermore, we were able to show anti-proliferative effects of the JAK2- and STAT5 blockers in primary neoplastic MC obtained from 3 MCT patients. Drug effects obtained with primary MCT cells were comparable to drug effects seen in the 2 cell lines tested. The different potencies among the drugs tested are best explained by additional targets recognized by these drugs. Similarly, R763 is well known to block a number of different kinase-targets including Aurora kinases, KIT, FLT3 and JAK2.47
JAK2 and STAT5 are well-known regulators of survival and cell cycle progression in neoplastic cells.20,28,48 In order to define the mechanism of action of the JAK2- and STAT5 inhibitors tested, we examined their effects on apoptosis and cell cycle progression in the MC lines. Indeed, most of the inhibitors applied decreased survival in C2 and NI-1 cells albeit with variable potency. As expected, the most potent inducer of apoptosis in C2 and NI-1 cells was R763. One explanation for the effect of R763 on cell cycle progression may be its inhibitory effect on Aurora kinases. All drugs applied in this study showed effects at a pharmacologically relevant range except for pimozide, piceatannol and ruxolitinib (Table 1). The 2 STAT5 blockers applied were only effective in inducing apoptosis in canine MC at very high concentrations but not in suppressing cell cycle progression. This observation is consistent with the weak effect of piceatannol in human MC.26 Unexpectedly, although ruxolitinib was found to decrease expression of pSTAT5 in NI-1 cells and to a lower extent in C2 cells in our flow cytometric experiments, the drug was unable to influence MC survival, cell cycle progression or proliferation of both cell lines at pharmacologically relevant concentrations. These discrepant results may be explained by additional (STAT5-independent) mechanisms of drug resistance in canine MC. Alternatively, the effects of ruxolitinib on STAT5 activation were too weak to lead to a visible growth-inhibitory or anti-survival effect. However, in our Western blot experiments the effect of ruxolitinib on pSTAT5 expression in NI-1 cells was stronger than in our flow cytometric analysis.
Whereas several different effective JAK2 blockers are available, pharmacologic inhibition of STAT5 remains a challenging task mainly because the development of specific STAT5 inhibitors is difficult.36–39
In the current study, 2 STAT5 blockers were used, pimozide and piceatannol. However, both drugs are rather weak and non-specific inhibitors of STAT5, which may explain the weak effects on canine neoplastic MC observed in this study.
In contrast to STAT5-targeting drugs, the development of JAK2 blockers is more advanced. The Aurora kinase- and JAK2 blocker R763 has been tested in clinical trials in human hematological malignancies as well as in a dose escalation trial in human patients suffering from solid tumors.50,51 The JAK2-targeting drug ruxolitinib has recently been approved by the FDA for treatment of human PMF and HU-resistant or -intolerant PV.22,23 One concern with these and other JAK2 blockers is toxicity. Therefore, one general goal is to develop less toxic JAK2 inhibitors. Another option would be to reduce single compound concentrations by applying drug combinations. Indeed, it has been shown that various combinations of kinase blockers can produce synergistic growth-inhibitory effects on canine and human MC.52,53 In this study, we were interested to learn whether similar drug combination-effects can be achieved in canine neoplastic MC when combining STAT5- (pimozide), JAK2- (R763, TG101348, AZD1480, ruxolitinib) or KIT inhibitors (imatinib, masitinib, midostaurin, nilotinib, toceranib). In these experiments, the most potent synergistic effects on survival of NI-1 cells were obtained with drug combinations consisting of pimozide and either toceranib, masitinib, nilotinib or midostaurin as well as combinations consisting of TG101348 and pimozide or TG101348 and toceranib (CI < 1).
In summary, we found that canine neoplastic MC express phosphorylated JAK2 as well as STAT5 and that inhibition of the JAK/STAT pathway is associated with decreased proliferation and survival of these cells. Based on our results, we hypothesize that the JAK2/STAT5 pathway is a potential new target in canine MCT. This hypothesis needs to be tested in forthcoming in vivo studies. In case of JAK2 inhibition, the application of novel targeted drugs is a straightforward approach whereas pharmacologic STAT5 blockers are currently being developed and will hopefully enter clinical application in the near future.
Acknowledgements
The study was supported by the Oesterreichische Nationalbank: Jubliaeumsfondsprojekt 14835 and the Austrian Science Fund (FWF): F 4704-B20, F 4707-B20, F 6105-B20 and P25937-B13. C2 cells were kindly provided by Dr. Warren Gold (Cardiovascular Research Institute, University of California, San Francisco, CA, USA). R763 (AS703569) was kindly provided by Dr. Lisa Bliss (Merck Serono, Darmstadt, Germany). We would like to thank Gabriele Stefanzl for skillful technical assistance.
Funding information
Oesterreichische Nationalbank: Jubliaeumsfondsprojekt 14835; Austrian Science Fund (FWF): F 4704-B20 F 4707-B20, F 6105-B20 and P25937-B13.
Footnotes
Alexandra Keller: 0000-0001-8843-7001
References
- 1.Priester WA. Skin tumors in domestic animals. Data from 21 US and Canadian colleges of veterinary medicine. J Natl Cancer Inst. 1973;50(2):457–466. doi: 10.1093/jnci/50.2.457. [DOI] [PubMed] [Google Scholar]
- 2.Cohen D, Reif JS, Brodey RS, Keiser H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 1974;34(11):2859–2868. [PubMed] [Google Scholar]
- 3.Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber M. Survery of animal neoplasms in Alameda and Contra Costa counties, California II. Cancer morbidity in dogs and cats from Almeda County. J Natl Cancer Inst. 1968;40(2):307–318. [PubMed] [Google Scholar]
- 4.Welle MM, Bley CR, Howard J, Rüfenacht S. Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 2008;19(6):321–339. doi: 10.1111/j.1365-3164.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumors: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21(5):469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 6.Misdorp W. Mast cells and canine mast cell tumors. A review. Vet Q. 2004;26(4):156–169. doi: 10.1080/01652176.2004.9695178. [DOI] [PubMed] [Google Scholar]
- 7.Gross TL, Ihrke PJ, Walder EJ, Affolter VR. Epithelial neoplasms and other tumors. In: Gross TL, Ihrke PJ, Walder EJ, Affolter VR, editors. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Oxford, UK: Blackwell Publishing; 2005. pp. 641–654. [DOI] [Google Scholar]
- 8.Webster JD, Yuzbasiyan-Gurkan V, Miller RA, Kaneene JB, Kiupel M. Cellular proliferation in canine cutaneous mast cell tumors: associations with c-KIT and its role in prognostication. Vet Pathol. 2007;44(3):298–308. doi: 10.1354/vp.44-3-298. [DOI] [PubMed] [Google Scholar]
- 9.London CA, Kisseberth WC, Galli SJ, Geissler EN, Helfand SC. Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. 1996;115(4):399–414. doi: 10.1016/s0021-9975(96)80074-0. [DOI] [PubMed] [Google Scholar]
- 10.Furitsu T, Tsujimura T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of the c-kit product. J Clin Invest. 1993;92(4):1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibi S, Arslanhan MD, Langenfeld F, et al. Co-operating STAT5 and AKT signaling pathways in chronic myeloid leukemia and mastocytosis: possible new targets of therapy. Haematologica. 2014;99(3):417–429. doi: 10.3324/haematol.2013.098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harir N, Boudot C, Friedbichler K, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112(6):2463–2473. doi: 10.1182/blood-2007-09-115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor ML, Metcalfe DD. Kit signal transduction. Hematol Oncol Clin North Am. 2000;14(3):517–535. doi: 10.1016/S0889-8588(05)70294-X. [DOI] [PubMed] [Google Scholar]
- 14.Dobson JM, Scase TJ. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J Small Anim Pract. 2007;48(8):424–431. doi: 10.1111/j.1748-5827.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 15.FDA. U.S. Food and Drug Administration Database. [Accessed March 6, 2017]; [updated 14 July 2014]. http://www.fda.gov/downloads/animalveterinary/products/approvedanimaldrugproducts/foiadrugsummaries/ucm245243.pdf.
- 16.Hahn KA, Ogilvie G, Rusk T, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22(6):1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 17.FDA. U.S. Food and Drug Administration Database. [Accessed March 6, 2017]; [updated 18, 2013]. http://www.fda.gov/downloads/AnimalVeterinary/Products/Approved AnimalDrugProducts/FOIADrugSummaries/UCM164091.pdf.
- 18.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 19.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 20.Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21(21):3334–3358. doi: 10.1038/sj.onc.1205398. [DOI] [PubMed] [Google Scholar]
- 21.Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care. 2011;1(1):3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 23.FDA. U.S. Food and Drug Administration Database. [Accessed March 6, 2017]; [updated 12, 2014]. http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm425732.htm.
- 24.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90(2):162–173. doi: 10.1002/ajh.23895. [DOI] [PubMed] [Google Scholar]
- 25.Beurlet S, Krief P, Sansonetti A, et al. Identification of JAK2 mutations in canine primary polycythemia. Exp Hematol. 2011;39(5):542–545. doi: 10.1016/j.exphem.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner C, Cerny-Reiterer S, Sonneck K, et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis. Am J Pathol. 2009;175(6):2416–2429. doi: 10.2353/ajpath.2009.080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8(4):945–954. [PubMed] [Google Scholar]
- 28.Wang Z, Bunting KD. STAT5 in hematopoietic stem cell biology and transplantation. JAKSTAT. 2013;2(4):e27159. doi: 10.4161/jkst.27159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3(5):413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 30.Hadzijusufovic E, Peter B, Herrmann H, et al. NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy. 2012;67(7):858–868. doi: 10.1111/j.1398-9995.2012.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peter B, Gleixner KV, Cerny-Reiterer S, et al. Polo-like kinase-1 as a novel target in neoplastic mast cells: demonstration of growth-inhibitory effects of small interfering RNA and the Polo-like kinase-1 targeting drug BI 2536. Haematologica. 2011;96(5):672–680. doi: 10.3324/haematol.2010.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadovnik I, Lierman E, Peter B, et al. Identification of Ponatinib as a potent inhibitor of growth, migration and activation of neoplastic eosinophils carrying FIP1L1-PDGFRA. Exp Hematol. 2014;42(4):282–293. doi: 10.1016/j.exphem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedbichler K, Themanns M, Mueller KM, et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology. 2012;55(3):941–952. doi: 10.1002/hep.24765. [DOI] [PubMed] [Google Scholar]
- 34.Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125. doi: 10.1186/1471-2407-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frost D, Lasota J, Miettinen M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40(1):42–54. doi: 10.1354/vp.40-1-42. [DOI] [PubMed] [Google Scholar]
- 36.Buchert M, Burns CJ, Ernst M. Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene. 2016;35(8):939–951. doi: 10.1038/onc.2015.150. [DOI] [PubMed] [Google Scholar]
- 37.Weber A, Borghouts C, Brendel C, et al. The inhibition of Stat5 by a peptide aptamer ligand specific for the DNA binding domain prevents target gene transactivation and the growth of breast and prostate tumor cells. Pharmaceuticals (Basel) 2013;6(8):960–987. doi: 10.3390/ph6080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page BD, Khoury H, Laister RC, et al. Small molecule STAT5-SH2 domain inhibitors exhibit potent antileukemia activity. J Med Chem. 2012;55(3):1047–1055. doi: 10.1021/jm200720n. [DOI] [PubMed] [Google Scholar]
- 39.Nam S, Scuto A, Yang F, et al. Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling. Mol Oncol. 2012;6(3):276–283. doi: 10.1016/j.molonc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim WS, Lee MJ, Kim DH, et al. 5′-OH-5-nitro-Indirubin oxime (AGM130), an Indirubin derivative, induces apoptosis of Imatinib-resistant chronic myeloid leukemia cells. Leuk Res. 2013;37(4):427–433. doi: 10.1016/j.leukres.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Lasho T, Tefferi A, Pardanani A. Inhibition of JAK-STAT signaling by TG101348: a novel mechanism for inhibition of KITD816V-dependent growth in mast cell leukemia cells. Leukemia. 2010;24(7):1378–1380. doi: 10.1038/leu.2010.109. [DOI] [PubMed] [Google Scholar]
- 42.Harir N, Pecquet C, Kerenyi M, et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood. 2007;109(4):1678–1686. doi: 10.1182/blood-2006-01-029918. [DOI] [PubMed] [Google Scholar]
- 43.Weiler SR, Mou S, DeBerry CS, Keller JR, Ruscetti FW, Ferris DK. JAK2 is associated with the c-kit proto-oncogene product and is phosphorylated in response to stem cell factor. Blood. 1996;87(9):3688–3693. [PubMed] [Google Scholar]
- 44.Dowse R, Ibrahim M, McLornan DP, Moonim MT, Harrison CN, Radia DH. Beneficial effects of JAK inhibitor therapy in systemic mastocytosis. Br J Haematol. 2017;176(2):324–327. doi: 10.1111/bjh.13951. [DOI] [PubMed] [Google Scholar]
- 45.Yacoub A, Prochaska L. Ruxolitinib improves symptoms and quality of life in a patient with systemic mastocytosis. Biomark Res. 2016;4:2. doi: 10.1186/s40364-016-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardanani A, Vannucchi AM, Passamonti F, Cervantes F, Barbui T, Tefferi A. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia. 2011;25(2):218–225. doi: 10.1038/leu.2010.269. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin J, Markovtsov V, Li H, et al. Preclinical characterization of Aurora kinase inhibitor R763/AS703569 identified through an image-based phenotypic screen. J Cancer Res Clin Oncol. 2010;136(1):99–113. doi: 10.1007/s00432-009-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109(9):1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinelli G, Iacobucci I, Papayannidis C, Soverini S. New targets for Ph+ leukaemia therapy. Best Pract Res Clin Haematol. 2009;22(3):445. doi: 10.1016/j.beha.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 50.U.S. National Institutes of Health. Clinical Trials Database. [Accessed March 6, 2017]; [updated 14 December 2015]. https://www.clinicaltrials.gov/ct2/show/NCT01080664?term=AS703569&rank=3.
- 51.U.S. National Institutes of Health. Clinical Trials Database. [Accessed March 6, 2017]; [updated 14 December 2015]. https://www.clinicaltrials.gov/ct2/show/NCT00391521? term=as703569&rank=3.
- 52.Gleixner KV, Rebuzzi L, Mayerhofer M, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Exp Hematol. 2007;35(10):1510–1521. doi: 10.1016/j.exphem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Gleixner KV, Peter B, Blatt K, et al. Synergistic growth-inhibitory effects of ponatinib and midostaurin (PKC412) on neoplastic mast cells carrying KIT D816V. Haematologica. 2013;98(9):1450–1457. doi: 10.3324/haematol.2012.079202. [DOI] [PMC free article] [PubMed] [Google Scholar]