Introduction
The vascular endothelial growth factor (VEGF) pathway has emerged as an important target for cancer therapy, with VEGF signaling pathway (VSP) inhibitors having been approved for a number of different malignancies. Less anticipated has been the multitude of cardiovascular and renal effects of these therapies. Hypertension occurs in at least a quarter of patients starting VSP inhibitors, with virtually every patient having an absolute increase in blood pressure. Patients can also develop proteinuria, renal dysfunction, vascular events or cardiomyopathy. While these toxicities have been generally manageable, they have introduced cardiovascular and renal considerations for patient care. On the other hand, these sequelae have provided a growing appreciation for the critical role of VEGF in vascular and renal homeostasis in human biology. While several recent articles have addressed practical considerations with respect to patient care with this class of drugs,1–4 this review will explore mechanisms associated with the vascular toxicities arising from VEGF signaling inhibition in cancer treatment.
The VEGF Signaling Pathway (VSP) and VSP Inhibitors
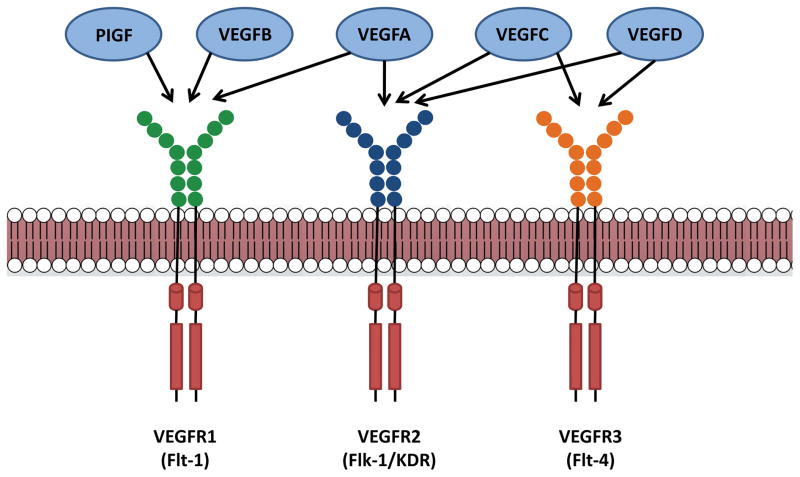
The human VEGF family consists of 5 related glycoproteins: VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor (PIGF). These are secreted to form homodimers, which interact with a family of 3 receptor tyrosine kinases: VEGF receptor (VEGFR) 1, VEGFR2, and VEGFR3 (Figure 1). VEGFA and VEGFB bind to VEGFR1, VEGFA binds to VEGFR2, and VEGFC and VEGFD bind to both VEGFR2 and VEGFR3.1, 5 PIGF primarily interacts with VEGFR1. The VEGFRs are found on a wide variety of cell types. VEGFR1, also called fms-like tyrosine kinase (Flt) 1, is found on vascular endothelial cells, hematopoietic stem cells, monocytes, and macrophages. VEGFR2, also called kinase insert domain (KDR) or fetal liver kinase (Flk1), is expressed on vascular and lymphatic endothelial cells; VEGFR3 (also called Flt-4) is restricted to lymphatic endothelial cells.5 Upon ligand binding, VEGFRs transduce intracellular signals through a variety of mediators. In the case of VEGFR2, which is the best characterized, these include phosphotidylinositol-3 kinase (PI3K)/Akt, mitogen-activated kinases, the non-receptor tyrosine kinase Src, as well as phospholipase C gamma (PLCγ)/protein kinase C (PKC), which promote angiogenesis, lymphangiogenesis, vascular permeability, and vascular homeostasis.1, 5
Figure 1. VEGF ligands and receptors.
Vascular endothelial growth factors (VEGFs) and placental growth factor (PIGF) can interact with a combination of various VEGF receptors (VEGFRs), which are a part of the receptor tyrosine kinase superfamily. VEGFR1 can interact with PIGF, VEGFA, and VEGFB. VEGFR2 interacts with VEGFA, VEGFC, and VEGFD, while VEGFR3 can bind VEGFC and VEGFD.
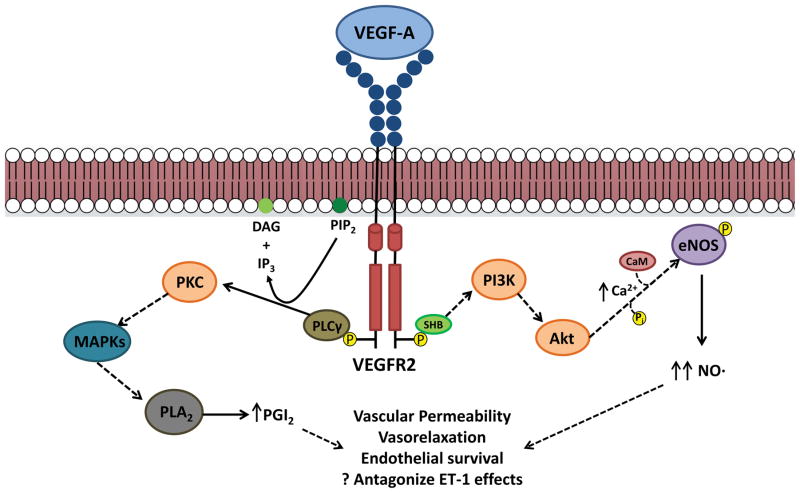
VEGF primarily exerts its effect through the production of vasodilatory mediators. VEGF signaling through VEGFR increases nitric oxide (NO) production, the key downstream mediator of the VEGF signaling pathway (Figure 2). Upon VEGF binding, VEGFR2 undergoes autophosphorylation, and through PI3K/Akt, increases intracellular calcium. Acutely, this activates calmodulin, which binds to and activates endothelial nitric oxide synthase (eNOS).6 Downstream signaling from PI3K/Akt can lead to direct phosphorylation of eNOS as well, which provides a more sustained, calcium-independent stimulus to increase eNOS activity. VEGF signaling also increases eNOS mRNA and protein levels, enhancing long-term eNOS expression.6 The resultant increase in NO production promotes vascular permeability and endothelial cell survival; NO also diffuses to adjacent vascular smooth muscle cells and mediates endothelium-dependent vasodilation.7 In addition to NO, VEGF signaling promotes production of the vasodilatory prostanoid prostacyclin (PGI2) through activation of phospholipase A2 via PLCγ/PKC.8
Figure 2. Intracellular signaling pathways for VEGFA/VEGFR2.
Upon ligand binding, VEGFR2 dimerizes and activates its receptor tyrosine kinase activity, which results in auto-phosphorylation of the intracellular domains. This can then lead to activation of a variety of signaling pathways. Intracellular calcium levels increase through activation of PI3K/Akt signaling, which activate endothelial nitric oxide synthase (eNOS) through calmodulin (CaM) binding as well as direct phosphorylation, resulting in increased nitric oxide (NO) production. VEGFR2 signaling also activates phospholipase C gamma (PLCγ), which converts phosphotidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 can also mediate increases in intracellular calcium, while DAG can activate protein kinase C (PKC). One downstream target of PKC signaling, through mitogen activated protein kinases (MAPKs), is phospholipase A2, ultimately leading to elevated levels of prostacyclin (PGI2). Both PGI2 and NO mediate many of the biological consequences of VEGF signaling, including enhanced vascular permeability, vasorelaxation, and endothelial survival. These may also antagonize the effects of vasoconstrictive mediators such as endothelin-1 (ET-1).
Several strategies have been utilized to inhibit VEGF signaling (Table 1). Broadly, these can be specific agents antagonizing the VEGFA/VEGFR2 axis, or they are small molecule tyrosine kinase inhibitors (TKIs) exhibiting potent anti-VEGFR activity. Direct neutralization of VEGFA was the initial strategy for VSP inhibition and led to the development of bevacizumab, a humanized monoclonal antibody. Soluble decoy receptors (“receptor traps”) sequester circulating VEGF to prevent downstream receptor activation; aflibercept functions in this role by mimicking the extracellular domains of VEGFR1 and VEGFR2, thus inhibiting the effects of VEGFA, VEGFB, and PIGF. Specific anti-VEGFR2 agents include ramucirumab, a fully human monoclonal antibody against VEGFR2. On the other hand, a number of small molecular multi-targeted kinase inhibitors with potent activity against all VEGF receptors have been approved. There are currently ten such agents currently in use (apatinib, axitinib, cabozantinib, lenvatinib, nintedanib, pazopanib, regorafinib, sorafenib, sunitinib, and vandetanib) with several others in development. The TKIs in this class can target more than one kinase receptor allowing for a broader indication for use but with a greater potential for off-target effects.
Table 1.
| VSP Inhibitor Sub-Class | Approved and Investigational Drugs |
|---|---|
| VEGFA monoclonal antibody | bevacizumab |
| VEGF Trap | alfibercept |
| VEGFR2 monoclonal antibody | Ramucirumab |
| Tyrosine kinase inhibitor | |
| FDA Approved: | apatinib, axitinib, cabozantinib, lenvatinib, nintedanib, pazopanib, regorafinib, sorafenib, sunitinib, vandetanib |
| Under Investigation: | cediranib, lucitanib, semaxanib, tivozanib |
VSP inhibitors have been investigated in the last decade as anti-cancer therapies. Solid tumors were known to secrete a pro-angiogenic factor, which, if inhibited, could slow and restrict tumor growth.9–10 Bevacizumab was initially approved in 2004 as an add-on to standard chemotherapy for metastatic colorectal cancer; it has since been utilized as adjunctive therapy in a number of other solid malignancies, including renal cell carcinoma, non-small cell lung cancer, and glioblastoma multiforme. The VEGFR TKIs are currently indicated for use in renal cell carcinoma, hepatocellular carcinoma, colorectal cancer, gastrointestinal stromal tumor, pancreatic neuroendocrine tumor, soft tissue sarcomas, and medullary thyroid cancer. In addition to their use in cancer treatment, VSP inhibitors are also commonly used in proliferative diseases of the retina; however, the toxicity profile with intraocular injection likely differs significantly compared with systemic delivery.
VSP Inhibitors and Hypertension
Hypertension is the most common vascular toxicity associated with VSP inhibition, occurring in around 25% of patients treated with the first approved drugs bevacizumab and sunitinib.11–12 However, because of varying definitions for hypertension used in the initial trials, the true incidence may have been underestimated.13 In early-phase clinical trials, newer VSP inhibitors such as lucitanib and lenvatinib appear to cause hypertension in up to 70–90% of patients.14–15 Early data from these newer agents also suggest an increased association with very elevated blood pressures (greater than 160 mmHg systolic or 100 mmHg diastolic) and evidence of end-organ injury.14–15 Irrespective of the specific drugs, the majority of patients experience an absolute increase in blood pressure that occurs within one week after starting treatment and appears to be reversible. An appreciation of VEGF receptor signaling provides biological plausibility for these observations.
VSP inhibitors have been found to reduce NO pathway metabolites and NO-dependent processes. In a pre-clinical mouse study, VEGFR2 blockade decreased expression of eNOS, and direct pharmacologic inhibition of eNOS mimicked the blood pressure-raising effects of VEGFR2.16 In a study of patients on the TKI regorafenib, plasma nitrate and nitrite levels, the stable oxidation products of NO, were decreased when patients were on the drug, with levels recovering during the week off therapy.17 Kidney cancer patients exposed to VSP inhibitors, including bevacizumab and TKIs, showed decreased urinary cyclic guanosine monophosphate (cGMP), the major downstream second messenger of NO, with a trend towards decreased urinary nitrate.18 In breast cancer patients treated with vandetanib in addition to standard chemotherapy, plasma nitrite was reduced.19 The decrease in NO metabolites is mirrored by impairment in endothelium-dependent vasodilation in the microvasculature, a process that requires NO. In a study of patients with metastatic colon cancer treated concurrently with bevacizumab for 6 months, microvascular endothelium-dependent flow dilation decreased in response to pilocarpine.20 A study with the TKI telatinib given to patients with solid organ tumors similarly found impaired microvascular responses to flow-mediated dilatation (endothelium-dependent) and nitroglycerin (endothelium-independent) after 5 weeks of therapy.21 In both of these studies, patients developed elevated blood pressures over the treatment period.
There are intriguing data that VSP inhibitors may lead to hypertension by enhancing vasomotor tone through the endothelin system, although specifically how VEGF-endothelin cross-talk occurs is less clear. Endothelin-1 (ET-1), acting through its receptor ETA, is a potent vasoconstrictor.22 Treatment with VSP inhibitors increases circulating ET-1 levels in both preclinical models and clinical studies. In patients with renal cell carcinoma or gastrointestinal stromal tumors treated with sunitinib, plasma ET-1 levels increased after therapy.23–24 Similarly, in patients with gastrointestinal stromal tumors treated with the TKI regorafenib, ET-1 levels increased and decreased with administration and withdrawal of therapy, respectively, mirroring the changes seen in blood pressure.17 On the other hand, in a small study of renal cell carcinoma patients treated with sorafenib, circulating ET-1 levels did not change from baseline after three weeks of therapy despite the development of hypertension.25 More convincing evidence regarding a causal role for the endothelin system comes from rat studies in which sunitinib increased plasma and urinary levels of ET-1 and increased blood pressure; importantly, the rise in blood pressure could be prevented by the non-specific ET receptor antagonist macitentan.23 Despite these intriguing observations, it is unclear how VEGF inhibition increases ET-1. NO and PGI2, downstream products of VEGF signaling, inhibit ET-1 expression and secretion in cultured endothelial cells.26–27 Thus, VSP inhibitors, by decreasing these vasodilator products of VEGF signaling, could increase ET-1 levels, causing vasoconstriction, leading to elevated blood pressure. Additional studies are needed to better understand the mechanisms by which VEGF inhibition enhances endothelin expression and action.
There is limited evidence of a role of the renin-angiotensin-aldosterone system in VSP-inhibitor-induced hypertension. One pre-clinical study in which mice were treated with an anti-VEGFA antibody demonstrated a near doubling in circulating angiotensin II levels.28 However clinical data provide conflicting evidence. In a study of patients with solid tumors who were not candidates for chemotherapy but who were receiving the TKI sorafenib, there was no change in plasma aldosterone or renin levels, although angiotensin II levels were not directly measured.25 In another study of patients with renal cell carcinoma or gastrointestinal stromal tumor receiving sunitinib, plasma renin activity and concentration were decreased after 4 weeks and recovered after 8 weeks of therapy; aldosterone levels were unchanged over the study period.24
VSP inhibitors can also promote changes in the microvasculature, leading to an increase in vascular resistance at the tissue level and contributing to hypertension. Microvascular rarefaction, where there is a physical decrease in small arteriolar and capillary density, or functional rarefaction, where increased vasomotor tone results in decreased blood flow through the vascular bed, can lead to hypertension.29 Preliminary data suggest vascular pruning of structurally and functionally abnormal vessels supplying solid tumors by anti-VEGF therapies.30 There is clinical evidence of rarefaction of non-tumor, systemic microvessels in patients treated with VSP inhibitors. In a series of metastatic colon cancer patients being treated with bevacizumab, both anatomic and functional decreases in skin capillary density and blood flow were noted after six months of therapy, with patients receiving higher cumulative doses showing greater capillary rarefaction.20
Emerging evidence suggests that VSP inhibitors are associated with a salt-sensitive form of hypertension. Interstitial sodium accumulation and resultant osmotic stress, particularly in the skin, is being recognized as an important feature of salt-sensitive hypertension.31–32 Sodium accumulation is sensed by tissue macrophages, which then signal through VEGFC/VEGFR3 to promote lymphatic vessel expansion and eNOS expression in order to improve local sodium/chloride ion clearance and counter-balance elevated vascular resistance. Moreover, skin-specific depletion of VEGFC in animal models recapitulates salt-sensitive hypertension. Increased tissue sodium also appears to increase ET-1 expression.33 In a pre-clinical study, sunitinib treatment increased tissue sodium concentrations and led to a salt-sensitive increase in blood pressure.34 Taken together, these data suggest an interesting avenue for further inquiry.
Treatment of hypertension during VSP inhibitor therapy is largely focused on recognition, monitoring and treatment with standard anti-hypertensive medications.35–36 Patients with pre-existing hypertension should be identified and optimally treated before beginning VSP inhibitor therapy, as these patients may experience a worsening in blood pressure control. During the VSP inhibitor treatment period, blood pressure should be monitored regularly and therapy initiated or adjusted according to standard blood pressure treatment guidelines, with the choice of agent individualized to the patient’s co-morbidities. Only very limited data are available comparing anti-hypertensive treatment regimens and additional studies are needed before any formal recommendations may be made about specific therapies. Interestingly, one retrospective study showed that in metastatic renal cell cancer patients treated with VSP inhibitors, concomitant use of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers was associated with longer overall survival.37 Keeping in mind that the hypertension is reversible, the blood pressure needs to be closely monitored after stopping VSP inhibitors and anti-hypertensive medications down-titrated if necessary.
Renal Effects of VSP Inhibition leading to Hypertension
VSP inhibitors can lead to deleterious effects on renal function and renovascular homeostasis. These perturbations in kidney function, in conjunction with the vasoconstrictive effects of VSP inhibitors, can shift the pressure natriuresis curve to higher values and contribute to the maintenance of hypertension over the span of treatment. In a study of sixteen patients with colorectal cancer treated with adjunctive bevacizumab, dynamic contrast magnetic resonance imaging demonstrated decreased renal perfusion, decreased renal vascular permeability and increased interstitial pressure.38 That same study found increased plasma concentrations of renin, angiotensin II, and aldosterone in patients. Several studies have found a significant prevalence of proteinuria with VSP inhibitor therapy, ranging from 15–36% in patients treated with bevacizumab39–41 and TKIs.42–43 In these studies, the incidence of severe proteinuria (defined as more than 3.5 g in a 24-hour urine collection or 4+ on a urine dipstick) was generally less than 10%. It remains unclear whether renal injury is a consequence of elevated blood pressure created by systemic vascular toxicities, or whether renovascular injury is a direct side effect of VSP inhibitor therapy. Nonetheless, these effects likely stem from a combination of renovascular effects, structural changes at the level of the glomerular filtration apparatus, and humoral changes.
VEGF signaling and downstream NO production is important for maintaining normal glomerular structure and function, as NO signaling plays a critical role in modulating both renal perfusion and sodium reabsorption.44 VEGF is expressed by podocytes and tubular epithelial cells and acts on surrounding endothelial and mesangial cells. Glomerular membrane endothelial cell fenestrations and podocyte integrity are dependent on VEGF-induced NO production and contribute to normal filtration and barrier function.45 The critical role of VEGF expression in the glomerulus was demonstrated in a study utilizing mice with a podocyte-specific deletion of the VEGF-A gene; these mice developed endotheliosis followed by loss of podocyte foot processes and endothelial fenestrations, ultimately leading to the development of nephrotic syndrome.46
In pre-clinical studies, VSP inhibitor therapy causes glomerular level injury. In vitro studies in cultured human glomerular endothelial cells show that TKI therapy can blunt VEGF-induced eNOS protein expression.47 Animal models similarly show deleterious effects of VSP inhibitor therapy on kidney function. Mice treated with an anti-VEGF antibody developed albuminuria within 24 hours of initiating therapy; kidney biopsies demonstrated glomerular endothelial hypertrophy, cellular detachment from the endothelium, and disruption of podocytes and slit membranes.48 Longer-term therapy with an anti-VEGFA antibody in mice - for up to five weeks - similarly resulted in increased urinary albumin excretion, increased serum creatinine, and diffuse glomeruloscerlosis, characterized by increased fibrin deposition in the mesangium and glomerulus.28 These changes are associated with a decrease in the key podocyte protein, nehprin. Nephrin is a component of the podocyte slit diaphragms that has both structural roles and anti-apoptotic effects; loss of nephrin is associated with podocyte foot process effacement, with eventual glomerular injury and proteinuria.49 VSP inhibitor therapy may exhibit a dose-dependent effect on kidney injury. In rats treated with varying doses of sunitinib for 8 days, all doses lead to hypertension and proteinuria, but it was only with intermediate and high doses that glomerular endotheliosis was observed on histology.50 This was also associated with decreased urinary cyclic GMP, increased circulating ET-1, and decreased renal nephrin expression. It may be that lower doses of VSP inhibitor therapy cause more functional declines in glomerular function, while higher doses lead to both structural and functional derangements.
Human pathologic studies support a direct structural impact on the kidneys from VSP inhibitor therapy. In a study of six patients treated with bevacizumab, characteristic microangiopathic changes, including endotheliosis, microthrombi, and mesangiolysis were observed.51 Further confirmation of the direct effect of VEGF signaling on glomeruli was demonstrated using an inducible podocyte-specific VEGFA-gene deletion mouse model. Podocyte-specific deletion of the VEGFA gene produced hypertension, proteinuria, and thrombotic microangiopathy as seen with bevacizumab therapy in humans.51 A larger study of patients who underwent kidney biopsy for renal toxicity from VSP inhibitors showed similar renovascular pathologic changes, including thrombotic microangiopathy, minimal change disease and focal segmental glomerulosclerosis.52
There are striking clinical and pathophysiologic similarities in the hypertension and renal injury observed with VSP inhibitor therapy and that seen in preeclampsia. In pregnancy, PIGF and VEGF expression are important for the normal development and vascularization of the placenta.53 In pre-eclampsia, this process is impaired due to excess production of soluble Flt-1 receptor by the placenta, which sequesters and lowers circulating levels of PIGF and VEGF.54–55 This altered balance between angiogenic and anti-angiogenic factors ultimately leads to systemic endothelial dysfunction and the downstream clinical manifestations of hypertension, renovascular disease, and coagulopathy. Besides these mechanistic similarities, there are also histopathologic correlates between the two entities, particularly relating to renal microangiopathic changes.23 Moreover, the proteinuria seen with anti-VEGF antibodies given in mice can be recapitulated by administering soluble VEGFR1/Flt-1.48 Thus, the pathologic and clinical similarities between VSP inhibitor-induced hypertension and pre-eclampsia, including the reversible nature of the disease course upon withdrawal of the offending agent (VSP therapy in the former and delivery of the placenta in the latter), support a central role of VEGF in the development of renovascular dysfunction with VSP inhibitors.
Thrombotic Complications of VSP Inhibitor Therapy
VSP inhibitor therapy is consistently associated with an increase in arterial and venous thrombosis. Bevacizumab is associated with the greatest incidence of venous thromboembolism (VTE), with VTEs occurring in nearly 12% of patients.56 The decoy VEGFR aflibercept is also associated with a VTE incidence of 9.3%.57 VTE occurs in 2–6% of patients treated with TKIs.2 Nearly half of these events are high grade VTEs, defined as thrombotic events leading to clinical events, medical interventions, or death. The rates of arterial thromboembolic disease are lower, ranging from 1–5% in most studies.2 One important consideration is that arterial events associated with VSP inhibitors may also be due to other arterial pathological processes such as atherosclerosis or vasospasm.
At first glance, increased vascular events due to VSP inhibitors may not be surprising given the vascular protective roles of VEGF. Through its downstream mediators NO and PGI2, VEGF maintains endothelial health, inhibits leukocyte adhesion to endothelial cells, prevents platelet aggregation, and exerts anti-mitogenic effects on smooth muscle cells.58 NO limits endothelial activation, thus preventing interleukin-1-induced expression of adhesion molecules such vascular cell adhesion molecule-1, E-selectin, and intercellular adhesion molecule-1 in cultured human umbilical vein cells.59 In animal models, VEGF can attenuate thrombin-induced leukocyte rolling in an NO-dependent manner, and VEGF inhibition augments leukocyte rolling with increased expression of endothelial P-selectin.60–61 VSP inhibitors may additionally promote defects in the endothelial lining, due to impaired endothelial cell turnover and regeneration, which can lead to activation of the coagulation cascade due to exposure of the subendothelial compartment.62
The anti-platelet effects of VEGF are primarily exerted through NO and PGI2, although bevacizumab may have a direct role in activating platelets. NO in platelets can decrease function of the platelet thromboxane A2 receptor, decreasing platelet activation and aggregation.63–64 Endogenous NO production by platelet eNOS can also serve as a negative feedback mechanism on hemostatic plug formation by acting as a brake for further platelet activation and recruitment at sites of injury.65 The mechanisms by which PGI2 may inhibit platelet function are less elucidated. Overall, the effects of PGI2 on platelets are antagonistic to thromboxane A2; thus VEGF-mediated increase in PGI2 shift the balance towards decreasing platelet activation. PGI2 likely acts through cell surface and intracellular receptors, generating the second messenger cAMP, to decrease free intracellular calcium and inhibit platelet activation.66 There is in vitro evidence that bevacizumab immune complexes may directly bind platelet Fc gamma receptors, leading to platelet activation.67 To what extent this mechanism may lead to in vivo platelet aggregation in patients receiving bevacizumab remains to be fully established.
VEGF signaling has been found to promote both anti- and pro-thrombotic pathways. VEGF augments the anti-thrombotic factors urokinase, tissue plasminogen activator, and urokinase receptor expression in endothelial cells.68–69 However, VEGF also increases plasminogen activator inhibitor 1 (PAI-1) levels, a pro-thrombotic mediator; in addition, VEGF has been found to increase expression of tissue factor and von Willebrand factor in cultured endothelial cells.70–71 Indeed, the net effect of these seemingly opposing actions has not been fully determined. However, there is evidence that in vivo, the anti-thrombotic effects of VEGF may predominate. In a rabbit model of intra-arterial VEGF gene therapy following bare metal stent placement, delivery of a VEGF-expressing plasmid was associated with a ten-fold reduction in stent-associated thrombus formation, suggesting a substantial anti-thrombotic effect of enhanced local VEGF expression.72 Additionally, in a mouse model of endothelial-specific VEGF deletion, microinfarcts were observed in several vascular territories.73 These studies provide a rationale for the increased incidence of thrombotic events with VSP inhibitor therapy.
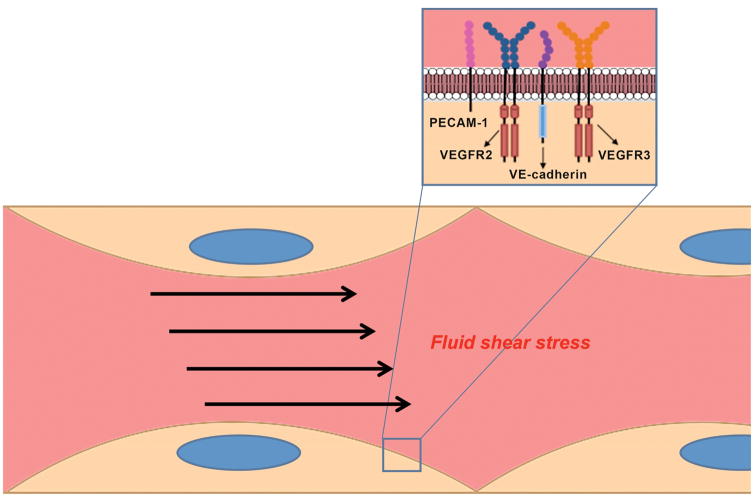
Impaired endothelial mechanosensation, mediated in part by VEGFR2 and VEGFR3, may serve as another mechanism for thrombus formation by VSP inhibitors. Fluid shear stress is an important determinant of endothelial function. Alterations in the normal laminar flow can result in proinflammatory changes in endothelial cells. This can inhibit anti-thrombotic and anti-oxidant pathways and upregulate pro-thrombotic mediators, leading to atherosclerotic plaque development as well as vascular malformations.74–75 Mechanotransduction is the process by which endothelial cells sense fluid shear stress and convert it into cellular responses. While the full pathway for mechanotransduction has not been elucidated, one well-characterized unit consists of VEGFR2, VEGFR3, platelet endothelial cell adhesion molecule 1 (PECAM-1), and vascular endothelial cadherin (VE-cadherin) (Figure 3).76–77 In this complex, PECAM-1 transmits alterations of the endothelial cytoskeleton caused by changes in laminar shear stress and activates VEGFR2 and VEGFR3 in a ligand-independent manner, leading to downstream production of NO through the PI3K/Akt pathway.77–78 Additionally, VEGFR3 has been shown to be important in maintaining the shear stress “set point” in endothelial cells of different vascular territories, alterations in which can lead to pathologic vascular remodeling.79 Thus, inhibition of VEGFR2/3 signaling by VSP inhibitors could lead to altered endothelial cell signaling, with activation of pro-thrombotic pathways, and eventual vascular injury and thrombosis. Further studies are needed to better understand how alterations in VEGFR-mediated mechanotransduction play a role in the vascular pathologies seen with VSP inhibitors.
Figure 3. Potential model for the role of VEGFR in the endothelial response to fluid shear stress.
Fluid shear stress activates mechanosensory complexes to affect endothelial activation and function. In one mechanosensory unit, platelet endothelial cell adhesion molecule 1 (PECAM-1) transmits changes in endothelial cytoskeletal structure to VEGFR2 and VEGFR3, through vascular endothelial cadherin (VE-cadherin). VEGFR2 and VEGFR3 are then activated in a ligand-independent fashion to mediate intracellular signaling changes.
Future Directions and Conclusions
Additional studies are needed to identify how inhibition of VEGF signaling leads to vascular dysfunction, kidney injury, and hypertension. Furthermore, downstream signaling mediators of VEGF need to be better studied, particularly with regards to ET-1. How VSP inhibition may affect lymphatic function, salt sensitivity and kidney function may provide newer appreciation of the role of VEGF in intra-organ communications. Finally, the consequences of VEGF system inhibition on non-ligand-dependent VEGFR activity need to be more fully elucidated.
As VSP inhibitors are increasingly used in cancer treatment, an appreciation of basic VEGF signaling is critical to understanding the clinical cardiovascular and renal sequalae of this class of therapy. Such a mechanistic approach to evaluating the VEGF/VEGFR axis lends important clues towards uncovering the cause of hypertension, renal dysfunction, and thrombosis seen with VSP inhibitor therapy. These insights can help refine our management of side effects and may aide in the development of more targeted therapies that can selectively inhibit only those aspects of VEGF signaling that augment tumor growth, while preserving the critical cardiovascular and renal homeostatic effects of the VEGF/VEGFR system.
Footnotes
Disclosures
None.
Sources of Funding
None.
References
- 1.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: Focus on kinase inhibitors. J Am Coll Cardiol. 2015;66:1160–1178. doi: 10.1016/j.jacc.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 4.Touyz RM, Lang NN, Herrmann J, van den Meiracker AH, Danser AHJ. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension. 2017;70:220–226. doi: 10.1161/HYPERTENSIONAHA.117.08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. Vegf receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 6.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999;41:773–780. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase a2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 10.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: Mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30:591–601. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 15.Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dienstmann R, Delmonte A, Cereda R, Isaacson J, Litten J, Allen A, Dubois F, Saba C, Robert R, D’Incalci M, Zucchetti M, Camboni MG, Tabernero J. Phase i/iia study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25:2244–2251. doi: 10.1093/annonc/mdu390. [DOI] [PubMed] [Google Scholar]
- 16.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jesus-Gonzalez N, Robinson E, Penchev R, von Mehren M, Heinrich MC, Tap W, Wang Q, Demetri G, George S, Humphreys BD. Regorafenib induces rapid and reversible changes in plasma nitric oxide and endothelin-1. Am J Hypertens. 2012;25:1118–1123. doi: 10.1038/ajh.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson ES, Khankin EV, Choueiri TK, Dhawan MS, Rogers MJ, Karumanchi SA, Humphreys BD. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension. 2010;56:1131–1136. doi: 10.1161/HYPERTENSIONAHA.110.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension. 2011;58:85–92. doi: 10.1161/HYPERTENSIONAHA.110.168120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 21.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 22.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 24.Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 25.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O’Dwyer PJ. Mechanisms of hypertension associated with bay 43-9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 26.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L984–991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- 27.Prins BA, Hu RM, Nazario B, Pedram A, Frank HJ, Weber MA, Levin ER. Prostaglandin e2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. J Biol Chem. 1994;269:11938–11944. [PubMed] [Google Scholar]
- 28.Belcik JT, Qi Y, Kaufmann BA, Xie A, Bullens S, Morgan TK, Bagby SP, Kolumam G, Kowalski J, Oyer JA, Bunting S, Lindner JR. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012;60:618–625. doi: 10.1016/j.jacc.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: A new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 31.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 32.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speed JS, Heimlich JB, Hyndman KA, Fox BM, Patel V, Yanagisawa M, Pollock JS, Titze JM, Pollock DM. Endothelin-1 as a master regulator of whole-body na+ homeostasis. FASEB J. 2015;29:4937–4944. doi: 10.1096/fj.15-276584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lankhorst S, Severs D, Marko L, Rakova N, Titze J, Muller DN, Danser AH, van den Meiracker AH. Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: Role of interstitial sodium accumulation? Hypertension. 2017;69:919–926. doi: 10.1161/HYPERTENSIONAHA.116.08565. [DOI] [PubMed] [Google Scholar]
- 35.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: Focus on hypertension. Circulation. 2011;124:1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230. [DOI] [PubMed] [Google Scholar]
- 37.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:2471–2479. doi: 10.1158/1078-0432.CCR-14-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Akiyoshi K, Horita Y, Nagashima K, Shirao K. New analysis of the hypertension mechanism in bevacizumab-treated pateints using 3-tesla dynamic contrast-enhanced magnetic resonance imaging. J Clin Oncol. 2011;29(Suppl 4) abstr 450. [Google Scholar]
- 39.Launay-Vacher V, Janus N, Selle F, Goldwasser F, Mir O, Spano J-P, Thery JC, Beuzeboc P, Rey J, Jouannaud C, Morere JF, Oudard S, Gligorov J, Azizi M, Dorent R, Deray G, Ray-Coquard I, Scotte F. Results of the mars study on the management of antiangiogenics’ renovascular safety in ovarian cancer. J Clin Oncol. 2013;31(suppl) abstr 5567. [Google Scholar]
- 40.Launay-Vacher V, Aapro M, De Castro G, Jr, Cohen E, Deray G, Dooley M, Humphreys B, Lichtman S, Rey J, Scotte F, Wildiers H, Sprangers B. Renal effects of molecular targeted therapies in oncology: A review by the cancer and the kidney international network (c-kin) Ann Oncol. 2015;26:1677–1684. doi: 10.1093/annonc/mdv136. [DOI] [PubMed] [Google Scholar]
- 41.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorich MJ, Rowland A, Kichenadasse G, Woodman RJ, Mangoni AA. Risk factors of proteinuria in renal cell carcinoma patients treated with vegf inhibitors: A secondary analysis of pooled clinical trial data. Br J Cancer. 2016;114:1313–1317. doi: 10.1038/bjc.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang ZF, Wang T, Liu LH, Guo HQ. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: A systematic review and meta-analysis. PLoS One. 2014;9:e90135. doi: 10.1371/journal.pone.0090135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep. 1999;1:178–186. doi: 10.1007/s11906-999-0016-7. [DOI] [PubMed] [Google Scholar]
- 45.Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: Update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of vegf-a expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu JW, Manning RD, Jr, Young E, Shparago M, Sartin B, Bailey AP. Vascular endothelial growth factor receptor inhibitor enhances dietary salt-induced hypertension in sprague-dawley rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R142–148. doi: 10.1152/ajpregu.90972.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (vegf) by anti-vegf antibodies and soluble vegf receptor 1 (sflt-1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 49.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:p26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 50.Lankhorst S, Baelde HJ, Kappers MH, Smedts FM, Hansen A, Clahsen-van Groningen MC, Sleijfer S, Mathijssen RH, Danser AH, van den Meiracker AH. Greater sensitivity of blood pressure than renal toxicity to tyrosine kinase receptor inhibition with sunitinib. Hypertension. 2015;66:543–549. doi: 10.1161/HYPERTENSIONAHA.115.05435. [DOI] [PubMed] [Google Scholar]
- 51.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. Vegf inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izzedine H, Escudier B, Lhomme C, Pautier P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R, Hollebecque A, Sahali D, Soria JC. Kidney diseases associated with anti-vascular endothelial growth factor (vegf): An 8-year observational study at a single center. Medicine (Baltimore) 2014;93:333–339. doi: 10.1097/MD.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 55.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 57.Cutsem EV, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, Hazel GAv, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase iii randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. Journal of Clinical Oncology. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 58.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:C1375–1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 59.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scalia R, Booth G, Lefer DJ. Vascular endothelial growth factor attenuates leukocyte-endothelium interaction during acute endothelial dysfunction: Essential role of endothelium-derived nitric oxide. FASEB J. 1999;13:1039–1046. doi: 10.1096/fasebj.13.9.1039. [DOI] [PubMed] [Google Scholar]
- 61.Walshe TE, Dole VS, Maharaj AS, Patten IS, Wagner DD, D’Amore PA. Inhibition of vegf or tgf-{beta} signaling activates endothelium and increases leukocyte rolling. Arterioscler Thromb Vasc Biol. 2009;29:1185–1192. doi: 10.1161/ATVBAHA.109.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol. 2003;21:3542. doi: 10.1200/JCO.2003.99.046. author reply 3543. [DOI] [PubMed] [Google Scholar]
- 63.Trepakova ES, Cohen RA, Bolotina VM. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum ca2+-atpase-dependent refilling of ca2+ stores. Circ Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- 64.Wang GR, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic gmp-dependent protein kinase. Proc Natl Acad Sci U S A. 1998;95:4888–4893. doi: 10.1073/pnas.95.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gkaliagkousi E, Ritter J, Ferro A. Platelet-derived nitric oxide signaling and regulation. Circ Res. 2007;101:654–662. doi: 10.1161/CIRCRESAHA.107.158410. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 67.Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, Amaya M, Francis JL, Amirkhosravi A. Bevacizumab immune complexes activate platelets and induce thrombosis in fcgr2a transgenic mice. J Thromb Haemost. 2009;7:171–181. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 68.Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem. 1995;270:9709–9716. doi: 10.1074/jbc.270.17.9709. [DOI] [PubMed] [Google Scholar]
- 69.Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (vegf) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902–906. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 70.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 71.Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K, Shamamian P, Mignatti P. Expression of von willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000;85:281–288. doi: 10.1002/(sici)1097-0215(20000115)85:2<281::aid-ijc21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Van Belle E, Tio FO, Chen D, Maillard L, Kearney M, Isner JM. Passivation of metallic stents after arterial gene transfer of phvegf165 inhibits thrombus formation and intimal thickening. J Am Coll Cardiol. 1997;29:1371–1379. doi: 10.1016/s0735-1097(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine vegf signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. Intramembrane binding of ve-cadherin to vegfr2 and vegfr3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 78.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of pecam-1 in the shear-stress-induced activation of akt and the endothelial nitric oxide synthase (enos) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 79.Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, Humphrey JD, Schwartz MA. Vascular remodeling is governed by a vegfr3-dependent fluid shear stress set point. Elife. 2015:4. doi: 10.7554/eLife.04645. [DOI] [PMC free article] [PubMed] [Google Scholar]