Abstract
Background
Globally, the rate of reduction in delivery-associated maternal and perinatal mortality has been slow compared to improvements in post-delivery mortality in children under five. Improving clinical readiness for basic obstetric emergencies is crucial for reducing facility-based maternal deaths. Emergency readiness is commonly assessed using tracers derived from the maternal signal functions model.
Objective-method
We compare emergency readiness using the signal functions model and a novel clinical cascade. The cascades model readiness as the proportion of facilities with resources to identify the emergency (stage 1), treat it (stage 2) and monitor-modify therapy (stage 3). Data were collected from 44 Kenyan clinics as part of an implementation trial.
Findings
Although most facilities (77.0%) stock maternal signal function tracer drugs, far fewer have resources to practically identify and treat emergencies. In hypertensive emergencies for example, 38.6% of facilities have resources to identify the emergency (Stage 1 readiness, including sphygmomanometer, stethoscope, urine collection device, protein test). 6.8% have the resources to treat the emergency (Stage 2, consumables (IV Kit, fluids), durable goods (IV pole) and drugs (magnesium sulfate and hydralazine). No facilities could monitor or modify therapy (Stage 3). Across five maternal emergencies, the signal functions overestimate readiness by 54.5%. A consistent, step-wise pattern of readiness loss across signal functions and care stage emerged and was profoundly consistent at 33.0%.
Significance
Comparing estimates from the maternal signal functions and cascades illustrates four themes. First, signal functions overestimate practical readiness by 55%. Second, the cascade’s intuitive indicators can support cross-sector health system or program planners to more precisely measure and improve emergency care. Third, adding few variables to existing readiness inventories permits step-wise modeling of readiness loss and can inform more precise interventions. Fourth, the novel aggregate readiness loss indicator provides an innovative and intuitive approach for modeling health system emergency readiness. Additional testing in diverse contexts is warranted.
Introduction
Protracted elevations in global labor-related deaths persist despite 71% of births now being attended by skilled professionals [1–3]. Between 1990 and 2015, global labor-related deaths declined much more slowly compared to post-partum deaths. Further, the under-five mortality ratio shrunk by 2.12% annually during this period while maternal mortality reductions were much slower at 1.80% [1] and perinatal mortality reductions were essentially stagnant [4–10]. The majority of peripartum deaths are driven by labor-related disorders including maternal hemorrhage, eclampsia and maternal/perinatal sepsis [4, 7, 11, 12]. Given this protracted facility-based mortality, mobilizing clinical resources for labor-related emergency care may be a crucial step for reducing persistent facility-based mortality [13–15]. Mobilizing and distributing such resources is based on accurate measurement of emergency readiness.
In 1993, the World Health Organization (WHO) identified essential resources for managing common obstetric emergencies [16]. The WHO’s Mother-Baby Package codified these resources into eight facility-based actions to treat the major causes of global maternal death at facilities [17]. This pioneering approach evolved into the signal functions framework and emergency obstetric care (EOC) [18–23]. The basic maternal signal functions include six clinical actions used during obstetric emergencies; three are medical treatment signal functions and three are manual procedure functions [22]. The three medical signal functions involve administration of: parenteral antibiotics, parenteral anticonvulsants and parenteral oxytocics. The three manual procedure functions are: assisted vaginal delivery, removal of retained products of conception and removal of retained placenta.
Specific items—or tracers—used for each signal function have been used as proxy measures for overall emergency readiness—or a facility’s ability to manage obstetric emergencies [23–29]. This signal function method has become the dominant approach for measuring global readiness at facilities [30–32]. The WHO created a Service Availability and Readiness Assessment tool (SARA) used to summarize the readiness—or resource availability—for a broad range of clinical services [33]. The obstetric-specific Service Readiness Index (SRI) defines a facility’s aggregate obstetric emergency readiness using the mean number of 11 tracers present on the day of observation at the facilities [32, 33]. This WHO method has been applied in multiple global contexts [30, 31, 33].
There has been growing consensus, however, on the need to revise or modify readiness assessments derived from the signal functions [25, 27, 34–38]. To our knowledge, signal functions, tracer items and readiness scores have not yet been used to predict a facility’s ability to manage specific emergencies or to predict labor-related health outcomes [34, 36]. Consequently, to substantively reduce delivery-related mortality on a global scale, additional work is needed to define intuitive, relevant, measurable indicators [39] that simultaneously predict survival [37] and are simultaneously relevant for clinics and health systems.
The capacity hierarchy of needs model identifies a predictable, interdependent relationship between health systems, facilities, clinician skill and the tools clinicians use to provide clinical care [40]. Clinical tools—such as drugs—can only be utilized when staff posses the tools, skills, knowledge and infrastructure required to administer those drugs. In health care systems, a predictable cascade of patient loss has been documented between initial diagnosis and sustained treatment for HIV and other diseases [41, 42].
Study rationale, research question and context
As part of a baseline facility inventory of a larger obstetric quality improvement implementation trial in Kakamega County, Kenya, we designed a nested descriptive study to 1) measure the signal functions ability to practically describe a facility’s clinical readiness to manage basic obstetric emergencies and 2) test a novel emergency obstetric readiness model. In Kenya, the ratio of pregnant women dying as a consequence of pregnancy has remained stable for over 30 years (maternal mortality ratio = 400 (MMR)) [43, 44]. Among all nations, Kenya bears the eighth highest total number of deaths [43, 45, 46]. A recent service readiness inventory suggests elevations in mortality may be partially attributed to limited clinical resources for managing emergencies. Across 7,995 facilities, Kenya’s preparedness for managing obstetric emergencies (as measured by the proportion of facilities with specified signal function resources) was 32%. Similar trends were present among public facilities (32%) and in Kakamega County (38%) [31]. One region in Kakamega County was used to test the proposed obstetric emergency clinical cascades at Ministry of Health (MoH) facilities and to compare the new model’s performance with readiness estimates from the standard signal functions model.
Materials and methods
Setting
Kakamega County was selected by the Government of Kenya for a parent implementation trial testing the impact of a package of community- and clinical quality interventions on the uptake and quality of facility-based care. Kakamega was selected by the MoH based on government data indicating the county’s MMR of 800 was nearly double the country’s ratio of 413 [44, 47]. Forty four facilities and their catchment areas were selected for intervention using four criteria: primary care clinics with KEPH Level 2–3 designation (Kenya Essential Package of Health [47]), providing basic emergency obstetric services as defined by the MoH (BEmOC) [48], conducting 10 or more deliveries in the previous calendar year (2011) and being located in one of five sub-counties within Kakamega County (for the purpose of analysis, two facilities that were formerly part of Kakamega Central prior to post-constitutional rezoning were retained in the study based on the intent-to-treat principle. Thus, the results report on five sub-counties since the newly designated Navakholo county was retained with Kakamega Central for the parent trial.
Study design
The cross-sectional analysis of facility readiness is nested within a non-equivalent group design pre-post implementation trial evaluating a facility- and community-intervention package in Kakamega County, Kenya [49]. In the parent trial, 756 facility-specific variables were collected at 44 primary care facilities; this nested study used 80 obstetric-specific variables collected from facilities prior to the start of the intervention.
Emergency readiness
Obstetric emergency readiness at the facility-level has been defined by the proportion of specified clinical items that are present at a facility on the day a facility inventory is conducted [33]. Although there is no universal consensus on the number of tracers that should be used to measure emergency readiness as defined by the signal functions model [30–33, 50], WHO’s Service Readiness Index (SRI) defines basic emergency obstetric readiness using 7 tracers (composed of 9 discrete items). These tracers are measured using observation and/or interview during facility visits [33]. This signal function-based approach to estimating emergency readiness uses 3 parenteral drugs (uterotonic, antibiotics, anticonvulsant), 3 intravenous items (including IV solution and a 2-part IV infusion kit), 1 durable good (manual vacuum apparatus) and 2 multipurpose items (gloves and light source).
The WHO-SRI standardized tool was used to create a signal function-based estimate of emergency readiness at the 44 primary care clinics. Next, we measured readiness using a novel emergency obstetric clinical cascade model derived from Potter’s hierarchy of needs framework [40] and the HIV care cascade model [42]. The resulting obstetric clinical cascade quantifies resources required to sequentially identify, treat and manage basic obstetric emergencies as they present clinically at primary care facilities. Consequently, emergency obstetric readiness is reported as the percentage of facilities with all of the related clinical tools for managing obstetric emergencies (as defined by the two models). The higher the percentage is, the higher the facilities’ readiness is to manage basic obstetric emergencies. Although facility-level estimates of readiness could be calculated, this study reports the percent readiness aggregated across all 44 clinics.
Emergency readiness using signal functions
The current signal function readiness estimates are reported for all basic maternal emergency obstetric signal functions as a single indicator—the proportion of facilities with tracer items for all manual procedures and all medical treatments. Standard signal function estimates and the WHO’s obstetric service readiness index (SRI) do not measure readiness for each clinical disorder. However, if one estimated emergency-specific readiness using the signal function tracer items alone, many resources required to practically deliver care would be absent. For example, signal function estimates for eclampsia would be defined as the proportion of facilities with IV solution/infusion set, hydralazine and magnesium sulfate [33]. Using these three items to model eclampsia emergency readiness alone does not account for the resources required to first identify if the emergency is present (i.e., sphygmomanometer, stethoscope, urine collection device and urine protein test). Also, it does not explicitly model all necessary drugs or ancillary resources required to practically deliver the first-line treatment. Although consumable supplies are required to deliver treatment drugs, consumable resources are often omitted from the signal functions for most emergencies (for example, the required refrigeration for oxytocin is not modeled in the oxytocic function and IV tubing, IV catheter and IV solution are discrete, interdependent items that are only measured as one item in signal function estimates. Consequently, reporting capacity when one or more of the three interdependent items are missing is not precise or accurate). Further, although durable goods are essential for conducting procedures or delivering drugs, they are often excluded from signal function estimates (i.e., IV poles, syringes or needles for delivering drugs). For some emergencies, the specific drug required for the emergency is not modeled by the signal functions (For example, in the oxytocic signal function, the cause of post-partum hemorrhage (PPH) and the varied drugs for treating hemorrhage based on its underlying cause are not modeled).
Emergency readiness using cascades
In contrast, the proposed cascade model is a clinically-oriented approach to measuring readiness. It is based on a practical, step-wise cascading relationship between resources [40, 42]. The resources for identifying the emergency (Stage 1) are required first before accurate treatments can be administered to patients (Stage 2). Further, the cascade explicitly models the consumable supplies and durable goods required to practically deliver treatment drugs in clinical practice (for example, in eclampsia, the cascades model the interrelationship between all clinical resources required to first identify the disorder and then deliver the treatment drug). Thus, emergency readiness in the cascade model is the proportion of facilities with the treatment drug that can first identify the disorder (stage 1, Identify) and then have the durable and consumable resources to administer the treatment drug (stage 2, Treat). Since the signal functions do not measure care quality, the third cascade stage for monitoring and modifying therapy as clinically indicated (Stage 3) is not used to compare the signal functions and cascade models (Fig 1).
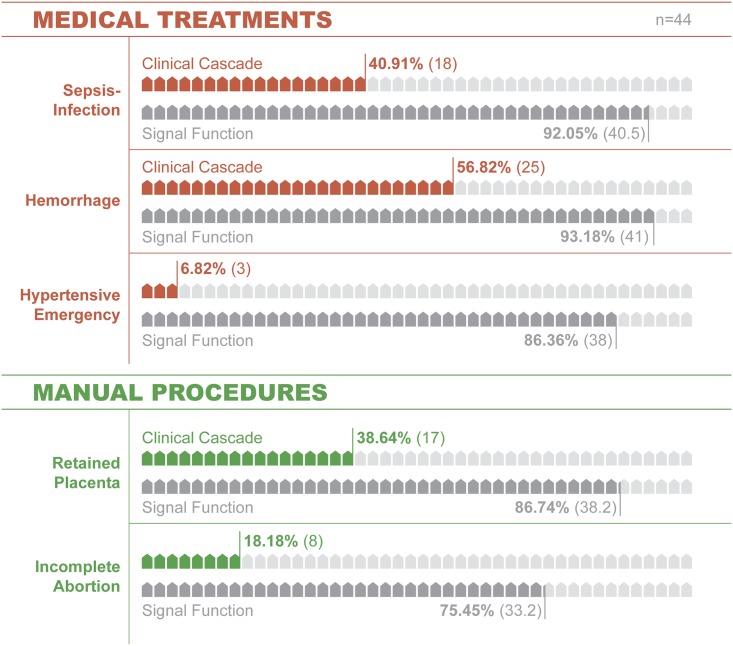
Fig 1. Signal function versus clinical cascade estimates of emergency readiness.
Clinical logic of cascade readiness estimates
Each emergency cascade’s title is based on the underlying clinical disorder and paired with existing signal functions as follows: Manage Sepsis-Infection (the parallel signal function is parenteral antibiotics), Hemorrhage (oxytocics), Hypertensive Emergency (anticonvulsant), Retained Placenta (manual removal of retained placenta), Incomplete Abortion (removal of retained products of conception) [22]. A facility’s ability to monitor or modify the primary treatment based on a patient’s clinical response (Stage 3) is a proposed indicator for measuring clinical quality but not for evaluating signal function performance (Fig 2).
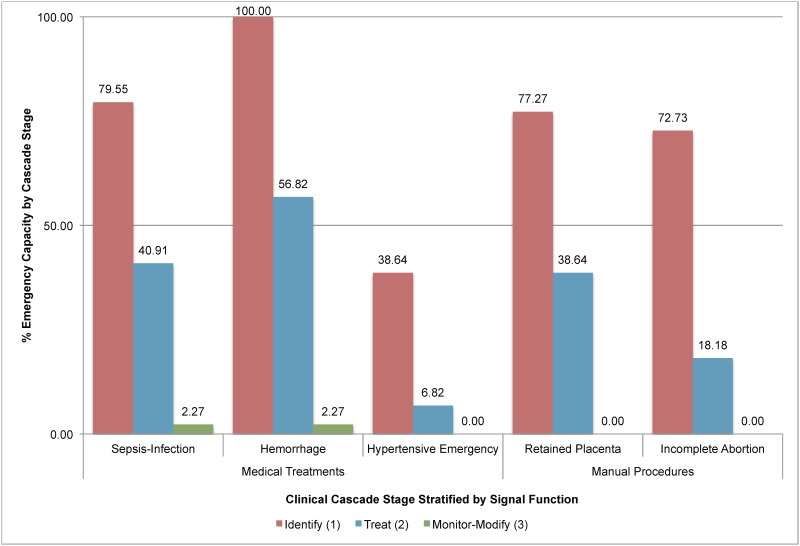
Fig 2. Emergency readiness estimates by emergency cascade and stage.
Operational definitions for ambiguous signal function tracers
Tracers were precisely defined to minimize ambiguity in signal function estimates and to facilitate comparison between models. When tracers were not explicitly defined by the signal functions, the WHO first-line recommendations for obstetric care were used [23, 51, 52] to make a general tracer from the signal function model (i.e., parenteral antibiotics) more specific (i.e., parenteral ampicillin and/or penicillin alternative). Four other ambiguous tracers included light source, IV supplies, drugs and emergency protocols. Light was defined as functional electric lights or functional flashlights. The IV kits tracer includes three discrete resources: drug-compatible fluids, tubing and a venous access device/cannula. We modeled this using the cannula and fluids since data on tubing were absent. Fluid was modeled as Lactated Ringer’s/Hartman solution or normal saline since both fluids are compatible with emergency drugs available at the study facilities.
For the cascades, the first-line clinically-indicated drug was used to model readiness when the drug was not defined by the signal function model. The oxytocics signal function does not specify the precise utertonic drug required for various hemorrhage emergencies. When managing obstetric hemorrhage, the preferred first-line drug is oxytocin [23, 51]. However, in its absence, misoprostol or ergometrine could be substituted (with blood pressure monitoring). In contrast, when managing incomplete abortion, the preferred uterotonic is a non-oxytocin agent such as ergometrine or misoprostol. Therefore, the presence of any first- or second-line uterotonic specific to the emergency was used to model readiness in the cascade model since the cascades are based on specific clinical emergencies [23, 51].
Further, the parentral antibiotic signal function does not define the tracer antibiotic drugs required. We used the WHO’s 3-step sequence of obstetric antibiotic therapy escalation based on the type of suspected infection to define readiness: Step 1—ampicillin, 2—gentamicin and 3—metronidazole [51]. Since most facilities lack ampicillin, the presence of ampicillin or any of three alternative penicillin drugs (benzathine, procaine or crystalline) was used to model this WHO step 1 antibiotic readiness (the primary study’s clinical inventory did not capture metronidazole availability, so WHO’s step 3 antibiotic readiness was not modeled).
Emergency readiness and clinical quality
Although some emergency protocols are tracers in the signal function model, they were selectively used to model the quality of clinical care but not general emergency readiness for four reasons: 1) individual clinician knowledge and skill vary so having a protocol does not guarantee readiness, 2) by extension, protocol absence does not guarantee a lack of emergency readiness, 3) signal functions do not define the protocol required [33] and 4) several protocols may actually be required to manage the primary emergency’s sequella. For example, when a patient presents with a retained placenta, a subset of patients may develop post-partum hemorrhage (PPH), endometritis and/or sepsis while another subset may resolve with first-line treatment.
Study assumptions
Identifying some clinical disorders is based primarily on clinician skill. Although clinician skills vary widely [53], a 100% skill level was assumed for all cascades since skill assessment is not include the signal function estimates of facility emergency readiness [22, 33]. Some items required for comprehensively modeling readiness in all six maternal signal functions were absent from the baseline facility inventory. This analysis focuses on three basic medical and two manual functions since data on assisted vaginal delivery supplies were absent [21, 22]. However, the expanded cascades in supplemental tables include resources for all six maternal cascades.
Data collection
In parallel with the WHO- readiness estimates based on the signal functions, this study measured the cross-sectional availability of routine and emergency obstetric resources during facility visits conducted by study staff between February and May 2013. Three trained research assistants used standardized forms to visually identify emergency resource availability and ask clinic mangers about resource availability when items were not initially located using observation. This method of survey data collection matches the WHO-SRI approach used to quantify signal function estimates of emergency readiness [33]. 80 variables from the inventory describe facility demographics, staff, consumable medical supplies, durable goods and obstetric drugs. Mean estimates of maternal signal function readiness are derived from 396 observations (9 tracer items from 44 facilities). Cascade estimates of readiness utilized 1,364 observations (31 variables from 44 facilities).
One author (JD) trained all staff on this the facility inventory instrument. The author also provided periodic in-person and remote instrument coaching and data quality assurance in-services. A trained clerk entered these data into the RedCap’s online database (Institute for Translational Health Sciences, 2007–2015). Accuracy of these data were confirmed using a standard double-entry technique where two assistants entered data from one quarter of the paper forms. Data clerks resolved any discrepancies between the two RedCap entries by reviewing the original paper forms. Thus, any discrepant REDCap entries were reconciled with the original paper records. The resulting validated database was used for analysis. RedCap data were exported to STATA for analysis (version 11.2, College Station, Texas, 1985–2009).
Analysis
We described obstetric variables with standard descriptive statistics; point estimates for the availability of each resource are reported as percentages. Since the variables in this dataset had fewer than 100 observations, skewed distributions or did not follow symmetric Gaussian distribution, non-parametric descriptive and inferential statistics with two-sided tests significance were used for all analyses. Drop-offs in readiness between each stage were quantified with percentages. Central tendency was typically reported as the median. Means were used primarily for estimates of overall emergency readiness estimates in both models for two reasons: 1) the SRI methodology uses means and 2) since this measure is based on few observations the median would not capture the range of observations effectively or accurately. Variability was primarily summarized using absolute ranges since facilities varied widely in the obstetric resources available. Standard deviation (SD) and interquartile ranges (IQR) were selectively used as measures of variance when variation in the central tendency and range was of interest (for example using both metrics for the number of monthly deliveries illustrates wide variability in delivery volume by study site). Since global variability in urban-rural obstetric care is well-documented [3, 39, 54–56], we statistically quantified periurban/rural differences in the facility characteristics based on a facility’s rural status using Kenya MoH definitions.
To test differences between proportions, we used Pearson’s chi-square test of independence or Fischer’s exact test (for cell counts less than five). When comparing a variable’s distribution across unpaired categories, we used Wilcoxon-ranked sum test (for two categories) or Kruskal-wallis’ h-test (more than two categories). We used the unmatched median test to compare medians across two unpaired categories. The ‘signal function overestimate’ indicator is calculated by subtracting the novel cascade estimate of readiness from the standard signal function estimate of readiness (signal function estimate [–] clinical cascade estimate [=] readiness overestimate by signal function).
Ethics
The activities and analysis of this nested study were all contained in the parent study approved by the University of Washington Institutional Review Board (43069) and the University of Nairobi Ethical Review Committee (P57/05/2012). The trial is registered in the PanAfrican Clinical Trials Registry (PACTR0121200045732, available from: http://www.pactr.org). Since the intervention targeted clinics and not individual clinical providers, prior to the clinic-level intervention, individual clinicians were verbally informed of the study and provided the opportunity to opt-out of the clinical training or assessments; no one opted out. Further, the MoH provided authorization to collect these data at the MoH facilities as part of the implementation trial. Consequently, the facility inventory data did not require individual informed consent.
Results
Facility characteristics
60% of facilities were rural and located within four Kakamega sub-counties: Khwisero, Butere, Matungu and Navakholo (S1 Table). 38% were open 24-hours and facilities conducted between 2–61 deliveries each month (S2 Table; mean = 10.50, median = 5.83, IQR = 4.67–13.50). In 24 hours, facilities had a median of 4 obstetric nursing staff on site (including licensed nurses, midwives and nurse auxiliaries) and 1 clinical officer/advanced practice clinician (S3 Table).
Emergency obstetric resource availability
There was high variability in the availability of consumable supplies (range = 6.82–93.18%) and durable goods by facility (2.27–100%; S4 and S5 Tables). The presence of WHO first-line emergency medications varied by drug class [23, 51]. While most facilities stocked oxytocin (93.18%) and magnesium sulfate (72.73%), far fewer stocked ampicillin (4.55%) or hydralazine (9.09%; S6 Table). Except for a higher proportion of rural facilities with flashlights (72.00 versus 29.41%, Fisher’s exact p = 0.013), no statistically significant differences in resources availability were present based on a facility’s degree of urbanization (S3–S6 Tables).
Signal function estimates of emergency readiness
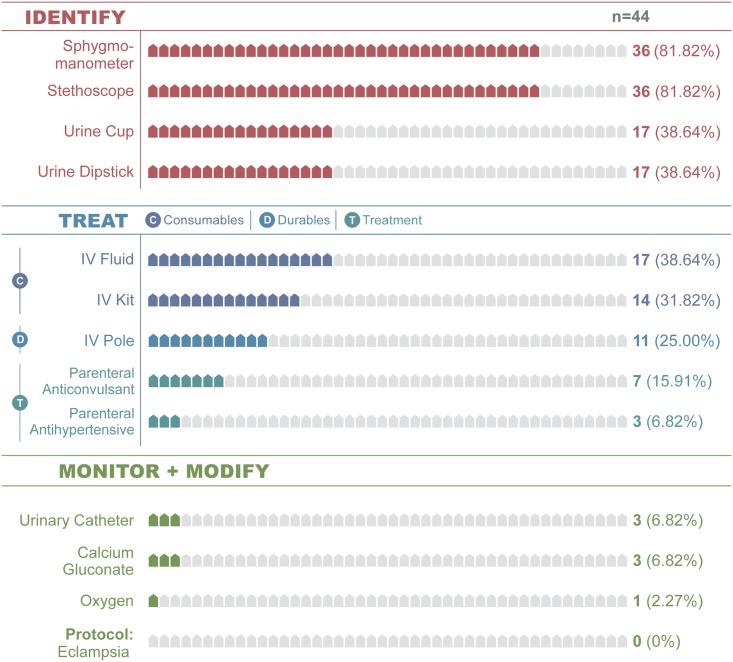
Maternal signal function estimates of emergency readiness were quantified using nine standard tracers (S6 Table) [28, 33]. Readiness ranged from 75.45% (retained products of conception) to 93.18% (oxytocic; Tables 1–3; S1 and S2 Figs). However, readiness estimates based on tracers alone do not model how multiple resources are required sequentially or simultaneously for practical clinical management. For example, in hypertensive emergencies, facilities can treat the disorder only when all needed resources to identify and treat the emergency are simultaneously present (Fig 3). Of 44 facilities, 36 had sphygmomanometers and stethoscopes (88.12%). Of those, 17 (38.64%) had the testing supplies necessary to identify eclampsia or pre-eclampsia (urine collection cups and dipsticks to test for proteinuria). Although 72.73% stocked the magnesium sulfate tracer drug, a much lower proportion had resources to identify the emergency and administer the drug (38.64%, stage 2 emergency readiness; Figs 1 and 2). Far fewer also stocked the antihypertensive drug hydralazine that should be simultaneously administered with magnesium sulfate (6.82%, Table 1, Fig 3).
Table 1. Cascade emergency readiness stratified by medical signal function.
| Clinical Cascade (Signal Function) | Cascade Stage | Item | % | n 1 |
|---|---|---|---|---|
| Manage Sepsis-Infection (Antibiotic) | Identify | Thermometer | 91.38% | 41 |
| Stethoscope | 91.38 | 41 | ||
| Sphygmomanometer | 79.55 | 35 | ||
| Treat (Consumables) | IV Fluid 2 | 77.27 | 34 | |
| IV Kit 3 | 70.45 | 31 | ||
| Treat (Durables) | IV Pole | 52.27 | 23 | |
| Treat (Treatments) | Parenteral Antibiotic-1 7 | 47.73 | 21 | |
| Parenteral Antibiotic-2 8 | 40.91 | 18 | ||
| Monitor-Modify | Protocol: Infection | 2.27 | 1 | |
| Manage Hemorrhage (Oxytocic) | Identify | Staff skill 9 | 100.00 | 44 |
| Treat (Consumables) | Gloves, aseptic | 93.18 | 41 | |
| IV Fluid 2 | 88.64 | 39 | ||
| IV Kit 3 | 81.82 | 36 | ||
| Treat (Durables) | IV Pole | 59.09 | 26 | |
| Refrigeration | 56.82 | 25 | ||
| Treat (Treatments) | Parenteral Uterotonic 10 | 56.82 | 25 | |
| Monitor-Modify | Sphygmomanometer | 47.73 | 21 | |
| Stethoscope | 47.73 | 21 | ||
| Uterotonic, Secondary 11 | 47.73 | 21 | ||
| Urinary catheter | 36.36 | 16 | ||
| Oxygen | 6.82 | 3 | ||
| Protocol: Hemorrhage | 2.27 | 1 | ||
| Manage Hypertensive Emergencies (Anticonvulsant) | Identify | Sphygmomanometer | 81.82 | 36 |
| Stethoscope | 81.82 | 36 | ||
| Urine Cup | 38.64 | 17 | ||
| Urine Dipstick | 38.64 | 17 | ||
| Treat (Consumables) | IV Fluid 2 | 38.64 | 17 | |
| IV Kit 3 | 31.82 | 14 | ||
| Treat (Durables) | IV Pole | 25.00 | 11 | |
| Treat (Treatments) | Parenteral Anticonvulsant 12 | 15.91 | 7 | |
| Parenteral Antihypertensive 13 | 6.82 | 3 | ||
| Monitor-Modify | Urinary catheter | 6.82 | 3 | |
| Calcium Gluconate | 6.82 | 3 | ||
| Oxygen | 2.27 | 1 | ||
| Protocol: Eclampsia | 0.00 | 0 |
(1) Total sample n = 44 facilities;
(2) Either normal saline (NS) or lactated ringer’s (LR);
(3) IV cannula;
(7) Parenteral ampicillin or parenteral penicillin (procaine, benzathine or cystalline);
(8) Parentral gentamicin;
(9) 100% staff skill for identifying the emergency disorder was assumed
(10) oxytocin or misoprostol;
(11) oxytocin, misoprostol or egometrine;
(12) magnesium sulfate;
(13) hydralazine
Table 3. Comparison of emergency readiness using clinical cascades and signal functions.
| Readiness Estimates by Model | |||
|---|---|---|---|
| Clinical Cascade | Signal Functions | Clinical Cascades | Overestimated Readiness |
| (Signal Function) | % Readiness, Tracer Items | % Readiness, Stage 2 | [Signal Functions (-) Cascade] |
| Medical Treatments | |||
| Manage Sepsis-Infection (Antibiotic) | 92.05% | 40.91% 1 | 51.14 |
| (IV fluids, IV kit, ampicillin and/or penicillin, gentamicin) | |||
| Manage Hemorrhage (Oxytocic) | 93.18 | 56.82 | 36.36 |
| (Aseptic gloves, IV fluids, IV kit, oxytocin and/or misoprostol) | |||
| Manage Hypertensive Emergency (Anticonvulsant) | 86·36 | 6.82 | 79.54 |
| (IV fluid, IV kit, magnesium sulfate) | |||
| Mean Medical Readiness | 82.58 | 34.85 | 55.68 |
| Manual Procedures | |||
| Manage Retained Placenta (Manual removal of retained placenta) | 86.74 | 38.64 | 48.10 |
| (Flashlight, IV fluids, IV kit, oxytocin, ampicillin and/or penicillin) | |||
| Manage Incomplete Abortion (Manual removal of retained products of conception) | 75.45 | 18.18 | 57.27 |
| (Flashlight, MVA, IV fluids, IV kit, ampicillin and/or penicillin) | |||
| Mean Manual Readiness | 81.10 | 38.64 | 42.64 |
| Mean Overall Readiness: | 86.76% | 32.27% | 54.48% |
| Signal Function Estimate | Cascade Estimate | % Overestimated by Signal Functions | |
(1) n = 44 facilities
Fig 3. Hypertensive emergency clinical cascade.
Clinical cascades
Overall readiness
The signal function model overestimated basic obstetric emergency readiness by 54.48% (Table 3; Figs 1 and 2). It estimated a mean 80.94% readiness for all five emergencies (range = 75.45–93.18). However, a facility’s practical readiness as measured at the 2nd stage of the cascade (emergency treatment) was substantively lower at 32.27% (range = 6.82–56.82%, Table 3; Fig 1). In addition, across clinical emergencies, signal functions consistently overestimate readiness by 33.03% with only moderate variance (SD = 18.94; Table 4). By signal function, the overall mean overestimates of readiness are: Antibiotic (51.14%), Oxytocic (36.36%), Anticonvulsant (79.54%), Placenta removal (48.10%) and Retained products removal (57.27%; Figs 2–4; S1–S3 Figs).
Table 4. Mean readiness loss by cascade and stage.
| Readiness Loss by Stage1 | Readiness Loss by Cascade | |||||
|---|---|---|---|---|---|---|
| Loss by Clinical Cascade | 1: | 2: | 3: | Mean Loss Across 3 Cascade Stagese Stages | SD | Range |
| Identify | Treat | Monitor-Modify | ||||
| -- | -- | -- | 33.03 | 18.94 | 35.91 | |
| Sepsis-Infection | 20.45% | 38.64% | 38.64% | 32.58 | 10.50 | 18.19 |
| Hemorrhage | 0.00 | 43.18 | 54.55 | 32.58 | 28.78 | 54.55 |
| Hypertensive Emergency | 61.36 | 31.82 | 6.82 | 33.33 | 27.30 | 54.54 |
| Retained Placenta | 22.73 | 38.63 | 38.64 | 33.33 | 9.18 | 15.91 |
| Incomplete Abortion | 27.27 | 54.55 | 18.18 | 33.33 | 18.93 | 36.37 |
| Overall Loss by Stage | ||||||
| Mean Loss Across Cascade | 22.31 | 33.71 | 23.72 | |||
| SD | 25.18 | 20.39 | 22.55 | 0.41 | ||
(1) n = 44 facilities
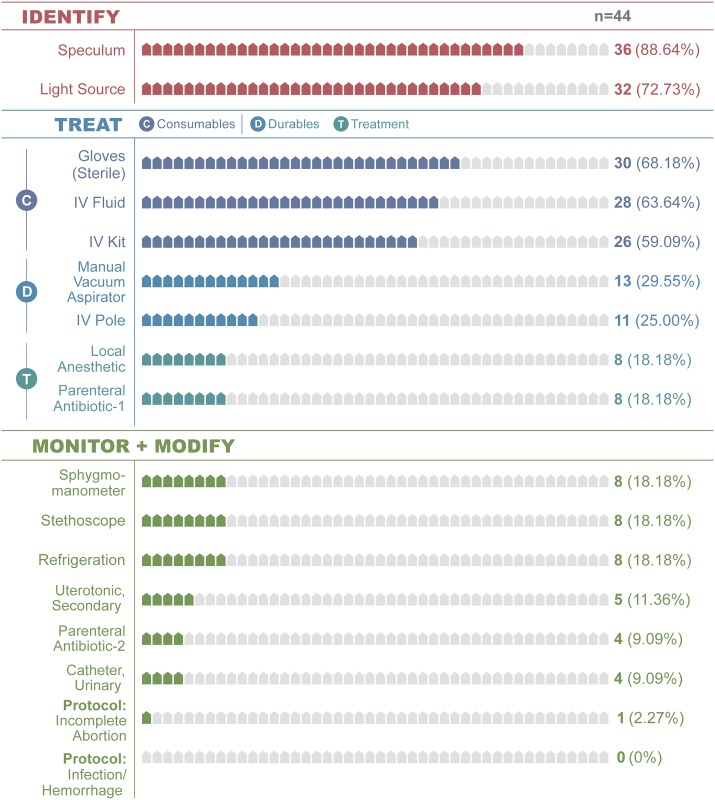
Fig 4. Incomplete abortion clinical cascade.
Readiness loss by cascade
There were notable differences in readiness loss along the cascades from identification of the disorder (stage 1) through monitoring-modifying therapy (stage 3). It varied least for the sepsis-infection (range = 18.19) and retained placenta cascades (range = 15.91) and most for hemorrhage and hypertensive emergencies (range = 54.55 and 61.36% respectively; Table 4; Fig 1; S4 Fig). There was also variability in when readiness was lost along the cascade of clinical care. For hemorrhage, the majority of readiness is lost in the monitor-modify stage (54.55%; Table 4; S1 Fig); in contrast, the hypertensive emergency cascade lost most readiness when identifying the disorder (61.36%; Table 4; Fig 3).
Readiness loss by stage
Across all five emergencies, the mean loss of readiness was 33.03% at each of three stages (Table 4). The loss across all five cascades was: 26.36% for emergency identification (stage 1), 41.36% for treatment (stage 2) and 31.37% for monitoring-modifying therapy (stage 3; Fig 2; S4 Fig). There was a profoundly consistent pattern of 33.03% overall readiness loss across emergencies and stages (SD = 0.41, Table 4) despite moderate variability in how loss occurred across these stages (SD across stages = 18.94).
Discussion
Using the cascades and its family of novel indicators (practical emergency readiness, readiness loss by cascade, aggregate readiness loss across all cascades, readiness loss by stage) can provide multiple benefits for global program planners, health system policy makers, practice scholars and clinicians. The cascades offer a clinically-oriented yet population-health relevant, rapid, intuitive estimate of emergency readiness that is more precise than historic indicators. By quantifying where readiness loss occurs in the cascade of clinical care or by clinical emergency can more strategically guide morbidity and mortality-reducing interventions at the facility-, system- or global-level. Further, aggregate readiness loss across cascades is the first know indicator that can simultaneously quantifying a health system’s overall readiness and be used as a standardized comparison of readiness between systems, countries or regions.
Although broad facility inventories [28] and discrete tracer items are widely used globally, they are unable to accurately quantify a facility’s practical emergency readiness. The obstetric Service Readiness Index (SRI) partially rectifies these limitations by providing an overall summary measure of readiness for all six maternal signal functions [33]. However, neither inventories nor the readiness index provide a clinically relevant assessment of a facility’s practical ability to identify and manage common emergencies. To quantify practical readiness, cascades explicitly identify the interdependent relationship among resources [57, 58]. It models the relationship between identifying emergencies, treating them and then monitoring-modifying therapy based on clinical response (Tables 1 and 2; Figs 3 and 4). Although no known standards exist for measuring readiness for each maternal emergency signal function, the cascades provide emergency-specific readiness indicators (Tables 1, 2 and 4, Figs 3 and 4).
Table 2. Cascade emergency readiness stratified by manual signal function.
| Clinical Cascade (Signal Function) | Cascade Step | Item | % | n 1 |
|---|---|---|---|---|
| Manage Retained Placenta (Manual removal of retained placenta) | Identify | Staff Skill 9 | 100.00 | 44 |
| Light Source 4 | 77.27 | 34 | ||
| Treat (Consumables) | Gloves, Aseptic | 72.73 | 32 | |
| IV Fluid 2 | 68.18 | 30 | ||
| IV Kit 3 | 63.64 | 28 | ||
| Treat (Durables) | IV Pole | 47.73 | 21 | |
| Refrigeration | 45.45 | 20 | ||
| Treat (Treatments) | Parenteral Uterotonic (oxytocin) | 45.45 | 20 | |
| Parenteral Sedative (diazepam) | 43.18 | 19 | ||
| Parenteral Antibiotic-1 7 | 38.64 | 17 | ||
| Monitor-Modify | Sphygmomanometer | 31.82 | 14 | |
| Stethoscope | 31.82 | 14 | ||
| Uterotonic, Secondary 11 | 13.64 | 6 | ||
| Parenteral Antibiotic-2 8 | 9.09 | 4 | ||
| Urinary catheter | 9.09 | 4 | ||
| Protocol: Retained Placenta | 0 | 0 | ||
| Protocol: Infection | 0 | 0 | ||
| Protocol: Hemorrhage | 0 | 0 | ||
| Manage Incomplete Abortion (Manual removal of retained products of conception) | Identify | Speculum | 88.64 | 39 |
| Light Source | 72.73 | 32 | ||
| Treat (Consumables) | Gloves, Sterile | 68.18 | 30 | |
| IV Fluid 2 | 63.64 | 28 | ||
| IV Kit 3 | 59.09 | 26 | ||
| Treat (Durables) | Manual Vacuum Aspirator | 29.55 | 13 | |
| IV Pole | 25.00 | 11 | ||
| Treat (Treatments) | Local Anesthetic (e.g., lidocaine) | 18.18 | 8 | |
| Parenteral Antibiotic-1 9 | 18.18 | 8 | ||
| Monitor-Modify | Sphygmomanometer | 18.18 | 8 | |
| Stethoscope | 18.18 | 8 | ||
| Refrigeration | 18.18 | 8 | ||
| Uterotonic, Secondary 11 | 11.36 | 5 | ||
| Parenteral Antibiotic-2 8 | 9.09 | 4 | ||
| Catheter, Urinary | 9.09 | 4 | ||
| Protocol: Incomplete Abortion | 2.27 | 1 | ||
| Protocol: Infection | 0.00 | 0 | ||
| Protocol: Hemorrhage | 0.00 | 0 |
(1) n = 44 facilities;
(2) normal saline (NS) or lactated ringer’s (LR);
(3) IV cannula;
(4) Functioning flashlight or functioning electric lights;
(7) Parenteral ampicillin or parenteral penicillin (procaine, benzathine or crystalline);
(8) Parenteral gentamicin;
(9) 100% staff skill for identifying the emergency disorder assumed
(11) Misoprostol or ergometrine
For reductions in facility-based obstetric mortality to occur on a global scale, a strategy-oriented approach to measuring readiness is needed. By adding 26 variables to existing inventories derived from the maternal signal functions [33], modeling resource interdependence and precisely defining variable ambiguities, the cascade achieves four goals. First, it identifies a 55% disparity between the high maternal signal function estimates of readiness (86.76%) and a facility’s actual readiness (32.27%; Table 4; Fig 1). Second, it summarizes a facility’s practical emergency readiness for each presenting clinical disorder (Figs 3 and 4, S1–S3 Figs). Third, it identifies points of readiness loss that predictably occur between identifying emergencies, treating them and monitoring-modifying therapy (Table 4, S4 Fig). Fourth, it offers a set of indicators for simultaneously measuring facility- and health system readiness. By defining treatment readiness as having all of the consumables, durables and treatments/drugs required to identify emergencies and give treatment, this model contains precise details about how and where readiness is lost (Tables 1, 2 and 3; Figs 1 and 2) and standardizes global estimates of readiness. Consequently, the cascade model could serve as a quantifiable, generalizable strategy for jointly assessing readiness and guiding strategic planning.
Since Stage 3 (monitor-modify therapy) is a benchmark for quality and not used to estimate readiness in the signal functions, it was not used to critique the existing maternal signal functions model. Including protocols to assess quality requires multiple protocols be used as tracers which complicates modeling and limits comparability with other studies since most existing facility readiness research does not include protocols (range = 2.27–11.36%, S6 Table). However, to maximize comprehensive emergency capacity, future work should model both the ability to provide essential emergency care (readiness indicator—stages 1–2) and to monitor-modify therapy (quality indicator—stage 3). These indicators can be combined into a single estimate of overall emergency capacity (stages 1–3 together measure quality which includes readiness).
Despite the existence of diverse facility inventory tools [28], little work has identified the variables most predictive of emergency readiness or produced guidance on how to strategically allocate emergency obstetric resources [13, 14, 59, 60]. The clinical cascade identifies discrete variables that can be used to measure and compare both readiness and quality by cascade, stage or specific resource with a minimal increase in data collection requirements compared to existing inventories based on the maternal signal functions (S7 and S8 Tables). The cascade approach also enables one to derive a summary of common resources required for multiple emergencies (S9 Table). By using this summary of common resources, one could strategically allocate resources that expand emergency readiness for multiple emergencies simultaneously.
These data are cross-sectional from 44 primary health centers in one region of east Africa. Since inventory data were collected for an intervention trial, some variables necessary for complete cascade modeling were not available (S7 and S8 Tables). However, any data gaps non-differentially affected both models so any potential misclassification bias in this study would simply underestimate the actual limitations of the maternal signal functions model [61, 62].
Ambiguities in how the maternal signal functions model defines tracer items may partially limit comparability between existing published estimates of global emergency readiness and this study. This suggests a model with more precise definitions is warranted to standardize global readiness estimates. The maternal signal functions poorly define some items (i.e., single parenteral antibiotic tracer does not differentiate between WHO stage 1, 2 and 3 condition-specific antibiotics) while some tracers actually contain multiple items (i.e., IV kits contains three discrete items). Ambiguous tracers were explicitly defined to maximize comparability between maternal signal function and cascade estimates of readiness. For example, although ampicillin is the stage 1 antibiotic tracer, few clinics stocked it (4.55%). Consequently, stage 1 readiness is modeled using any clinically indicated alternative drugs. The approach increased the number of clinics with stage 1 antibiotic readiness from 4.55 to 93.18% (Table 1; S2 Fig).
Future research could use the expanded cascades to compare the two estimates of readiness in additional contexts (S7 and S8 Tables). Process research could compare the cascade with others methods for measuring health system readiness using cost, cost-effectiveness or item response burden analyses. Outcome studies could test the cascade’s ability to predict facility-based morbidity, mortality, severe maternal outcomes or labor-related health outcomes for neonates [63–67]. The cascade logic could be applied to other obstetric situations [41, 68–79] including perinatal emergencies or routine clinical care for uncomplicated deliveries [35].
Conclusions
Scholars and practitioners are calling for revisions in the signal functions in order to more precisely estimate global obstetric emergency readiness. This analysis demonstrates how maternal signal functions overestimate practical readiness by at least 55% and a consistent, predictable pattern of 33% capacity loss across all stages of emergency care exist in this context. This aggregate loss of readiness across cascades, stages and facilities provides the first known indicator for measuring, tracking and comparing health system emergency obstetric readiness. The cascades provide a quantitative, step-wise approach to clearly define basic obstetric emergency readiness. Practical, quantifiable assessments of readiness derived from the cascades can guide facility- and health system-level strategies for improving readiness and promoting maternal and neonatal survival at health facilities in low and middle income countries.
Supporting information
(TIF)
(TIF)
(TIF)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Felicitous Ekutu, the regional Kenyan MoH reproductive health coordinator for her assistance with the design and implementation of the primary trial and Dr. Onesmus Gachuno for his project oversight in Kenya. Kenneth Sisimwo and Stephen Kamau provided administrative oversight for the PRONTO intervention. Peter Onyango, Lucy Natecho and Jane Okungu collected the facility data. We are grateful to Damien Scogin (dls4.com) for his skillful graphic design of the cascade visual art and insights on linking key data messages with the images. We are grateful to Drs. Kenneth Hepburn, LisaMarie Wands and Imelda Reyes for constructive editorial input.
Data Availability
Data are available in DANS at the following URL: https://doi.org/10.17026/dans-zgg-6wu.
Funding Statement
The MicroNutrient Initiative funded the research activities of the Linda Mama study with a grant from the Canadian International Development Agency (http://www.micronutrient.org). A portion of the JNC’s research activities were sponsored by Fogarty International Center of NIMH & NIH office of the director (#R25 TW009345) in a grant to the Northern Pacific Global Health Research Fellows Training Consortium (https://www.fic.nih.gov/Pages/Default.aspx and http://fogartyfellows.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MI-Kenya staff participated in the program implementation design and data collection plan.
References
- 1.United Nations (UN). The Millennium Development Goals Report 2015. In: Economic and Social Affairs, editor.: United Nations; 2015.
- 2.Souza JP, Gulmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet. 2013;381(9879):1747–55. Epub 2013/05/21. doi: 10.1016/S0140-6736(13)60686-8 . [DOI] [PubMed] [Google Scholar]
- 3.Ronsmans C, Graham WJ, Lancet Maternal Survival Series steering g. Maternal mortality: who, when, where, and why. Lancet. 2006;368(9542):1189–200. [DOI] [PubMed] [Google Scholar]
- 4.Kinney MV, Kerber KJ, Black RE, Cohen B, Nkrumah F, Coovadia H, et al. Sub-Saharan Africa’s mothers, newborns, and children: where and why do they die? PLoS Med. 2010;7(6):e1000294 doi: 10.1371/journal.pmed.1000294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn JE, Osrin D, Adler A, Cousens S. Four million neonatal deaths: counting and attribution of cause of death. Paediatr Perinat Epidemiol. 2008;22(5):410–6. doi: 10.1111/j.1365-3016.2008.00960.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3.6 million neonatal deaths—what is progressing and what is not? Semin Perinatol. 2010;34(6):371–86. doi: 10.1053/j.semperi.2010.09.011 . [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1 . [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6 . [DOI] [PubMed] [Google Scholar]
- 9.Moran AC, Kerber K, Sitrin D, Guenther T, Morrissey CS, Newby H, et al. Measuring coverage in MNCH: indicators for global tracking of newborn care. PLoS Med. 2013;10(5):e1001415 doi: 10.1371/journal.pmed.1001415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudan I, Lawn J, Cousens S, Rowe AK, Boschi-Pinto C, Tomaskovic L, et al. Gaps in policy-relevant information on burden of disease in children: a systematic review. Lancet. 2005;365(9476):2031–40. Epub 2005/06/14. doi: 10.1016/S0140-6736(05)66697-4 . [DOI] [PubMed] [Google Scholar]
- 11.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global health. 2014;2(6):e323–33. doi: 10.1016/S2214-109X(14)70227-X . [DOI] [PubMed] [Google Scholar]
- 12.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1 . [DOI] [PubMed] [Google Scholar]
- 13.Das J. The quality of medical care in low-income countries: from providers to markets. PLoS Med. 2011;8(4):e1000432 doi: 10.1371/journal.pmed.1000432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das JK, Kumar R, Salam RA, Lassi ZS, Bhutta ZA. Evidence from facility level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11 Suppl 2:S4 doi: 10.1186/1742-4755-11-S2-S4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berendes S, Heywood P, Oliver S, Garner P. Quality of private and public ambulatory health care in low and middle income countries: systematic review of comparative studies. PLoS Med. 2011;8(4):e1000433 doi: 10.1371/journal.pmed.1000433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO). Mother-Baby Package: Implementing Safe Motherhood in Countries. In: Maternal Health and Safe Motherhood Programme, editor. Geneva, Switzerland1994.
- 17.WHO. Indicators to Monitor Maternal Health Goals: Report of a Technical Working Group 8–12 November 1993. Geneva, Switzerland: WHO, 1994.
- 18.Maine D A Z, Ward VM, Kamara A. The design and evaluation of maternal mortality programs. New York, NY: Center for Population & Family Health, Columbia University; 1997. [Google Scholar]
- 19.UNICEF. Guidelines for Monitoring the Availability and Use of Obstetric Services. New York, NY, USA, 1997. [Google Scholar]
- 20.Koblinsky M. Essential Obstetrical Care and Subsets—Basic and Emergency Care: What’s the Difference? Washington, DC: John Snow Institute, 1999. Contract No.: M750. [Google Scholar]
- 21.Koblinsky MA, Campbell O, Heichelheim J. Organizing delivery care: what works for safe motherhood? Bull World Health Organ. 1999;77(5):399–406. Epub 1999/06/11. [PMC free article] [PubMed] [Google Scholar]
- 22.Hussein J, Clapham S. Message in a bottle: sinking in a sea of safe motherhood concepts. Health Policy. 2005;73(3):294–302. Epub 2005/07/26. doi: 10.1016/j.healthpol.2004.11.021 . [DOI] [PubMed] [Google Scholar]
- 23.WHO. Monitoring Emergency Obstetric Care: A Handbook. Geneva, Switzerland: Department of Reproductive Health and Research, 2009. [Google Scholar]
- 24.Nesbitt RC, Lohela TJ, Manu A, Vesel L, Okyere E, Edmond K, et al. Quality along the continuum: a health facility assessment of intrapartum and postnatal care in Ghana. PLoS One. 2013;8(11):e81089 doi: 10.1371/journal.pone.0081089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxton A, Bailey P, Lobis S. The United Nations Process Indicators for emergency obstetric care: Reflections based on a decade of experience. Int J Gynaecol Obstet. 2006;95(2):192–208. doi: 10.1016/j.ijgo.2006.08.009 . [DOI] [PubMed] [Google Scholar]
- 26.WHO. Monitoring the Building Blocks of Health Systems: A Handbook of Indicators and their Measurement Strategies. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 27.Gabrysch S, Civitelli G, Edmond KM, Mathai M, Ali M, Bhutta ZA, et al. New signal functions to measure the ability of health facilities to provide routine and emergency newborn care. PLoS Med. 2012;9(11):e1001340 doi: 10.1371/journal.pmed.1001340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickerson JW, Adams O, Attaran A, Hatcher-Roberts J, Tugwell P. Monitoring the ability to deliver care in low- and middle-income countries: a systematic review of health facility assessment tools. Health Policy Plan. 2015;30(5):675–86. doi: 10.1093/heapol/czu043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill Z, Bailey P, Waxman R, Smith JB. A tool for assessing ‘readiness’ in emergency obstetric care: the room-by-room ‘walk-through’. Int J Gynaecol Obstet. 2005;89(2):191–9. doi: 10.1016/j.ijgo.2004.12.043 . [DOI] [PubMed] [Google Scholar]
- 30.Leone GoS. Sierra Leone Servica Avaliability and Readiness Assessment 2011 Report. Ministry of Health and Sanitation, 2011.
- 31.Government of Kenya. Kenya Serivce Avaliability and Assessment Mapping (SARAM) Report 2013. Nairiboi, Kenya: Ministiry of Health, 2013.
- 32.O’Neill K, Takane M, Sheffel A, Abou-Zahr C, Boerma T. Monitoring service delivery for universal health coverage: the Service Availability and Readiness Assessment. Bull World Health Organ. 2013;91(12):923–31. doi: 10.2471/BLT.12.116798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Service Availability and Readiness Assessment (SARA): An annual monitoring system for service delivery. Geneva, Switzerland: WHO, July 2015. [Google Scholar]
- 34.Spangler SA. Assessing skilled birth attendants and emergency obstetric care in rural Tanzania: the inadequacy of using global standards and indicators to measure local realities. Reprod Health Matters. 2012;20(39):133–41. doi: 10.1016/S0968-8080(12)39603-4 . [DOI] [PubMed] [Google Scholar]
- 35.Brenner S, De Allegri M, Gabrysch S, Chinkhumba J, Sarker M, Muula AS. The quality of clinical maternal and neonatal healthcare—a strategy for identifying ‘routine care signal functions’. PLoS One. 2015;10(4):e0123968 doi: 10.1371/journal.pone.0123968 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey P, Paxton A, Lobis S, Fry D. The availability of life-saving obstetric services in developing countries: an in-depth look at the signal functions for emergency obstetric care. Int J Gynaecol Obstet. 2006;93(3):285–91. doi: 10.1016/j.ijgo.2006.01.028 . [DOI] [PubMed] [Google Scholar]
- 37.Collender G, Gabrysch S, Campbell OM. Reducing maternal mortality: better monitoring, indicators and benchmarks needed to improve emergency obstetric care. Research summary for policymakers. Trop Med Int Health. 2012;17(6):694–6. doi: 10.1111/j.1365-3156.2012.02983.x . [DOI] [PubMed] [Google Scholar]
- 38.Gabrysch S, Zanger P, Seneviratne HR, Mbewe R, Campbell OM. Tracking progress towards safe motherhood: meeting the benchmark yet missing the goal? An appeal for better use of health-system output indicators with evidence from Zambia and Sri Lanka. Trop Med Int Health. 2011;16(5):627–39. doi: 10.1111/j.1365-3156.2011.02741.x . [DOI] [PubMed] [Google Scholar]
- 39.Bhutta ZA, Salam RA, Lassi ZS, Austin A, Langer A. Approaches to improve quality of care (QoC) for women and newborns: conclusions, evidence gaps and research priorities. Reprod Health. 2014;11 Suppl 2:S5 doi: 10.1186/1742-4755-11-S2-S5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter C, Brough R. Systemic capacity building: a hierarchy of needs. Health Policy Plan. 2004;19(5):336–45. Epub 2004/08/18. . [DOI] [PubMed] [Google Scholar]
- 41.Lourenco L, Hull M, Nosyk B, Montaner JS, Lima VD. The need for standardisation of the HIV continuum of care. Lancet HIV. 2015;2(6):e225–6. doi: 10.1016/S2352-3018(15)00086-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO. Trends in Maternal Mortality: 1990 to 2013 Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. In: Reproductive Health and Reserach, editor. Geneva, Switzerland: WHO; 2014.
- 44.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010. Epub 2010/04/13. doi: 10.1016/S0140-6736(10)60518-1 . [DOI] [PubMed] [Google Scholar]
- 45.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980–1004. doi: 10.1016/S0140-6736(14)60696-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassebaum NJ, Lopez AD, Murray CJ, Lozano R. A comparison of maternal mortality estimates from GBD 2013 and WHO. Lancet. 2014;384(9961):2209–10. doi: 10.1016/S0140-6736(14)62421-1 . [DOI] [PubMed] [Google Scholar]
- 47.Ministry of Health. Reversing the Trends: The Second National Health Sector Strategic Plan of Kenya, NHSSP II 2005–2010. In: Ministry of Health, Health Sector Reform Sectariat, ed. Nairobi, Kenya: Ministry of Health; 2005.
- 48.Were S, Kizito, P, Ndei, C. Chapter 2: Overview of the Health System in Kenya. In: National Coordinating Agency for Population and Development (NCAPD) [Kenya] Mo, Medical Services (MOMS) [Kenya] MoPHaSMK, Kenya National Bureau of Statistics (KNBS) [Kenya] IM, editors. Kenya Service Provision Assessment 2011 (SPA). Nairobi, Kenya: National Coordinating Agency for Population and Development, Ministry of Medical Services, Ministry of Public Health and Sanitation, Kenya National Bureau of Statistics, and ICF Macro.; 2011.
- 49.Micronutrieint Initiative. MI and Partners Launch “Linda Afya ya mama na Mtoto” in Mumias District of Kenya: The Micronutrient Initiative; 2013 [March 24, 2015]. Available from: http://www.micronutrient.org/english/view.asp?x=869
- 50.WHO. Practical guidance for scaling up health service innovations. Geneva, Switzerland: WHO, Department of Reproductive Health, 2009. [Google Scholar]
- 51.WHO. Pregnancy, Childbirth, Postpartum and Newborn Care: A guide for essential practice. Geneva, Switzerland: WHO, 2003. 2003. Report No. [PubMed] [Google Scholar]
- 52.WHO. Essential Interventions, Commodities and Guidelines for Reproductive, Maternal Newborn and Childhealth. Geneva, Switzerland: 2012.
- 53.Mutungi A, Harvey, S., Kibaru, J., Lugina, H., Kinoti, S., Jennings, J., Bornstein, T., Hizza, E. Kenya: Assessment of Health Workforce Competency and Facility Readiness to Provide Quality Maternal Health Services. Bethesda, MD: The United States Agency for International Development, September 2008.
- 54.Echoka E, Dubourg D, Makokha A, Kombe Y, Olsen OE, Mwangi M, et al. Using the unmet obstetric needs indicator to map inequities in life-saving obstetric interventions at the local health care system in Kenya. Int J Equity Health. 2014;13:112 doi: 10.1186/s12939-014-0112-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen A, Dutta, A, Maina, T. Assessing the Quality of Primary Healthcare Services in Kenya. USAID Kenya, Health Policy Project FG; 2014.
- 56.Echoka E, Kombe Y, Dubourg D, Makokha A, Evjen-Olsen B, Mwangi M, et al. Existence and functionality of emergency obstetric care services at district level in Kenya: theoretical coverage versus reality. BMC Health Serv Res. 2013;13:113 doi: 10.1186/1472-6963-13-113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pokhrel S. Enhancement of Capacity to further improve Health System in Nepal Potential use of the Capacity Pyramid in Nepal to support NHSP-IP II. Ministry of Health and Population—Government of Nepal, Ministerial Leadership Initiative for Global Health and World Health Organisation Nepal Country Office, January 2011.
- 58.Potter CC, Harries J. The determinants of policy effectiveness. Bull World Health Organ. 2006;84(11):843 . [PMC free article] [PubMed] [Google Scholar]
- 59.Lassi ZS, Das JK, Salam RA, Bhutta ZA. Evidence from community level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11 Suppl 2:S2 doi: 10.1186/1742-4755-11-S2-S2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salam RA, Lassi ZS, Das JK, Bhutta ZA. Evidence from district level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11 Suppl 2:S3 doi: 10.1186/1742-4755-11-S2-S3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koepsell TD W N. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 62.Flegal KM, Keyl PM, Nieto FJ. Differential misclassification arising from nondifferential errors in exposure measurement. Am J Epidemiol. 1991;134(10):1233–44. . [DOI] [PubMed] [Google Scholar]
- 63.Penny J. Severe acute maternal morbidity: a pilot study of a definition of a near-miss. Br J Obstet Gynaecol. 1999;106(4):397 . [DOI] [PubMed] [Google Scholar]
- 64.Say L, Pattinson RC, Gulmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health. 2004;1(1):3 doi: 10.1186/1742-4755-1-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Souza JP, Cecatti JG, Haddad SM, Parpinelli MA, Costa ML, Katz L, et al. The WHO maternal near-miss approach and the maternal severity index model (MSI): tools for assessing the management of severe maternal morbidity. PLoS One. 2012;7(8):e44129 doi: 10.1371/journal.pone.0044129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souza JP, Cecatti JG, Parpinelli MA, de Sousa MH, Serruya SJ. [Systematic review of near miss maternal morbidity]. Cad Saude Publica. 2006;22(2):255–64. /S0102-311X2006000200003. . [DOI] [PubMed] [Google Scholar]
- 67.Menezes FE, Galvao LP, de Mendonca CM, Gois KA, Ribeiro RF Jr., Santos VS, et al. Similarities and differences between WHO criteria and two other approaches for maternal near miss diagnosis. Trop Med Int Health. 2015;20(11):1501–6. doi: 10.1111/tmi.12568 . [DOI] [PubMed] [Google Scholar]
- 68.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS. 2015;10(6):430–8. doi: 10.1097/COH.0000000000000192 . [DOI] [PubMed] [Google Scholar]
- 69.Alemnji G, Fonjungo P, Van Der Pol B, Peter T, Kantor R, Nkengasong J. The centrality of laboratory services in the HIV treatment and prevention cascade: The need for effective linkages and referrals in resource-limited settings. AIDS Patient Care STDS. 2014;28(5):268–73. doi: 10.1089/apc.2013.0356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lourenco L, Colley G, Nosyk B, Shopin D, Montaner JS, Lima VD, et al. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS One. 2014;9(12):e115277 doi: 10.1371/journal.pone.0115277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lourenco L, Nohpal A, Shopin D, Colley G, Nosyk B, Montaner J, et al. Non-HIV-related health care utilization, demographic, clinical and laboratory factors associated with time to initial retention in HIV care among HIV-positive individuals linked to HIV care. HIV Med. 2015. doi: 10.1111/hiv.12297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nosyk B, Krebs E, Eyawo O, Min JE, Barrios R, Montaner JS. Cost-effectiveness analysis along the continuum of HIV care: how can we optimize the effect of HIV treatment as prevention programs? Curr HIV/AIDS Rep. 2014;11(4):468–78. doi: 10.1007/s11904-014-0227-7 . [DOI] [PubMed] [Google Scholar]
- 73.Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–9. doi: 10.1016/S1473-3099(13)70254-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turan JM, Onono M, Steinfeld RL, Shade SB, Owuor K, Washington S, et al. Implementation and Operational Research: Effects of Antenatal Care and HIV Treatment Integration on Elements of the PMTCT Cascade: Results From the SHAIP Cluster-Randomized Controlled Trial in Kenya. J Acquir Immune Defic Syndr. 2015;69(5):e172–81. doi: 10.1097/QAI.0000000000000678 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watson-Jones D, Balira R, Ross DA, Weiss HA, Mabey D. Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;7(7):e40091 doi: 10.1371/journal.pone.0040091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mountain E, Pickles M, Mishra S, Vickerman P, Alary M, Boily MC. The HIV care cascade and antiretroviral therapy in female sex workers: implications for HIV prevention. Expert Rev Anti Infect Ther. 2014;12(10):1203–19. doi: 10.1586/14787210.2014.948422 . [DOI] [PubMed] [Google Scholar]
- 77.Nosyk B, Krebs E, Min JE, Ahamed K, Buxton J, Goldsmith C, et al. The ‘Expanded HIV care in opioid substitution treatment’ (EHOST) cluster-randomized, stepped-wedge trial: A study protocol. Contemp Clin Trials. 2015. doi: 10.1016/j.cct.2015.08.020 . [DOI] [PubMed] [Google Scholar]
- 78.Tang EC, Segura ER, Clark JL, Sanchez J, Lama JR. The syphilis care cascade: tracking the course of care after screening positive among men and transgender women who have sex with men in Lima, Peru. BMJ open. 2015;5(9):e008552 doi: 10.1136/bmjopen-2015-008552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One. 2014;9(5):e97317 doi: 10.1371/journal.pone.0097317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are available in DANS at the following URL: https://doi.org/10.17026/dans-zgg-6wu.