Abstract
We examined the association between statin use and the risk of rheumatoid arthritis (RA), with special focus on describing the patterns of risks of RA during statin exposure in a large population-based cohort in the United Kingdom. In the Clinical Practice Research Datalink, patients aged ≥40 years with at least one prescription of statins (1995–2009) were selected, and matched by age (+/-5 years), sex, practice and date of first prescription of statins to non-users. The follow-up period of statin use was divided into periods of current, recent and past exposure, with patients moving between these three exposure categories over time. Time-dependent Cox models were used to derive hazard ratios (HRs) of RA, adjusted for disease history and previous drug use. The study population included 1,023,240 patients, of whom 511,620 were statin users. No associations were found between RA and current (HRadj,1.06;99%CI:0.88–1.27) or past statin users (HRadj,1.18;99%CI:0.88–1.57). However, in patients who currently used statins, hazard rates were increased shortly after the first prescription of statins and then gradually decreased to baseline level. The risk of developing RA was increased in recent statin users, as compared to non-users (HRadj,1.39;99%CI:1.01–1.90). The risk of RA is substantially increased in the first year after the start of statins and then diminishes to baseline level. These findings may suggest that statins might accelerate disease onset in patients susceptible to develop RA, but in other patients, statins are probably safe and well tolerated, even after prolonged use. Alternatively, we cannot rule out that confounding by cardiovascular risk factors and ascertainment bias may have influenced the findings.
Introduction
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are widely prescribed drugs to reduce the risk of cardiovascular morbidity and mortality [1]. Statins exert, next to their well-known cholesterol-lowering activity, anti-inflammatory and immunomodulatory effects, and may be beneficial in the treatment of immune-mediated disorders other than atherosclerosis. Indeed, beneficial effects of statins were observed in clinical trials and experimental models of RA [2–4]. The anti-inflammatory effects of statins have been studied in several clinical trials by measuring C-reactive protein (CRP) [5,6]. In these clinical trials it has been shown that statins decrease levels of CRP [5,6]. Since statin therapy reduces the incidence of acute and chronic rejection in heart and renal transplant patients [7,8], the immunomodulating effects have been further studied. Statins have been reported to suppress interferon-γ (IFN-γ)-inducible expression of major histocompatibility complex (MHC) class II proteins in endothelial cells, monocytes/macrophages and T cells [9,10]. Another beneficial effect of statins is the effect on the T helper cells (Th1)/Th2 balance by inducing the secretion of Th2 cytokines (IL-4, IL-5 and IL-10) and transforming factor β (TGF-β), or by suppressing secretion of Th1 cytokines (IL-2, IL-12, IFN-γ and TNF-α) [11,12]. Recently, it has been suggested that statins skew T cell differentiation towards regulatory T cell (T reg) and away from pro-inflammatory Th17 cells via geranylgeranylation of proteins, resulting in promoting Treg differentiation in the periphery, while blocking Th17 cell differentiation [13]. Statins can also down-regulate expression of the co-stimulatory molecule CD40 in various cell types, e.g., endothelial cells, smooth muscle cells and macrophages [14]. Interestingly, certain types of statins selectively block the β-2 integrin, leukocyte function antigen-1 (LFA-1), thereby blocking binding to intercellular adhesion molecule-1 (ICAM-1) and thus reducing T cell activation [15].
The immunomodulating effects may, on the other hand, facilitate the development of autoimmunity potentially resulting in autoimmune diseases, such as RA [16–21]. It has been suggested that statins induce a shift from a Th1 to Th2 immune response by their direct effect on T cells. Promoting a shift from Th1 to Th2 immune responses may dysregulate the immune homeostasis and can lead to the breakdown of self-tolerance, precipitating autoimmunity [16,22]. In addition, statins are potent pro-apoptotic agents and may trigger or exacerbate cellular apoptosis [23], thereby releasing nuclear antigens into the circulation, which may foster the production of pathogenic autoantibodies [24].
Apart from our studies [17–19,21,25], six other studies have described the risk of developing RA or connective tissue disease (CTD) (the majority of the patients were coded with RA) during statin treatment, and have shown conflicting results [26–31]. Possible explanations for these conflicting findings may be attributed to the lag-time between statin use and incident RA [27,29], using different definitions of exposure to statins [19,26–31], using different definitions of RA [19,27–31], comparing to a control group of non-persistent statin users [27] or lowest duration weighted average statin intensity [31] instead of non-users, controlling for other confounders [19,26–31], shifting the date of incident RA [19,26], propensity score matching on baseline characteristics [30], or conducting separate analyses in patients with or without a medical history of cardiovascular risk factors (S1 Table) [19,26].
Cardiovascular risk factors, including smoking and hormone replacement therapy have been associated with RA [32–36]. Several studies have demonstrated an unfavourable lipid profile before a patient is diagnosed with RA [32,37]. If statin use is a proxy for hyperlipidaemia then the increased risk of developing RA in our previous study [19] might be explained by hyperlipidaemia rather than by an immunomodulating effect of statins. Otherwise, subclinical RA may have been present before the initiation of statin treatment since it is well-known that autoantibodies and non-specific symptoms may be present long before patients are diagnosed as having RA [38].
At present, the previous observational studies have not shown a conclusive relationship between statin use and the risk of developing RA. It is unclear whether the association between statin use and the risk of developing RA is related to the use of statins or whether it is merely an association with hyperlipidaemia. Moreover, none of the previous studies [19,26–31] studied the pattern of risks of RA with changes in statin exposure over time. Therefore, we examined the association between the use of statins and the risk of developing RA, with special focus on describing the patterns of risks of RA with changes in statin exposure over time and confounding by cardiovascular co-morbidities in a large population-based cohort.
Materials and methods
Data source
Data were derived from the Clinical Practice Research Datalink (CPRD), previously known as the General Practice Research Database, which contains computerised medical records of all patients under the care of 625 general practices (GPs) in the United Kingdom, representing 8% of the population. The CPRD has been described in detail elsewhere [39–40]. The database provides detailed information on demographics, diagnoses, prescription details, preventive care provided, specialist referrals, and hospital admissions [40]. Several independent validation studies have shown that the CPRD database has a high level of completeness and validity [41,42].
The CPRD Group has obtained ethical approval from a National Research Ethics Service Committee (NRES) for all purely observational research using anonymised CPRD data; namely, studies which do not include patient involvement (which is the vast majority of CPRD studies). Independent Scientific Advisory Committee (ISAC) (https://www.cprd.com/ISAC/members.asp) is responsible for reviewing protocols for scientific quality, but may recommend that study-specific ethical approval is sought if ethical issues arise in relation to an individual study. For the present study a separate ethical approval was not required since the patients were not directly involved in formulating the research question, nor were patients actively involved in the design and/or conduct of the research (https://www.cprd.com/isac/otherinfo.asp).
Study population
We conducted a matched cohort study with prospectively collected data which has been described previously [25]. All patients who had at least one prescription of statins at least one year after the start of data collection (period: 1995–2009) were included. The date of the first prescription of statins was defined as the index date. Statin users were matched by age (+/-5 years), sex, and practice to a single control (non-users of statins), with the index date of the control being the same as that of the statin user (i.e. matching on calendar time). After using a matched random sampling approach, statin users and non-users who were younger than 40 years, had ever been diagnosed with RA, and/or had used disease modifying anti-rheumatic drugs (DMARDs) before or at index date were excluded.
Exposure to statins
Exposure to statins was determined by all prescriptions, and each prescription length was calculated by dividing the number of prescribed tablets by the number of days prescribed daily dose. Compliance to statins declines substantially over time [43], and therefore the time of follow-up was divided into periods of current, recent and past exposure to statins, with patients moving between these three exposure categories over time [25]. Current exposure was defined as the days from the start date of a prescription until the start date of the consecutive prescription for statins. When the start date of the consecutive prescription of statins was prescribed within these 3 months after the start date of the prescription, patients continued to be ‘current users’. The expected duration between consecutive prescriptions was defined as 3 months, which reflects the prescribing regimen for long-term use of statins. Since patients can move between different categories of exposure to statins over time, patients can be defined more than once as ‘current users’ [25]. The duration of current statin use was calculated by estimating the number of days within each category of ‘current users’, and then the days per category were added up. We divided the duration of statin use into ≤1 and >1 year use.
We believe that ‘current users’ who initially started their statin therapy have another risk profile than ‘current users’ who restarted their therapy; therefore, ‘current users’ were divided into ‘de novo’ and ‘restart’ users. ‘De novo’ statin users were defined as patients who were not moving between the three periods of statin exposure over time. Recent exposure was defined as the period of time from 3 to 12 months after the end date of the most recent prescription, and past exposure was the period of time from 12 months or longer after the end date of the most recent prescription of statins. Three examples of time-dependent exposure to statins were illustrated elsewhere [25].
When the event (RA) occurred in a category of ‘current users’, the patient was defined at the time of the event as a current user. If not, the statin user was a recent user or a past user at the time of occurrence of the event.
Clinical outcome
Each patient was followed from the index date up to the date of the first diagnosis of RA (identified from the CPRD Read codes), or the date when the patient left the general practice, died, or the end date of data collection, whichever date came first. Patients were considered as having a diagnosis of RA if the first diagnosis of RA registered by a GP was verified by at least one prescription of a DMARD during follow-up, adapted from an algorithm proposed by Thomas et al [44]. When a patient was previously referred to a rheumatologist, the date of the first referral was defined as the event date.
We carried out four sensitivity analyses to evaluate the impact of potential case misclassification and we changed the definition of RA:
into patients with a first diagnosis of RA with a referral to a rheumatologist or with at least one prescription of a DMARD;
into the first diagnosis of RA with a referral to a rheumatologist, or at least one prescription of a DMARD and/or at least two prescriptions of corticosteroids, a definition we used in a previous study [19];
into having another medical record of RA after the first diagnosis as proposed by Kim et al [45].
into patients with a medical record of RA who were treated with at least one prescription of a DMARD within a time span of two years after their first-time diagnosis, limiting bias due to “peeking” into the future to define RA.
In another sensitivity analysis, a lag time between the onset of RA and the diagnosis was considered [27,29], and therefore we excluded the first year of every patient following the initiation of statin treatment, thereby excluding the events of RA (S1 Fig) In addition, we examined the effect of potential late manifestation of the clinically apparent symptoms of RA by changing the event date of RA exactly one year before the first diagnosis of RA, as has been suggested by Jick et al. (S1 Fig) [26]. We studied the effect of setting the event date of RA when the date of referral to a rheumatologist was more than 2 years before the first diagnosis of RA. We changed the referral date into the date of the first diagnosis for RA. Finally, we examined residual confounding due to omission of confounding variables from the adjusted model by including all potential confounders in the model.
Statistical analysis
We controlled for false discovery rates to compensate for the problem of finding statistically significant results by chance. We considered the effect of statins on the risk of developing RA to be significant at the 0.01 level. We estimated the hazard ratios (HRs) and 99% confidence intervals (CIs) for the risk of developing RA among current, recent and past statin users (versus non-users), using a time-dependent Cox proportional hazards model (SAS 9.2. PHREG procedure).
The following risk factors were considered as potential confounders: use of non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, proton pump inhibitors (PPIs), antibiotics, hormone replacement therapy, antidepressants, anticonvulsants, anti-psychotics, anti-arrhythmic and other lipid-lowering agents within 6 months before the index date [19,29,30,46,47]. In addition, a diagnosis of hypertension, diabetes mellitus, hyperlipidaemia, cardiovascular disease, asthma, inflammatory bowel and thyroid disease, and body mass index (BMI), smoking and alcohol intake (a record of currently smoking or drinking, ex-smoker or -drinker, or never smoked or drank) ever before index date were considered as potential confounders [19,26–31,33,46,48,49]. Next to the matching variables, covariables were included in the final model if they independently changed the β-coefficient for statin use by at least 5%.
We used multiple imputation to address missing data for BMI, smoking and alcohol status. The missing values were imputed by the multiple imputation method using the fully conditional specification method [50]. Twenty imputation were created, analysed and pooled. Results from the complete case and multiple imputation analyses were compared. No difference in the results from both analysed was observed, and multiple imputation analyses are presented.
A descriptive analysis of the pattern of changes in the risk of RA (hazard rates) in current statin users compared to non-users was performed. Non-users included never users but also past and recent users, defined as time = 0. The duration of current use was divided into 10 periods (using every 10th percentile), and hazard rates were calculated within each period. For each period, the risk of RA was plotted against the median time since the first prescription of current statin use and visualised using smoothing spline regression [51], which has been advocated as an alternative to categorical analysis [52].
For all eight sensitivity analyses, we conducted descriptive analyses of the pattern of changes in the risk of RA in current statin users compared to non-users.
Pre-specified subgroup analyses based on the presence of cardiovascular diseases or related risk factors were conducted since previous studies suggested different associations between statin use and the risk of developing RA in patients with a history of cardiovascular diseases, hypertension and diabetes [33,34]. Statins could also have been prescribed to patients with only a diagnosis of diabetes mellitus, or to patients with a low socioeconomic status, or to patients with a family history of cardiovascular disease or to patients with a high-risk ethnicity [53], regardless of their lipid levels, and therefore; we conducted a subgroup analysis in patients with or without a medical history of hyperlipidaemia.
According to previous studies, older or female patients are more likely to experience adverse events of statins than younger or male patients [54,55]. In attempt to examine for this potential bias, we conducted subgroup analyses by age and sex.
We tested for interactions between statin use and age, sex, cardiovascular diseases and related risk factors. Significant interaction terms (Pvalue<0.05) were included in the model.
Results
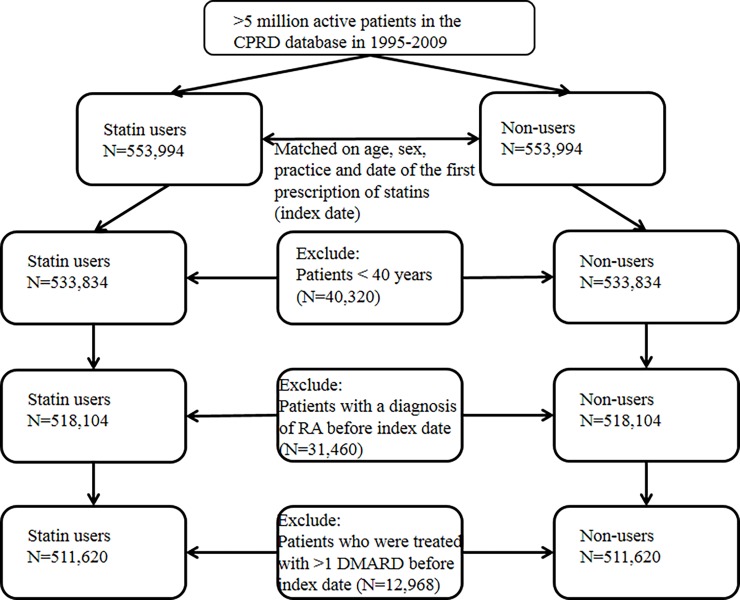
The study population included 1,107,988 patients. After excluding 40,320 patients who were younger than 40 years, 31,460 patients with a medical history of RA and 12,968 patients with prescriptions of DMARDs before the index date, 511,620 statin users and 511,620 non-users were enrolled in the study (Fig 1).
Fig 1. Flow chart study population.
Legend: CPRD, Clinical Practice Research Datalink; RA, rheumatoid arthritis; DMARD, disease modifying anti-rheumatic drug.
Due to matching, statin users and non-users had similar distributions of age (statin users: mean age, 63.0 years and non-users: 62.8 years) and sex (statin users and non-users: 48% women). Statin users were more often diagnosed with cardiovascular diseases, hyperlipidaemia, hypertension, diabetes and cerebrovascular events than non-users. Remarkably, smoking was not different between statin users and non-users. Statin users were more likely to have used aspirin, antihypertensive and anti-diabetic agents, NSAIDs, PPIs, antibiotics and antidepressants than non-users (Table 1).
Table 1. Baseline characteristics of statin users and non-statin users.
| Baseline characteristics | Statin users (n = 511,620) | Non-users (n = 511,620) |
|---|---|---|
| Duration of follow-up (years) | ||
| Mean (SD) | 3 (2.5) | 3 (2.6) |
| Sex, n (%) | ||
| Women | 244,870 (47.9) | 244,870 (47.9) |
| Age (years) | ||
| Mean (SD) | 63.0 (12.1) | 62.8 (12.5) |
| BMI (kg/m2) | ||
| Mean (SD) | 26.9 (8.4) | 21.0 (11.6) |
| Missing | 27,760 (5.4) | 104,435 (20.4) |
| Smoking status, n (%) | ||
| Non-smoker | 213,102 (41.7) | 230,927 (45.1) |
| Ex-smoker | 161,885 (31.6) | 109,645 (21.5) |
| Smoker | 114,085 (22.3) | 99,340 (19.4) |
| Missing | 22,548 (4.4) | 71,708 (14.0) |
| Drinking status, n (%) | ||
| Non-drinker | 63,872 (12.5) | 53,309 (10.4) |
| Ex-drinker | 32,104 (6.3) | 20,384 (4.0) |
| Drinker | 352,827 (68.9) | 317,067 (62.0) |
| Missing | 62,817 (12.3) | 120,860 (23.6) |
| Drug use within previous 6 months, n (%) | ||
| Antihypertensive agents | 317,494 (62.1) | 121,220 (23.7) |
| Fibrates | 8,436 (1.6) | 881 (0.2) |
| Ezetimibe | 1,943 (0.4) | 130 (0.03) |
| Anti-diabetic agents | 120,353 (23.5) | 18,200 (3.6) |
| Anti-arrhythmic agents | 20,207 (3.9) | 11,051 (2.2) |
| Aspirin | 142,209 (27.8) | 36,003 (7.0) |
| NSAIDs | 197,750 (38.7) | 86,106 (16.8) |
| Proton pump inhibitors | 82,939 (16.2) | 46,820 (9.2) |
| Hormone replacement therapy or oral contraceptives | 21,219 (4.1) | 20,598 (4.0) |
| Oral corticosteroids | 16,815 (3.3) | 14,684 (2.9) |
| Antibiotics | 46,267 (9.0) | 35,564 (7.0) |
| Anticonvulsants | 10,648 (2.1) | 7,957 (1.6) |
| Antipsychotics | 5,355 (1.0) | 6,025 (1.2) |
| Antidepressants | 113,390 (22.2) | 93,400 (18.3) |
| History of disease ever before, n (%) | ||
| Hypertension a | 317,523 (72.4) | 194,097 (33.2) |
| Hyperlipidaemia | 151,380 (29.6) | 12,492 (2.4) |
| Diabetesb | 120,681 (23.6) | 18,355 (3.6) |
| Cardiovascular diseases | 171,581 (33.5) | 46,357 (9.1) |
| Stroke or TIA | 52,336 (10.2) | 13,671 (2.7) |
| Psoriasis | 19,719 (3.9) | 16,212 (3.2) |
| Inflammatory bowel disease | 5,074 (1.0) | 5,034 (1.0) |
| Cancer | 34,369 (6.7) | 39,229 (7.7) |
| Thyroid Disease | 52,212 (10.2) | 35,735 (7.0) |
| COPD | 20,583 (4.0) | 20,283 (4.0) |
| Asthma | 60,252 (11.8) | 52,152 (10.2) |
| Dementia | 4,937 (1.0) | 8,342 (1.6) |
| Depression | 71,029 (13.9) | 48,201 (9.4) |
NSAIDs, non-steroidal anti-inflammatory drugs; COPD, Chronic Obstructive Pulmonary Disease; TIA, Transient Ischaemic Attack; SD, standard deviation
a Diagnosis of hypertension or use of antihypertensive agents
b Diagnosis of diabetes mellitus or use of anti-diabetic therapy
The incidence rate for RA is 4.2 per 10,000 person-years. Current users had a risk of developing RA comparable to that of non-users (HRadjusted (adj), 1.06; 99% CI, 0.88 to 1.27) (Table 2). The HRadj for ‘de novo’ users and ‘restart’ users were 1.04; 99% CI, 0.89 to 1.20 and 1.00; 99% CI, 0.86 to 1.50, respectively. Importantly, current statin users who continued the therapy for ≤1 year had a 1.3-fold increased risk of developing RA, (HRadj, 1.27; 99% CI, 1.00 to 1.61). Risk of RA was 1.4-fold increased with recent statin use, as compared to non-users (HRadj, 1.39; 99% CI, 1.01 to 1.90). No association was found between past statin users and incident RA.
Table 2. Risk of rheumatoid arthritis in statin users compared to non-statin users.
| RA (n) | IR a | age- and sex-adjusted HR (99% CI) | fully adjusted HR (99% CI) b | |
|---|---|---|---|---|
| No statin use | 579 | 3.7 | 1.00 | 1.00 |
| Past statin use | 105 | 4.9 | 1.33 (1.08 to 1.64) | 1.18 (0.88 to 1.57) |
| Recent statin use | 101 | 5.6 | 1.60 (1.27 to 2.02) | 1.39 (1.01 to 1.90) |
| Current statin use | 837 | 4.3 | 1.24 (1.10 to 1.38) | 1.06 (0.88 to 1.27) |
| ≤ 1 year | 386 | 11.5 | 1.47 (1.25 to 1.73) | 1.27 (1.00 to 1.61) |
| > 1 year | 451 | 2.8 | 1.15 (1.02 to 1.31) | 0.98 (0.80 to 1.19) |
| ‘de novo’ statin use | 464 | 4.1 | 1.16 (1.02 to 1.31) | 1.04 (0.89 to 1.20) |
| ‘restart’ statin use | 373 | 4.7 | 1.34 (1.17 to 1.54) | 1.00 (0.86 to 1.50) |
RA, rheumatoid arthritis; IR, incidence rate (per 10 000 person-years); HR, hazard ratio; CI, confidence interval
a Incidence rate is calculated for each recency of statin use by dividing the number of events by the person time within each given recency of use.
bAdjusted for age, sex, practice, smoking, cardiovascular diseases, hyperlipidaemia, hypertension, diabetes and use of non-steroid anti-inflammatory drugs.
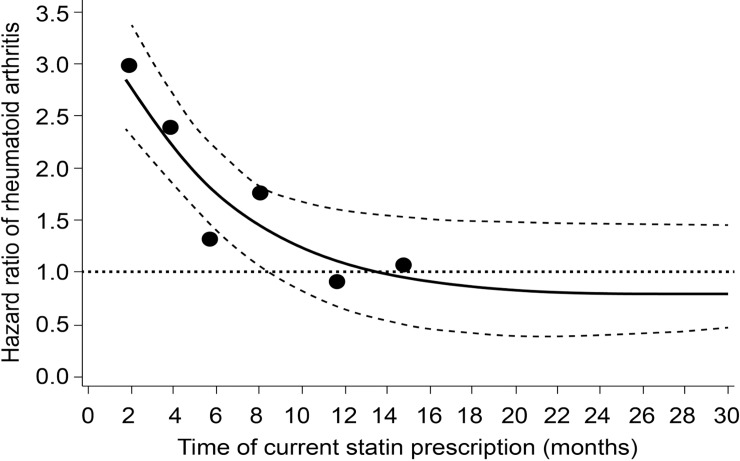
Fig 2 shows that the risk of RA was substantially increased in the first year after the first prescription of statins compared to non-users. The HRadj was 2.93 (99% CI, 2.46 to 3.49) at the start of statin therapy. After one year of statin exposure, the risk of RA declined to baseline level (>1 year: HRadj, 0.95; 99% CI, 0.74 to 1.16).
Fig 2. Risk of rheumatoid arthritis in current statin users versus non-users, by time since the first statin prescription.
Legend: Solid bold line and circles: adjusted hazard ratios. Dotted lines: 95% confidence bands. Spline regression plot of time since the first prescription of statins and the risk of RA in current statin users vs. matched non-users. HRs are adjusted for confounders as shown in Table 2.
In Table 3, we present several potential risk factors that may have influenced the risk of developing RA after statin exposure. We observed a tendency towards an increased risk of developing RA in women and patients without a medical record of hyperlipidaemia, hypertension and diabetes who currently used statins for less than one year, or who were recent users. However, for none of these potential risk factors, the interaction term did reach significance. No effect modifiers for the association between current, recent and past statin exposure and incident RA were found.
Table 3. Risk of rheumatoid arthritis risk in statin users vs. non-statin users according to different populations.
| adjusted HR (99% CI) b | |||||||
|---|---|---|---|---|---|---|---|
| RA | IR a | Past statin use | Recent statin use | Current statin use | Current statin use | Current statin use | |
| (n) | ≤ 1 year | > 1 year | |||||
| By age, y | |||||||
| 40–50 | 131 | 2.6 | 2.23 (0.95 to 5.18) | 1.57 (0.49 to 5.00) | 1.29 (0.63 to 2.65) | 1.06 (0.43 to 2.64) | 1.39 (0.66 to 2.93) |
| 51–60 | 475 | 4.1 | 1.11 (0.66 to 1.90) | 1.60 (0.91 to 2.82) | 0.94 (0.66 to 1.33) | 1.28 (0.82 to 2.02) | 0.80 (0.55 to 1.18) |
| 61–80 | 938 | 5.1 | 1.08 (0.73 to 1.60) | 1.19 (0.77 to 1.84) | 1.01 (0.79 to 1.28) | 1.21 (0.89 to 1.65) | 0.93 (0.72 to 1.20) |
| >80 | 78 | 2.0 | 1.21 (0.38 to 3.79) | 1.49 (0.43 to 5.13) | 0.93 (0.43 to 2.02) | 1.06 (0.40 to 2.76) | 0.86 (0.36 to 2.05) |
| By sex | |||||||
| Women | 1,021 | 5.4 | 1.19 (0.83 to 1.69) | 1.68 (1.14 to 2.50) | 1.17 (0.93 to 1.47) | 1.49 (1.09 to 2.02) | 1.06 (0.83 to 1.36) |
| Men | 601 | 3.0 | 1.19 (0.74 to 1.93) | 0.99 (0.58 to 1.71) | 0.89 (0.66 to 1.21) | 0.99 (0.68 to 1.44) | 0.85 (0.61 to 1.18) |
| By any previous history of disease | |||||||
| No previous cardiovascular disease | 1,138 | 3.9 | 1.24 (0.90 to 1.72) | 1.35 (0.91 to 1.99) | 1.00 (0.80 to 1.24) | 1.19 (0.90 to 1.57) | 0.91 (0.72 to 1.16) |
| Previous cardiovascular disease | 484 | 4.8 | 0.96 (0.50 to 1.83) | 1.32 (0.72 to 2.46) | 1.06 (0.68 to 1.64 | 1.28 (0.77 to 2.15) | 0.99 (0.63 to 1.55) |
| No previous cardiovascular risk factorc | 649 | 4.0 | 1.18 (0.72 to 1.93) | 1.52 (0.87 to 2.63) | 1.04 (0.77 to 1.39) | 1.37 (0.93 to 2.01) | 0.84 (0.58 to 1.21) |
| Previous cardiovascular risk factor | 973 | 4.3 | 1.05 (0.73 to 1.53) | 1.19 (0.87 to 1.61) | 0.94 (0.73 to 1.21) | 1.09 (0.79 to 1.49) | 0.89 (0.69 to 1.16) |
| No previous hyperlipidaemia | 1,254 | 4.1 | 1.11 (0.79 to 1.57) | 1.59 (1.12 to 2.28) | 1.05 (0.86 to 1.29) | 1.24 (1.01 to 1.57) | 0.98 (0.79 to 1.23) |
| Previous hyperlipidaemia | 368 | 4.5 | 1.81 (0.69 to 4.70) | 1.32 (0.47 to 3.71) | 1.48 (0.61 to 3.58) | 1.96 (0.77 to 5.00) | 1.34 (0.55 to 3.28) |
| No previous hypertension | 857 | 4.1 | 1.25 (0.84 to 1.85) | 1.29 (0.81 to 2.05) | 1.12 (0.87 to 1.44) | 1.50 (1.08 to 2.07) | 0.94 (0.70 to 1.26) |
| Previous hypertension | 765 | 4.3 | 0.99 (0.64 to 1.51) | 1.29 (0.91 to 1.81) | 0.88 (0.67 to 1.17) | 0.96 (0.67 to 1.36) | 0.86 (0.64 to 1.15) |
| No previous diabetes | 1,394 | 4.2 | 1.16 (0.86 to 1.58) | 1.39 (0.98 to 1.98) | 1.07 (0.88 to 1.30) | 1.31 (1.02 to 1.70) | 0.97 (0.78 to 1.20) |
| Previous diabetes | 228 | 4.1 | 1.10 (0.41 to 2.94) | 1.14 (0.44 to 2.97) | 0.89 (0.41 to 1.91) | 0.92 (0.40 to 2.13) | 0.88 (0.40 to 1.91) |
RA, rheumatoid arthritis; IR, incidence rate (per 10,000 person-years); HR, hazard ratio; CI, confidence interval
a Incidence rate is calculated for each recency of statin use by dividing the number of events by the person time within each given recency of use.
bAdjusted for confounders as shown in Table 2.
cCardiovascular risk factor included previous hyperlipidaemia, hypertension and diabetes
Sensitivity analyses
Performing different sensitivity analyses did alter our findings slightly. The sensitivity analysis where we excluded the first year after initiation of statin treatment showed that current use, who were divided by the duration of their therapy was not associated with a risk of RA. All other sensitivity analyses showed similar results as the main analysis, although they were slightly attenuated. When we depicted the patterns of changes in the risk of RA during current statin use of all the sensitivity analyses, no differences in the patterns of all the sensitivity analyses were observed. The results of the eight sensitivity analyses are presented online (S2 Table).
Discussion
This study demonstrated a 1.3-fold increased risk of developing RA during the first year of statin use. The risk of developing RA was increased shortly after the first prescription of statins and then gradually decreased to baseline level. In recent users, statin use was associated with incident RA whereas in past users no such effect was found.
Two population-based cohort studies showed no association between statin use and the risk of developing RA [28,29]. Furthermore, in a nested case-control study of 313 incident RA patients and 1,252 matched controls, no association between current statin use and incident RA was found [26]. The same study showed, however, in a subsample of only patients with hyperlipidaemia a decreased risk of developing RA in current users [26]. We found no association between current statin use and incident RA in patients with hyperlipidaemia, although the sample size was too small to draw a definite conclusion. However, the differences in findings may be explained by the difference in defining statin exposure. Jick et al. defined current statin use as receiving two prescriptions in the year prior to the first diagnosis of RA [26] whereas we defined it as the time from the date of a prescription until three months after its expected duration of use (time-dependent variable).
In a population-based cohort study of 211,627 new statin users, statin use was associated with a reduction in the risk of developing RA. This effect was only present for those who used statins for more than one year [27]. In contrast, we found no association between statin use and incident RA in current users who continued statin therapy for more than one year. Another population-based cohort study of 528,654 new statin users showed that high-intensity statin treatment was associated with a reduced risk of incident RA in comparison to low-intensity statins [31]. In our study we did not observe the potential beneficial effects of statins. The difference between our study and Tascilar et al. may be explained by the selection of the reference group [31]. In a propensity score matched cohort study of 6,956 pairs of statin users and non-users, statin use was associated with a lower risk of connective tissue disease (CTD) [30]. The possible protective effect of statins was not observed in our study. The discrepancies may be partially explained by the difference in defining statin exposure and defining RA. We classified statin exposure by the recency of use, and modelled it as a time-dependent variable. Chodick et al. and Tascilar et al., however, defined statin exposure as the proportion of follow-up days covered with statins [27,31], whereas Schmidt et al. defined statin use as receiving at least a 90-day supply at baseline [30]. Also, our definition of RA may have been more specific than the ones used by other three studies [27,30,31]. We verified patients’ electronic records with at least one prescription of a DMARD after the diagnosis of RA. Tascilar et al. used the same approach of defining RA as we did, but on top of that, they included patients with two electronic records for RA at least three months apart [31]. In the study by Chodick et al., patients were included when they had a diagnostic code for RA with or without the use of DMARDs [27], whereas Schmidt et al. included patients with CTD, including RA [30]. Using a more specific definition of RA may have influenced the association between statin use and incident RA.
Another explanation for the conflicting results between these six observational studies [26–31] and ours may be the selection of the non-users. In two other studies non-users were also selected at the date of the first prescription of statins, although these non-users were matched or adjusted for propensity scores, including age, sex and other baseline characteristics [29,30]. Smeeth et al. excluded [29], however, the first year of every patient following the initiation of statin treatment. In another study, non-users were again selected at the date of the first prescription of statins, however, no matching was conducted. Instead, the analyses were adjusted for age, BMI, ethnicity, smoking and hypothyroidism in men and women separately [28]. The other three studies selected the reference group as patients who were non-persistent statin users [27], or patients who were exposed to low-intensity statin treatment [31], or controls as patients without a diagnosis of RA at the date of the first diagnosis of RA [26]. We believe that the selection of the reference group (non-users) may have influenced the study results. To address the possible differences in baseline characteristics between statin users and non-users, we did consider the use of a propensity-matched cohort of non-users. However, recent research has found that propensity matching does not always improve the adjustment of measured confounding [56]. Besides this, unmeasured confounding is not addressed by propensity score matching [56]. We used a study population comprising a matched sample of the general population (i.e. matched on age, sex, general practice and calendar time) to reduce bias due to differences between the statin users and non-users. In addition, we adjusted our models for potential confounders. Further, we conducted a sensitivity analysis where we included all potential confounders in the model. The effect estimates were similar to those presented in the main analysis. By using various methods for handling the potential differences between the statin users and non-users, we believe that we have selected a representable reference group. Furthermore, the incidence rate of RA in the non-users of our study is almost in line with the incidence rate of RA as presented in other studies [57,58]. Therefore, we suggest that the non-users may be a good reflection of the general population.
In our previous study [19], we found an increased risk of developing RA within six months of statin use, which is in line with the results of the present study. In this study, we found that the risk of developing RA disappeared after one year. Statins may accelerate the onset of RA in patients susceptible to develop RA as previously demonstrated in an animal model of arthritis [20]. In line with this hypothesis, the majority of cases of statin-associated lupus-like syndrome developed this syndrome within one year after starting statin therapy [16]. However, in patients not prone to develop RA, statins are probably safe and well tolerated, even after prolonged use. In this case, it should be stressed that patients with a high (>20% risk) 10-year risk of cardiovascular disease or intermediate (>10% risk) 10-year risk should continue statin treatment. In a recent study, it has been shown that statin cessation after media debates regarding safety could result in increased statin cessation and at least 2,173 excess cardiovascular events over 10 years in the UK [59].
RA may have been present and not well documented before the start of statins, which may have introduced bias (protopathic) in this study. In both our studies, we defined the onset date of RA by the first record or specialist referral. Unfortunately, the onset date of symptoms is unknown in our studies. One study reported a median time between onset of symptoms to diagnosis of RA of approximately 36 weeks (range: 4 weeks to >10 years) [60]. As two population-based studies considered a lag-time of one year between statin use and incident RA [27,29], we performed a sensitivity analysis where we excluded the first year following the initiation of statin treatment. We found no increased risk of RA in the first year after the excluded year. However, the descriptive analysis of this sensitivity analysis showed a similar pattern of risks of RA during statin use but was slightly attenuated.
Importantly, cardiovascular risk factors (e.g. hyperlipidaemia) may have influenced the association between statin use and incident RA. Several studies have demonstrated an unfavourable lipid profile in patients with RA [32,37]. Hyperlipidaemia may induce leukocyte activation and possibly complement activation [61–63], which may result in an earlier diagnosis of RA in patients prone to develop RA. When we conducted subgroup analyses in patients with and without a medical record for hyperlipidaemia, patients with hyperlipidaemia showed high risks of developing RA with current, recent and past statin use, although the sample sizes were too small for conclusions.
The mechanism and time course by which statins may facilitate RA are unknown. According to one review, the mean time of exposure before disease onset ranges from one month to six years [16]. Despite the unknown time course, it has been suggested that statins may promote a shift in Th1/Th2 balance [16,22] or affect regulatory T cells [13,64,65], or lead to unstable regulatory T cells in the periphery [66,67], and thus may promote autoimmunity. Based on these findings, we hypothesise that statins do not themselves cause autoimmunity but they may promote a pre-existing autoimmune-prone condition to progress towards a clinical manifest disease such as RA. Another possible hypothesis is that the self-tolerance is lost due to persistence of infectious agents in individuals who were treated with statins. Since statins may reduce Th1 responses [9], infectious agents may not be cleared as efficiently as under normal circumstances [68].
Strengths of this study include its large sample size, representativeness of the population, completeness of follow-up and information on matched non-users, and detailed information on confounders, such as smoking status was available [35,48]. Further, data are prospectively collected in the CPRD, and thus not subjected to recall bias.
Some drawbacks of our study should be considered.
First, the information about statin exposure was based on prescription data rather than on actual drug use, which could have resulted in an overestimation of statin use.
Second, although we have used a relatively specific diagnosis of RA, we had limited information on rheumatoid factor and anti-cyclic citrullinated peptide antibodies [38]. In this study, we have used the diagnostic algorithm as postulated by Thomas et al [44]. The proposed diagnostic algorithm resulted in a diagnostic specificity of 96% and sensitivity of 78% [44]. By applying this diagnostic algorithm, we believe we have used a relatively accurate diagnosis of RA. Furthermore, we performed three sensitivity analyses regarding the definition of RA. All of them consistently showed similar results.
Third, no data on dietary intake, physical activity, and limited data were available on other examinations such as lipid, blood pressure and glucose levels, and inflammatory markers (e.g. C-reactive protein), which may be important confounders. Especially, in the subgroup analyses based on the cardiovascular risk factors, lack of clinical data may have affected our results. It is likely that we have included patients with high lipid, glucose or high blood pressure levels in the group of patients without a medical history of hyperlipidaemia, hypertension or diabetes.
Fourth, our study cannot be considered as a definitive study. A randomised trial evaluating the effects of statins on the development of RA would be ideal, but that this is unlikely to occur due to infrequency of RA and thus need for a very large study.
Fifth, the increased risk of RA in the first year after the initiation of statin therapy may be explained by ascertainment bias, as some patients initiating statin therapy may experience myalgia or other muscle-related adverse effects [69]; they may tend to visit their GP more often, be more likely to be referred to a rheumatologist and may have been more carefully examined (blood tests) [28,70], therefore; these patients may be more likely to be diagnosed with RA than non-users. In the sensitivity analysis where we excluded the first year following the initiation of statin treatment, we found no increased risk of RA in the first year of statin use after the excluded year; nor did we observe an increased risk of RA in recent and past statin users. Therefore, we believe that ascertainment bias may have influenced our findings, and therefore; it is more than likely that the association between statin use and the increased risk of developing RA in the first year after initiating statin treatment, is not causal.
Conclusions
To our knowledge, this is the first study evaluating risks of RA in statin users over time. In patients who use statins, the risk of RA is substantially increased in the first year after initiation of statins and then diminishes to baseline, suggesting an association between statin use and an increased risk of RA in the first year after initiating statin treatment. Our finding may suggest that statins can accelerate disease onset in patients susceptible to develop RA, but in other patients, statins are probably safe and well tolerated, even after prolonged use. Another explanation for this increased risk of RA shortly after starting statins is ascertainment bias with increased diagnostic monitoring around the time of initiation of statin therapy. Although more research is needed, this study supports our previous finding showing an increased risk of developing RA shortly after starting statin treatment.
Supporting information
Index date: the date of the first prescription
Sensitivity analysis 1: exclude the first year of every patient following the initiation of statin treatment (index date), thereby excluding the events of RA
Sensitivity analysis 2: change the date of the first diagnosis of RA to exactly one year before this date.
(TIFF)
(DOCX)
Legend:
RA, rheumatoid arthritis; IR, incidence rate (per 10,000 person-years); HR, hazard ratio; CI, confidence interval
aIncidence rate is calculated for each sensitivity analysis by dividing the number of events by the person time within each given recency of use.
bAdjusted for age, sex, smoking, cardiovascular diseases, hyperlipidaemia, hypertension, diabetes and the use of NSAIDs.
(DOCX)
Acknowledgments
The authors thank MSc A.M. Gallagher for providing the data for the present study and Dr S.L. Thomas for sharing the Read codes for RA.
Data Availability
We share our list of Read codes for defining rheumatoid arthritis, but we have no permission from CPRD to share our data. Researchers can apply for access to data subject to approval of the scientific protocol and adherence to licensing conditions [www.cprd.com]. Data are owned by CPRD and Medicines and Healthcare products Regulatory Agency (MHRA) (third parties). There is a standard process for accessing data [www.cprd.com]. Researchers need to get scientific approval of the protocol by the independent scientific advisory committee (ISAC) of CPRD, sign a licence agreement/contract for data use and pay fees for data. The authors did not have special privileges.
Funding Statement
This work was supported by the National Institute for Public Health and the Environment [research grant S340040]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267–78. doi: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 2.Okamoto H, Koizumi K, Kamitsuji S, Inoue E, Hara M, Tomatsu T, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. J Rheumatol 2007; 34(5):964–8. [PubMed] [Google Scholar]
- 3.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004;363(9426):2015–21. doi: 10.1016/S0140-6736(04)16449-0 [DOI] [PubMed] [Google Scholar]
- 4.Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 2004;63(12):1571–5. doi: 10.1136/ard.2003.018333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373(9670):1175–82. doi: 10.1016/S0140-6736(09)60447-5 [DOI] [PubMed] [Google Scholar]
- 6.Crouse JR 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007; 297(12): 1344–53. doi: 10.1001/jama.297.12.1344 [DOI] [PubMed] [Google Scholar]
- 7.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333(10):621–7. doi: 10.1056/NEJM199509073331003 [DOI] [PubMed] [Google Scholar]
- 8.Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Steinbeck G, et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation 1997;96(5):1398–402. [DOI] [PubMed] [Google Scholar]
- 9.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med 2005;15(6):202–6. doi: 10.1016/j.tcm.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med 2000;6(12):1399–402. doi: 10.1038/82219 [DOI] [PubMed] [Google Scholar]
- 11.Shimada K, Miyauchi K, Daida H. Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench. Circulation 2004;109(18):e213–4. doi: 10.1161/01.CIR.0000127616.70152.5D [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Yokota K, Kohno C, Sawada T, Sato K, Yamaguchi M, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol 2007;17(5):364–8. doi: 10.1007/s10165-007-0589-4 [DOI] [PubMed] [Google Scholar]
- 13.Kagami S, Owada T, Kanari H, Saito Y, Suto A, Ikeda K, et al. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol 2009;21(6):679–89. doi: 10.1093/intimm/dxp037 [DOI] [PubMed] [Google Scholar]
- 14.Mulhaupt F, Matter CM, Kwak BR, Pelli G, Veillard NR, Burger F, et al. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res 2003;59(3):755–66. [DOI] [PubMed] [Google Scholar]
- 15.Weitz Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 2001;7(6):687–92. doi: 10.1038/89058 [DOI] [PubMed] [Google Scholar]
- 16.Noël B. Lupus erythematosus and other autoimmune diseases related to statin therapy: a systematic review. J Eur Acad Dermatol Venereol 2007;21(1):17–24. doi: 10.1111/j.1468-3083.2006.01838.x [DOI] [PubMed] [Google Scholar]
- 17.de Jong HJ, Tervaert JW, Saldi SR, Vandebriel RJ, Souverein PC, Meyboom RH, et al. Association between statin use and lupus-like syndrome using spontaneous reports. Semin Arthritis Rheum 2011;41(3):373–81. doi: 10.1016/j.semarthrit.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 18.de Jong HJ, Saldi SR, Klungel OH, Vandebriel RJ, Souverein PC, Meyboom RH, et al. Statin-Associated Polymyalgia Rheumatica. An Analysis Using WHO Global Individual Case Safety Database: A Case/Non-Case Approach. PLoS One 2012;7(7):e41289 doi: 10.1371/journal.pone.0041289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong HJ, Klungel OH, van Dijk L, Vandebriel RJ, Leufkens HG, van der Laan JW, et al. Use of statins is associated with an increased risk of rheumatoid arthritis. Ann Rheum Dis 2012;71(5):648–54. doi: 10.1136/ard.2011.155622 [DOI] [PubMed] [Google Scholar]
- 20.Vandebriel RJ, de Jong HJ, Gremmer ER, Klungel OH, Tervaert JW, Slob W, et al. Statins accelerate the onset of collagen type II-induced arthritis in mice. Arthritis Res Ther 2012;14(2):R90 doi: 10.1186/ar3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong HJ, Meyboom RH, Helle MJ, Klungel OH, Niskanen L, Cohen Tervaert JW. Giant cell arteritis and polymyalgia rheumatica after reexposure to a statin: a case report. Ann Intern Med 2014;161(8):614–5. doi: 10.7326/L14-5020-6 [DOI] [PubMed] [Google Scholar]
- 22.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420(6911):78–84. doi: 10.1038/nature01158 [DOI] [PubMed] [Google Scholar]
- 23.Knapp AC, Huang J, Starling G, Kiener PA. Inhibitors of HMG-CoA reductase sensitize human smooth muscle cells to Fas-ligand and cytokine-induced cell death. Atherosclerosis 2000;152(1):217–27. [DOI] [PubMed] [Google Scholar]
- 24.Noël B. Statins and lupus erythematosus. Rheumatology 2004;43(3):397–8. doi: 10.1093/rheumatology/keh035 [DOI] [PubMed] [Google Scholar]
- 25.de Jong HJ, van Staa TP, Lalmohamed A, de Vries F, Vandebriel R, Van Loveren H, Klungel OH, Cohen Tervaert JW. Pattern of risks of systemic lupus erythematosus among statin users: a population-based cohort study. Ann Rheum Dis 2017. July 6. Pii: annrheumdis-2016-210936 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Jick SS, Choi H, Li L, McInnes IB, Sattar N. Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann Rheum Dis 2009;68(4):546–51. doi: 10.1136/ard.2008.091967 [DOI] [PubMed] [Google Scholar]
- 27.Chodick G, Amital H, Shalem Y, Kokia E, Heymann AD, Porath A, et al. Persistence with statins and onset of rheumatoid arthritis: a population-based cohort study. PLoS Med 2010;7(9):e1000336 doi: 10.1371/journal.pmed.1000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197 doi: 10.1136/bmj.c2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009;67(1):99–109. doi: 10.1111/j.1365-2125.2008.03308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt T, Battafarano DF, Mortensen EM, Frei CR, Mansi I. Frequency of development of connective tissue disease in statin-users versus nonusers. Am J Cardiol 2013;112(6):883–8. doi: 10.1016/j.amjcard.2013.04.059 [DOI] [PubMed] [Google Scholar]
- 31.Tascilar K, Dell’Aniello S, Hudson M, Suissa S. Statins and risk of rheumatoid arthritis–a nested case-control study. Arthritis Rheumatol 2016;68(11):2603–11. doi: 10.1002/art.39774 [DOI] [PubMed] [Google Scholar]
- 32.van Halm VP, Nielen MM, Nurmohamed MT, van Schaardenburg D, Reesink HW, Voskuyl AE, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis 2007;66(2):184–8. doi: 10.1136/ard.2006.051672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurmohamed MT. Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev 2009;8(8):663–7. doi: 10.1016/j.autrev.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med 2008;121(10 suppl 1):S21–31. doi: 10.1016/j.amjmed.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 35.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol 1999;26(1):47–54. [PubMed] [Google Scholar]
- 36.Vandenbroucke JP. Oral contraceptives and rheumatoid arthritis. Lancet 1983;2(8343):228–9. [DOI] [PubMed] [Google Scholar]
- 37.Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum 2009;38(5):372–81. doi: 10.1016/j.semarthrit.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 38.Nielen MM, Van Schaardenburg D, Reesink HW, Twisk JW, van den Stadt RJ, van der Horst-Bruinsma IE et al. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis 2006;65(4):535–7. doi: 10.1136/ard.2005.040659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol 1998;45(5):419–25. doi: 10.1046/j.1365-2125.1998.00701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walley T, Mantgani A. The UK General Practice Research Database. Lancet 1997;350(9084):1097–9. doi: 10.1016/S0140-6736(97)04248-7 [DOI] [PubMed] [Google Scholar]
- 41.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 1991;302(6779):766–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf 2000;9(5):359–66. doi: 10.1002/1099-1557(200009/10)9:5<359::AID-PDS507>3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 43.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288(4):455–61. [DOI] [PubMed] [Google Scholar]
- 44.Thomas SL, Edwards CJ, Smeeth L, Cooper C, Hall AJ. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis Rheum 2008;59(9):1314–21. doi: 10.1002/art.24015 [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther 2011;13(1):R32 doi: 10.1186/ar3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther 2006;8(4):R133 doi: 10.1186/ar2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsen N. Drug-induced autoimmunity. Best Pract Res Clin Rheumatol 2004;18(5):677–88. doi: 10.1016/j.berh.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 48.Hazes JM, Dijkmans BA, Vandenbroucke JP, De Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis 1990;49(12):980–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Innala L, Sjöberg C, Möller B, Ljung L, Smedby T, Södergren A, et al. Co-morbidity in patients with early rheumatoid arthritis–inflammation matters. Arthritis Res Ther 2016;18:33 doi: 10.1186/s13075-016-0928-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16(3):219–42. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 51.Ramlau-Hansen H. Smoothing counting process intensities by means of kernel functional. Ann Stastist 1983;11(2):453–66. [Google Scholar]
- 52.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 1995;6(4):356–65. [DOI] [PubMed] [Google Scholar]
- 53.National Collaborating Centre for Primary Care. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease NICE clinical guideline 67. London, UK: National Institute for Health and Clinical Excellence, 2008. (reissued 2010). [PubMed] [Google Scholar]
- 54.Bhardwaj S, Selvarajah S, Schneider EB. Muscular effects of statins in the elderly female: a review. Clin Interv Aging 2013;8:47–59. doi: 10.2147/CIA.S29686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291(18):2243–52. doi: 10.1001/jama.291.18.2243 [DOI] [PubMed] [Google Scholar]
- 56.Lalmohamed A, van Staa TP, Vestergaard P, Leufkens HGM, de Boer A, Emans P, et al. Statins and risk of lower limb revision surgery: the influence of differences in study design using electronic health records from the United Kingdom and Denmark. Am J Epidemiol 2016;184(1):58–66. doi: 10.1093/aje/kwv311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006;36(3):182–8. doi: 10.1016/j.semarthrit.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 58.Humphreys JH, Verstappen SM, Hyrich KL, Chipping JR, Marshall T, Symmons DP. The incidence of rheumatoid arthritis in the UK: comparisons using the 2010 ACR/EULAR classification criteria and the 1987 ACR classification criteria. Results from the Norfolk Arthritis Register. Ann Rheum Dis 2013;72(8):1315–20. doi: 10.1136/annrheumdis-2012-201960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthews A, Herrett E, Gasparrini A, van Staa T, Goldacre B, Smeeth L, et al. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016;353:i3283 doi: 10.1136/bmj.i3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan KW, Felson DT, Yood RA, Walker AM. The lag time between onset of symptoms and diagnosis of rheumatoid arthritis. Arthritis Rheum 1994;37(6):814–20. [DOI] [PubMed] [Google Scholar]
- 61.Alipour A, van Oostrom AJ, Izraeljan A, Verseyden C, Collins JM, Frayn KN, et al. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 2008;28(4):792–7. doi: 10.1161/ATVBAHA.107.159749 [DOI] [PubMed] [Google Scholar]
- 62.van Oostrom AJ, Rabelink TJ, Verseyden C, Sijmonsma TP, Plokker HW, De Jaegere PP, et al. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis 2004;177(1):175–82. doi: 10.1016/j.atherosclerosis.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 63.Meijssen S, Van Dijk H, Verseyden C, Erkelens DW, Cabezas MC. Delayed and exaggerated postprandial complement component 3 response in familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol 2002;22(5):811–6. [DOI] [PubMed] [Google Scholar]
- 64.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol 2009;5(10):560–5. doi: 10.1038/nrrheum.2009.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang TT, Song Y, Ding YJ, Liao YH, Yu X, Du R, et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res 2011;52(5):1023–32. doi: 10.1194/jlr.M010876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009;10(9):1000–7. doi: 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komatsu N, Mariotti-Fernandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A 2009;106(6):1903–8. doi: 10.1073/pnas.0811556106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity—friends or foes? Trend Immunol 2009;30(8):409–14. [DOI] [PubMed] [Google Scholar]
- 69.Sathasivam S, Lecky B. Statin induced myopathy. BMJ 2008;337:a2286 doi: 10.1136/bmj.a2286 [DOI] [PubMed] [Google Scholar]
- 70.Mansi I, Mortensen E. The controversy of a wider statin utilization: why? Expert Opin Drug Saf 2013;12(3):327–37. doi: 10.1517/14740338.2013.779667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Index date: the date of the first prescription
Sensitivity analysis 1: exclude the first year of every patient following the initiation of statin treatment (index date), thereby excluding the events of RA
Sensitivity analysis 2: change the date of the first diagnosis of RA to exactly one year before this date.
(TIFF)
(DOCX)
Legend:
RA, rheumatoid arthritis; IR, incidence rate (per 10,000 person-years); HR, hazard ratio; CI, confidence interval
aIncidence rate is calculated for each sensitivity analysis by dividing the number of events by the person time within each given recency of use.
bAdjusted for age, sex, smoking, cardiovascular diseases, hyperlipidaemia, hypertension, diabetes and the use of NSAIDs.
(DOCX)
Data Availability Statement
We share our list of Read codes for defining rheumatoid arthritis, but we have no permission from CPRD to share our data. Researchers can apply for access to data subject to approval of the scientific protocol and adherence to licensing conditions [www.cprd.com]. Data are owned by CPRD and Medicines and Healthcare products Regulatory Agency (MHRA) (third parties). There is a standard process for accessing data [www.cprd.com]. Researchers need to get scientific approval of the protocol by the independent scientific advisory committee (ISAC) of CPRD, sign a licence agreement/contract for data use and pay fees for data. The authors did not have special privileges.