Abstract
Su(Hw) belongs to the class of proteins that organize chromosome architecture and boundaries/insulators between regulatory domains. This protein contains a cluster of 12 zinc finger domains most of which are responsible for binding to three different modules in the consensus site. Su(Hw) forms a complex with CP190 and Mod(mdg4)-67.2 proteins that binds to well-known Drosophila insulators. To understand how Su(Hw) performs its activities and binds to specific sites in chromatin, we have examined the previously described su(Hw)f mutation that disrupts the 10th zinc finger (ZF10) responsible for Su(Hw) binding to the upstream module. The results have shown that Su(Hw)f loses the ability to interact with CP190 in the absence of DNA. In contrast, complete deletion of ZF10 does not prevent the interaction between Su(Hw)Δ10 and CP190. Having studied insulator complex formation in different mutant backgrounds, we conclude that both association with CP190 and Mod(mdg4)-67.2 partners and proper organization of DNA binding site are essential for the efficient recruitment of the Su(Hw) complex to chromatin insulators.
Introduction
Eukaryotic gene expression is regulated by a complex interplay of different regulatory elements. Recently, it has been found that chromosomes in the genomes of higher eukaryotes are organized into a series of discrete topologically associating domains (TADs). [1–5]. In addition, specific interactions between enhancers and promoters, between several promoters, or more complex interactions between multiple enhancers and promoters have been described [6–9]. Mechanisms involved in the formation of chromosome architecture are not yet completely elucidated. However, a model has arisen that a special class of architectural proteins is involved in the organization of specific long-distance interactions between different types of regulatory elements [10–12].
Transcription factors involved in the activity of insulators have been attributed to the category of architectural proteins. Insulators, or chromatin boundaries, are genomic regulatory elements (nucleoprotein complexes) that can block the action of an enhancer on a promoter when interposed between them [12–16]. It was also shown in transgenic lines, that the majority of insulators can support specific distant interactions between enhancers or silencers and promoters [17–22]. Some insulators are able to interpose a barrier between the transcriptionally active chromatin and heterochromatin [21,23–26].
The best studied DNA binding architectural/insulator proteins belong to the large and poorly characterized class of transcription factors with clusters of zinc fingers (ZFs) of C2H2 type [10,27]. The CTCF protein, evolutionary conserved among bilateral metazoans, contains a cluster of 11 ZFs, while the Su(Hw) protein specific to insects contains 12 ZFs also organized into one cluster [28,29]. The C2H2 ZF clusters recognize long DNA binding motif in which each ZF recognizes three nucleotides [30,31]. As follows from limited experimental data, only part of C2H2 domains are usually involved in DNA binding, with the rest being involved in interactions with proteins or RNAs [32,33]. Most of genomic Su(Hw) binding regions have one or two consensus site(s) [34,35], while studies on transgenic lines have shown that only four reiterated binding sites for Su(Hw) can function affectively [36].
The Su(Hw) protein directly interacts with three proteins characterized in detail previously: Mod(mdg4)-67.2, CP190, and E(y)2/Sus1 [37–43]. In addition, several new interactions of the Su(Hw) insulators have recently been described [44–46].
Mod(mdg4)-67.2 and CP190 are BTB/POZ domain proteins that are recruited to chromatin by the Su(Hw) protein [37,40,43]. Mod(mdg4)-67.2 is one of protein isoforms produced by the mod(mdg4) gene, also known as Evar3-93D, which encodes a large set of proteins containing the BTB domain at the N-terminus and different C-terminal domains [37,40,41]. As shown previously, Mod(mdg4)-67.2 specifically interacts with Su(Hw) through an isoform-specific C-terminal acidic domain. As most of the described BTB domains in higher eukaryotes, the BTB/POZ domain of CP190 forms stable homodimers, while the BTB domain of Mod(mdg4)-67.2 belongs to a specific class of BTB domains that form multimeric complexes [47–51]. Su(Hw) and many other insulator proteins are involved in recruiting of CP190 to the chromatin [43,51–55].
The genome-wide studies have shown that binding sites for the Su(Hw), Mod(mdg4)-67.2, and CP190 proteins could be grouped into two main classes which are characterized by the binding of either Su(Hw) alone (SBS-O) or of all the three proteins (SBS-CM) [35,56,57]. In the interphase nucleus the Su(Hw), CP190, and Mod(mdg4)-67.2 proteins colocalize in speckles, named insulator bodies [43,58–62]. It was speculated [60] that protein complexes are formed in the insulator bodies before binding to the chromatin.
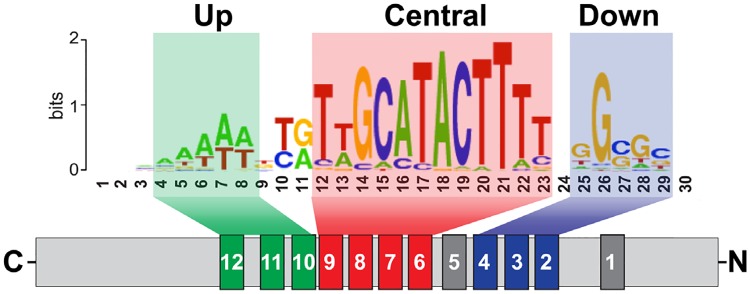
The recent data on the ‘Su(Hw) code’ [56,57] show that Su(Hw) binds to a compound consensus sequence (approximately 26 nucleotides) comprised of three modules. The ZF6–ZF9 cluster binds to the main, central module; the ZF2–ZF4 cluster, to the downstream CG-rich module; and the ZF10–ZF12 cluster, to the upstream AT-rich module (Fig 1). It was shown that the Su(Hw)f protein failed to bind to the gypsy insulator consisting from 12 binding sites organized mainly by up and central modules [56,57]. The su(Hw)f mutation involves the point substitution of the first cysteine in ZF10 with tyrosine [63], which affects the critical Zn-coordinating residue and thereby radically alters the structure of the C2H2 domain. As a result, the Su(Hw)f has inactive the ZF10-ZF12 cluster that is essential for binding to the up AT-rich module in the site. Thus, the Su(Hw)f protein can’t effectively bind to the gypsy insulator by using only interaction between the ZF6-ZF9 cluster and the central module in the 12 reiterated sites.
Fig 1. Scheme of Su(Hw) binding with a full consensus binding site, showing which ZFs are involved in recognition of specific cores (from Baxley et al. 2017) [57].
Here we have found that Su(Hw)f loses the ability to interact with CP190 in the absence of DNA. However, deletion of ZF10 (Su(Hw)Δ10) restores the CP190–Su(Hw) interaction, suggesting that the point mutation in ZF10 affect conformation of Su(Hw)f leading to loss of interaction with CP190. The Su(Hw)Δ10 mutant binds to the gypsy insulator better than does Su(Hw)f and partially restores its enhancer-blocking activity but, in contrast to the wild-type Su(Hw) protein, fails to interact with the gypsy insulator in the mod(mdg4)u1 mutant background. These results suggest that CP190 and Mod(mdg4)-67.2 are critical for binding of the mutant Su(Hw)Δ10 (lacking ZF10) to the gypsy insulator consisting mainly of the upstream and central modules. Like the wild-type Su(Hw), the mutant Su(Hw)f protein can efficiently bind to four reiterated binding sites that each consist of three modules. Thus, stable binding of Su(Hw) depends on association with CP190 and Mod(mdg4)-67.2 partners and proper organization of DNA binding site.
Materials and methods
The constructs for yeast two-hybrid assay, transgenic constructs, and details of experimental and analytical procedures are described in the Supplementary Data (S1 Appendix).
Germ-line transformation, genetic crosses, and phenotypic analysis
All flies were maintained at 25°C on the standard yeast medium. To obtained transgenic lines carrying a random P transposon insertion, the construct together with P25.7wc—a P element with defective inverted repeats used as a transposase source [64]–were injected into y ac w1118 preblastoderm embryos. The resulting flies were crossed with y ac w1118 flies, and the transgenic progeny were identified by their eye color. Chromosome localization of various transgene insertions was determined by crossing the transformants with the y ac w1118 balancer stock containing dominant markers, In(2RL),CyO for chromosome 2 and In(3LR)TM3,Sb for chromosome 3. The transformed lines were examined by Southern blot hybridization to check for transposon integrity and copy number.
The lines with excisions of Su(Hw) binding sites were obtained by crossing flies carrying the transposons with the Cre recombinase-expressing line y1, w1118; CyO, P[w+,cre]/Sco; +. All excisions were confirmed by PCR analysis.
To test the effects of Su(Hw) protein on yellow gene expression, lines containing the yellow transposons were crossed into su(Hw)v/su(Hw)f or su(Hw)v/su(Hw)e04061 mutant background as described previously [38]. Su(Hw)v is a deletion of the su(Hw) gene; su(Hw)f encodes a protein with a point substitution in ZF10 that retains some ability to bind to DNA; and su(Hw)e04061 is a mutation generated by inserting the PiggyBac element near the start codon [65].
The yellow (y) phenotype was determined from the level of pigmentation of the abdominal cuticle and wings in 3- to 5-day-old males developing at 25°C. The level of pigmentation (i.e., of y expression) was estimated on an arbitrary five-grade scale, with the wild-type expression and the absence of expression assigned scores 5 and 1, respectively. The pigmentation of bristles on the thorax and head was also assessed in 3- to 5-day-old males developing at 25°C. Its variegation was scored on a five-grade scale, with score 1 denoting the loss of pigmentation in all bristles on the thorax and head, score 5 denoting the wild-type pigmentation of all bristles, and intermediate scores being as follows: e-v, extreme variegation (only 1–3 bristles on the thorax and head are pigmented); m-v, moderate variegation (about half of bristles are yellow); and w-v, weak variegation (only 1–3 bristles on thorax and head are yellow). No less than 50 flies from each y line were independently scored by two observers.
The white (w) phenotype was determined from eye pigmentation in adult flies. Wild-type white expression determined the bright red eye color (R); in the absence of white expression, the eyes were white (W). Intermediate levels of white expression (in increasing order) were reflected in the eye color ranging from yellow (Y), through orange (Or), to brown (Br).
To obtain transgenic flies with insertion in 38D, the DNA of reporter constructs was injected into preblastoderm embryos of y1 M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP'}ZH-38D genotype [66]. The resulting flies were crossed with y2scD1w1118 ct6 flies, and the progeny carrying the transgene in the 38D region were identified by their eye color. The generation of transgenic lines and construct introduction into the mod(mdg4)u1 or Su(Hw)v/ Su(Hw)e04061 background were performed as described [38].
Cells lysate preparation and immunoprecipitation experiments
The S2 cells culture was obtained from the stock center of Vavilov Institute of General Genetics, Russian Academy of Sciences (http://vigg.ru/index.php?id=337) [67,68]. The S2 cells cultured as described [69] were transformed using the Effectene Transfection Reagent (Qiagen) as recommended by the manufacturer. To prepare cell lysate, approximately 107 cells were scraped from the plate and washed with two portions of PBS at 4°C, with pelleting at 1000 g for 5 min after each wash. The washed pellet was resuspended in 300 μL of lysis buffer (10 mM HEPES, pH 7.9; 450 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM DTT, Protease Inhibitor Cocktail (Roche) and 1U/mL DNase I); incubated on ice for 45 min, with pipetting up and down three to four times to disrupt cell clumps; centrifuged at 15 000 g, 4°C, for 15 min; and the supernatant was transferred to a new tube. An aliquot of the supernatant (10% of total volume) was stored to be used as input control #1, and the rest was diluted with four volumes of dilution buffer (10 mM HEPES, pH 7.9; 5 mM MgCl2, 1 mM DTT, Protease Inhibitor Cocktail). The diluted lysate was again centrifuged at 15 000 g, 4°C, for 15 min, and the supernatant was transferred to a new tube, with an aliquot (10% of total volume) of it being stored as input control #2. The lysate was then supplemented with 40 μL of FLAG Sepharose beads (Sigma) and incubated overnight at 4°C on a rotary shaker. The beads were gently pelleted by centrifugation (700–1000 rpm at 4°C, ~1 min), an aliquot of the supernatant was stored as output control, and the beads were washed with three portions of IP150 buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl, 10mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.3 mM DTT, 0.1% NP-40, 10% glycerol), 10 min each, with pelleting between washes. The resulting immunoprecipitate was boiled with 1× Laemmli buffer (25 μL per sample) for 10 min, resolved by SDS-PAGE (20 μL per lane), and immunoblotted with appropriate antibodies (see below).
Yeast two-hybrid assay
Experiments were performed using plasmids and protocols from Clontech. For growth assays, plasmids were transformed into yeast pJ69-4A cells, which were plated onto media without tryptophan and leucine and grown at 30°C for 3 days. Thereafter, the cells were plated onto selective media without tryptophan, leucine, histidine, and adenine (high stringency conditions) or without tryptophan, leucine, histidine and 5 mM 3-aminotriazole (medium stringency conditions), and their growth was compared after 2–3 days.
Chromatin preparation and ChIP analysis in pupae
The material (about 150–200 mg of adult male flies, sufficient for four to five independent immunoprecipitations) was homogenized in 5 ml of buffer IP-S+ (10 mM Tris-HCl, pH 7.5; 10 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 250 mM sucrose with PMSF, leupeptin, and pepstatin A) at 4°C using a Dounce homogenizer with type A pestle. The homogenate was filtered through a BD Falcon filter into a 50-mL tube and pelleted by centrifugation at 4000 g, 4°C for 5 min. The supernatant was discarded, and the pellet was washed with three 3-mL portions of buffer IP-S+, with pelleting at 4000 g for 5 min after each wash. The final pellet was resuspended in 0.5 mL of IP-10+ buffer (10 mM Tris-HCl, pH 7.5; 10 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1mM DTT, 0,1% NP-40, 10% glycerol, and Roche Complete Protease Inhibitor Cocktail) and homogenized at 4°C using a Dounce homogenizer with type B pestle. The homogenate was supplemented with an equal volume of IP-850 buffer (10 mM Tris-HCl, pH 7.5; 850 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0,1% NP-40, 10% glycerol, and Roche Complete Protease Inhibitor Cocktail), mixed gently, and left on ice for 30–60 min. This was followed by 4 rounds of centrifugation at 14 000 g for 15 min, with the supernatant being each time transferred to a new tube without disturbing the pellet. Immediately before experiments, the lysate was diluted with three volumes of IP-0 buffer (10 mM Tris-HCl, pH 7.5; 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1% NP-40, 10% glycerol, and Roche Complete Protease Inhibitor Cocktail), centrifuged as above, and transferred to a new tube. To reduce nonspecific background, supernatant was pre-cleared by incubation with protein A or protein G agarose beads at 4°C for 30 min, with constant stirring. Agarose was pelleted by brief centrifugation, and the supernatant was collected for chromatin immunoprecipitation with appropriate antibodies (see below). After overnight incubation at 4°C on a rotary shaker, protein A or protein G agarose beads were added to collect the precipitated complexes, and incubation was continued for 2 h under the same conditions. Agarose was pelleted by centrifugation (700–1000 rpm at 4°C, ~1 min), the supernatant was carefully removed, and the pellet was washed with the following buffers (1 mL each, for 3–5 min on a rotary shaker): Low Salt Wash Buffer (20 mM Tris-HCl, pH 8.0, with 0.1% SDS, 1% Triton X-100, 2 mM EDTA, and 150 mM NaCl), High Salt Wash Buffer (20 mM Tris-HCl, pH 8.0, with 0.1% SDS, 1% Triton X-100, 2 mM EDTA, and 500 mM NaCl), LiCl Wash Buffer (10 mM Tris-HCl, pH 8.0, with 0.25 M LiCl, 1% NP40, 1% deoxycholate, and 1 mM EDTA,), and two portions of TE Buffer. The complex was removed from the agarose by elution with two 250-μL portions of elution buffer (0.1 M NaHCO3 with 1% SDS), 15 min each, at room temperature with rotation. The eluates were pooled, supplemented with 20 μL of 5 M NaCl, and heated at 65°C for 4 h to reverse the complex–DNA crosslinks. Then 20 μL of 1 M Tris-HCl (pH 6.5), 10 μL of 0.5 M EDTA, and 2 μL of Proteinase K solution (10 mg/mL) were added, and the mixture was incubated for 1 h at 45°C. DNA was recovered by phenol/chloroform extraction and ethanol precipitation and solubilized in water for PCR. PCR products were amplified from at least two separate immunoprecipitates from three different chromatin preparations. Primer sequences used in PCR for ChIP analysis are shown in the S1 Table.
Antibodies
Specific antibodies and working dilutions were as follows: monoclonal mouse anti-FLAG (1:300) from Sigma (# F 1804), and polyclonal rat anti-CP190 (1:500), polyclonal rabbit anti-Mod(mdg4)-67.2 (1:500), rabbit anti-Su(Hw) N-terminal domain (1:200), rabbit anti-Chromator (1;300), and rat anti-Skeletor (1:200) raised in our lab and described previously [59,60].
Results
The Su(Hw)f protein interacts with Mod(mdg4)-67.2 but not with CP190 in vitro
The Su(Hw)f protein in the ovaries fails to bind to 60% of genomic Su(Hw) sites but can still bind to a consensus site in vitro, although with a weaker efficiency than the wild-type protein [56]. Since Mod(mdg4)-67.2 and CP190 may be involved in Su(Hw) recruitment to chromatin [35,41,60], we tested the possibility that Su(Hw)f loses the ability to interact with any of these proteins.
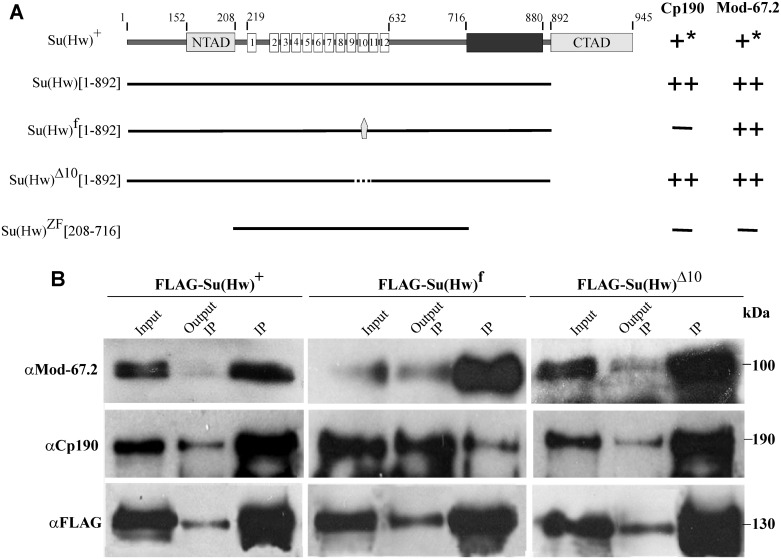
The Su(Hw) protein (Fig 2A) contains an N-terminal acidic domain (152 aa– 208 aa), 12 ZF domains (219 aa– 632 aa), and a C-terminal acidic domain (892 aa– 945 aa) [37,63]. It has been shown that Mod(mdg4)-67.2 interacts with the N-terminal domain [70] and the C-terminal domain, including the aa 716 to 880 region [40] (Fig 2A). The BTB domain of CP190 interacts with two adjacent regions in the N-terminal part of Su(Hw) [71].
Fig 2. Role of ZF10 in Su(Hw) for interaction between the insulator proteins.
A structural schematic of Su(Hw). The borders between the Su(Hw) domains (NTAD, N-terminal acidic domain; CTAD, C-terminal acidic domain; ZF, zinc-finger domain) are indicated by numbers. Gray pentagon indicates H-to-Y substitution in the Su(Hw)f mutant; dotted line, deletion of ZF10 in the Su(Hw)Δ10 mutant. The number of plus signs indicates the relative strength of interaction in the Y2H assay (S1 Fig); the minus sign denoted an absence of interactions; an asterisk, a reduction in the Y2H signal due to the repressive effect of Su(Hw) C-terminal domain on the transcription in yeast [72]. All the results were reproduced in three independent experiments. Numbers in brackets refer to the amino acid residues that flank protein regions included in the analysis. (B) Co-immunoprecipitation between different Su(Hw) variants fused to the FLAG epitope and the insulator proteins under normal conditions. The FLAG-Su(Hw)+, FLAG-Su(Hw)f, and FLAG-Su(Hw)Δ10 were expressed in the S2 cells. The immunoprecipitated complexes were washed with buffers containing 150 mM NaCl before loading onto the SDS-PAGE for Western blot analysis. The PVDF membrane was consecutively probed with antibodies against the indicated proteins (CP190 or Mod-67.2) or FLAG epitope. Each column represents a single FLAG-immunoprecipitation experiment with the particular mutant variant. Each lane shows the result of a subsequent hybridization of each immunoprecipitate with different antibodies on the same membrane. "Input" is the input fraction (10% of the lysate used for the immunoprecipitation); "Output IP," the supernatant after the immunoprecipitation; "IP," the immunoprecipitate. The results were obtained in three independent experiments.
To test for the interaction between the insulator proteins, we used the yeast two-hybrid assay (Y2H), which is effective for studying complete proteins that are difficult to express in a bacterial in vitro system (S1 Fig). The method is based on fusing the GAL4 activation and DNA binding domains to the N- or C-end of the test protein or its part. We compared the interactions of the wild-type and Su(Hw)f mutant with CP190 and Mod(mdg4)-67.2 and found that Su(Hw)f did not interact with Cp190 (Fig 2A), but still interacted with Mod(mdg4)-67.2.
The loss of association between CP190 and Su(Hw) might be explained by a change in Su(Hw) conformation due to a defect in the structure of ZF10. To test this possibility, we used the mutant Su(Hw)Δ10 protein in which ZF10 was completely deleted and found that Su(Hw)Δ10 interacted with both CP190 and Mod(mdg4)-67.2 like the wild-type protein (Fig 2A). These results suggest that ZF10 is not directly required for the Su(Hw)–CP190 interaction, but structurally defective ZF10 affects the ability of mutant Su(Hw) to interact with CP190.
To confirm the results of Y2H assay, we tested the above interactions in co-IP experiments on S2 cells in which Su(Hw), Su(Hw)f, and Su(Hw)Δ10 tagged with a triple FLAG epitope were expressed (Fig 2B and S2 Fig). We observed co-immunoprecipitation between FLAG-Su(Hw)+ or FLAG-Su(Hw)Δ10 and endogenous Mod(mdg4)-67.2 and CP190 proteins. At the same time, FLAG-Su(Hw)f effectively co-precipitated with Mod(mdg4)-67.2 but only weakly co-precipitated with endogenous CP190. Taken together, these results show that structural alteration of ZF10 by point mutation in the Su(Hw)f protein interferes with the ability of the mutant to interact with CP190.
Normal ZF10 and interaction with CP190 and Mod(mdg4) are essential for binding of Su(Hw) to chromatin
To further compare the role of ZF10 and CP190/Mod(mdg4)-67.2 partners in the binding of Su(Hw) to chromatin sites, we produced transgenic lines expressing either wild-type FLAG-tagged protein (Su(Hw)+) or its FLAG-tagged mutant variants (Su(Hw)f and Su(Hw)Δ10) under control of the Actin5C (Act5C) promoter. As shown previously, Su(Hw)f in the ovaries and larvae binds only to the “f-retained” sites (such as 62D, 50A, 87E), but not to “f-lost” sites (such as 1A2, 66E, and gypsy) [56]. To test the affinity of Su(Hw)f mutant for the “f-lost” sites we also expressed this protein under control of the ubiquitin-63E (Ubi) promoter. A phiC31-based integration system was used to insert the transgenes at the 38D site. After that, the transgenes were introduced into the su(Hw)− background (su(Hw)v/su(Hw)e04061). The expression of the Su(Hw) transgenes was verified by the Western blot analysis with the anti-FLAG antibodies (S3 Fig) The Su(Hw) variants under control of Act5C were expressed at similar levels, while the expression of Su(Hw)f under control of the Ubi promoter was at least two to three times stronger than in that lines.
The interactions between the Su(Hw) variants and Mod(mdg4)-67.2 or CP190 were also assayed by co-IPs from the extract prepared from the 2-day old males of the corresponding transgenic lines (S4 Fig). This experiment confirmed the results obtained in S2 cells: Su(Hw)f showed only weak co-precipitation with endogenous CP190 but normal co-precipitation with Mod(mdg4)-67.2, while Su(Hw)Δ10 effectively co-precipitated with both proteins.
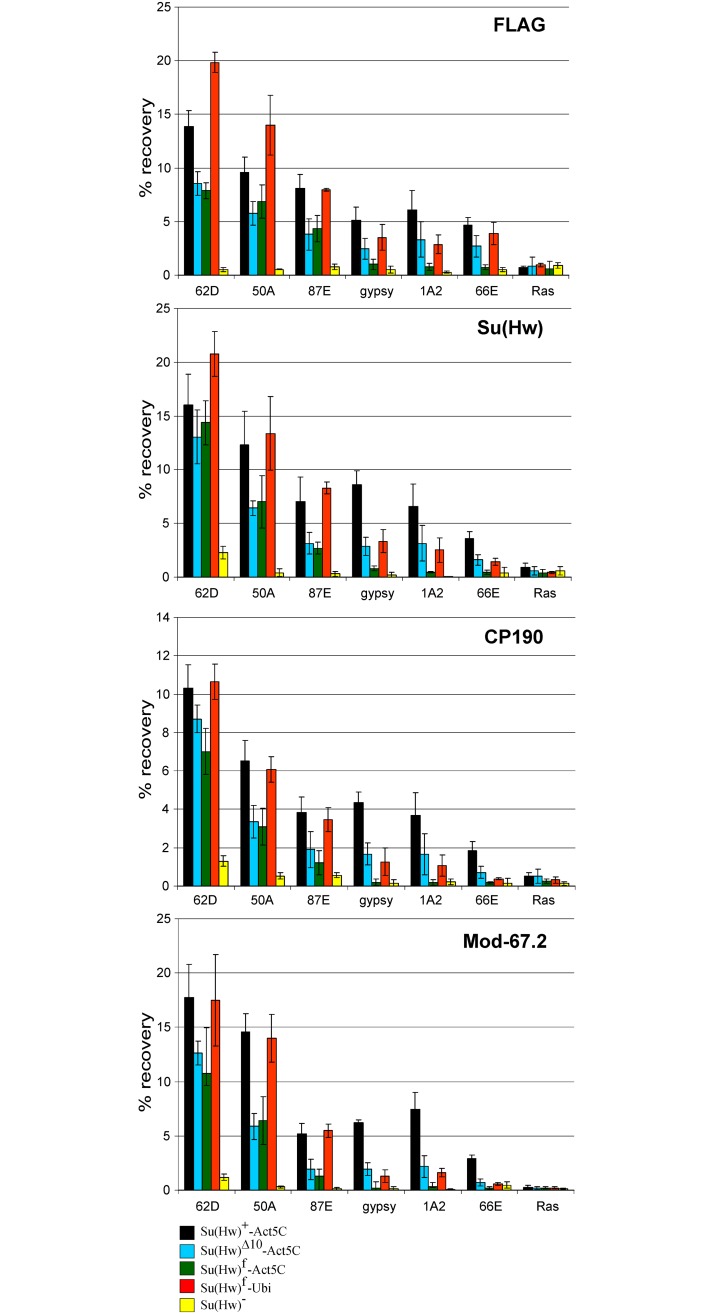
Next, we studied binding of the insulator proteins by ChIP at the previously characterized Su(Hw) sites [73] in pupae of the transgenic lines (Fig 3). As expected, we observed a positive Su(Hw) binding signal at all test sites in the Su(Hw)+ transgenic line. In the su(Hw)− pupae, we still observed residual Su(Hw) binding to insulator site 62D, which was correlated with Mod(mdg4)-67.2 binding to the same site. Residual binding of CP190 was observed at 62D, 50A and 87E sites. These results are in agreement with data on the critical role of Su(Hw) in recruiting of the Mod(mdg4)-67.2 isoform [40], while CP190 is known to be recruited to chromatin by many DNA-binding proteins [35,54].
Fig 3. Evaluation of binding of the insulator protein in the mod(mdg4)+ transgenic lines.
Variants of the Su(Hw) protein were expressed in the y2scD1ct6; P{Su(Hw)}-38D/P{Su(Hw)}-38D; su(Hw)v/su(Hw)e04061 lines, where P{Su(Hw)} are Su(Hw)+-Act5C –P{w+;WAB-Su(Hw)1-945-FLAG}/ P{w+;WAB-Su(Hw)1-945-FLAG}; Su(Hw)Δ10-Act5C –P{w+;WAB-Su(Hw)Δ10-FLAG}/ P{w+;WAB-Su(Hw)Δ10-FLAG}; Su(Hw)f-Act5C –P{w+;WAB-Su(Hw)f -FLAG}/ P{w+;WAB-Su(Hw)f -FLAG}; Su(Hw)f-Ubi–P{w+;UbqW-Su(Hw)f -FLAG}/ P{w+;UbqW-Su(Hw)f -FLAG}. The y2scD1ct6; su(Hw)v/su(Hw)e04061 line is designated as Su(Hw)−. Quantitative PCR (qPCR) was performed at the selected Su(Hw) regions. The ras64B coding region (Ras) was used as a negative control. The percent recovery of immunoprecipitated DNA (Y axis) was calculated relative to the amount of input DNA. Error bars indicate standard deviation of three independent biological replicates.
In the transgenic line expressing Su(Hw)f under control of the Act5C promoter, this protein was found to bind to the f-retained 62D, 50A, and 87E sites, similar to wild-type Su(Hw), but not to the f-lost sites 1A2, 66E, and gypsy (Fig 3). We also observed that the Mod(mdg4)-67.2 and CP190 proteins were recruited to the 62D, 50A, 87E sites but not to the 1A2, 66E and gypsy sites, suggesting that Su(Hw)f was able to recruit the CP190 and Mod(mdg4)-67.2 proteins to chromatin. Thus, Su(Hw)f failed to bind the gypsy and 1A2 sites in female gonads [56], larvae [73] and pupae (this study).
In contrast, Su(Hw)Δ10 bound not only to the 62D, 50A, 87E sites but also (at a medium level) to the 1A2, 66E, and gypsy insulators. The C190 and Mod(mdg4)-67.2 proteins were also recruited to the 1A2, 66E, and gypsy sites. These results showed that Su(Hw)Δ10, compared to Su(Hw)f, can better bind to the f-lost sites. However, when Su(Hw)f was expressed at a higher level (under control of the Ubi promoter), we observed not only better binding of Su(Hw)f to the 62D, 50A, 87E sites, but also moderate binding of the mutant protein to the 1A2, 66E and gypsy sites Thus, the increasing expression of Su(Hw)f provides for its binding to the f-lost sites (Fig 3).
These results correlated with phenotypes of the y2 allele in different Su(Hw) backgrounds (Fig 4A). In the y2 mutation (Fig 4A), gypsy is inserted between the yellow promoter and the enhancers controlling expression in the wings and body cuticle [74]. The Su(Hw) insulator blocks activity of the enhancers that results in a weak yellow expression in wing blades and body cuticle (y2 phenotype) [74,75]. In flies bearing a combination of Su(Hw)f and y2 yellow expression was completely restored, like in y2; su(Hw)− flies. In contrast, the expression of Su(Hw)Δ10 supported the enhancer-blocking activity of the gypsy insulator in the y2 allele, as in the case of Su(Hw)+. Thus, Su(Hw)Δ10 binds to the gypsy sequences and almost completely restores its enhancer-blocking activity. On the other hand, the overexpression of Su(Hw)f failed to restore even partial blocking of the yellow enhancers by the gypsy insulator in the y2 allele.
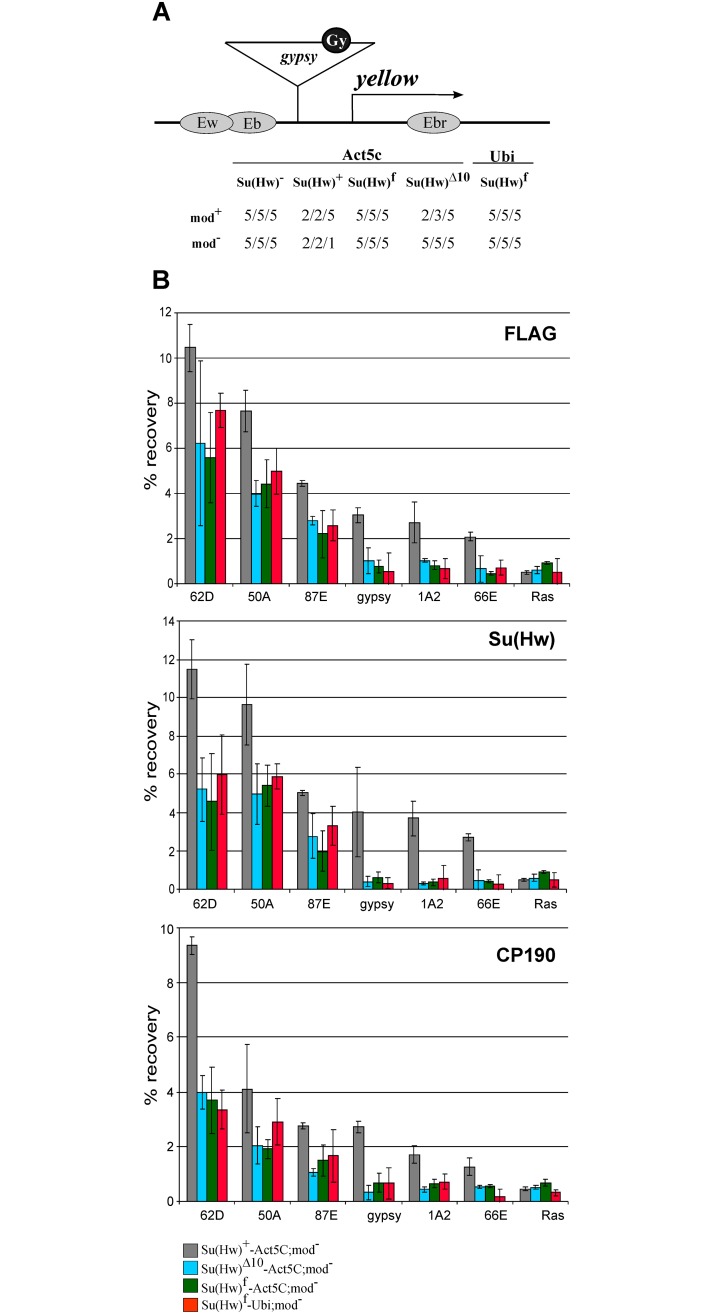
Fig 4. Effects of the su(Hw) mutations on the activity of gypsy insulator in the mod(mdg4)u1 background.
(A) Effects of the mod(mdg4)u1 mutation on yellow expression in transgenic lines. A schematic of the y2 allele (drawn not to scale): the yellow wing (Ew) and body (Eb) enhancers are shown as partially overlapping gray ovals; the bristle enhancer (Ebr), as a gray oval in the yellow intron; the transcription start site is indicated by an arrowhead. The gypsy insertion is shown as a triangle with the black circle (Gy) marking Su(Hw) binding sites. Analysis of the transgenic lines was performed in the y2scD1ct6; mod(mdg4)+/mod(mdg4)+ background (mod+) or in the y2scD1ct6; mod(mdg4)u1/mod(mdg4)u1 background (mod−). Numbers in the "mod+" and "modȢ" lines indicate a relative level of yellow expression in the body cuticle/wing blades/bristles, which ranged from 2 (pigmentation as in the y2 allele) to 5 (pigmentation as in the wild-type flies). (B) ChIP-qPCR analysis of Su(Hw), Mod-67.2, and CP190 binding at the mid-pupal stage in the transgenic lines expressing different variants of Su(Hw) in the y2scD1ct6; P{Su(Hw)}-38D/P{Su(Hw)}-38D; su(Hw)v mod(mdg4)u1/ su(Hw)e04061 mod(mdg4)u1 lines (mod− in designations of the lines). The abbreviations of transgenes P{Su(Hw)} are as in Fig 3. Quantitative PCR (qPCR) was performed on the intergenic regions bound by Su(Hw). PCR products were amplified from two separate immunoprecipitates of three different chromatin preparations. The ras64B coding region (Ras) was used as a control devoid of Su(Hw) binding sites. The recovery percentage of immunoprecipitated DNA (Y axis) was calculated relative to the amount of input DNA. Error bars indicate standard deviation of three independent biological replicates.
We also tested the effect of different Su(Hw) variants on the activity of the gypsy insulator in cut and scute loci (S5 Fig). In the ct6 allele, gypsy is located between the cut promoter and the wing margin enhancer separated by 85 kb [37]. The mutant phenotype in the ct6 allele is a consequence of complete blocking of the margin enhancer by the insulator. In scD1 allele, downstream insertion of gypsy inhibits scute expression in several specific areas of the epithelium and, in particular, blocks the formation of several types of bristles [76]. All Su(Hw) mutant variants expressed in the su(Hw)− background (su(Hw)v/su(Hw)e04061) failed to support the ability of the gypsy insulator to block cut or scute enhancer in the ct6 and scD1 alleles, showing that Su(Hw)Δ10, like Su(Hw)f, does not support the enhancer-blocking activity of the gypsy insulator in tissues where the cut or scute genes are required.
Taken together, these results indicated that the CP190–Su(Hw) interaction has an effect on binding of the Su(Hw) complex to the chromatin. The Su(Hw)–Mod(mdg4)-67.2 interaction was also suggested to have a role in this process [60]. To test the role of Mod(mdg4)-67.2 in the binding of Su(Hw)Δ10 and Su(Hw)f, the Su(Hw) variants were expressed in the su(Hw)−(su(Hw)v/su(Hw)e04061); mod(mdg4)u1 background (Fig 4B). The result showed that inactivation of Mod(mdg4)-67.2 only slightly affected the binding of Su(Hw)+ to the chromatin but almost completely prevented Su(Hw)Δ10 and Su(Hw)f binding to the f-lost sites and strongly reduced their binding to the f-retained sites.
As shown previously, the mod(mdg4)u1 mutation leads to a direct repression of yellow in y2 flies that results in the nonpigmented bristles and a variegated pigmentation in the abdominal segments. We found that mod(mdg4)u1 in combination with Su(Hw)Δ10 transgene almost completely suppressed the y2 mutant phenotype, indicating that Su(Hw)Δ10 failed to bind the gypsy sequence in the absence of the Mod(mdg4)-67.2 protein (Fig 4B).
Comparing the functional activity of the gypsy insulator and four Su(Hw) binding sites
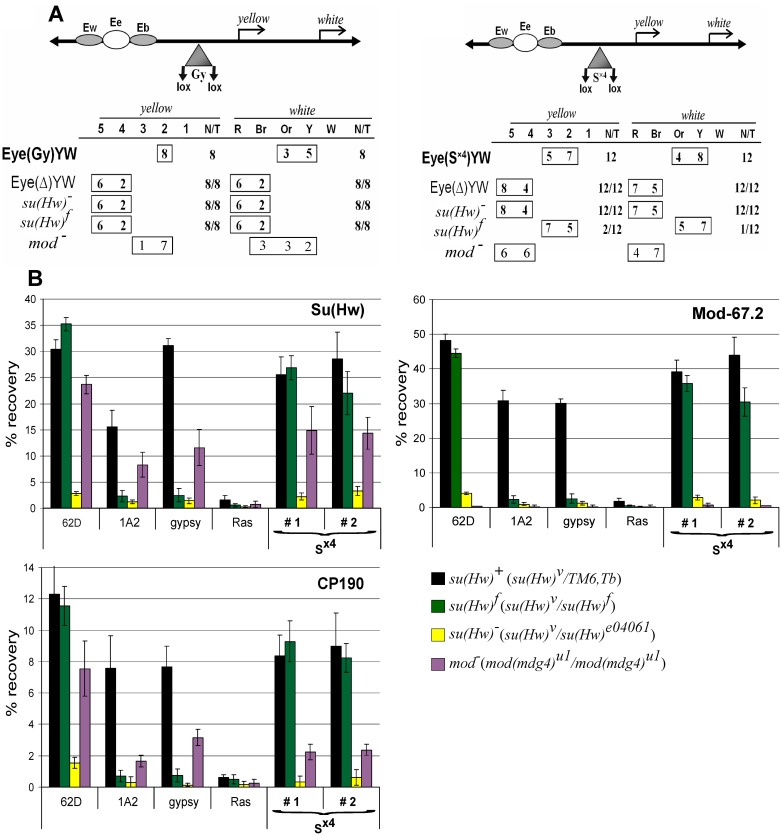
The Su(Hw) protein binds to the sites integrated in the regulatory regions that are also bound by unknown transcription factors, which may facilitate the binding of Su(Hw)f to DNA. To find out whether the presence of an appropriate DNA binding motif is sufficient for recruiting the Su(Hw)f protein, we used a DNA fragment with four reiterated Su(Hw) motifs (S×4) corresponding to site 3 in the gypsy insulator (S6 Fig). This fragment contained all three modules corresponding to the consensus site.
We compared the Su(Hw)+ and Su(Hw)f proteins for binding to the S×4 element and the ability to mediate the enhancer-blocking and barrier activity of the Su(Hw) sites. As reporters, we used the yellow gene and the white gene that was inserted on the 3’ side of yellow in tail-to-head orientation. The white gene accounts for eye pigmentation and is regulated by the eye-specific enhancer [77]. To test the enhancer-blocking activity, constructs were made (Fig 5A), in which the eye enhancer was inserted between the yellow wing and body enhancers, and the S×4 fragment flanked by loxP sites was inserted between the enhancers and promoters at –893 relative to the yellow transcription start site. As a control, we used the previously characterized transgenic line carrying the construct with the same configuration of the regulatory elements and the gypsy insulator flanked by the loxP sites (Fig 5A). We obtained 12 transgenic lines, each carrying one copy of the construct with the S×4 sites. The S×4 fragment effectively blocked the yellow and white enhancers, with deletion of S×4 restoring the expression of the reporter genes. These results showed that the S×4 sites blocked the enhancers at a comparable level with the gypsy insulator (Fig 5A and S7 Fig). In the su(Hw)v/ su(Hw)f background, the Su(Hw)f protein completely blocked the enhancers in transgenic lines carrying the S×4 but not in transgenic lines carrying the gypsy insulator (Fig 5A). As expected, both test elements lost their enhancer-blocking activity in the su(Hw)−(su(Hw)v/ su(Hw)e041064) background. Taken together, these results showed that S×4 sites were fully active in the transgenic lines expressing the mutant Su(Hw)f protein.
Fig 5. Comparison of the enhancer-blocking activity of the gypsy (Gy) and Sx4 insulators.
(A) Effects of the su(Hw) and mod(mdg4) mutations on yellow and white expression in transgenic lines. In the scheme of the construct (drawn not to scale), the yellow wing (Ew) and body (Eb) enhancers are shown as shaded ovals; the eye enhancer (Ee) inserted between them, as a white oval; the yellow (Y) and white (W) genes, as arrows indicating the direction of transcription; and the gypsy and S×4 insulators, as shaded triangles. Downward arrows indicate loxP target sites for the Cre recombinase; the same sites in the construct names are denoted by parentheses. The “yellow” column shows the numbers of transgenic lines with the yellow pigmentation level in the abdominal cuticle (reflecting the activity of the body enhancer); in most of the lines, the pigmentation levels in wing blades (reflecting the activity of the wing enhancer) closely correlated with these scores. The level of pigmentation (i.e., of y expression) was estimated on an arbitrary five-grade scale, with wild-type expression and the absence of expression assigned scores of 5 and 1, respectively. Wild-type white expression determined the bright red eye color (R); in the absence of white expression, the eyes were white (W). Intermediate levels of pigmentation, with the eye color ranging from pale yellow to yellow (Y) from dark yellow to orange (Or), from dark orange to brownish red (Br) reflect the increasing levels of white expression. In the N/T ratio, N is the number of lines in which flies acquired a new y phenotype upon deletion (Δ) of the specified DNA fragment, or on the mutant background, and T is the total number of lines examined for each particular construct. (B) ChIP-qPCR analysis of insulator proteins binding to the gypsy or S×4 insulator in transgenic lines. The Eye(S×4)YW lines included in the analysis are designated # 1 and # 2. The ras64B coding region (Ras) was used as a control devoid of Su(Hw) binding sites. The percent recovery of immunoprecipitated DNA (Y axis) was calculated relative to the amount of input DNA. Error bars indicate standard deviation of three independent biological replicates. The abbreviations of mutant backgrounds: su(Hw)+—su(Hw)v/TM6,Tb; su(Hw)‒—su(Hw)v/su(Hw)e04061; su(Hw)f–su(Hw)v/su(Hw)f; mod-–mod(mdg4)u1/mod(mdg4)u1.
In two independent S×4 transgenic lines, binding of the Su(Hw), Mod(mdg4)-67.2, and CP190 proteins was tested by means of ChIP, with the 62D and 1A2 sites being used as an internal control. The binding of these proteins was also tested in the control transgenic line carrying the gypsy insulator. Chromatin was isolated from pupae of transgenic lines with either su(Hw)v/TM6,Tb, su(Hw)v/su(Hw)e041064 or su(Hw)v/su(Hw)f background (Fig 5B). The results showed that all three proteins—Su(Hw), CP190 and Mod(mdg4)-67.2– bound to the S×4 sites in the two selected transgenic lines and to the gypsy insulator in the su(Hw)v/TM6,Tb background, while the Su(Hw)f protein effectively bound to the S×4 sites but not to the gypsy insulator. Thus, Su(Hw)f proved to bind to the reiterated four sites containing all three modules (S5 Fig). Importantly, CP190 bound to the S×4 sites with the same efficiency in both su(Hw)+ and su(Hw)f backgrounds. These results confirm that CP190 is able to interact with the Su(Hw) sites after its binding to DNA.
According to the ‘Su(Hw) code’ model, the DNA binding site determines which of the ZF domains are not involved in DNA binding and may therefore be available for association with additional proteins such as E(y)2/Sus1 [42,56,57]. To test whether gypsy and S×4 display different properties, we compared their activity in transgenic lines crossed into the mod(mdg4)u1 background (Fig 5A). The inactivation of Mod(mdg4)-67.2 in the mod(mdg4)u1 mutation had little effect on the activity of the gypsy insulator, which was manifested in the partial release of the eye enhancer, while the yellow enhancers remained inactive. In contrast, the mod(mdg4)u1 mutation led to almost complete inactivation of the enhancer-blocking activity of the S×4 insulator. Interestingly, inactivation of mod(mdg4)u1 only moderately affected the binding of Su(Hw) and CP190 to both gypsy and S×4 insulators (Fig 5B). Thus, the Su(Hw) and CP190 proteins still bound to the sites, but their ability to block enhancers was lost only in the case of the S×4 sites.
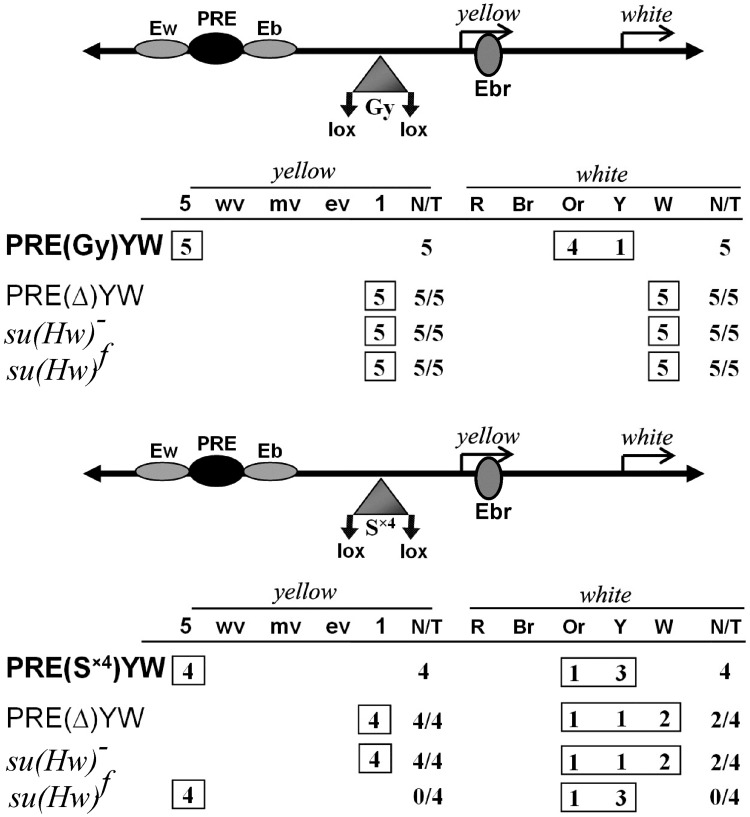
In addition to blocking the enhancers, one copy of the gypsy insulator can also counteract the repressive effect of the Polycomb Response Element (PRE) [17,78,79]. PREs are bound by proteins of the Polycomb group (PcG), inducing the silencing of both endogenous target genes and reporter genes [80]. As shown previously, E(y)2/Sus1 is recruited to the Su(Hw) insulators and is required for blocking of PRE-mediated repression by the gypsy insulator [42]. Since ZFs 7–12 were mapped to interact with E(y)2/Sus1, we ask whether S×4 functions differently from the gypsy insulator in protecting reporter expression from PRE-mediated repression. In the constructs (Fig 6), the 660-bp core PRE from the bxd region of homeotic gene Ultrabithorax [79] was inserted between the wing and body enhancers. The loxP-flanked S×4 fragment was inserted at –893 between the yellow promoter and PRE. Only those transgenic lines were included in this experiment in which the PRE repressed the yellow and white genes after deletion of the insulators. As a result, we found that S×4 effectively blocked PRE-mediated silencing of the yellow and white expression (Fig 6). Crossing into the su(Hw)− background resulted in the silencing of yellow and white expression. Once again, S×4 could protect yellow and white expression from PRE-mediated silencing in the su(Hw)v/su(Hw)f background. Thus, inactivation of ZF10 in the Su(Hw)f protein does not affect the ability to block PRE-mediated silencing.
Fig 6. Comparison of the antisilencing activity of gypsy and S×4 insulators.
The 660-bp PRE is shown as a black oval; the bristle enhancer (Ebr), as a gray oval in the intron of the yellow gene. The “yellow” column shows the numbers of transgenic lines with the yellow pigmentation level in bristles. The degree of variegation in bristles of the thorax and head: 1, loss of pigmentation in all bristles at thorax and head; e-v, extreme variegation (only 1–3 bristles on thorax and head are pigmented); m-v, moderate variegation (about half of bristles are yellow); w-v, weak variegation (only 1–3 bristles on thorax and head are yellow); 5, pigmentation of all bristles as in wild-type flies. Other designations are as in Fig 5.
Discussion
Here we have found that the Su(Hw)f protein cannot interact with CP190 in the absence of DNA. It is likely that structural alteration of ZF10 leads to a change in the conformation of the Su(Hw) protein that prevents the interaction of its N-terminal domain with CP190. The proper conformation of Su(Hw) is restored after binding to DNA, which allows the interaction between its N-terminal domain and the BTB domain of CP190. This finding makes it possible to estimate the role of the Su(Hw)–CP190 interaction in recruiting the insulator complex to chromatin.
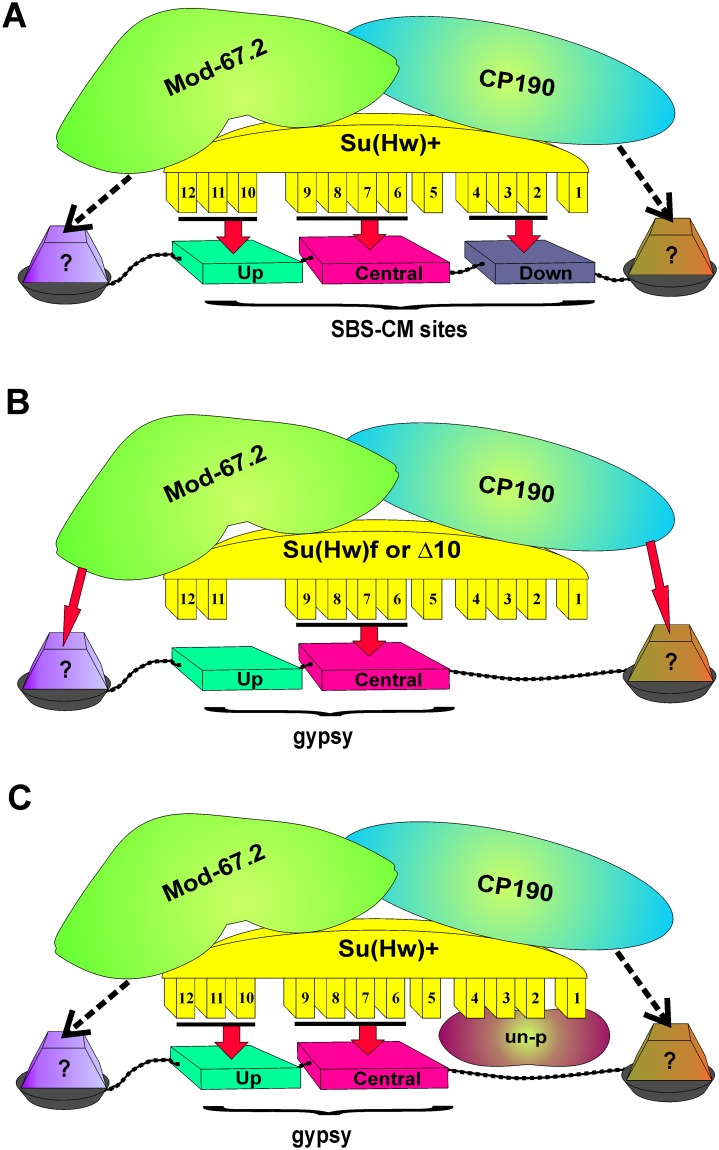
In the Drosophila interphase cell nucleus, the Su(Hw), Mod(mdg4)-67.2, and CP190 proteins colocalize in speckles (insulator bodies) that might function as a depot of proteins involved in the regulation of transcription and insulation [60,61,81,82]. The insulator bodies could facilitate a formation of complexes between Su(Hw)–Mod(mdg4)-67.2–CP190 and other transcription factors. It appears that Mod(mdg4)-67.2 and CP190 interact with neighboring DNA binding (architectural) proteins and in this way facilitate the recruitment of ‘mature’ Su(Hw) insulator complexes to the corresponding chromatin sites. The efficiency of Su(Hw) complex recruiting depends on the presence of essential modules in the consensus site, the number of such sites, and the interaction with neighboring partners (Fig 7A). According to the ‘Su(Hw) code’ [56,57], ZF10 and ZF4 of the Su(Hw) protein are required for interaction with the upstream AT-reach module and the downstream CG-reach module of the consensus binding site (Fig 1). The Su(Hw)f protein looses the ability to recognize the upstream module at DNA and at the same cannot interact with CP190 before recruitment to chromatin (Fig 7B). For this reason, only overexpressed Su(Hw)f can weakly bind to the 12 sites in the gypsy insulator. It seems likely (although not confirmed) that Su(Hw)f binds only to the third site in the gypsy insulator that contains all three modules. This may explain the inability of overexpressed Su(Hw)f to block the yellow enhancers, in contrast to Su(Hw)Δ10 that interacts with CP190. The relatively weak binding of Su(Hw)Δ10 to the gypsy insulator is indicative of the main contribution of ZF10 to the Su(Hw) complex recruitment to the gypsy sites. Moreover, inactivation of Mod(mdg4)-67.2 also completely blocks the recruitment of Su(Hw)Δ10 to the gypsy insulator and other “f-lost” sites. Thus, the recruitment of the mutant Su(Hw) protein incapable of recognizing the upstream module in the consensus site is completely dependent on Mod(mdg4)-67.2 and CP190 partners.
Fig 7. A model explaining differences in the Su(Hw) complex recruitment to the consensus sites and to sites in the gypsy insulator.
(A) The Su(Hw) complex is recruited to consensus site(s) consisting of three modules. Most of ZFs are involved in stable interaction with the consensus site. The interactions of C190 and Mod(mdg4) with neighboring proteins (dotted arrows) are not critical for recruiting the insulator complex. (B) The insulator complex carrying Su(Hw) with mutated ZF10 is recruited to the sites in the gypsy insulator. The binding of the Su(Hw) complex to DNA is unstable and strongly depends on CP190 and Mod(mdg4)-67.2 (thick arrows) that may stabilize interaction with surrounding proteins. (C) The insulator complex with the Su(Hw)+ protein is recruited to the gypsy sites. ZFs 1–4 are not involved in binding to these sites and may recruit unknown proteins/complex (un-p) that improve the enhancer-blocking activity of the Su(Hw) complex in the absence of the Mod(mdg4)-67.2 protein.
The Su(Hw) code model [57] predicts that the consensus site determine which of Su(Hw) ZF domains are not involved in DNA binding and, hence, are free to interact with other proteins and/or complexes (Fig 7C). For example, the ZF domains of the Su(Hw) and dCTCF proteins are required for recruitment of E(y)2/Sus1, which is involved in blocking of PRE-mediated silencing [11,42]. The interaction of E(y)2/Sus1 with Su(Hw) was previously mapped to the region of ZFs 7–12 [42]. Here we found that ZF10 is not required for blocking of PRE by the Su(Hw) sites. Thus, it appears that E(y)2/Sus1 interacts with ZF11 and/or ZF12, which are not highly required for DNA binding [57].
Interestingly, the gypsy insulator (12 sites with different modules structure) and four Su(Hw) sites (S×4) with three modules show the same efficiency in blocking the yellow enhancers in transgenic lines. Inactivation of Mod(mdg4)-67.2 did not significantly change the enhancer-blocking activity of the gypsy insulator. At the same time, Mod(mdg4)-67.2 is critical for the insulator activity of the S×4 sites. Since Mod(mdg4)-67.2 inactivation only weakly affected binding of the Su(Hw) and CP190 proteins to both gypsy and S×4 sites, this difference in insulation may be explained by the existence of an unknown protein/complex involved in enhancer blocking that is recruited to the gypsy but not to the S×4 site. For example, this unknown protein/complex can supposedly interact with ZF4, which is essential for binding to the S×4 sites but not to gypsy. Further study is required for testing the relevance of the code model.
Conclusions
The mechanisms regulating the chromatin binding of architectural proteins, such as Su(Hw), are not yet well understood. Our results support the model that the recruitment of the Su(Hw) complex depends on many protein–protein and DNA–protein interactions at the target chromatin sites.
Supporting information
(DOCX)
(DOC)
(1)—no interaction « − », (2)—« + », (3)—« ++ ».
(TIF)
The FLAG-Su(Hw)+, FLAG-Su(Hw)f, and FLAG-Su(Hw)Δ10 were expressed in the S2 cells. The immunoprecipitated complexes were washed with buffers containing 150 mM NaCl before loading onto the SDS-PAGE for Western blot analysis. The PVDF membrane was probed with antibodies against the Skeletor protein. Each panel represents a single FLAG immunoprecipitation experiment with the particular Su(Hw) variant. Su(Hw) variants do not interact with the Skeletor protein. The bottom panel shows the result of immunoprecipitation between Chromator and Skeletor proteins that interact with each other [83]. All results were reproduced in two independent experiments.
(TIF)
Su(Hw) isolated from adult flies as described (Gdula et al., 1997) [84] was resolved by electrophoresis in 7.5% SDS-PAAG, electroblotted onto a PVDF membrane, and probed with antibodies against Su(Hw) or FLAG epitope (designated αSu(Hw) and αFLAG, respectively). Anti-tubulin staining (αTub) was used as loading control. The name of alleles included in analysis are indicated above the figure. Expression analysis of transgenic lines was performed on the y2scD1ct6; su(Hw)v/ su(Hw)e04061 background.
(TIF)
The immunoprecipitated complexes were washed with 150 mM NaCl-containing buffers before loading onto SDS-PAGE for Western blot analysis. The PVDF membrane was consecutively probed with antibodies against the indicated proteins (CP190 or Mod-67.2) or FLAG epitope. "Input" is the input fraction (10% of lysate used for immunoprecipitation); "Output IP," the supernatant after immunoprecipitation; "IP," the immunoprecipitate. The names of alleles included in analysis are indicated above the figure. Analysis of transgenic lines was performed on the y2scD1ct6; su(Hw)v/ su(Hw)e04061 background. All results were reproduced in three independent experiments.
(TIF)
(A) Schemes (not to scale) of the ct6 allele. The gray oval (Wme) indicates the wing margin enhancer controlling cut expression in the wings. The transcription start site indicated by arrowheads. The gypsy insertion is shown as a triangle with the black circle (Gy) marking Su(Hw) binding sites. The names of alleles included in analysis are under the photos showing the cut wing phenotype. (B) Scheme of the yellow/ac/sc region in scD allele. The coordinates of the AS-C region are as defined by Campuzano et al. (1985) [76]. The gypsy (scD1) insertion is shown as a triangle with an arrow. Thick horizontal gray arrows show the positions and direction of yellow and AS-C gene transcripts. Filled oval indicate the endogenous Su(Hw) insulator. The standard nomenclature for each bristle is as follows: HU, humeral; AOR, anterior orbital; PS, presutural; ASA, anterior supra-alar; OC, ocellar; PV, postvertical; ANP, anterior notopleural; SC, scutellar. Only the bristles affected in the ac and sc mutations are shown. Empty boxes indicate that the corresponding bristles are present (wild-type phenotype). Filled boxes indicate the absence of the corresponding bristle(s) in more 90% of the flies.
(TIF)
This oligonucleotide contains gypsy insulator sequences corresponding to binding site 3 and includes the consensus sequence with three modules that represents the highest-affinity Su(Hw) binding site [57].
(TIF)
Photos represent the abdominal and eyes pigmentation in the 3-day-old males. Numbers indicate the scores of yellow expression in the abdominal segments, which ranged from 1 (pigmentation as in y1 allele) to 5 (pigmentation as in wild-type flies). Wild-type white expression determined the bright red eye color (R); in the absence of white expression, the eyes were white (W). Intermediate levels of pigmentation are yellow (Y), orange (Or), and brownish red (Br).
(TIF)
Acknowledgments
We are grateful to N.A. Gorgolyuk and V.A. Mogila for his help in preparing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
All the experiments, except the genetic analysis experiments were supported by the Russian Science Foundation (http://www.rscf.ru/), project no. 14-24-00166 (to P.G.). Genetic analysis experiments were supported by Molecular and Cellular Biology Program of the Russian Academy of Sciences (http://molbiol.edu.ru/data/index.html) (a grant to A.G.).
References
- 1.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326: 289–93. doi: 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485: 376–80. doi: 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148: 458–72. doi: 10.1016/j.cell.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Ulianov SV, Khrameeva EE, Gavrilov AA, Flyamer IM, Kos P, Mikhaleva EA, et al. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 2016;26: 70–84. doi: 10.1101/gr.196006.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulianov SV, Tachibana-Konwalski K, Razin SV. Single-cell Hi-C bridges microscopy and genome-wide sequencing approaches to study 3D chromatin organization. Bioessays. 2017. doi: 10.1002/bies.201700104 [DOI] [PubMed] [Google Scholar]
- 6.Dixon JR, Gorkin DU, Ren B. Chromatin Domains: The Unit of Chromosome Organization. Mol Cell. 2016;62: 668–80. doi: 10.1016/j.molcel.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hnisz D, Day DS, Young RA. Insulated Neighborhoods: Structural and Functional Units of Mammalian Gene Control. Cell. 2016;167: 1188–200. doi: 10.1016/j.cell.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulianov SV, Gavrilov AA, Razin SV. Nuclear compartments, genome folding, and enhancer-promoter communication. Int Rev Cell Mol Biol. 2015;315: 183–244. doi: 10.1016/bs.ircmb.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Ghavi-Helm Y, Klein FA, Pakozdi T, Ciglar L, Noordermeer D, Huber W, et al. Enhancer loops appear stable during development and are associated with paused polymerase. Nature. 2014;512: 96–100. doi: 10.1038/nature13417 [DOI] [PubMed] [Google Scholar]
- 10.Fedotova AA, Bonchuk AN, Mogila VA, Georgiev PG. C2H2 Zinc Finger Proteins: The Largest but Poorly Explored Family of Higher Eukaryotic Transcription Factors. Acta Naturae. 2017;9: 47–58. [PMC free article] [PubMed] [Google Scholar]
- 11.Maksimenko O, Georgiev P. Mechanisms and proteins involved in long-distance interactions. Front Genet. 2014;5: 28 doi: 10.3389/fgene.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz YB, Cavalli G. Three-Dimensional Genome Organization and Function in Drosophila. Genetics. 2017;205: 5–24. doi: 10.1534/genetics.115.185132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali T, Renkawitz R, Bartkuhn M. Insulators and domains of gene expression. Curr Opin Genet Dev. 2016;37: 17–26. doi: 10.1016/j.gde.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 14.Chetverina D, Fujioka M, Erokhin M, Georgiev P, Jaynes JB, Schedl P. Boundaries of loop domains (insulators): Determinants of chromosome form and function in multicellular eukaryotes. Bioessays. 2017;39(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyrchanova O, Georgiev P. Chromatin insulators and long-distance interactions in Drosophila. FEBS Lett. 2014;588: 8–14. doi: 10.1016/j.febslet.2013.10.039 [DOI] [PubMed] [Google Scholar]
- 16.Matzat LH, Lei EP. Surviving an identity crisis: a revised view of chromatin insulators in the genomics era. Biochim Biophys Acta. 2014;1839: 203–14. doi: 10.1016/j.bbagrm.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comet I, Savitskaya E, Schuettengruber B, Negre N, Lavrov S, et al. PRE-mediated bypass of two Su(Hw) insulators targets PcG proteins to a downstream promoter. Dev Cell. 2006;11: 117–124. doi: 10.1016/j.devcel.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 18.Fujioka M, Mistry H, Schedl P, Jaynes JB. Determinants of Chromosome Architecture: Insulator Pairing in cis and in trans. PLoS Genet. 2016;12: e1005889 doi: 10.1371/journal.pgen.1005889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyrchanova O, Chetverina D, Maksimenko O, Kullyev A, Georgiev P. Orientation-dependent interaction between Drosophila insulators is a property of this class of regulatory elements. Nucleic Acids Res. 2008;36: 7019–7028. doi: 10.1093/nar/gkn781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyrchanova O, Ivlieva T, Toshchakov S, Parshikov A, Maksimenko O, et al. Selective interactions of boundaries with upstream region of Abd-B promoter in Drosophila bithorax complex and role of dCTCF in this process. Nucleic Acids Res. 2011;39: 3042–3052. doi: 10.1093/nar/gkq1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyrchanova O, Mogila V, Wolle D, Deshpande G, Parshikov A, et al. Functional Dissection of the Blocking and Bypass Activities of the Fab-8 Boundary in the Drosophila Bithorax Complex. PLoS Genet. 2016;12: e1006188 doi: 10.1371/journal.pgen.1006188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zolotarev N, Fedotova A, Kyrchanova O, Bonchuk A, Penin AA, et al. Architectural proteins Pita, Zw5,and ZIPIC contain homodimerization domain and support specific long-range interactions in Drosophila. Nucleic Acids Res. 2016;44: 7228–7241. doi: 10.1093/nar/gkw371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahanger SH, Shouche YS, Mishra RK. Functional sub-division of the Drosophila genome via chromatin looping: the emerging importance of CP190. Nucleus. 2013;4: 115–22. doi: 10.4161/nucl.23389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chetverina D, Aoki T, Erokhin M, Georgiev P, Schedl P. Making connections: insulators organize eukaryotic chromosomes into independent cis-regulatory networks. Bioessays. 2014;36: 163–72. doi: 10.1002/bies.201300125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghirlando R, Felsenfeld G. CTCF: making the right connections. Genes Dev. 2016;30: 881–91. doi: 10.1101/gad.277863.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyrchanova O, Mogila V, Wolle D, Magbanua JP, White R, Georgiev P, et al. The boundary paradox in the Bithorax complex. Mech Dev. 2015;138: 122–32. doi: 10.1016/j.mod.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razin SV, Borunova VV, Maksimenko OG, Kantidze OL. Cys2His2 zinc finger protein family: classification, functions, and major members. Biochemistry (Mosc). 2012;77: 217–26. [DOI] [PubMed] [Google Scholar]
- 28.Heger P, George R, Wiehe T. Successive gain of insulator proteins in arthropod evolution. Evolution. 2013;67: 2945–56. doi: 10.1111/evo.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauli T, Vedder L, Dowling D, Petersen M, Meusemann K, Donath A, et al. Transcriptomic data from panarthropods shed new light on the evolution of insulator binding proteins in insects: Insect insulator proteins. BMC Genomics. 2016;17: 861 doi: 10.1186/s12864-016-3205-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252: 809–17. [DOI] [PubMed] [Google Scholar]
- 31.Persikov AV, Singh M. De novo prediction of DNA-binding specificities for Cys2His2 zinc finger proteins. Nucleic Acids Res. 2014;42: 97–108. doi: 10.1093/nar/gkt890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iuchi S. Three classes of C2H2 zinc finger proteins. Cell Mol Life Sci. 2001;58: 625–35. doi: 10.1007/PL00000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldana-Meyer R, Gonzalez-Buendia E, Guerrero G, Narendra V, Bonasio R, Recillas-Targa F, et al. CTCF regulates the human p53 gene through direct interaction with its natural antisense transcript, Wrap53. Genes Dev. 2014;28: 723–34. doi: 10.1101/gad.236869.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negre N, Brown CD, Shah PK, Kheradpour P, Morrison CA, Henikoff JG, et al. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 2010;6: e1000814 doi: 10.1371/journal.pgen.1000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz YB, Linder-Basso D, Kharchenko PV, Tolstorukov MY, Kim M, Li HB, et al. Nature and function of insulator protein binding sites in the Drosophila genome. Genome Res. 2012;22: 2188–98. doi: 10.1101/gr.138156.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott KC, Taubman AD, Geyer PK. Enhancer blocking by the Drosophila gypsy insulator depends upon insulator anatomy and enhancer strength. Genetics. 1999;153: 787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gause M, Morcillo P, Dorsett D. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol Cell Biol. 2001;21: 4807–17. doi: 10.1128/MCB.21.14.4807-4817.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgiev P, Kozycina M. Interaction between mutations in the suppressor of Hairy wing and modifier of mdg4 genes of Drosophila melanogaster affecting the phenotype of gypsy-induced mutations. Genetics. 1996;142: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerasimova TI, Gdula DA, Gerasimov DV, Simonova O, Corces VG. A Drosophila protein that imparts directionality on a chromatin insulator is an enhancer of position-effect variegation. Cell. 1995;82: 587–97. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh D, Gerasimova TI, Corces VG. Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 2001;20: 2518–27. doi: 10.1093/emboj/20.10.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golovnin A, Mazur A, Kopantseva M, Kurshakova M, Gulak PV, Gilmore B, et al. Integrity of the Mod(mdg4)-67.2 BTB domain is critical to insulator function in Drosophila melanogaster. Mol Cell Biol. 2007;27: 963–74. doi: 10.1128/MCB.00795-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurshakova M, Maksimenko O, Golovnin A, Pulina M, Georgieva S, Georgiev P, et al. Evolutionarily conserved E(y)2/Sus1 protein is essential for the barrier activity of Su(Hw)-dependent insulators in Drosophila. Mol Cell. 2007;27: 332–8. doi: 10.1016/j.molcel.2007.05.035 [DOI] [PubMed] [Google Scholar]
- 43.Pai CY, Lei EP, Ghosh D, Corces VG. The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol Cell. 2004;16: 737–48. doi: 10.1016/j.molcel.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 44.Alekseyenko AA, Gorchakov AA, Zee BM, Fuchs SM, Kharchenko PV, Kuroda MI. Heterochromatin-associated interactions of Drosophila HP1a with dADD1, HIPP1, and repetitive RNAs. Genes Dev. 2014;28: 1445–60. doi: 10.1101/gad.241950.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King MR, Matzat LH, Dale RK, Lim SJ, Lei EP. The RNA-binding protein Rumpelstiltskin antagonizes gypsy chromatin insulator function in a tissue-specific manner. J Cell Sci. 2014;127: 2956–66. doi: 10.1242/jcs.151126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matzat LH, Dale RK, Moshkovich N, Lei EP. Tissue-specific regulation of chromatin insulator function. PLoS Genet. 2012;8: e1003069 doi: 10.1371/journal.pgen.1003069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonchuk A, Denisov S, Georgiev P, Maksimenko O. Drosophila BTB/POZ domains of "ttk group" can form multimers and selectively interact with each other. J Mol Biol. 2011;412: 423–36. doi: 10.1016/j.jmb.2011.07.052 [DOI] [PubMed] [Google Scholar]
- 48.Oliver D, Sheehan B, South H, Akbari O, Pai CY. The chromosomal association/dissociation of the chromatin insulator protein Cp190 of Drosophila melanogaster is mediated by the BTB/POZ domain and two acidic regions. BMC Cell Biol. 2010;11: 101 doi: 10.1186/1471-2121-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogelmann J, Le Gall A, Dejardin S, Allemand F, Gamot A, Labesse G, et al. Chromatin insulator factors involved in long-range DNA interactions and their role in the folding of the Drosophila genome. PLoS Genet. 2014;10: e1004544 doi: 10.1371/journal.pgen.1004544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plevock KM, Galletta BJ, Slep KC, Rusan NM. Newly Characterized Region of CP190 Associates with Microtubules and Mediates Proper Spindle Morphology in Drosophila Stem Cells. PLoS One. 2015;10: e0144174 doi: 10.1371/journal.pone.0144174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonchuk A, Maksimenko O, Kyrchanova O, Ivlieva T, Mogila V, Deshpande G, et al. Functional role of dimerization and CP190 interacting domains of CTCF protein in Drosophila melanogaster. BMC Biol. 2015;13: 63 doi: 10.1186/s12915-015-0168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartkuhn M, Straub T, Herold M, Herrmann M, Rathke C, Saumweber H, et al. Active promoters and insulators are marked by the centrosomal protein 190. EMBO J. 2009;28: 877–88. doi: 10.1038/emboj.2009.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuartero S, Fresan U, Reina O, Planet E, Espinas ML. Ibf1 and Ibf2 are novel CP190-interacting proteins required for insulator function. EMBO J. 2014;33: 637–47. doi: 10.1002/embj.201386001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maksimenko O, Bartkuhn M, Stakhov V, Herold M, Zolotarev N, Jox T, et al. Two new insulator proteins, Pita and ZIPIC, target CP190 to chromatin. Genome Res. 2015;25: 89–99. doi: 10.1101/gr.174169.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohan M, Bartkuhn M, Herold M, Philippen A, Heinl N, Bardenhagen I, et al. The Drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. The EMBO J. 2007;26: 4203–14. doi: 10.1038/sj.emboj.7601851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soshnev AA, He B, Baxley RM, Jiang N, Hart CM, Tan K, et al. Genome-wide studies of the multi-zinc finger Drosophila Suppressor of Hairy-wing protein in the ovary. Nucleic Acids Res. 2012;40: 5415–31. doi: 10.1093/nar/gks225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baxley RM, Bullard JD, Klein MW, Fell AG, Morales-Rosado JA, Duan T, et al. Deciphering the DNA code for the function of the Drosophila polydactyl zinc finger protein Suppressor of Hairy-wing. Nucleic Acids Res. 2017. doi: 10.1093/nar/gkx040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buxa MK, Slotman JA, van Royen ME, Paul MW, Houtsmuller AB, Renkawitz R. Insulator speckles associated with long-distance chromatin contacts. Biol Open. 2016;5: 1266–74. doi: 10.1242/bio.019455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golovnin A, Melnikova L, Volkov I, Kostuchenko M, Galkin AV, Georgiev P. 'Insulator bodies' are aggregates of proteins but not of insulators. EMBO Rep. 2008;9: 440–5. doi: 10.1038/embor.2008.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golovnin A, Volkov I, Georgiev P. SUMO conjugation is required for the assembly of Drosophila Su(Hw) and Mod(mdg4) into insulator bodies that facilitate insulator complex formation. J Cell Sci. 2012;125: 2064–74. doi: 10.1242/jcs.100172 [DOI] [PubMed] [Google Scholar]
- 61.Melnikova L, Shapovalov I, Kostyuchenko M, Georgiev P, Golovnin A. EAST affects the activity of Su(Hw) insulators by two different mechanisms in Drosophila melanogaster. Chromosoma. 2017;126: 299–311. doi: 10.1007/s00412-016-0596-3 [DOI] [PubMed] [Google Scholar]
- 62.Schoborg T, Rickels R, Barrios J, Labrador M. Chromatin insulator bodies are nuclear structures that form in response to osmotic stress and cell death. J Cell Biol. 2013;202: 261–76. doi: 10.1083/jcb.201304181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison DA, Gdula DA, Coyne RS, Corces VG. A leucine zipper domain of the suppressor of Hairy-wing protein mediates its repressive effect on enhancer function. Genes Dev. 1993;7: 1966–78. [DOI] [PubMed] [Google Scholar]
- 64.Karess RE, Rubin GM. Analysis of P transposable element functions in Drosophila. Cell. 1984;38: 135–46. [DOI] [PubMed] [Google Scholar]
- 65.Baxley RM, Soshnev AA, Koryakov DE, Zhimulev IF, Geyer PK. The role of the Suppressor of Hairy-wing insulator protein in Drosophila oogenesis. Dev Biol. 2011;356: 398–410. doi: 10.1016/j.ydbio.2011.05.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104: 3312–7. doi: 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panteleev D, Kakpakov VT, Gorelova TV, Andrianov BV. [Variability of continuous insect cell lines and their identification]. Tsitologiia. 2006;48: 653–60. [PubMed] [Google Scholar]
- 68.Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27: 353–65. [PubMed] [Google Scholar]
- 69.Georgieva SG, Nabirochkina EN, Ladygina NG, Georgiev PG, Soldatov AV. [Nuclear protein e(y)2 from Drosophila melanogaster participates in transcription control]. Genetika. 2001;37: 24–8. [PubMed] [Google Scholar]
- 70.Melnikova L, Kostyuchenko M, Molodina V, Parshikov A, Georgiev P, Golovnin A. Multiple interactions are involved in a highly specific association of the Mod(mdg4)-67.2 isoform with the Su(Hw) sites in Drosophila. Open Biol. 2017; pii: 170150. doi: 10.1098/rsob.170150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melnikova L, Kostyuchenko M, Molodina V, Parshikov A, Georgiev P, Golovnin A. Interactions between BTB domain of CP190 and two adjacent regions in Su(Hw) are required for the insulator complex formation. Chromosoma. 2017. doi: 10.1007/s00412-017-0645-6 [DOI] [PubMed] [Google Scholar]
- 72.Mazur AM, Georgiev PG, Golovnin AK. The acid domain located at the C-terminus of the Su(Hw) protein represses transcription in the yeast two-hybrid system. Dokl Biochem Biophys. 2005;400: 1–3. [DOI] [PubMed] [Google Scholar]
- 73.Kuhn-Parnell EJ, Helou C, Marion DJ, Gilmore BL, Parnell TJ, Wold MS, et al. Investigation of the properties of non-gypsy suppressor of hairy-wing-binding sites. Genetics. 2008;179: 1263–73. doi: 10.1534/genetics.108.087254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geyer PK, Spana C, Corces VG. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986;5: 2657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geyer PK, Corces VG. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987;1: 996–1004. [DOI] [PubMed] [Google Scholar]
- 76.Campuzano S, Carramolino L, Cabrera CV, Ruiz-Gomez M, Villares R, Boronat A, et al. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985;40: 327–38. [DOI] [PubMed] [Google Scholar]
- 77.Qian S, Varjavand B, Pirrotta V. Molecular analysis of the zeste-white interaction reveals a promoter-proximal element essential for distant enhancer-promoter communication. Genetics. 1992;131: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mallin DR, Myung JS, Patton JS, Geyer PK. Polycomb group repression is blocked by the Drosophila suppressor of Hairy-wing [su(Hw)] insulator. Genetics. 1998;148: 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sigrist CJ, Pirrotta V. Chromatin insulator elements block the silencing of a target gene by the Drosophila polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics. 1997;147: 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kassis JA, Kennison JA, Tamkun JW. Polycomb and Trithorax Group Genes in Drosophila. Genetics. 2017;206: 1699–725. doi: 10.1534/genetics.115.185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerasimova TI, Byrd K, Corces VG. A chromatin insulator determines the nuclear localization of DNA. Mol Cell. 2000;6: 1025–35. [DOI] [PubMed] [Google Scholar]
- 82.Golovnin A, Melnikova L, Shapovalov I, Kostyuchenko M, Georgiev P. EAST Organizes Drosophila Insulator Proteins in the Interchromosomal Nuclear Compartment and Modulates CP190 Binding to Chromatin. PLoS One. 2015;10: e0140991 doi: 10.1371/journal.pone.0140991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rath U, Wang D, Ding Y, Xu YZ, Qi H, et al. Chromator, a novel and essential chromodomain protein interacts directly with the putative spindle matrix protein skeletor. J Cell Biochem. 2004;93: 1033–1047. doi: 10.1002/jcb.20243 [DOI] [PubMed] [Google Scholar]
- 84.Gdula DA, Corces VG. Characterization of functional domains of the su(Hw) protein that mediate the silencing effect of mod(mdg4) mutations. Genetics. 1997;145: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(1)—no interaction « − », (2)—« + », (3)—« ++ ».
(TIF)
The FLAG-Su(Hw)+, FLAG-Su(Hw)f, and FLAG-Su(Hw)Δ10 were expressed in the S2 cells. The immunoprecipitated complexes were washed with buffers containing 150 mM NaCl before loading onto the SDS-PAGE for Western blot analysis. The PVDF membrane was probed with antibodies against the Skeletor protein. Each panel represents a single FLAG immunoprecipitation experiment with the particular Su(Hw) variant. Su(Hw) variants do not interact with the Skeletor protein. The bottom panel shows the result of immunoprecipitation between Chromator and Skeletor proteins that interact with each other [83]. All results were reproduced in two independent experiments.
(TIF)
Su(Hw) isolated from adult flies as described (Gdula et al., 1997) [84] was resolved by electrophoresis in 7.5% SDS-PAAG, electroblotted onto a PVDF membrane, and probed with antibodies against Su(Hw) or FLAG epitope (designated αSu(Hw) and αFLAG, respectively). Anti-tubulin staining (αTub) was used as loading control. The name of alleles included in analysis are indicated above the figure. Expression analysis of transgenic lines was performed on the y2scD1ct6; su(Hw)v/ su(Hw)e04061 background.
(TIF)
The immunoprecipitated complexes were washed with 150 mM NaCl-containing buffers before loading onto SDS-PAGE for Western blot analysis. The PVDF membrane was consecutively probed with antibodies against the indicated proteins (CP190 or Mod-67.2) or FLAG epitope. "Input" is the input fraction (10% of lysate used for immunoprecipitation); "Output IP," the supernatant after immunoprecipitation; "IP," the immunoprecipitate. The names of alleles included in analysis are indicated above the figure. Analysis of transgenic lines was performed on the y2scD1ct6; su(Hw)v/ su(Hw)e04061 background. All results were reproduced in three independent experiments.
(TIF)
(A) Schemes (not to scale) of the ct6 allele. The gray oval (Wme) indicates the wing margin enhancer controlling cut expression in the wings. The transcription start site indicated by arrowheads. The gypsy insertion is shown as a triangle with the black circle (Gy) marking Su(Hw) binding sites. The names of alleles included in analysis are under the photos showing the cut wing phenotype. (B) Scheme of the yellow/ac/sc region in scD allele. The coordinates of the AS-C region are as defined by Campuzano et al. (1985) [76]. The gypsy (scD1) insertion is shown as a triangle with an arrow. Thick horizontal gray arrows show the positions and direction of yellow and AS-C gene transcripts. Filled oval indicate the endogenous Su(Hw) insulator. The standard nomenclature for each bristle is as follows: HU, humeral; AOR, anterior orbital; PS, presutural; ASA, anterior supra-alar; OC, ocellar; PV, postvertical; ANP, anterior notopleural; SC, scutellar. Only the bristles affected in the ac and sc mutations are shown. Empty boxes indicate that the corresponding bristles are present (wild-type phenotype). Filled boxes indicate the absence of the corresponding bristle(s) in more 90% of the flies.
(TIF)
This oligonucleotide contains gypsy insulator sequences corresponding to binding site 3 and includes the consensus sequence with three modules that represents the highest-affinity Su(Hw) binding site [57].
(TIF)
Photos represent the abdominal and eyes pigmentation in the 3-day-old males. Numbers indicate the scores of yellow expression in the abdominal segments, which ranged from 1 (pigmentation as in y1 allele) to 5 (pigmentation as in wild-type flies). Wild-type white expression determined the bright red eye color (R); in the absence of white expression, the eyes were white (W). Intermediate levels of pigmentation are yellow (Y), orange (Or), and brownish red (Br).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.