Abstract
Objective
To describe a group of children with co-incident pulmonary vein stenosis and Smith-Lemli-Opitz syndrome and to generate hypotheses as to the shared pathogenesis of these disorders.
Design
Retrospective case series
Patients
Five subjects in a pulmonary vein stenosis cohort of 170 subjects were diagnosed with Smith-Lemli-Opitz syndrome soon after birth.
Results
All five cases were diagnosed with Smith-Lemli-Opitz syndrome within six weeks of life, with no prior family history of either disorder. All cases had pathologically elevated 7-dehydrocholesterol levels and two of the five cases had previously reported pathogenic 7-dehydrocholesterol reductase mutations. Smith-Lemli-Opitz syndrome severity scores ranged from mild to classical (2–7). Gestational age at birth ranged from 35 to 39 weeks. Four of the cases were male by karyotype. Pulmonary vein stenosis was diagnosed in all cases within two months of life, earlier than most published cohorts. All cases progressed to bilateral disease and three cases developed atresia of at least one vein. Despite catheter and surgical interventions, all subjects’ pulmonary vein stenosis rapidly recurred and progressed. Three of the subjects died, at 2 months, 3 months, and 11 months. Survival at 16 months after diagnosis was 43%.
Conclusions
Patients with pulmonary vein stenosis who have a suggestive syndromic presentation should be screened for Smith-Lemli-Opitz syndrome with easily obtainable serum sterol tests. Echocardiograms should be obtained in all newly diagnosed patients with Smith-Lemli-Opitz syndrome, with a low threshold for repeating the study if new respiratory symptoms of uncertain etiology arise. Further studies into the pathophysiology of pulmonary vein stenosis should consider the role of cholesterol-based signaling pathways in the promotion of intimal proliferation.
Keywords: Pulmonary Vein Stenosis, Pulmonary Veno-Occlusive Disease, Smith-Lemli-Opitz Syndrome
Introduction)
Primary pulmonary vein stenosis (PVS) is a rare and relentless disorder that occurs in normally-connected, surgically-untouched pulmonary veins, with an estimated incidence ranging from 1.7 per 100,000 in the general population under 2 years old to 30 per 100,000 patients undergoing congenital cardiac surgery or catheterization.1,2 This condition is associated with preterm birth, bronchopulmonary dysplasia (BPD), intracardiac left-to-right shunt lesions, and chromosomal abnormalities.1,3 Pathologic specimens demonstrate neo-intimal proliferation of myofibroblasts as the primary etiology for the progressive luminal stenosis and obliteration of the vessels,4 with immunohistochemistry demonstrating strong receptor tyrosine kinase expression, such as VEGF.5 In some children, obstruction to pulmonary venous flow can be improved with surgical procedures such as sutureless marsupialization repair or catheter-based interventions such as balloon dilation and stenting.6–8 More recently, drug therapies have been attempted to delay disease progression and to treat the intimal proliferation within the vessels.9,10 Many of our more recent PVS patients have been enrolled in a 48-week open-label FDA-regulated trial of imatinib mesylate with or without bevacizumab.11 Despite intervention, many patients experience both recurrence of disease (restenosis in a location with prior disease) and progression of disease (stenosis in new vessels, longer distal stenotic segments of previously diseased vessels, or complete venous atresia).12 In many cases, PVS is an end-stage condition, with mortality rates of up to 83% in patients with 3 or 4 stenosed veins.13 Another study notes a 2-year survival rate from diagnosis of 43%, but only 25% survival in children who underwent transcatheter interventions.1
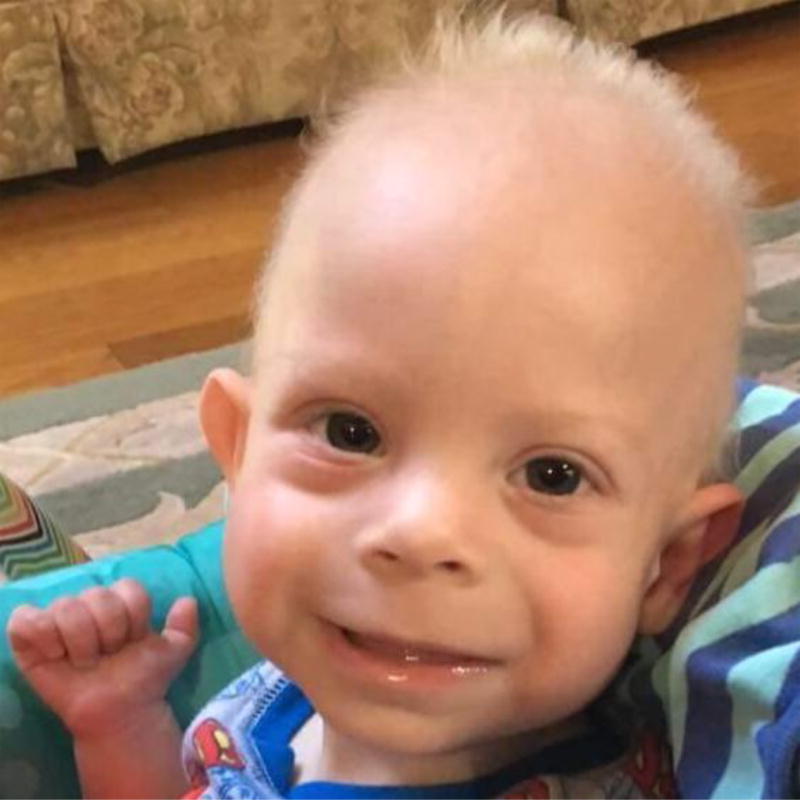
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive disorder of cholesterol metabolism that is associated with multiple congenital anomalies such as hypotonia, growth delays, cleft palate, post-axial polydactyly, thumb abnormalities, Y-shaped cutaneous 2/3 toe syndactyly, hypospadias, and undervirilization of male genitalia. Prematurity and breech presentation are common gestational complications. There are significant associated behavioral and intellectual deficits, in addition to distinctive facial features (Figure 1). The incidence of SLOS in the heterogeneous United States population is estimated at 2 in 100,000.14 In SLOS, loss-of-function mutations in both copies of 7-dehydrocholesterol reductase (DHCR7) prevent the final step of cholesterol synthesis and result in the accumulation of the precursor molecule 7-dehydrocholesterol (7-DHC).15 The diagnosis is typically made by serum analysis for total cholesterol (low or normal) and 7-DHC (elevated).16 The clinical severity of the disorder is inversely correlated with plasma cholesterol concentration and directly correlated with plasma 7-DHC levels.17,18 Cardiac manifestations of SLOS are found in approximately 50% of cases and most commonly include endocardial cushion defect, secundum atrial septal defect (ASD), patent ductus arteriosus (PDA), and anomalous pulmonary venous connection (APVC).19 Lung manifestations include abnormal lobation, hypoplasia, pulmonary hypertension, and laryngo/tracheomalacia.20–22 Cases of discrete pulmonary vein stenosis have not previously been described in the SLOS literature, but SLOS has been infrequently reported in limited case series of PVS outside of our institution’s cohort.1,23
Figure 1.
Subject C, at 15 months, demonstrating the characteristic SLOS facial features of bitemporal narrowing, hypertelorism, short nasal root, broad nasal bridge and base, anteverted nares, smooth long philtrum, and a small chin. Also notable is the short, proximally placed thumb. Parental consent was obtained for publication of this photograph.
Within our institutional cohort of 170 patients with PVS, we have diagnosed five patients with SLOS and have appreciated that this rate is notably different from the rate of SLOS in the general population (p < 0.0001 by Chi-square with Yates correction). We obtained institutional IRB approval for this retrospective case series. Pertinent records were reviewed for demographic and phenotypic information related to both diagnoses and interventions. The SLOS severity score, as described by Kelley and Hennekam,24 was calculated in the typical fashion, scoring for brain, oral, acral, cardiac, kidney, liver, lung, bowel, and genital abnormalities. The clinical phenotypes of all subjects are summarized in Table 1.
Table 1.
Clinical phenotype of the five PVS-SLOS subjects, including the SLOS Severity Score, as described by Kelley and Hennekam.24
| SLOS Severity Scoring Criteria |
Case A | Case B | Case C | Case D | Case E | |
|---|---|---|---|---|---|---|
| Prenatal and Neonatal Course | 36-week di-di twin gestation | 39-week gestation | 35-week gestation, pre-term labor, rupture of membranes, breech presentation, small for gestational age | 39-week gestation, breech presentation, fetal anomalies | 39-week gestation, intrauterine growth restriction | |
| Karyotype | XY | XY (undervirilized) | XY | XY | XX | |
| Severity Score | 2 (mild) | 7 (classical) | 4 (classical) | 6 (classical) | 4 (classical) | |
| Brain | seizures, CNS malformations, gyral defects | hypotonia, poor oromotor reflex | hypotonia, poor oromotor reflex, cerebellar vermis hypoplasia, prominent 4th ventricle, sacral pit, tethered cord | hypotonia, sacral pit, tethered cord, microcephaly | cebrebral atrophy, enlarged ventricles, low occipital and parietal lobe volumes, hypotonia | hypotonia, polymicrogyri, poor oromotor reflex |
| Oral | bifid uvula, cleft palate | posterior cleft palate, retrognathia | retrognathia | cleft soft palate, micrognathia (Pierre Robin) | high palate | |
| Acral | Y-shaped 2/3 toe syndactyly, polydactyly, club foot | 2/3 syndactyly | 2/3 syndactyly | 2/3 syndactyly | 2/3 syndactyly, bilateral finger polydactyly | 2/3 syndactyly, club foot |
| Eye | cataracts, microophthalmia | eyelid hemangioma | ||||
| Cardiac (excluding PVS) | Single chamber or vessel defect, complex cardiac malformation | PFO, PDA at 2mo | ASD, PDA | ASD, memVSD, mild PPS, dilated aortic root | PFO, PDA at 3mo | PFO, PDA |
| Kidney | cystic kidney disease, renal agenesis | pelvic kidney | horseshoe kidney, hydronephrosis | nephrocalcinosis, hydronephrosis | urethral obstruction, prostatic utricle | low-set malrotated kidneys, echogenic cortex |
| Liver | structural abnormality, progressive hepatic disease | |||||
| Lung | abnormal lobation, hypoplasia, pulmonary cysts | complete tracheal rings | ||||
| Bowel | pyloric stenosis, Hirschsprung disease | GE reflux, gastric dysmotility, failure to thrive, gastrostomy tube | inguinal hernia, gastrostomy tube | gastrostomy tube | ||
| Genitalia | hypospadias, ambiguous genitalia | cryptorchidism, hypospadias | undervirilized genitalia (XY), cryptochidism | hypospadias, bifid scrotum, cryptorchidism | hypospadias | |
| Facial Features | ptosis, short upturned nose, depressed nasal bridge, low-set posteriorly rotated ears, bitemporal hollowing | prominent nasal bridge, short anteverted nasal tip, low-set ears | upturned nose | small upturned nose | hypertelorism, large low- set ears, smooth philtrum, upturned nose, ptosis, bitemporal narrowing | |
| Endocrine | adrenal insufficiency | adrenal insufficiency | adrenal insufficiency (assumed) | |||
| Cholesterol Replacement | yes | yes | yes | yes | yes |
Abbreviations: ASD (atrial septal defect), memVSD (membranous ventricular septal defect), PDA (patent ductus arteriosus), PFO (patent foramen ovale), PPS (peripheral pulmonary stenosis).
Case A)
Patient A is currently 2 years 10 months old. He was conceived via intrauterine insemination and a sperm donor, with no known family history of either congenital heart disease or SLOS. The clinical suspicion of SLOS was raised at birth due to 2/3 syndactyly, facial dysmorphisms, and poor oromotor coordination. The diagnosis was biochemically confirmed in the first week of life with a 7-DHC level of 78.69 ug/mL (reference range 0.04–0.36 ug/mL) and a total cholesterol level less than 50 mg/dL. His severity score24 was in the mild range at 2 out of a total of 20 possible points (1 point for 2/3 syndactyly and 1 point for cryptorchidism/hypospadias).
Patient A’s initial echocardiogram after birth found normal anatomy with a PDA and patent foramen ovale (PFO). After an admission for respiratory distress at the age of 2 months, an echocardiogram was performed that demonstrated severe pulmonary artery hypertension with right ventricular (RV) hypertrophy and RV pressure likely greater than systemic pressure. The patient was transferred to our institution and a follow-up echocardiogram noted bilateral upper pulmonary vein stenosis, 20 mmHg in the right upper vein and 15 mmHg in the left upper vein. A catheterization revealed severe RUPV stenosis, near atresia of the right lower pulmonary vein (RLPV), moderate left upper pulmonary vein (LUPV) stenosis, and left lower pulmonary vein (LLPV) atresia. The initial RV pressure was 54 mmHg compared to an aortic pressure of 68 mmHg. All patent veins were balloon dilated and the final PA pressure was 71 mmHg compared to an aortic pressure of 94 mmHg.
Four days later, a sutureless repair of the pulmonary veins was performed, along with a fenestrated closure of the PFO and ligation of the PDA. At the time of surgery, the RV pressure was 30 mmHg, compared to an aortic pressure of 65 mmHg. The operative course was complicated by an MCA stroke and sepsis. Pathology from the resected veins was notable for proliferative fibromyxoid tissue. Two weeks after surgery, the patient began one year of medical PVS therapy with imatinib mesylate and bevacizumab.
Two subsequent catheterizations were performed at 4 months and 24 months of age. At 4 months, two months from the sutureless repair, there was significant proximal stenosis of all pulmonary veins, with recurrence of the LLPV atresia. The patent veins were again balloon dilated with improved pressure gradients and angiographic appearance. At 24 months, there was stable LLPV atresia and stable mild proximal narrowing of the LUPV. There was mild recurrence of RUPV stenosis that was successfully balloon dilated. The most recent echocardiogram at 25 months revealed a mean gradient of 3 mmHg across the RUPV with laminar flow from the RLPV. The left-sided veins were not evaluated. The RV pressure was estimated to be less than 50% systemic. He has been followed for a total of 32 months.
Case B)
Patient B was born with ambiguous genitalia, facial dysmorphisms, 2/3 toe syndactyly, and hypotonia, and SLOS was suspected. The 7DHC level was obtained hours after birth and was 41.2 ug/mL (reference range <2). There was no family history of SLOS, nor was there any consanguinity. Subsequent genetic testing demonstrated pathogenic gene mutations in the DHCR7 protein: IVS8-1 G>C (intron 8 splice acceptor/frameshift) and C380R (exon 9 missense mutation). This compound heterozygote combination has been previously described with a severe phenotype.25,26 The SLOS severity score24 was in the classical range at 7 (1 point for vermis hypoplasia, 2 for cleft palate, 1 for 2/3 syndactyly, 1 for horseshoe kidney, and 2 for ambiguous genitalia).
An echocardiogram obtained at birth due to the dysmorphisms and mild tachypnea noted a large ASD, a large PDA with bidirectional flow, and PVS. Ten days later, there was worsening tachypnea and bilateral PVS disease across stenotic membrane-like orifices. The right-sided pulmonary vein gradient was 20–25 mmHg and a left-sided gradient was 5–8 mmHg. At two weeks of life, the patient was taken to the catheterization lab, which revealed an RV pressure that was 75% systemic pressure. There was one nearly atretic and one stenosed LUPV. The accessible vein had a gradient of 40 mmHg that was unsuccessfully balloon dilated. There was a stenotic common right pulmonary venous orifice that was balloon dilated, resulting in a decrease in gradient from 29 to 21 mmHg. There was no significant change in the RV pressure compared to the systemic pressure. The LLPV was found to be widely patent. At five weeks of life, the patient acquired new tachypnea and an oxygen requirement, so he was taken back to the catheterization lab. The RV pressure was equivalent to the systemic pressure and increased to 150% of systemic pressure in the setting of a reactive vascular bed. Angiography demonstrated severe and rapid progression of PVS with new bilateral stenosis of the pulmonary veins. The previously nearly atretic LUPV was now completely atretic. The re-wired LUPV, the RLPV, and the newly stenosed LLPV were balloon dilated at their insertions into the left atrium (LA). The RUPV could not be accessed and was suspected of being atretic. After these findings were discussed with the family, the decision was made to halt further interventions. The patient was transferred to a local hospital and passed away ten days later at seven weeks of life.
Case C)
Patient C is currently 16 months old. He was 1.87 kg at birth, small for gestational age. Hypotonia, 2/3 syndactyly, hypospadias with a bifid scrotum, microcephaly, and an upturned nose prompted an evaluation (Figure 1)for SLOS. At two days of life, the 7-DHC level was 61 ug/mL (reference range 0.04–0.36), while the total cholesterol level was 40 mg/dL, confirming the diagnosis. Chromosomal analysis confirmed a normal 46, XY karyotype. There is no family history for SLOS or PVS, nor is there any reported consanguinity in the family. The patient’s total SLOS severity score24 was in the classical category at 4 (1 point for hypospadias and cryptorchidism, 1 for 2/3 syndactyly, 1 for microcephaly, 1 for VSD).
Patient C was found to have a persistent murmur after birth and was diagnosed with a membranous VSD, small ASD/PFO, dilated aortic root, and mild peripheral pulmonary stenosis. There was no pulmonary venous obstruction three weeks later, but at 6 weeks of life, there was 6–8 mmHg of obstruction at the RLPV, with normal RV pressure. At that time, the patient was without any respiratory symptoms but was experiencing slow weight gain. By 8 weeks of life, there was bilateral involvement of all four pulmonary veins. At four months of life, cardiac catheterization found proximal stenosis of three vessels, but the RV pressure was less than 33% systemic pressure. The stenosis of the RUPV was dilated down from 10 to 2 mmHg, the RLPV from 8 to 2, and the LUPV down to 6. There was no LLPV disease.
Six days later, the patient was taken to the operating room for a VSD closure, tricuspid valvuloplasty, ASD closure with fenestration, PDA ligation, and ostial resection of the RU/RL/LU pulmonary veins with a sutureless repair of the right-sided veins, leaving no residual VSD, a small patent atrial fenestration, and an RLPV gradient of 5 mmHg. Pathologic specimens were notable for intimal fibrosis and myxoid change within the veins. The patient began medical therapy with imatinib mesylate and bevacizumab one week later. Two weeks after the sutureless repair, a lung scan and echocardiogram suggested progression of disease, so he was taken back to the catheterization lab. There was only mild recurrent disease after 21 days from the initial cath, with unchanged RV pressure (33% systemic). The mild right middle pulmonary vein (RMPV) ostial obstruction was dilated with a cutting balloon, reducing the gradient from 6 to 2 mmHg. In total, he has had 7 catheterizations since diagnosis. All four vessels have demonstrated recurrence and progression, and the RV pressure peaked at 10 mmHg above the systemic pressure, which normalized to a degree when the vessels were dilated. The RMPV has become atretic. At 13 months of age, the RV pressure was 83% systemic at the start of the catheterization with recurrence of proximal ostial atresia in all other pulmonary veins. However, the LUPV had an 8–10 mmHg gradient due to a tortuous course, rather than intraluminal disease. All stenotic veins were balloon dilated with angiographic improvement. After dilation, the RV pressure fell to 50% of the systemic pressure. The patient remains under active surveillance (now 14 months since diagnosis) and has completed the imatinib mesylate and bevacizumab therapeutic protocol.
Case D)
Patient D was suspected of having SLOS at birth due to a small upturned nose, cleft soft palate, 2/3 toe syndactyly, bilateral polydactyly of his upper extremities, and hypospadias. At one month of life, the 7-DHC level was 155 ug/mL (reference range <2), and the final diagnosis was made. Genetic testing revealed that the patient was heterozygous for IVS8-1 G>C (intron 8 splice acceptor/frameshift) and V326L (exon 9 missense mutation in a highly conserved region) in the DHCR7 gene.26 There was no specific family history of SLOS, PVS, or consanguinity. The total SLOS Severity Score24 was in the classical category at 6 (2 points for cleft palate, 2 for syndactyly and polydactyly, 1 for brain atrophy, and 1 for hypospadias).
Due to his congenital anomalies, Patient D had a normal screening echocardiogram after birth that noted a PDA and a PFO. One month later, an echocardiogram was performed due to systemic hypertension that demonstrated possible pulmonary venous obstruction. A post-hoc review of his initial study appreciated somewhat turbulent pulmonary venous flows. One week later, additional imaging demonstrated a common, stenotic orifice of all four pulmonary veins, with a 9 mmHg gradient into the LA. Later studies reported a common left pulmonary venous confluence that drained into the right side of the LA and stenosis of the three right pulmonary veins as they entered the LA. By three months of life, there was a peak echocardiographic gradient of 12–18 mmHg on the left and 11 mmHg on the right, so he was taken to the catheterization lab. There, his RV pressure was 75% systemic, with near-total occlusion of his left pulmonary venous confluence. This vein was re-canalized and after dilation, there was a 12 mmHg gradient into the LA. The distal left veins were markedly hypoplastic and much of the pulmonary blood flow was decompressed by veno-venous collaterals. The right-sided veins drained together into the LA through a tight ring with a gradient of 6–8 mmHg and intervention was not attempted.
Five days later, he was taken to the operating room where he received a debriding of the waist of the right-sided pulmonary venous orifice and a sutureless repair of the nearly atretic common left PV, leaving a 5 mmHg gradient to the right veins and patent flow from the left-sided vein by epicardial echocardiogram. Pathology from the surgery noted increased elastic tissue fibers and mild intimal myxoid changes in the right vein tissue. There was normal tissue in the LPV stenosis excisional biopsy. On post-operative day 10, there was a 2–3 mmHg gradient across the bilateral lower veins, a patent RUPV, and an atretic LUPV by echocardiogram.
On a routine echocardiogram 7 weeks after discharge, there was significant recurrence of the stenosis with associated RV dilation and dysfunction. Other than intermittent episodes of tachypnea, he was generally asymptomatic. He returned to the catheterization lab two months after his first surgery and was found to have systemic RV pressures, with mean PA pressures of 41 mmHg, a PVR of 11 indexed Wood units, and diffuse PVS. There was atresia of the RLPV orifice, peripheral hypoplasia of the LPVs and the RLPV, and stenosis of all remaining orifices. The RLPV was recanalized and the other veins were dilated as well, resulting in the RV pressure falling to 66% systemic. In the days after the cath, additional studies were performed, including an airway evaluation, lung scan, and repeat echocardiogram. The family decided to defer additional interventions and brought their child home with hospice care. The patient passed away six months later at the age of 11 months.
Case E)
Patient E was born full term, but remained in the NICU for three weeks due to hypotonia and feeding issues. Features consistent with SLOS were noted at five weeks of life when she was admitted with tachypnea. Gene testing was not available at that time, but biochemical testing demonstrated a 7-DHC level of 87 ug/mL (reference range <2) with total cholesterol of 21 mg/dL. Her severity score24 was in the classical range at 4 (2 points for gyral defects, 1 point for syndactyly, and 1 point for club foot).
During her admission for tachypnea, a chest x-ray revealed an enlarged heart and pulmonary vascular congestion. A first-time echocardiogram was performed and demonstrated normal structural anatomy with a PFO, a PDA, suprasystemic RV pressures with RV hypertrophy, and significant PVS of all four veins. There was near atresia of the RPVs and the common orifice of the LPVs was covered by a membrane, creating a peak gradient of at least 12 mmHg. The LLPV was small in size and the LUPV was normal to large. Three days after admission, the patient was taken for catheterization. She was found to have RPV atresia with severely hypoplastic veins and LPV osteal stenosis with a mean pressure gradient of 4–5 mmHg. The RV pressure was 135% of the systemic pressure. No interventions were performed. Two days later, she was taken to the operating room where a sutureless repair of the bilateral pulmonary veins was performed. The ASD was patched with a fenestration. After bypass, there was no left-sided obstruction but the RPVs were very small with turbulent blood flow. Pathology from the resected veins demonstrated intimal proliferation of spindle cells in myxoid stroma that stained positive for smooth muscle actin and negative for CD34.
Three weeks after discharge from her month-long admission, an echocardiogram at a scheduled cardiology clinic visit demonstrated re-stenosis of the LPVs with a mean gradient of 8–9 mmHg and a peak gradient of 13–14 mmHg. Flow from the RPVs was not visualized and the RV pressure was systemic. Within 24 hours, she was admitted due to tachypnea and intubated two days later. At three months of age, nearly 2 months after her first surgery, Patient E was taken back to the operating room for left-sided sutureless marsupialization. Post-operatively, the RV pressure was 75% systemic and pathology demonstrated intimal spindle cell proliferation and focal scarring. There was significant post-operative bleeding in the first 24 hours. An EEG was performed on the second day after surgery that demonstrated diffuse cortical dysfunction with encephalopathy. Two days later, she was found to have a severe thrombotic SVC obstruction. Anti-thrombotics were initiated, but she died from cardiogenic shock. She had been followed for PVS for 57 days.
Discussion)
Herein we describe an unexpected association between children with Smith-Lemli-Opitz syndrome and the development of pulmonary vein stenosis. The five cases of PVS-SLOS were all diagnosed with SLOS soon after birth with no prior family history of the syndrome. The diagnosis was made clinically with confirmatory lab values (high 7-DHC and low cholesterol). Two of the five cases were genotyped, and those cases were compound heterozygous for previously reported pathogenic SLOS mutations. SLOS severity scores24 ranged from mild to classical (2–7), with no severe cases. Gestational age at birth ranged from 35 to 39 weeks, and none of the children were diagnosed with BPD. Only one case had suspected fetal anomalies. All but one of the five cases were male (confirmed by karyotype).
These five cases were identified at a single institution which has served as a referral institution for aggressive PVS. Treatment protocols have evolved from an early focus on surgical resection using sutureless techniques6,8 coupled with catheter-based intervention,7 to more recent attempts to suppress myofibroblast activity with biologic agents,4,5 initially with antiproliferative drugs,9 and more recently with agents targeted at receptor tyrosine kinases present on the myofibroblasts.5,12 Over time, there has been an increasing emphasis on aggressive surveillance to identify and treat recurrences with catheter-based interventions to maintain an adequate vascular bed.
Compared to several published groups of PVS subjects (Table 2), our subjects were diagnosed with primary PVS earlier than the typical mean age (birth to 2 months, versus mean ages greater than 7 months).1,2,23,27 Three of the diagnoses were incidental findings on screening echocardiogram, but the other two children were diagnosed in the setting of respiratory distress. Four cases had bilateral stenosis at diagnosis, and the other rapidly progressed from a single stenotic vein to bilateral disease in four vessels within two weeks. Three cases developed atresia of at least one vein. Additional cardiac anomalies included PDA, ASD, VSD, dilated aortic root, peripheral pulmonary stenosis, and a possible cor triatriatum, but there were no cases of abnormal pulmonary venous connections (i.e. total or partial APVC), heterotaxy, or single ventricle physiology. After diagnosis, all subjects were taken to the catheterization lab within 0 to 6 weeks. Four of the subjects underwent balloon dilation of the stenotic vessels. Soon afterward, four cases underwent sutureless repair of their PVS, but the stenosis rapidly recurred and progressed 2 to 6 weeks after intervention in all five cases. In total, the subjects were followed for 2 to 23 months, having gone to the catheterization lab 1 to 7 times. Due to severe, unrelenting PVS recurrence, three children died, at 2 months, 3 months, and 11 months. The other two subjects were enrolled in an anti-proliferative drug trial11 and are currently 16 and 34 months old. The older child had recurrence of single vessel PVS two months after completion of 48 weeks of imatinib mesylate/bevacizumab. Overall survival after diagnosis of PVS was 64% at 6 months, and 43% at 16 months.
Table 2.
| Case | A | B | C | D | E | Drossner1 | Holt2 | Seale23 | Song27 |
|---|---|---|---|---|---|---|---|---|---|
| Number | 26 | 31 | 58 | 34 | |||||
| Exclusion Criteria | APVC, HLHS | APVC, cor triatriatum, atrial switch | Single Ventricle, T APVC | APVC | |||||
| Gestational Age at Birth | 36 (twin) | 39 | 35 | 39 | 39 | 61% preterm (median 32 weeks) | 38% preterm (<37 weeks) | 21% preterm (median 35 weeks) | |
| SLOS Genotype | -- | IVS8-1 G>C, C380R | -- | IVS8-1 G>C, V326L | -- | 31% genetic syndromes, 1 subject with SLOS | 28% non-cardiac anomaly or syndrome, 1 subject with SLOS | ||
| Age at PVS Diagnosis | 2 months | birth, worse by day 6 | 6 weeks, progressed w/in 2wk | 1mo3d | 1m11d | median 7.4 months (0 – 35) | median 7.6 months (0 – 196) | median 12 months (0.2 – 14.6) | |
| Additional Cardiac Disease | PFO, PDA at 2 months | ASD, PDA | ASD, memVSD, mild PPS, dilated aortic root | PFO, tiny PDA at 3mo | PFO, PDA | 88% | 79% | 79% | |
| Veins Involved | 2 → 5 | 4 | 1 → 5 | 5 | 5 | median 2 (1 –4) | |||
| Age at First Cath | 2m6d | 0m11d | 4m6d | 3m20d | 1m12d | 51% any intervention | |||
| Bilateral Disease: at start /ever | yes / yes | yes / yes | no / yes | yes / yes | yes / yes | ~60% ever | 40% / 46% | 41% / 47% | |
| Total Catheterizations | 3 | 2 | 7 | 2 | 1 | ||||
| Age at PVS Surgery | 2m10d | -- | 4m12d | 3m25d | 1m14d | median 11.6mo (2.6 – 154) | |||
| Time to Recurrence | 2m8d | 26d | 15d | 2mo | 1m7d | 95% rate of recurrence | 74% rate of recurrence | ||
| Research Drugs | completed 12-month trial | -- | completed 12-month trial | -- | -- | ||||
| Age at Death | -- | 7 weeks | -- | 11 months | 3m8d | 57% at 2yr, 50% died at median 5.5mo | 52% total, 81% w/in 4 mo after diagnosis | 30% at 6mo 45% at 1yr 50% at 2yr | 47% total (median 10.8mo), median f/u 3mo |
| Follow-up Length/ Current Age | 32 mo / 2yr10mo | 7 weeks / -- | 14 mo / 16 months | 10 months / -- | 2 months / -- | median 18mo (2 – 185) |
Abbreviations: APVC (anomalous pulmonary venous connection), TAPVC (totally APVC), HLHS (hypoplastic left heart syndrome).
A pathophysiologic connection that explains the overrepresentation of patients with SLOS in our cohort of PVS has not yet been established. Studies investigating the genetic basis for PVS are limited. A single genome-wide linkage analysis study of a consanguineous Turkish family with PVS found a locus that mapped to chromosome 2q35–2q36.1. Although only 12 candidate genes of the 88 genes located in the region were sequenced, no disease-causing mutations were identified. Despite this, several genes were related to angiogenesis, including angio-associated migratory cell protein (AAMP), ephrin receptor A4 (EPHRA4), sphingosine-1-phosphate phosphatase-2 (SGPP2), C-terminal domain of small phosphatase 1 (CTDSP1), secretogranin II (SCG2), potassium voltage-gated channel, Isk-related family, member 4 (KCNE4), serine/threonine kinase 16 (STK16), serine/threonine protein kinase 36 (STK36), and serine/threonine protein kinase 11 interacting protein (STK11IP).28 An analysis of our institution’s PVS cohort revealed chromosomal abnormalities or a genetic diagnosis in nearly 70% of the subjects.3 To date, the genetic basis of PVS remains unknown.
In contrast, there has been significant progress in explaining how the metabolic derangement associated with SLOS translates into structural malformations and cognitive disabilities.15 Elevated levels of 7-DHC have been found to be cytotoxic to neurons due to increased oxidative stress induced by 7-DHC peroxidation,29 and to promote anti-proliferative effects.30 The anti-proliferative effects of 7-DHC are supported further by studies in a mouse model of SLOS that demonstrated altered pulmonary alveolar vascular development.31 7-DHC can also affect cellular signaling pathways by replacing cholesterol in lipid rafts, which contain a number of signaling molecules and receptors that regulate endothelial homeostatic functions and vascular smooth muscle cell/myofibroblast proliferation. These molecules and receptors include endothelial nitric oxide synthase, NADPH oxidase, the VEGF receptor, and the TGF-β receptor.32 TGF- β, in particular, has been shown to play a role in the pathophysiology of “upstream” PVS, by modulating endothelial-to-mesenchymal transformation and intimal thickening.33 In addition, some of the genes related to PVS are known to interact with and modulate these signaling intermediaries. It is also possible that one or more 7-DHC-derived oxysterols may be involved in signaling pathways that regulate myofibroblast proliferation and vascular remodeling.34
Intriguingly, many of the congenital malformations associated with SLOS can be linked to altered sonic hedgehog (SHH)-Patched/Smoothened-Gli signaling.35,36 SHH is a signaling molecule instrumental in pattern formation within the developing embryo and relies on post-translational modification with cholesterol in order to activate fully. SHH expression regulates cardiopulmonary mesoderm progenitors, such as the cardiac neural crest cells and second heart field cells, that are required for connecting the pulmonary vasculature to the heart.37 Alterations in the hedgehog signaling cascade could explain the association between SLOS and anomalous pulmonary venous connection. Genetic studies have identified STK36, which is a modifier of Gli function, in a PVS disease locus.28
Recent work using induced pluripotent stem cells derived from patients with SLOS identified downregulation of the canonical Wnt/β-catenin signaling pathway as a key mediator of neuronal abnormalities. This study demonstrated that 7-DHC has a direct inhibitory effect on formation of a functionally active Wnt receptor complex.38 Wnt signaling has been shown to regulate ephrin receptor expression (EPHRA4), which was identified as an important gene in the PVS disease locus.28,39
Finally, the association between premature birth and primary PVS may provide insight into the intimal proliferation seen in PVS. The premature infant is prone to diseases of inflammation, such as BPD and retinopathy of prematurity (ROP), and inflammation is a known factor in the initiation of vascular intimal proliferation.40 BPD is a neonatal inflammatory condition of immature pulmonary capillary beds41 that is also associated with abnormal pulmonary vasculature.42 ROP is caused by altered retinal vasculogenesis that can incited by the inflammatory state.43 Alterations in the mechanism of cell signaling and inflammation regulation due to the low cholesterol and elevated 7-DHC levels in SLOS may play a role in the predisposition of children with SLOS for the development of the vascular intimal proliferation seen in PVS. For example, cholesterol has been shown to play a role in VEGF-related signaling, which could impact vascular proliferation.44 In addition, the oxidative stress that is caused by 7-DHC34 is likely at its peak in the pulmonary venous bed, as the partial pressure of oxygen is highest in these vessels. This stress may promote intimal proliferation in the pulmonary veins.
Ultimately, we can only propose hypotheses on the pathophysiological and embryological connections between Smith-Lemli-Opitz syndrome and pulmonary vein stenosis. Despite ascertainment bias in our unique cohort of children with PVS who present for further treatment to our institution, the significantly increased incidence of SLOS in our cohort suggests that there are possible connections between the two conditions. Similar descriptive studies of PVS cohorts have also incidentally included rare subjects with SLOS.1,23 Further studies into the pathophysiology of PVS should consider the role of cholesterol-based signaling pathways in the promotion of intimal proliferation. There is insufficient evidence at this time to suggest that the natural history of PVS associated with SLOS is different than non-syndromic PVS. Infants newly diagnosed with PVS who have suggestive syndromic presentation should be screened for SLOS with easily obtainable serum sterol tests (cholesterol and 7-DHC levels). Additionally, clinical providers who care for children newly diagnosed with SLOS should be cognizant that an initially normal echocardiogram does not rule out the potential for rapid development and progression of pulmonary vein stenosis. As with non-syndromic PVS, early echocardiograms may be normal prior to the development of a life-threatening cardiac condition. Development of unexplained respiratory distress or poor growth should prompt repeat echocardiographic investigation and consideration should be made to repeat the study in children with SLOS at two to three months of life even without symptoms of PVS. Upon diagnosis of this complication, urgent referral to a PVS center will be necessary to initiate appropriate therapies.
Acknowledgments
Judith Geva, Christina Ireland, Kim Gavreau, David Kane, and Steve Fliesler for helpful discussions and suggestions.
Disclosure of Grants or Other Funding:
The efforts of A.R. Prosnitz are supported by the following grants:
NIH training grant “Research Methods in Pediatric Heart Disease Children’s Hospital Boston T32 (NIH 2 T32HL007572).”
The Program in Clinical and Translation Science (PCaTS) at Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University.
Footnotes
Conflict of Interest:
Dr. Jenkins: Novartis (Free drug for research)
Author Contributions:
Aaron Prosnitz, M.D.: Concept/Design, Data analysis/interpretation, Drafting article, Critical revision of article, Statistics, Data Collection
Jane Leopold, M.D.: Concept/Design, Critical revision of article, Approval of article
Mira Irons, M.D.: Concept/Design, Critical revision of article, Approval of article
Kathy Jenkins, M.D., M.P.H.: Concept/Design, Data analysis/interpretation, Critical revision of article, Approval of article
Amy Roberts, M.D.: Concept/Design, Data analysis/interpretation, Critical revision of article, Approval of article
Contributor Information
Aaron R. Prosnitz, Department of Cardiology, Boston Children’s Hospital, Boston, MA
Jane Leopold, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA
Mira Irons, American Board of Medical Specialties, Chicago, IL
Kathy Jenkins, Department of Cardiology, Boston Children’s Hospital, Boston, MA
Amy E. Roberts, Department of Cardiology, Boston Children’s Hospital, Boston, MA
References
- 1.Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: prematurity and associated conditions. Pediatrics. 2008;122(3):e656–61. doi: 10.1542/peds.2008-0075. [DOI] [PubMed] [Google Scholar]
- 2.Holt DB, Moller JH, Larson S, Johnson MC. Primary pulmonary vein stenosis. Am J Cardiol. 2007;99(4):568–572. doi: 10.1016/j.amjcard.2006.09.100. [DOI] [PubMed] [Google Scholar]
- 3.Chen MH, Debbaut C, McEnaney K, et al. Clinical and Genetic Features of Pulmonary Vein Stenosis [abstract] Journal of the American College of Cardiology. 2014;63:A524. doi: 10.1016/S0735-1097(14)60524-6. In: Journal of the American College of Cardiology. [DOI] [Google Scholar]
- 4.Sadr IM, Tan PE, Kieran MW, Jenkins KJ. Mechanism of pulmonary vein stenosis in infants with normally connected veins. Am J Cardiol. 2000;86(5):577–579. doi: 10.1016/S0002-9149(00)01022-5. [DOI] [PubMed] [Google Scholar]
- 5.Riedlinger WFJ, Juraszek AL, Jenkins KJ, et al. Pulmonary vein stenosis: expression of receptor tyrosine kinases by lesional cells. Cardiovasc Pathol. 2006;15(2):91–99. doi: 10.1016/j.carpath.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Quinonez LG, Gauvreau K, Borisuk M, et al. Outcomes of surgery for young children with multivessel pulmonary vein stenosis. J Thorac Cardiovasc Surg. 2015 Jul; doi: 10.1016/j.jtcvs.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 7.Peng LF, Lock JE, Nugent AW, Jenkins KJ, McElhinney DB. Comparison of conventional and cutting balloon angioplasty for congenital and postoperative pulmonary vein stenosis in infants and young children. Catheter Cardiovasc Interv. 2010;75(7) doi: 10.1002/ccd.22405. NA-NA. [DOI] [PubMed] [Google Scholar]
- 8.Shuhaiber J, Rehman M, Jenkins K, Fynn-Thompson F, Bacha E. The role of surgical therapy for pulmonary vein atresia in childhood. Pediatr Cardiol. 2011;32(5):639–645. doi: 10.1007/s00246-011-9942-7. [DOI] [PubMed] [Google Scholar]
- 9.Rehman M, Jenkins KJ, Juraszek AL, et al. A prospective phase II trial of vinblastine and methotrexate in multivessel intraluminal pulmonary vein stenosis in infants and children. Congenit Heart Dis. 2011;6(6):608–623. doi: 10.1111/j.1747-0803.2011.00574.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Ide H, Fu YY, et al. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J Thorac Cardiovasc Surg. 2014;148(6):2550–2558. doi: 10.1016/j.jtcvs.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Western K, Kieran M, Baird C, et al. 48 Week Outcomes Using Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Pulmonary Vein Stenosis. Circulation. 2014;130(Suppl 2):A12244-A12244. http://circ.ahajournals.org/content/130/Suppl_2/A12244.abstract. [Google Scholar]
- 12.Latson La, Prieto LR. Congenital and Acquired Pulmonary Vein Stenosis. Circulation. 2006;115(1):103–108. doi: 10.1161/CIRCULATIONAHA.106.646166. [DOI] [PubMed] [Google Scholar]
- 13.Breinholt JP, Hawkins JA, Minich L, et al. Pulmonary vein stenosis with normal connection: Associated cardiac abnormalities and variable outcome. Ann Thorac Surg. 1999;68(1):164–168. doi: 10.1016/S0003-4975(99)00311-2. [DOI] [PubMed] [Google Scholar]
- 14.Kelley RI. A new face for an old syndrome. Am J Med Genet. 1997;68(3):251–256. doi: 10.1002/(sici)1096-8628(19970131)68:3<251::aid-ajmg1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Nowaczyk MJM, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012;160C(4):250–262. doi: 10.1002/ajmg.c.31343. [DOI] [PubMed] [Google Scholar]
- 16.Porter FD. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2008;16(5):535–541. doi: 10.1038/ejhg.2008.10. [DOI] [PubMed] [Google Scholar]
- 17.Waterham HR, Hennekam RCMM. Mutational spectrum of Smith-Lemli-Opitz syndrome. Am J Med Genet C Semin Med Genet. 2012;160C(4):263–284. doi: 10.1002/ajmg.c.31346. [DOI] [PubMed] [Google Scholar]
- 18.Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, et al. Mutational Spectrum in the D7-Sterol Reductase Gene and Genotype- Phenotype Correlation in 84 Patients with Smith-Lemli-Opitz Syndrome. Am J Hum Genet. 2000;66:402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin AE, Ardinger HH, Ardinger RH, Cunniff C, Kelley RI. Cardiovascular malformations in Smith-Lemli-Opitz syndrome. Am J Med Genet. 1997;68(3):270–278. [PubMed] [Google Scholar]
- 20.Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am J Med Genet. 1997;68(3):315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Katheria AC, Masliah E, Benirschke K, Jones KL, Kim JH. Idiopathic persistent pulmonary hypertension in an infant with Smith-Lemli-Opitz syndrome. Fetal Pediatr Pathol. 2010;29(6):373–379. doi: 10.3109/15513815.2010.512045. [DOI] [PubMed] [Google Scholar]
- 22.Quélin C, Loget P, Verloes A, et al. Phenotypic spectrum of fetal Smith-Lemli-Opitz syndrome. Eur J Med Genet. 2012;55(2):81–90. doi: 10.1016/j.ejmg.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Seale AN, Webber SA, Uemura H, et al. Pulmonary vein stenosis: the UK, Ireland and Sweden collaborative study. Heart. 2009;95(23):1944–1949. doi: 10.1136/hrt.2008.161356. [DOI] [PubMed] [Google Scholar]
- 24.Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37(5):321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianconi SE, Conley SK, Keil MF, et al. Adrenal function in Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2011;155A(11):2732–2738. doi: 10.1002/ajmg.a.34271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, et al. Mutational spectrum in the Delta7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am J Hum Genet. 2000;66(2):402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song MK, Bae EJ, Jeong SI, et al. Clinical characteristics and prognostic factors of primary pulmonary vein stenosis or atresia in children. Ann Thorac Surg. 2013;95(1):229–234. doi: 10.1016/j.athoracsur.2012.08.104. [DOI] [PubMed] [Google Scholar]
- 28.van de Laar I, Wessels M, Frohn-Mulder I, et al. First locus for primary pulmonary vein stenosis maps to chromosome 2q. Eur Heart J. 2009;30(20):2485–2492. doi: 10.1093/eurheartj/ehp271. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82(3):496–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51(11):3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Wessels A, Chen J, et al. Late gestational lung hypoplasia in a mouse model of the Smith-Lemli-Opitz syndrome. BMC Dev Biol. 2004;4:1. doi: 10.1186/1471-213X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Front Physiol. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H, Fu YY, Zhu J, et al. Pulmonary vein stenosis and the pathophysiology of “upstream” pulmonary veins. J Thorac Cardiovasc Surg. 2014;148(1):245–253. doi: 10.1016/j.jtcvs.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ. The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Prog Retin Eye Res. 2015;45:111–131. doi: 10.1016/j.preteyeres.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter Ja, Young KE, Beachy Pa. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 36.Cooper MK, Wassif CA, Krakowiak PA, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33(4):508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 37.Peng T, Tian Y, Boogerd CJ, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500(7464):589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis KR, Ton AN, Xin Y, et al. Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/β-catenin defects in neuronal cholesterol synthesis phenotypes. Nat Med. 2016;22(4):388–396. doi: 10.1038/nm.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13(1):39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- 40.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4(1):104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balany J, Bhandari V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front Med. 2015;2:90. doi: 10.3389/fmed.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42(4):839–855. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Béliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14(1):334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]