Abstract
Trauma to either the central or peripheral nervous system often leads to significant loss of function and disability in patients. This high rate of long-term disability is due to the overall limited regenerative potential of nervous tissue, even though the peripheral nervous system (PNS) has more regenerative potential than the central nervous system (CNS). The supporting glial cells in the periphery, Schwann cells, are part of the reason for the improved recovery observed in the PNS. In the CNS, the glial populations, astrocytes and oligodendrocytes, do not have as much potential to promote regeneration and are at times inhibitory to neuronal growth. In particular, the inhibitory roles astrocytes play following trauma has led to a historical focus on neurons and oligodendrocytes instead of astrocytes. Recently, this focus has shifted as new, regenerative astrocyte phenotypes have been described. From these observations, glial cells clearly play critical roles in native recovery pathways in both the CNS and PNS. This makes the ability to manipulate both transplanted and native glial cell phenotypes a potentially successful strategy to improve nerve injury outcomes. This review focuses on factors that cause glial cells to adopt repair phenotypes and biomaterials that manipulate and/or harness these glial phenotypes.
Keywords: Astrocytes, Schwann Cells, Oligodendrocytes, Spinal Cord Injury
Introduction
Central and peripheral nerve trauma leads to a significant burden for patients due to the long-term loss of function that often results from these injuries. Nervous system injuries also have a high lifetime cost for patients because loss of function often leads to some level of disability that can be lifelong, particularly in the case of CNS injury. Peripheral nerve trauma is a relatively common injury, with about 100,000 cases annually in the United States and Europe and, despite the higher regeneration capacity of the peripheral system, repair surgeries are frequently not successful [1]. In the central nervous system (CNS), the most common form of injury is stroke, which represents the most common cause of disability in the world [2]. Another, significantly less common, form of CNS injury is spinal cord injury (SCI) with an annual incidence of 17,000 new cases a year in the United States with an estimated 243,000 to 347,000 Americans living with chronic SCI [3]. Due to the extreme long-term burden on patients, there is significant interest in the development of novel biomaterials and strategies to improve clinical outcomes from nervous system injuries. These strategies have focused on numerous modalities to improve neuronal growth including removal of inhibitory molecules, growth factor delivery, cell replacement therapies, and combinations of these approaches [4,5]. There, however, has been relatively little focus on the roles played by glial cells in neural regeneration, especially within the CNS.
Glia perform many important functions to support neurons within both the PNS and CNS. This review focuses on astrocytes, and oligodendrocytes (OLs) with some discussion of Schwann Cells (SCs) as well due to their higher native regenerative support. In the CNS, astrocytes are responsible for maintenance of the blood-brain barrier, disposal of toxic metabolites by neurons, signal transduction through tripartite synapses, and water homeostasis; and, OLs are responsible for myelination of the axons. In the PNS, SCs, which are generally divided into either myelinating or non-myelinating populations, are the major glial cell population and are responsible for both myelination and trophic support of axons. The higher regenerative potential of the PNS has been attributed to the ability of SCs to dedifferentiate into a pro-repair phenotype following injury [6]. In contrast to this recognized regenerative role of SCs, astrocytes have been historically thought to be predominantly inhibitory to nerve growth after injury. Recently; however, there is a growing body of work that shows astrocytes are necessary for recovery following SCI [7,8]. In contrast to astrocytes, the capacity for OLs to improve recovery following trauma has been much more extensively studied via cell transplantation studies that have found that human iPSCs and ESCs can be pre-differentiated into oligodendrocyte precursors (OPCs), and that these cells are able to promote myelination of spared, demyelinated fibers following SCI [9,10]. This review focuses on what is known of the pro-regenerative roles played by different glial cell phenotypes in central nervous system repair, and biomaterials that are designed to harness or manipulate these phenotypes.
Role of Schwann Cell Phenotypes in PNS Regeneration
It is important to consider the role of SCs in PNS regeneration since the significantly greater capacity for PNS repair is generally attributed to SCs. SCs are generally divided into either myelinating or non-myelinating populations, and have a well-defined, pro-regenerative response to injury. In the context of nerve trauma, SCs distal to the injury from both populations de-differentiate and adopt a pro-repair phenotype, sometimes called a Büngner SC. This phenotypic switch is dependent on the transcription factor c-Jun and is characterized by the suppression of myelin genes and the activation of trophic factors that support neurite extension. The dynamics of this SC phenotypic switch is nicely review by Jessen and Mirsky [6]. Following c-Jun-driven dedifferentiation, remyelination of the regenerated peripheral axons occurs in response to neuron-derived neuregulin-1 (NRG1)-type III binding to ErbB2 and ErbB3 receptors on the repair SCs [11]. The roles of different NRG1 isoforms in this remyelination process is reviewed by Gambarotta et al. [12]. This same repair pathway is believed to largely be absent in OL and astrocytes, leading to the decreased regenerative potential of the CNS. However, NRG1 has also be associated with the limited amount of remyelination that occurs following SCI, suggesting some similar regenerative pathways do exist centrally, even though they are harder to activate [13]. It is an area of interest in current CNS therapies to find ways to activate these OL and astrocyte regenerative pathways.
In addition to the well-defined SC repair program described above, it has been observed that there is a phenotypic preference for sensory neurons to grow on sensory SCs and motor neurons to grow on motor SCs. This effect was first observed in femoral nerve injury, where axons would regenerate specifically to either motor or sensory targets, even if an intentional mismatch was created [14]. Further investigation revealed that this targeting specificity was partially tied to SC phenotype with motor neurons extending longer neurites over motor SCs, while sensory neurons extended longer neurites over sensory SCs [15,16]. Considerably less is known about what causes these functional differences between sensory and motor SCs, making this an area of active investigation since many peripheral nerve grafts have a sensory/motor mismatch. What is known is that both sensory and motor SCs can differentiate away from the repair SC phenotype into myelinating (or non-myelinating) SCs in vitro through the use of glial-derived neurotrophic factor (GDNF) signaling via Fyn kinase [17]. Furthermore, it has been demonstrated that SCs maintain their motor/sensory phenotypic identity in vitro, even without the presence of axons; however, GDNF treatment prior to the introduction of neurons can be used to mitigate this effect in the case of a mismatch between axon and SC [18].
These differences between motor and sensory SCs in terms of neuronal support has been successfully mimicked with acellular materials. Specifically, the combination of collagen and laminin matrix along with nerve growth factor and neurotrophin-3 containing poly(lactic-co-glycolic acid) (PLGA) microspheres specifically increased the regeneration of sensory neurons and improved sensory recovery. In contrast, collagen and fibronectin with brain-derived neurotrophic factor in PLGA microspheres preferentially increased the regeneration of motor neurons and enhanced motor recovery following a sciatic nerve transection [19]. The use of acellular material systems is particularly appealing for clinical translation since these materials do not require patients to receive immune suppression, thereby eliminating many complications and contraindications. Hopefully, as the defining features of the different reactive astrocyte populations and OLs are elucidated, similar approaches can be used for CNS injury treatment, although the presence of multiple glia cell types within the CNS significantly complicates regeneration.
Role of Astrocyte Phenotypes in CNS Regeneration
There are many different astrocyte subpopulations found in different CNS regions that support normal neuronal function. Following trauma, astrocytes become reactive and participate in the formation of a glial scar that has historically been thought to be a primary inhibitor of CNS regeneration. The concept of astrocytes as inhibitors of regeneration is supported by the physical barrier created by astrocyte processes [20,21], as well as astrocytic production of molecules (e.g. chondroitin sulfate proteoglycans (CSPGs)) that are inhibitory to neuronal growth [22]. In order to remove astrocyte-associated inhibitory effects, astrocyte reactivity was knocked-out in mice prior to SCI. Astrocyte knockout, either via vimentin and glial fibrillary acidic protein (GFAP) double knock out or conditional knockout of STAT3 (a required factor for astrocyte reactivity) in astrocytes, was found to increase the size of the SCI lesion cavity and increase immune cell infiltration [23,24]. Similarly, GFAP (−/−) Vimentin (−/−) mice that have attenuated astrocyte reactivity have been found to have worse functional recovery following cerebral cortical stroke [25].
Since astrocytes clearly serve an important role in limiting secondary injury immediately following CNS injury, delayed astrocyte ablation was performed using a GFAP-driven thymidine kinase and ganciclovir injections. However, even in the case of delayed ablation, the lack of astrocytes led to decreased functional recovery [7,26]. These knockout studies suggest that astrocytes play a role in the formation of the inhibitory scar, and a role in the creation of an environment that is permissive to axon regeneration. The concept of pro-regenerative astrocytes is supported by the observation that neurites within a SCI lesion often co-localize with “GFAP+ bridge” in both mice [27] and zebrafish [28]. Overall these recent studies suggest that astrocytes are an overlooked cell population that plays a key role in promoting recovery following SCI.
Variability in Astrocyte Reactivity
One explanation of the observed duality in astrocytic roles following SCI is that different reactive astrocyte populations are involved in scar formation versus bridge formation. This hypothesis is supported by the inherent heterogeneity of astrocytes [29], and the known differences in the role and function of different astrocyte subpopulations within brain regions [30]. In support of the idea that there is heterogeneity in astrocyte reactivity, it has been observed that astrocyte gene expression changes depending on whether the CNS insult was ischemic or inflammatory. Ischemic injury has been found to lead pro-regenerative reactive astrocytes, while inflammatory insults leads to inhibitory reactive astrocytes [31]. This injury type-dependent reactivity has led to the concept that astrocytes may have two types of reactive polarization, similar to macrophages and microglia, which have been termed A1 or A2 reactive astrocytes. A1 (pro-inflammatory) astrocytes have “harmful” functions, such as synapse destruction, while A2 reactive astrocytes have “helpful” (pro-regenerative) functions. The heterogeneity of astrocyte reactivity is a newly appreciated concept and has been reviewed by Liddelow and Barres [32].
In addition to the differences in astrocyte reactivity depending on insult, it has also been found that different brain regions behave differently in response to injury. While there are many astrocyte subtypes in the CNS, astrocytes can be broadly defined as either fibrous (found in white matter) or protoplasmic (found in grey matter). Interestingly, studies looking at the reactivity of white matter and grey matter astrocytes following CNS injury have found significant differences in how astrocyte morphology changes in response to injury. Optic nerve crush and corpus callosum injury studies have shown that fibrous astrocytes initially retract their processes following insult and then re-extend them leading to a significant increase in the area covered by each astrocyte. This process re-extension and hypertrophy leads to significant process overlap, which disrupts the normal lamellar structure of the white matter [20]. In contrast to this, filling studies performed on resting and reactive protoplasmic astrocytes show that reactive protoplasmic astrocytes exhibit some process hypertrophy, but they do not have increased overlap between adjacent astrocytes [33]. These observations together suggest that fibrous astrocytes are more involved in the creation of the physical barrier found in the glial scar environment, and that potentially a subset of protoplasmic astrocytes may be responsible for the formation of GFAP+ bridges across lesion cavities.
Astrocyte reactivity can also be directly manipulated to increase the presence of pro-regenerative astrocytes with growth factors and other signaling molecules. Metallothionein is one such factor that has been shown to induce astrocytes to become more pro-regenerative through both intracellular and extracellular actions [34]. Furthermore, delivery of metallothionein has been found to improve neuronal regeneration following an optic nerve crush injury [35]. Endogenous glial cells can also be manipulated by fibroblast growth factor 2 (FGF-2). Studies in both mice and zebrafish have shown that FGF-2 signaling facilitates glial bridge formation following SCI [28,36]. In addition, knockout of spry4, a FGF signaling inhibitor, has been found to reduce inflammatory response and decrease gliosis following SCI [37]. FGF-2 within lipid microtubules has been incorporated into collagen-based hydrogels leading to increased astrocyte infiltration into hydrogels in vitro [38].
There is evidence that astrocytes exhibit plasticity of their reactive phenotype based on the local environment. Astrocytes transplanted acutely into an SCI lesion, but not healthy spinal cord, have been shown to adopt an inhibitory phenotype. This phenotypic switch has been shown to be dependent on integrin-binding to collagen I within the scar. Inhibition of collagen I binding with an anti-β1 integrin antibody leads to increase axon penetration into the SCI lesion and improved behavioral recovery following a spinal cord contusion injury in mice [39].
Consistent with the classification of reactive astrocytes using the same system as macrophages, astrocytes express receptors and cytokines that are associated with the immune system. In particular, astrocytes are known to express toll-like receptor 4 (TLR4), suggesting a pathway for activation in response to lipopolysaccharide (LPS) [40]. Loss-of-function and gain-of-function studies of TLR4 and triggering receptor expressed on myeloid cells-2 (TERM-2), a negative regulator of TLR signaling, have shown that increased TLR4 activation with LPS increases pro-inflammatory gene expression by astrocytes. In contrast, increased TERM-2 signaling has been found to modulate this response by decreasing NF-κB activation, suggesting that NF-κB signaling could be an important regulator of pro-inflammatory astrocytes [41].
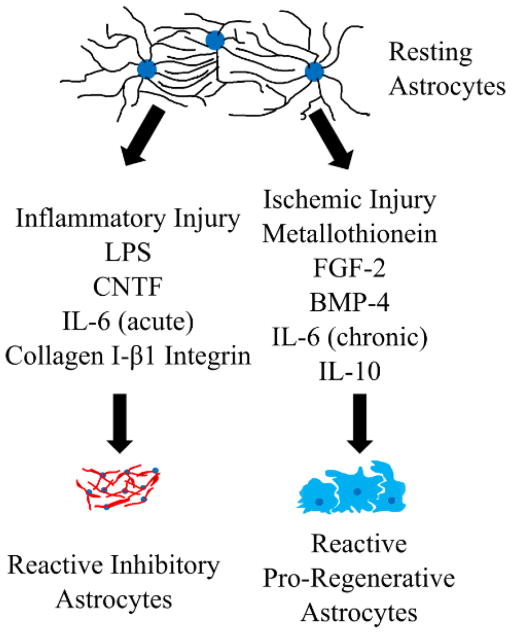
Astrocytes are also known to upregulate interleukin receptors in response to injury and to express some interleukins. Because of these expression profiles, it stands to reason that interactions with immune cells and their secreted factors may alter astrocyte phenotype. Two interleukins that have been extensively studied in astrocyte phenotype manipulation are IL-6 and IL-10. IL-6 is a pro-inflammatory cytokine that modulates CNS inflammation. IL-6 is produced by astrocytes after injury or infection [42], and the presence of IL-6 has been associated with astrocyte proliferation and scar formation, as well as immune cell infiltration in the acute phase following injury [43]. There is also evidence that in the subacute phase of SCI injury, IL-6 expression has pro-regenerative effects, suggesting a duality of roles for this molecule [44]. In contrast, IL-10 is an anti-inflammatory cytokine that is important for the resolution of the immune response throughout the body. In the context on CNS injury, IL-10 has been delivered intrathecally and intramuscularly to improve functional recovery [45]. Furthermore, astrocyte-specific production of IL-10 has been shown to increase immune cell infiltration, but also increases motor neuron survival following a facial nerve axotomy [46]. There has been some work using flavopiridol, a cell-cycle inhibitor, as a way to alter the interleukin expression from astrocytes. In particular, flavopiridol delivery from PLGA nanoparticles was found to reduce astrocytic synthesis of pro-inflammatory cytokines, including IL-6, as well as increasing astrocyte-based IL-10 expression [47]. These observations of the astrocytic roles in immunomodulation suggest that anti-inflammatory signaling cascades used to alter the immune response in other organ systems may be able to alter reactive astrocyte phenotypes as well. Overall the manipulation of astrocyte heterogeneity is still being actively investigated and hold great potential to harness astrocytes as a regenerative population. The factors discussed here that have been found to alter reactive astrocyte phenotype are summarized in Figure 1.
Figure 1. Factors found to alter reactive astrocyte phenotype.
Recent work has demonstrated that heterogeneity exists with astrocytes following trauma with some factors causing inhibitory astrocytes with overlapping processes to develop and others cause a pro-regenerative population to arise.
Astrocyte Phenotype Affects Transplant Outcomes
The inherent functional differences between astrocyte subtypes have also been observed in SCI transplant studies. Glial restricted progenitors (GRPs) are a population of primary cells that can differentiate into either fibrous or protoplasmic astrocytes as well as OLs, but not neurons [48]. These cells have been successfully isolated from mouse, rat, and human embryonic spinal cords. Pre-differentiation of these cells into astrocytes using FGF-2, bone morphogenetic protein 4 (BMP-4) and N2 media supplement showed improved recovery of when compared to the transplantation of undifferentiated GRPs in a right-sided cervical hemisection SCI [49,50]. These BMP-4 differentiated astrocytes have a protoplasmic-like phenotype. Interestingly, when GRPs were pre-differentiated into fibrous-like astrocytes using ciliary neurotrophic factor (CNTF), they have a detrimental effect on recovery leading to decreased axon penetration into the injury site and increased allodynia [51,52]. Similar to other studies using primary cells, there has been heterogeneity in these findings, likely due to variations in the methods used, that affects the outcome of transplantation. When the cells are in a more immature state, transplantation of GRP-derived astrocytes has been found to improve axon penetration into the injury site, regardless of phenotype at the time of transplantation [53]. Further investigation of these GRP populations has demonstrated that one factor responsible for these observed functional difference is periostin-1, which is produced by BMP-4 exposed astrocytes, but not CNTF-exposed astrocytes [54]. Overall there is significant promise in astrocyte-based therapies, but there is much left to be elucidated in terms of the reactive states of different astrocyte subtypes and how different astrocyte subtypes effect axon growth and regenerative potential [55].
Role of Oligodendrocytes in CNS repair
OLs are the other primary glial population within the CNS and are responsible for the myelination of CNS axons. Myelination is the final step of recovery in the PNS and is critical for appropriate transmission of action potentials and protection of the axons. For these reasons, central glial transplantation studies have largely focused on OLs in order to increase the myelination of the axons that are able to grow into the lesion cavity. Native remyelination in the CNS is more difficult to achieve than in the PNS because mature OLs, unlike SCs, lack the capacity to produce new myelin sheaths [56]. This means that central remyelination must be carried out by dividing and differentiating oligodendrocyte precursor cells (OPCs), or infiltrating SCs [57,58]. There has been some work looking to how to activate local remyelination pathways without requiring cell transplantation. One interesting finding is that treatment of the spinal cord with a synthetic TLR4 agonist (E6020) accelerated myelin debris clearance and remyelination following a demyelinating injury with lysolecithin. This data shows that there is a clear role of macrophage activation in remyelination, and it is also worth noting that astrocytes express TLR4 so they may also play a role [59].
Unfortunately, there are not many native OPCs and their migration distance is limited [60], so transplantation of OPCs and control of their differentiation in vivo has been heavily explored. OPCs themselves do not produce myelin, so they must be differentiated into mature, myelinating OLs. Unfortunately, OLs have proven more difficult to differentiate from stem cells or progenitors than either astrocytes or neurons and transplantation of OPCs alone is insufficient for myelin production. Thus, to facilitate myelination, different biomaterials and growth factor cocktails have been tested. The ability of different materials to support OL differentiation and myelination has been reviewed by Russell and Lampe [61]. In particular, myelination has been observed in vivo following transplantation of fibrin gels [62], heparin-modified PLGA bridges [63], or HA and gelatin crosslinked by polyethylene glycol (PEG) diacrylate [64].
Primary OPCs have been transplanted, after having been cultured and modified to express ciliary neurotrophic factor, and were found to improved functional recovery and remyelinate the axons [65]. Similarly, it has been found that human induced pluripotent stem cells can be pre-differentiated into OPCs and that those cells are able to promote myelination following a thoracic contusion SCI [9]. Likewise, human embryonic stem cells (hESCs) can be differentiated into OPCs and have been found to improve remyelination and functional repair following contusion or complete transection SCI [10,66]. Extensive study of these hESC-derived OPCs has indicated that they are safe for clinical trial [67], which has led to an ongoing Phase I/II clinical trial sponsored by Asterias Biotherapeutics that has reported promising initial efficacy data [68]. Despite these early successes with this OPC population in humans, it is worth noting that a review of all SCI treatment studies using rodent-derived remyelinating populations found that there is significant inconsistencies in recovery findings, showing that more work is needed on understanding and manipulating myelinating glia populations [69].
Biomaterial Manipulation of Glial Phenotypes
Given the complexities of glial cell response to trauma, with some glia providing a pro-regenerative support and others tending to inhibit regeneration, there is significant potential benefit to use materials to manipulate the phenotypes of both transplanted and native glia. The major approaches used for these manipulations are modifying the mechanical properties, composition, growth factor delivery, and alignment of the materials. Here what is known about how these biomaterial factors affect the phenotype and differentiation of the major CNS glial populations is discussed.
Material Properties affecting cell fate
Matrix stiffness is a powerful tool for the manipulation of cell phenotype and the differentiation of neural progenitor cells (NPCs). NPCs have been shown to differentiate into OLs and neurons on softer HA-methacrylate hydrogels (3 kPa compressive bulk modulus) and generally differentiate into astrocytes on stiffer matrices (5 kPa compressive bulk modulus) [70]. Matrix elasticity has also been found to alter NPC differentiation on methacrylamide chitosan with increased astrocytic and neuronal differentiation observed on materials with Young’s moduli <1 kPa, while materials with 7 kPa moduli demonstrate decreased neuronal and astrocytic differentiation and increased OL differentiation, but not maturation [71]. In addition to OL differentiation being dependent on matrix stiffness, myelination by either SCs or OLs is affected by matrix elasticity with low elasticity matrices (1.5 kPa elastic modulus) allowing for increased myelin production by OLs with in vivo OL process extension and myelin production observed to be increased in HA gelatin hydrogels with Young’s moduli of 13.8 Pa [64,72] In contrast, high matrix elasticity (30 kPa elastic modulus) increases SC myelin production [72]. These elasticity differences are the result of non-muscle myosin II which has been found to be a positive myelin regulator in the PNS, but a negative myelin regulator in the CNS [72,73]. These studies demonstrate that material properties are an important consideration when designing scaffolds for either the CNS or PNS.
Consistent with the observation that material properties affect NPC differentiation, there is also evidence that integrin signaling is important for astrocytic differentiation from neural progenitor populations. In particular, the exposure of NPCs to IKVAV peptide amphiphile (IKVAV-PA) has been shown to increase neuronal differentiation and decrease astrocyte differentiation [74]. IKVAV is a short peptide sequence from the C-terminal of laminin α1 that represents one of the primary functional sites of laminin, and this sequence has been found to promote cell adhesion and neurite outgrowth [75]. This functionality of the IKVAV peptide, demonstrates the importance of ECM in NPC differentiation, and the amphiphile structure used for presentation of the peptides allows for a significant increase in epitope density. Delivery of IKVAV-PA has also been shown to decrease glial scar density, and increase oligodendrocyte infiltration following a compressive SCI in mice [76]. The observed effect of IKVAV-PA particles has been partially attributed to β1-integrin signaling with both ESCs and subventricular zone NPCs demonstrating increased astrocyte differentiation in the presence of IKVAV when β1-integrin is knocked out. The importance of β1-integrin signaling in functional recovery following SCI is further suggested by observed behavioral improvements in mice treated with 2 other integrin binding peptide amphiphiles, RGD-PA or ADEGVFDNFVLK (Tenascin C)-PA [77].
Materials that increase oligodendrocyte myelination following CNS trauma
Since myelination is a critical step in recovery from nervous system trauma, the ability of implanted materials to increase the percent of myelinated fibers could have significant clinical utility. Since remyelination occurs late following SCI and native OLs don’t have a high capacity for remyelination, the ability of materials to promote local remyelination in vivo has not been extensively studied. A comparative study of different substrates effect on myelination in vitro by myelinating cultures composed of primary cells isolated from E15 rat spinal cords found that low molecular weight ε-polycaprolactone (PCL) increased myelination compared to polycarbonate, poly(methyl) methacrylate, polystyrene, poly-L-lactide, polydimethylsiloxane, and high molecular weight PCL. These studies also showed a clear effect on astrocyte phenotype on myelination with media conditioned by astrocytes cultured on PCL decreasing OL myelination, while media conditioned by astrocytes cultured on glass increased OL myelination [78]. This suggests that astrocyte phenotype should be considered when designing materials to increase remyelination following CNS injury and that the myelination observed in this study may be secondary to astrocyte phenotype alteration. Electrospun PCL has also been found to increase in vitro differentiation and myelination by OPCs. Furthermore, the use of PCL-gelatin nanofibers was found to increase the percentage of myelin wrapped fibers in vitro [79]. In the context of PNS injury, it has been observed that the implantation of electrospun PCL-PLGA conduits into a 10 mm sciatic nerve gap increased myelination and collagen IV deposition within the injury and improved behavioral recovery [80]. Further work is required to determine what mechanism explains these observed effects of PCL on both SC and OL myelination.
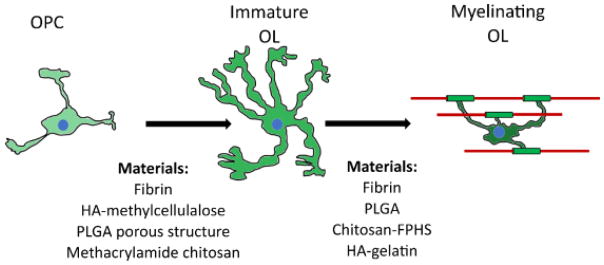
There have been some acellular in vivo implantation studies that have shown increased myelin production. One strategy is to deliver sonic hedgehog (SHH) and neurotrophin-3 (NT-3). These factors were delivered following a lateral hemisection SCI using lentivirus within a multiple channel bridge composed of PLGA. The inclusion of NT-3 was found to increase myelination by infiltrating SCs, while SHH over-expression significantly increased OL myelination [63]. Another material found to increase myelination is a Chitosan Fragmented Physical Hydrogel suspension (Chitosan-FPHS). This material was found to increase myelination in rats at 4, 8 and 10 weeks after a dorsal overhemisection SCI from both SCs and OLs. Interestingly, the OL myelination observed in this study was restricted to regions in which astrocytes were able to infiltrate the Chitosan-FPHS [81]. The materials that have been found to affect myelination and OL phenotype/differentiation are summarized in Figure 2.
Figure 2. Materials Altering Oligodendrocyte Differentiation and Myelination.
The materials known to impact OPC differentiation (left side) are drawn from Russell and Lampe [61]. Materials promoting oligodendrocyte myelination (right side) focuses on acellular implantation studies.
Materials that Alter Astrocyte Phenotypes
Astrocyte phenotype and penetration is an important consideration for CNS repair materials since astrocytes are known to support neuronal growth and to be required for the survival of certain neuronal populations. It has been found in many biomaterials that neuronal growth into the material is correlated with astrocytes or their processes [82,83]. Thus, it is crucial to consider astrocytic response to transplanted materials and how to push native astrocytes away from a scar phenotype toward a more pro-regenerative state. One material that has been shown to limit inhibitory astrocyte formation is high molecular weight (MW) hyaluronic acid (HA). Acute transplant of high MW HA following dorsal hemisection SCI decreased immune cell infiltration and CSPG deposition [84]. The same is not true of small (40–400 kDa) HA chains, which have been shown to activate NF-κB in astrocytes and so upregulate inhibitory reactive astrocytes [85]. Similar to this observation with HA, implantation of fibrin scaffolds has also be found to slow the accumulation of reactive astrocytes around a SCI lesion [62].
Matrix alignment has been shown to be a powerful tool in glial manipulations with glia cells, and neurons, aligning to a provided matrix. Unfortunately, alignment is extremely difficult to achieve in vivo, so much of what is known about alignment effects is based on in vitro data. Aligned cell morphology has been shown to result in increased neurite outgrowth in both 2 and 3 dimensions [86,87]. Furthermore it has been shown that, when aligned, astrocytes enhance neuronal growth [88]. In addition, randomly-aligned, electrospun polyamide nanofibers have been found to decrease astrocyte process hypertrophy and GFAP expression in vitro compared to poly-L-lysine on either glass or Aclar when exposed to an inflammatory stimulus (dibutyryladenosin cyclic monophosphate) [89,90]. This suggests that astrocytes may adopt a more quiescent phenotype when on a fibrillar surface in vitro, regardless of alignment.
Since electrospinning can be used to generate alignment, the benefit of fibrillar matrices and alignment has been widely used material starting point for astrocyte manipulations in vitro. Study of astrocytes cultured on aligned collagen fibers in vitro have found that astrocytes in an aligned environment decrease expression of GFAP, a hallmark of astrocyte reactivity, and elongated in the direction of alignment. Furthermore, these structures could be rolled into 3D conduits that maintain their alignment growth benefits [91]. Further manipulation of collagen fibers with other matrix proteins can be used to improve astrocyte alignment and decrease the expression of CSPGs, a major class of inhibitory proteins. In particular, fibrinogen coating significantly increased alignment with collagen fibers, while aggrecan, laminin, and fibrinogen, but not fibronectin decreased CSPG expression of cultured astrocytes [92].
Astrocytes cultured on aligned PLLA materials have also been found to elongated and upregulate the two major glutamate transporters, glutamate transporter 1 (GLT-1) and glutamate and aspartate transporter 1 (GLAST) [93]. The presence of these transporters is important to the support of excitatory, glutamatergic neurons that are unable to process glutamate, which leads to excitotoxicity. In addition to alignment effects, fiber size also impacts astrocyte phenotypes with 400 nm silk fibers inducing longer astrocyte process extension, increased area per astrocyte, and improved neuronal maturation when compared to 1200 nm fibers [94]. Increasing the stiffness of 400 nm cellulose acetate nanofibers from a tensile modulus of around 24 MPa to around 80 MPa resulted in an increase in astrocyte attachment, proliferation, and ECM deposition [95]. It is important to note that alignment is difficult to achieve in an implantation setting, and so the clinical utility of alignment is currently limited. However, randomly aligned, coated electrospun fibers could have significant potential as a strategy to manipulate glial populations. Electrospun, randomly aligned PCL scaffolds have been seeded with human endometrial stem cells and transplanted following dorsal hemisection in rats. The PCL scaffold implant was found to slightly increase neurite growth into the SCI lesion, demonstrating the potential of electrospun scaffolds [96]. The material effects on astrocyte reactivity discussed here are summarized in Table 1. There is still significantly more work required to fully define how astrocyte reactivity is regulated and how to promote pro-regenerative astrocytes following injury.
Table 1.
Materials found to Alter Astrocyte Reactivity
| Material | Astrocyte Effect | Source |
|---|---|---|
|
| ||
| High MW HA in vivo | Decreased CSPG | [84] |
| Low MW HA in vivo | Increased Axonal Inhibition | [85] |
| Fibrin in vivo | Decreased GFAP | [62] |
| Electrospun polyamide | Decreased GFAP and process hypertrophy | [89,90] |
| Fibrinogen coated aligned collagen fibers | Decreased CSPG and GFAP | [92] |
| Smaller fiber diameter | Longer processes, improve neuronal maturation | [94] |
| Increased matrix stiffness | Increased ECM deposition and proliferation | [95] |
Concluding Remarks
Glia are the primary supportive cells of the CNS and PNS and so represent a powerful tool in efforts to increase the support provided by transplanted materials to neurons following nervous system injury. Recent work has demonstrated that there is significant heterogeneity in glial responses to injury, with some glia being better suited to promoting regeneration than others. The manipulation of these glial phenotypes represents a wide new area for novel biomaterial approaches to explore.
Acknowledgments
The authors would like to thank the University of Texas at Austin, the Washington University Medical Scientist Training Program (NIH T32 GM07200), and the support of the NIH (NINDS R01 NS090617).
References
- 1.National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2013. [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Of A. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2013;37:479–80. doi: 10.1179/1079026814Z.000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Führmann T, Anandakumaran PN, Shoichet MS. Combinatorial Therapies After Spinal Cord Injury: How Can Biomaterials Help? Adv Healthc Mater. 2017;6:1601130. doi: 10.1002/adhm.201601130. [DOI] [PubMed] [Google Scholar]
- 5.Tsintou M, Dalamagkas K, Seifalian A. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10:726. doi: 10.4103/1673-5374.156966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–31. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukovic D, Valdés-Sanchez L, Sanchez-Vera I, Moreno-Manzano V, Stojkovic M, Bhattacharya SS, Erceg S. Brief report: Astrogliosis promotes functional recovery of completely transected spinal cord following transplantation of hESC-derived oligodendrocyte and motoneuron progenitors. Stem Cells. 2014;32:594–599. doi: 10.1002/stem.1562. [DOI] [PubMed] [Google Scholar]
- 9.Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M, Shibata S, Kohyama J, Iwanami A, Toyama Y, Matsumoto M, Nakamura M, Okano H. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Reports. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erceg S, Ronaghi M, Oria M, Roselló MG, Aragó MAP, Lopez MG, Radojevic I, Moreno-Manzano V, Rodríguez-Jiménez F-J, Bhattacharya SS, Cordoba J, Stojkovic M. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–9. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–59. doi: 10.1523/JNEUROSCI.17-05-01642.1997. http://www.ncbi.nlm.nih.gov/pubmed/9030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambarotta G, Fregnan F, Gnavi S, Perroteau I. Neuregulin 1 Role in Schwann Cell Regulation and Potential Applications to Promote Peripheral Nerve Regeneration. Int Rev Neurobiol. 2013:223–256. doi: 10.1016/B978-0-12-410499-0.00009-5. [DOI] [PubMed] [Google Scholar]
- 13.Bartus K, Galino J, James ND, Hernandez-Miranda LR, Dawes JM, Fricker FR, Garratt AN, McMahon SB, Ramer MS, Birchmeier C, Bennett DLH, Bradbury EJ. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain. 2016;139:1394–1416. doi: 10.1093/brain/aww039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16(18):5698–703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–55. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Höke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247:272–81. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jesuraj NJ, Marquardt LM, Kwasa JA, Sakiyama-Elbert SE. Glial cell line-derived neurotrophic factor promotes increased phenotypic marker expression in femoral sensory and motor-derived Schwann cell cultures. Exp Neurol. 2014;257:10–18. doi: 10.1016/j.expneurol.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquardt LM, Sakiyama-Elbert SE. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp Neurol. 2015;265:1–7. doi: 10.1016/j.expneurol.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos D, González-Pérez F, Giudetti G, Micera S, Udina E, Del Valle J, Navarro X. Preferential Enhancement of Sensory and Motor Axon Regeneration by Combining Extracellular Matrix Components with Neurotrophic Factors. Int J Mol Sci. 2016;18:65. doi: 10.3390/ijms18010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Lye-Barthel M, Masland RH, Jakobs TC. Structural remodeling of fibrous astrocytes after axonal injury. J Neurosci. 2010;30:14008–19. doi: 10.1523/JNEUROSCI.3605-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D, Jakobs TC. Structural Remodeling of Astrocytes in the Injured CNS. Neurosci. 2012;18:567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oohira A, Matsui F, Katoh-Semba R. Inhibitory effects of brain chondroitin sulfate proteoglycans on neurite outgrowth from PC12D cells. [accessed May 13, 2017];J Neurosci. 1991 11:822–7. doi: 10.1523/JNEUROSCI.11-03-00822.1991. http://www.ncbi.nlm.nih.gov/pubmed/2002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallén A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisén J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vime. [accessed March 10, 2015];J Cell Biol. 1999 145:503–14. doi: 10.1083/jcb.145.3.503. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2185074&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62:2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–61. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M, Currie PD. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci. 2012;32:7477–92. doi: 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung YKJ, Pankhurst M, Dunlop SA, Ray S, Dittmann J, Eaton ED, Palumaa P, Sillard R, Chuah MI, West AK, Chung RS. Metallothionein induces a regenerative reactive astrocyte phenotype via JAK/STAT and RhoA signalling pathways. Exp Neurol. 2009;221:98–106. doi: 10.1016/j.expneurol.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Siddiq MM, Hannila SS, Carmel JB, Bryson JB, Hou J, Nikulina E, Willis MR, Mellado W, Richman EL, Hilaire M, Hart RP, Filbin MT. Metallothionein-I/II Promotes Axonal Regeneration in the Central Nervous System. J Biol Chem. 2015;290:16343–16356. doi: 10.1074/jbc.M114.630574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldshmit Y, Frisca F, Pinto AR, Pébay A, Tang J-KKY, Siegel AL, Kaslin J, Currie PD. Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 2014;4:187–200. doi: 10.1002/brb3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldshmit Y, Frisca F, Kaslin J, Pinto AR, Tang J-KKY, Pébay A, Pinkas-Kramarski R, Currie PD. Decreased anti-regenerative effects after spinal cord injury in spry4−/− mice. Neuroscience. 2015;287:104–112. doi: 10.1016/j.neuroscience.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Macaya DJ, Hayakawa K, Arai K, Spector M. Astrocyte infiltration into injectable collagen-based hydrogels containing FGF-2 to treat spinal cord injury. Biomaterials. 2013;34:3591–3602. doi: 10.1016/j.biomaterials.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 39.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, Okada S. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N-cadherin pathway after spinal cord injury. Nat Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 40.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. [accessed July 5, 2017];J Neuropathol Exp Neurol. 2002 61:1013–21. doi: 10.1093/jnen/61.11.1013. http://www.ncbi.nlm.nih.gov/pubmed/12430718. [DOI] [PubMed] [Google Scholar]
- 41.Rosciszewski G, Cadena V, Murta V, Lukin J, Villarreal A, Roger T, Ramos AJ. Toll-Like Receptor 4 (TLR4) and Triggering Receptor Expressed on Myeloid Cells-2 (TREM-2) Activation Balance Astrocyte Polarization into a Proinflammatory Phenotype. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0618-z. [DOI] [PubMed] [Google Scholar]
- 42.Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. [accessed July 6, 2017];J Neuroimmunol. 1990 30:201–12. doi: 10.1016/0165-5728(90)90104-u. http://www.ncbi.nlm.nih.gov/pubmed/2121800. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Okada S, Toyama Y, Okano H. Role of IL-6 in Spinal Cord Injury in a Mouse Model. Clin Rev Allergy Immunol. 2005;28:197–204. doi: 10.1385/CRIAI:28:3:197. [DOI] [PubMed] [Google Scholar]
- 44.Codeluppi S, Fernandez-Zafra T, Sandor K, Kjell J, Liu Q, Abrams M, Olson L, Gray NS, Svensson CI, Uhlén P. Interleukin-6 secretion by astrocytes is dynamically regulated by PI3K-mTOR-calcium signaling. PLoS One. 2014;9:e92649. doi: 10.1371/journal.pone.0092649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336:173–183. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Villacampa N, Almolda B, Vilella A, Campbell IL, González B, Castellano B. Astrocyte-targeted production of IL-10 induces changes in microglial reactivity and reduces motor neuron death after facial nerve axotomy. Glia. 2015;63:1166–1184. doi: 10.1002/glia.22807. [DOI] [PubMed] [Google Scholar]
- 47.Ren H, Han M, Zhou J, Zheng Z-F, Lu P, Wang J-J, Wang J-Q, Mao Q-J, Gao J-Q, Ouyang HW. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35:6585–6594. doi: 10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 48.Rao MS, Noble M, Mayer-Pröschel M. A tripotential glial precursor cell is present in the developing spinal cord. [accessed March 10, 2015];Proc Natl Acad Sci U S A. 1998 95:3996–4001. doi: 10.1073/pnas.95.7.3996. /pmc/articles/PMC19951/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJA. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Y, Bouyer J, Shumsky JS, Haas C, Fischer I. Transplantation of neural progenitor cells in chronic spinal cord injury. Neuroscience. 2016;320:69–82. doi: 10.1016/j.neuroscience.2016.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies JE, Pröschel C, Zhang N, Noble M, Mayer-Pröschel M, Davies SJA. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies SJA, Shih C-H, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–32. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shih C-H, Lacagnina M, Leuer-Bisciotti K, Proschel C. Astroglial-Derived Periostin Promotes Axonal Regeneration after Spinal Cord Injury. J Neurosci. 2014;34:2438–2443. doi: 10.1523/JNEUROSCI.2947-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu T, Zhou H, Li F, Wang T, Lu L, Feng S. Astrocyte transplantation for spinal cord injury: Current status and perspective. Brain Res Bull. 2014;107:18–30. doi: 10.1016/j.brainresbull.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. [accessed July 7, 2017];J Neuropathol Exp Neurol. 1997 56:1191–201. doi: 10.1097/00005072-199711000-00003. http://www.ncbi.nlm.nih.gov/pubmed/9370229. [DOI] [PubMed] [Google Scholar]
- 57.Gensert JM, Goldman JE. Endogenous Progenitors Remyelinate Demyelinated Axons in the Adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/S0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 58.Brook GA, Plate D, Franzen R, Martin D, Moonen G, Schoenen J, Schmitt AB, Noth J, Nacimiento W. Spontaneous longitudinally orientated axonal regeneration is associated with the Schwann cell framework within the lesion site following spinal cord compression injury of the rat. J Neurosci Res. 1998;53:51–65. doi: 10.1002/(SICI)1097-4547(19980701)53:1<51::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 59.Church JS, Milich LM, Lerch JK, Popovich PG, McTigue DM. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, Schwann cell infiltration, and remyelination in the rat spinal cord. Glia. 2017;65:883–899. doi: 10.1002/glia.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/S0166-2236(00)01691-X. [DOI] [PubMed] [Google Scholar]
- 61.Russell LN, Lampe KJ. Engineering Biomaterials to Influence Oligodendroglial Growth, Maturation, and Myelin Production. Cells Tissues Organs. 2016;202:85–101. doi: 10.1159/000446645. [DOI] [PubMed] [Google Scholar]
- 62.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J Biomed Mater Res Part A. 2010;92A:152–163. doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas AM, Seidlits SK, Goodman AG, Kukushliev TV, Hassani DM, Cummings BJ, Anderson AJ, Shea LD. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb) 2014;6:694–705. doi: 10.1039/c4ib00009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Liu X, Cui L, Brunson C, Zhao W, Bhat NR, Zhang N, Wen X. Engineering an in situ crosslinkable hydrogel for enhanced remyelination. FASEB J. 2013;27:1127–36. doi: 10.1096/fj.12-211151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DSK, Xu X-M, Kim DH, Whittemore SR. Transplantation of Ciliary Neurotrophic Factor-Expressing Adult Oligodendrocyte Precursor Cells Promotes Remyelination and Functional Recovery after SpinalCord Injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faulkner J, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol. 2005;15:131–142. doi: 10.1016/j.trim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Priest CA, Manley NC, Denham J, Wirth ED, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 2015;10:939–58. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- 68.Biotherapeutics A. [accessed July 13, 2017];Dose Escalation Study of AST-OPC1 in Spinal Cord Injury - Full Text View - ClinicalTrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02302157.
- 69.Myers SA, Bankston AN, Burke DA, Ohri SS, Whittemore SR. Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Exp Neurol. 2016 Sep;283(Pt B):560–72. doi: 10.1016/j.expneurol.2016.04.009. https://doi.org/10.1016/j.expneurol.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 71.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Urbanski MM, Kingsbury L, Moussouros D, Kassim I, Mehjabeen S, Paknejad N, Melendez-Vasquez CV. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci Rep. 2016;6:33751. doi: 10.1038/srep33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. J Cell Biol. 2008;182:1171–84. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 75.Sephel GC, Tashiro KI, Sasaki M, Greatorex D, Martin GR, Yamada Y, Kleinman HK. Laminin A chain synthetic peptide which supports neurite outgrowth. [accessed November 1, 2017];Biochem Biophys Res Commun. 1989 162:821–9. doi: 10.1016/0006-291x(89)92384-x. http://www.ncbi.nlm.nih.gov/pubmed/2757641. [DOI] [PubMed] [Google Scholar]
- 76.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan L, North HA, Sahni V, Jeong SJ, Mcguire TL, Berns EJ, Stupp SI, Kessler JA. β1-Integrin and integrin linked kinase regulate astrocytic differentiation of neural stem cells. PLoS One. 2014;9:e104335. doi: 10.1371/journal.pone.0104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donoghue PS, Lamond R, Boomkamp SD, Sun T, Gadegaard N, Riehle MO, Barnett SC. The Development of a e-Polycaprolactone Scaffold for Central Nervous System Repair. Tissue Eng Part A. 2013;19:497–507. doi: 10.1089/ten.tea.2012.0382. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Ceylan M, Shrestha B, Wang H, Lu QR, Asmatulu R, Yao L. Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules. 2014;15:319–26. doi: 10.1021/bm401558c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, Vescovi A, Gelain F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chedly J, Soares S, Montembault A, von Boxberg Y, Veron-Ravaille M, Mouffle C, Benassy M-N, Taxi J, David L, Nothias F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91–107. doi: 10.1016/j.biomaterials.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Pawar K, Prang P, Müller R, Caioni M, Bogdahn U, Kunz W, Weidner N. Intrinsic and extrinsic determinants of central nervous system axon outgrowth into alginate-based anisotropic hydrogels. Acta Biomater. 2015;27:131–139. doi: 10.1016/j.actbio.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 83.Taylor SJ, Rosenzweig ES, McDonald JW, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–35. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8:46033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- 85.Pandey MS, Baggenstoss BA, Washburn J, Harris EN, Weigel PH. The Hyaluronan Receptor for Endocytosis (HARE) Activates NF-κB-mediated Gene Expression in Response to 40–400-kDa, but Not Smaller or Larger, Hyaluronans. J Biol Chem. 2013;288:14068–14079. doi: 10.1074/jbc.M112.442889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, Martin DC. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res Part A. 2007;83A:636–645. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 87.East E, de Oliveira DB, Golding JP, Phillips JB. Alignment of Astrocytes Increases Neuronal Growth in Three-Dimensional Collagen Gels and Is Maintained Following Plastic Compression to Form a Spinal Cord Repair Conduit. Tissue Eng Part A. 2010;16:3173–3184. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biran R, Noble MD, Tresco PA. Directed nerve outgrowth is enhanced by engineered glial substrates. Exp Neurol. 2003;184:141–152. doi: 10.1016/S0014-4886(03)00253-X. [DOI] [PubMed] [Google Scholar]
- 89.Tiryaki VM, Ayres VM, Ahmed I, Shreiber DI. Differentiation of reactive-like astrocytes cultured on nanofibrillar and comparative culture surfaces. Nanomedicine. 2015;10:529–545. doi: 10.2217/nnm.14.33. [DOI] [PubMed] [Google Scholar]
- 90.Tiryaki VM, Ayres VM, Khan AA, Ahmed I, Shreiber DI, Meiners S. Nanofibrillar scaffolds induce preferential activation of Rho GTPases in cerebral cortical astrocytes. Int J Nanomedicine. 2012;7:3891–905. doi: 10.2147/IJN.S32681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu T, Houle JD, Xu J, Chan BP, Chew SY. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng Part A. 2012;18:1057–66. doi: 10.1089/ten.TEA.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsiao TW, Tresco PA, Hlady V. Astrocytes alignment and reactivity on collagen hydrogels patterned with ECM proteins. Biomaterials. 2015;39:124–130. doi: 10.1016/j.biomaterials.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuidema JM, Hyzinski-García MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers. Biomaterials. 2014;35:1439–49. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qu J, Wang D, Wang H, Dong Y, Zhang F, Zuo B, Zhang H. Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration. J Biomed Mater Res A. 2013;101:2667–78. doi: 10.1002/jbm.a.34551. [DOI] [PubMed] [Google Scholar]
- 95.Min SK, Jung SM, Ju JH, Kwon YS, Yoon GH, Shin HS. Regulation of astrocyte activity via control over stiffness of cellulose acetate electrospun nanofiber. Vitr Cell Dev Biol - Anim. 2015;51:933–940. doi: 10.1007/s11626-015-9925-8. [DOI] [PubMed] [Google Scholar]
- 96.Terraf P, Kouhsari SM, Ai J, Babaloo H. Tissue-Engineered Regeneration of Hemisected Spinal Cord Using Human Endometrial Stem Cells, Poly ε-Caprolactone Scaffolds, and Crocin as a Neuroprotective Agent. Mol Neurobiol. 2017;54:5657–5667. doi: 10.1007/s12035-016-0089-7. [DOI] [PubMed] [Google Scholar]