Abstract
Objectives
1) Examine angular insertion depths (AID) and scalar location of MED-EL electrodes; and 2) determine the relationship between angular insertion depth (AID) and audiologic outcomes controlling for scalar position.
Study Design
Retrospective review.
Methods
Post-lingually deafened adults undergoing cochlear implantation with Flex 24, Flex 28, and Standard electrode arrays were identified. Patients with preoperative and postoperative CT scans were included, such that electrode location and AID could be determined. Outcome measures were 1) speech perception in the CI-only condition and 2) short-term hearing preservation.
Results
Forty-eight implants were included; all electrodes (48/48) were positioned entirely within the scala tympani. The median AID was 408°(IQ range 373-449°) for Flex 24, 575°(IQ range 465-584°) for Flex 28, and 584°(IQ range 368-643°) for Standard electrodes. The mean postoperative CNC score was 43.7%±21.9. A positive correlation was observed between greater AID and better CNC performance (r=0.48, p<0.001). Excluding patients with post-operative residual hearing, a strong correlation between AID and CNC persisted (r=0.57, p<0.001). In patients with pre-operative residual hearing, mean low-frequency PTA shift was 27 dB ± 14. A correlation between AID and low-frequency PTA shift at activation was noted (r=0.41, p=0.04).
Conclusions
Favorable rates of scala tympani insertion (100%) were observed. In the CI only condition, a direct correlation between greater AID and CNC score was noted regardless of post-operative hearing status. Deeper insertions were, however, associated with worse short-term hearing preservation. When patients without post-operative residual hearing were analyzed independently, the relationship between greater insertion depth and better performance was strengthened.
Keywords: cochlear implant, electrode location, scalar translocation, speech perception, audiologic outcomes, Flex 24, Flex 28
INTRODUCTION
Minimizing trauma during cochlear implant (CI) surgery has a number of potential audiologic benefits. First, it increases the likelihood of preserving residual low-frequency hearing, which can allow for concurrent electric-acoustic stimulation (EAS).1 Second, minimizing insertion trauma has also been shown to result in superior speech perception in the CI only condition.2-4 Lastly, decreasing intracochlear trauma may allow for the application of future technologies such as cellular regeneration.2 For these reasons, soft surgery and atraumatic electrode insertion have become fundamental concepts of CI surgery.
While histologic evaluation remains the gold-standard for evaluating cochlear trauma, advancements in imaging techniques have allowed clinicians to determine scalar electrode location in vivo. Scalar translocation from the scala tympani to the scala vestibuli is generally considered severe trauma, as it inherently disrupts cochlear partitions. Recent studies have demonstrated that scalar electrode translocation has important clinical implications.3-7 Specifically, lower speech perception scores have been observed for electrodes that cross into the scala vestibuli.
Another variable that has the potential to impact cochlear trauma and audiologic performance is insertion depth. Intuitively, shallow insertions would inflict less trauma to the apical region of the cochlea. This concept led to the development of shorter electrode arrays designed to minimize trauma and maximize hearing preservation. Clinical studies have corroborated this notion, demonstrating that residual hearing is more frequently preserved with shorter electrodes.8,9 The relationship between insertion depth and audiologic performance, however, is less clear. When testing patients in the CI only condition, recent studies have suggested that greater insertion depths are associated with better audiologic outcomes.3,5,10-12 If these data are consistently replicated, the predicament in which clinicians are striving to preserve hearing with shorter less traumatic electrodes, while attempting to maximize cochlear coverage in the event that residual hearing is lost, will become increasingly relevant.
Given these issues, the primary objective of this study was to further assess the relationship between electrode insertion depth and speech perception in the CI only condition. We secondarily sought to examine hearing preservation in relation to insertion depth. In order to capture a range of insertion depths but also minimize variability introduced secondary to electrode design, only patients who underwent implantation with MED-EL (GmbH Innsbruck, Austria) Flex 24, Flex 28, and Standard arrays were studied.
MATERIALS AND METHODS
Patient Selection and Clinical Information
Prior to initiation of the study, Institutional Review Board (IRB) approval was obtained. Post-lingually deafened adults implanted with MED-EL devices were identified. Patients received one of three electrode arrays: Flex 24 (24mm), Flex 28 (28mm), and Standard (31.5mm). The electrode type was chosen by the patient’s surgeon with consideration given to cochlear anatomy and residual hearing.
Patient demographics (age, gender, race) and electrode type (Flex 24, Flex 28, Standard) were recorded. Consenting patients underwent preoperative and postoperative temporal bone computed tomography (CT) studies to determine electrode location and angular insertion depth (AID), as detailed below. If information regarding electrode location or AID was unavailable, or the patient lacked post-operative speech perception testing, the patient was excluded from the study.
Electrode Location
AID of the electrode array was measured as the maximum angular depth of all of the contacts using the coordinate system defined by Verbist et al.13 The methodology used to determine scalar electrode location has previously been reported (Figure 1).4,14-16 In short, the scala tympani and scala vestibuli are identified on pre-operative CT imaging through application of statistical shape model of the cochlea generated from micro CT scans of cadaveric cochlea. The post-operative CT scan is used to locate the electrode array and individual contacts. The post-operative scan is then rigidly registered to the pre-operative scan, such that scalar location of each electrode contact can be determined. This approach has been validated using cadaveric models and has been shown to be highly accurate in predicting electrode location.17
Figure 1.
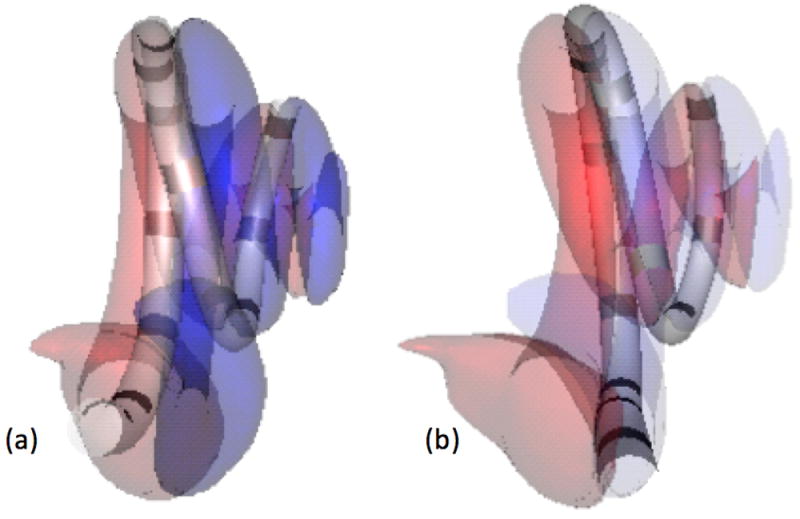
Shown are 3D renderings of electrode arrays and cochlear scala generated from CT images. (A) In this case, the electrode array is fully inserted into the scala tympani (red), (B) an electrode array with contacts in the scala vestibuli (blue) is depicted.
Outcome Measures
The primary outcome measure of interest was speech perception. Speech perception performance was assessed using the Consonant-Nucleus-Consonant (CNC) word recognition test, which consists of monosyllabic words preceded by a carrier word, which are arranged into lists of 50 words (ex. “Ready, bird”).18 CNC test was administered preoperatively and between 6 and 18 months post-activation. CNC scores obtained at the time point closest to one year after activation were analyzed. All speech perception testing was completed in a double-walled, sound treated booth. Stimuli was presented at 60 dBA through a single loudspeaker positioned at zero degrees azimuth approximately one meter from the listener. Testing was completed in quiet, in the CI only condition. Patients were instructed to repeat as much of each word as possible and encouraged to guess when necessary.
The secondary outcome measure was hearing preservation. The authors assert that functional hearing—hearing that can be successfully aided acoustically— should be an important consideration. Therefore, residual hearing was defined as an unaided air-conduction threshold ≤80 dB at 250 Hz. Residual hearing was assessed pre-operatively and at activation. We chose ≤80 dB given that target gain with acoustic amplification would theoretically be achievable assuming a half-gain rule and typical low-frequency gain limits for conventional amplification in the range of 40 to 45 dB. The 250 Hz frequency was chosen because it is the lowest frequency for which we are able to reliably amplify to prescriptive targets, thus it serves as a marker for whether or not residual hearing is functionally useful.
Low-frequency pure-tone average (PTA), defined as the average of air conduction thresholds at 125, 250, and 500 Hz, was also recorded. Shift in low-frequency PTA was calculated by subtracting the pre-operative value low-frequency PTA from that at activation. The impact of AID on speech perception, maintenance of functional residual hearing, and low-frequency PTA shift was examined.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, California). Nominal data were analyzed using a Fisher exact or Chi-square test. For continuous variables, values were determined to follow a normal distribution using a D’Agostino and Pearson omnibus normality test. Normally distributed data are presented as means ± standard deviations, while non-parametric data are presented as medians with interquartile (IQ) ranges.
For normally distributed data, one-way analysis of variance (ANOVA) followed by a Holm-Sidak’s multiple comparisons test was performed. For non-parametric data, the Kruskal-Wallis test followed by Dunn’s multiple comparisons test was performed. Correlations for parametric and nonparametric data were examined using a Pearson or Spearman correlation analysis. P values <0.05 were considered indicative of statistical significance.
RESULTS
Demographics and Clinical Characteristics
Forty patients (48 implants) were included in the analysis. Patients were predominantly male (62.5%) and had a median age at the time of surgery of 69 years (range 32-91). Of the 48 implants, 5 electrodes were Flex 24 (10.4%), 28 electrodes were Flex 28 (58.3%), and 15 were Standard (31.3%) electrode arrays.
Pre-operative air-conduction thresholds correlated with the type of electrode array implanted (p=0.04). Patients undergoing implantation with Flex 24 electrodes all demonstrated residual hearing (4/4, 100%) compared to only 36% (5/14) patients with Standard electrode arrays; this difference failed to reach statistical significance (p=0.08). When comparing Flex 28 (18/28, 64.3%) electrodes to either Flex 24 or Standard electrode arrays, there were no differences in residual hearing (p=0.28 and p=0.15, respectively). Two patients lacked unaided pre-operative air-conduction thresholds and were excluded from these analyses.
Intracochlear Position of Electrode Arrays
All electrode arrays (48/48) were located entirely within the scala tympani, and therefore none were excluded from analyses based on translocation. The overall mean AID was 514° ± 110.6 (range 317-710). AID was analyzed in relation to electrode array (Figure 2). The median AID was 408° (IQ Range 373-449°) for Flex 24, 575° (IQ Range 465-584°) for Flex 28, and 584° (IQ Range 368-643°) for Standard electrode arrays. These differences in AID were not statistically significant on ANOVA (p=0.07).
Figure 2.
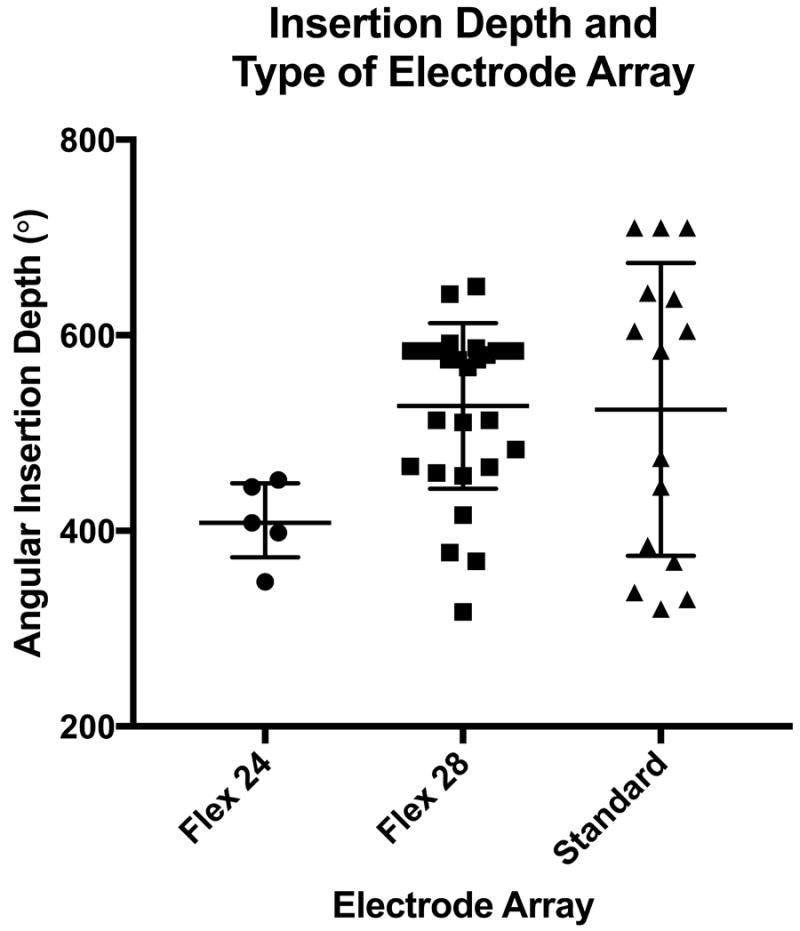
Median angular insertion depths (AID) with interquartile ranges for Flex 24, Flex 28, and Standard electrode arrays are depicted; each dot represents an individual patient.
Speech Perception Performance
Speech perception using the CNC word test was assessed at an average of 11.7 ± 2.4 months post-operatively in the CI only condition. The average CNC score was 43.7% ± 21.9. Age at implantation (r= -0.23, p=0.10), pre-operative residual hearing (p=0.16), and post-operative residual hearing (p=0.93) did not significantly impact CNC scores.
The association between performance and electrode type was then examined. CNC scores trended toward higher (better) scores for the longer electrodes, but did not reach statistical significance when comparing Flex 24 (25.2% ± 21.5), Flex 28 (47.4% ± 20.1), and Standard (42.9% ± 23.7) electrode arrays (p=0.15)(Figure 3).
Figure 3.

Mean Consonant-Nucleus-Consonant (CNC) score and standard deviations are plotted in relation to the type of electrode array; each dot represents an individual patient.
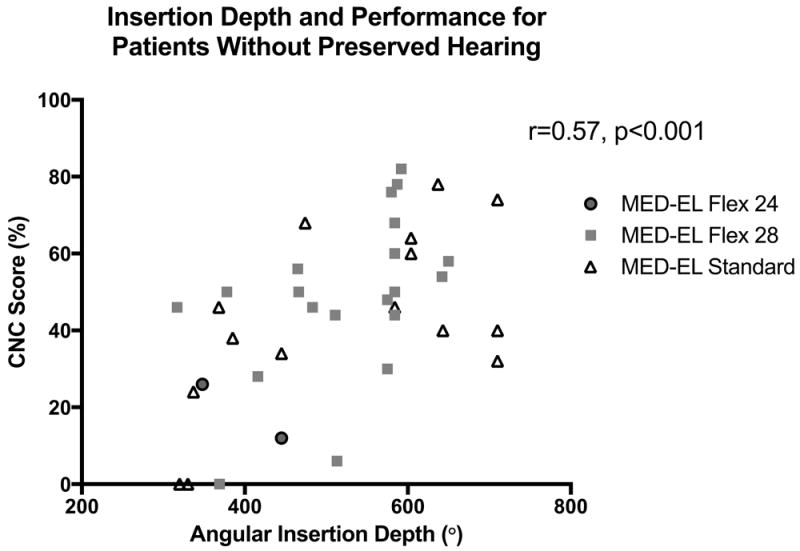
The relationship between speech perception and AID was then examined. A significant positive correlation was observed between greater AIDs and better CNC performance (r=0.48, p<0.001)(Figure 4). Patients with residual post-operative hearing were then excluded, as there is data to suggest a relation between access to acoustic hearing and better speech perception in the CI only condition.19 A strong correlation between AID and CNC score persisted in those patients without residual hearing (n=38)(r=0.57, p<0.001)(Figure 5).
Figure 4.
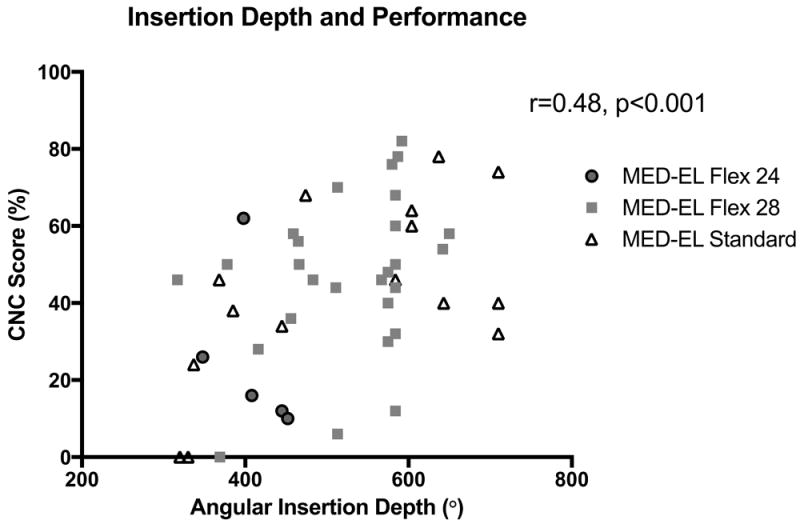
A correlation between greater angular insertion depth (AID) and Consonant-Nucleus-Consonant (CNC) score was observed (r=0.48, p<0.001).
Figure 5.
A strong correlation between greater angular insertion depth (AID) and Consonant-Nucleus-Consonant (CNC) score was observed in the subset of patients without preserved functional hearing post-operatively (r=0.57, p<0.001).
Post-operative Hearing Preservation
Of the 27 patients with residual hearing pre-operatively, 25 had post-operative unaided air-conduction thresholds available at activation and were included in the following analyses. Eight (32%) patients maintained functional residual hearing post-operatively. At activation, rates of achieving AC thresholds ≤80 dB at 250 Hz were generally better for shorter electrodes (Flex 24- 3/4 75%, Flex 28- 5/16 31%, Standard- 0/5 0%), but differences between groups did not reach statistical significance (p=0.05). Mean AID did not significantly differ between those patients with post-operative residual hearing (517° ± 114) and those without residual hearing (495° ± 76)(p=0.63).
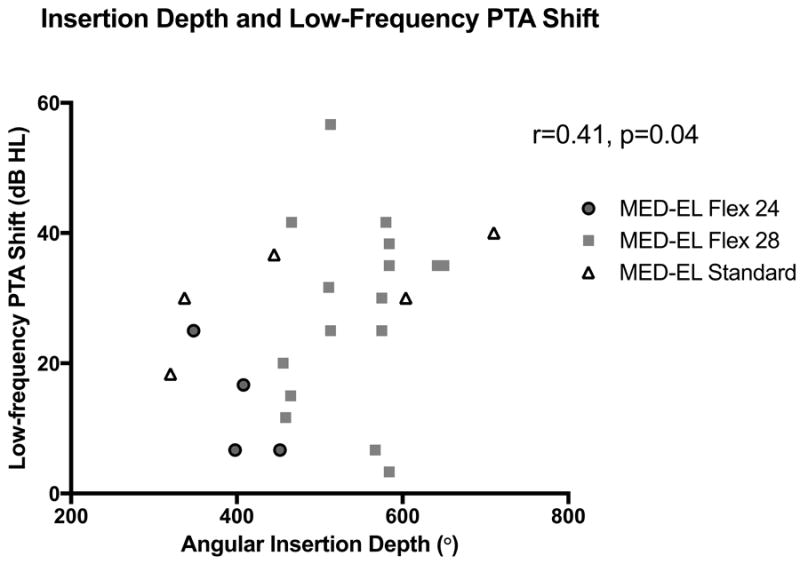
In patients with residual hearing pre-operatively, the mean low-frequency PTA shift at activation was 27 dB ± 14. Changes in low-frequency PTA tended to be less (i.e. better preservation of hearing) for Flex 24 electrodes (14 dB ± 9) than Flex 28 (28 dB ± 14) and Standard arrays (31 dB ± 8), but these differences did not achieve significance (p=0.10). A significant correlation was noted between AID and low-frequency PTA shift (r=0.41, p=0.04)(Figure 6). In other words, deeper insertions were associated with greater losses in low-frequency hearing. Age at implantation did not impact low-frequency PTA shift (r=0.11, p=0.60).
Figure 6.
A direct correlation between angular insertion depth (AID) and shift in low-frequency pure-tone average (PTA) was noted in patients with residual hearing (r=0.41, p=0.04).
DISCUSSION
This study was primarily designed to assess the relationship between AID and speech perception performance. To decrease variability in electrode design between subjects, we only studied patients implanted with lateral wall electrodes from a single device manufacturer. Furthermore, given that scalar translocation during CI surgery has proven to result in shallower insertion, poorer speech perception performance, and worse hearing preservation, we ensured that all electrode contacts were implanted entirely within the scala tympani prior to analysis.3,4,20 This was case for all electrodes studied (48/48, 100%), and is consistent with prior reports which have shown that the majority of lateral wall electrodes reside entirely within the scala tympani.3,4,21 The most notable finding of this study, however, is that greater AIDs conferred speech perception benefit in the CI-only condition for lateral wall electrodes positioned entirely within the scala tympani.
There are a number of potential explanations for why deeper insertions would be associated with better speech recognition. Deeper insertions distribute electrode coverage over the entire length of the cochlea, which extends the range of possible pitch percepts. It is also possible that a closer match is achieved with greater insertion depths between the frequency bands assigned to electrode contacts by the speech processor and the tonotopic organization of the cochlea.10 It needs to be emphasized that the observed positive correlation between AID and performance was noted only for insertion depths available in this cohort (317-710°). To that end, very shallow insertions are not modeled. Furthermore, while we strive for full insertions, electrodes are not inserted beyond the first point of resistance in an effort to minimize trauma, therefore the deepest insertions are also unlikely to be included. To that end, the authors urge caution in interpreting these results and discourage forceful insertions intended to maximize insertion depth. Whether there is 1) an upper limit to insertion depth beyond which speech scores decline, or 2) a minimum insertion depth beyond which speech scores do not improve with further electrode advancement, remains unclear and warrants further investigation in larger populations.
Other groups have published similar findings regarding the relationship between increased cochlear coverage and improved outcomes with electrical stimulation,.3,5,10-12 Skinner et al. (2002) studied 26 patients implanted with perimodiolar arrays, and demonstrated that word scores were significantly correlated with insertion depth as a percentage of total cochlear length.5 Yukawa et al. (2004) examined the impact that insertion length and AID had on post-operative speech perception in 48 patients who received either perimodiolar or lateral wall arrays.10 Controlling for duration of deafness, implant type, pre-operative speech perception scores, and post-operative pure-tone averages, they found that greater insertion length and AID were associated with better CNC performance. Furthermore, AID was the best depth-related predictor of postoperative speech perception. The authors agree with this notion-- that AID accounts for variance in both linear insertion depth and length of the cochlear duct.
More recently, the relationship between insertion depths and speech recognition has been further corroborated in both large retrospective studies and prospective analyses. Our group retrospectively examined 220 ears controlling for age, electrode type, surgical approach, scalar electrode, and cochlear volume.3 Using multivariate analysis, CNC score was shown to increase 0.6% with every 10 degree increase in AID. Given the wide variance in overall insertion depths observed for electrode arrays in this study (393°), such multivariate modeling would account for large differences in CNC score. Perhaps the most compelling evidence in support of the positive correlation between depth of insertion and performance was reported in a prospective study by Buchman et al (2014).12 Thirteen patients were randomized to receive either standard or medium-length lateral wall electrodes, and the relationship between greater insertion depth and better speech perception neared significance (p=0.07). Analysis was repeated with six additional subjects added retrospectively, and statistical significance was reached. In light of these findings suggesting an apparent speech recognition benefit associated with longer electrodes, the IRB discontinued the study for ethical reasons.
Contrary to the aforementioned studies, van der Marel et al. (2015) did not find a significant relationship between insertion depth and audiologic performance.22 Patients were implanted with either an Advanced Bionics HiFocus 1 (n=14) or HiFocus 1J (n=189) electrode, which are almost identical in length. Given that the electrodes were essentially the same length, less variation in insertion depths may explain why they did not find any significant relation between depth and performance. Finley et al. (2008) suggested that lower speech perception scores were associated with deeper insertions, but their results could be negatively affected by a low sample size (14 patients), of which, all had at least one electrode contact that crossed over to the scala vestibuli.6 The same group later analyzed 114 patients undergoing implantation, the majority of whom were implanted with a perimodiolar array (n=102).23 Their data again suggested an inverse relationship between insertion depth and audiologic performance. These conflicting findings may suggest that the impact of insertion depth on speech performance differs for perimodiolar arrays and lateral wall arrays. Perimodiolar electrodes are pre-curved arrays inserted via advance off-stylet (AOS) techniques, while lateral wall electrodes are straight and generally more flexible. Given that cochleae vary in size, the one size fits all technique of AOS insertion for perimodiolar arrays may cause insertion trauma that confounds analyses regarding the impact of insertion depth. Alternatively, increased proximity to the modiolus with perimodiolar arrays may allow for adequate cochlear stimulation regardless of insertion depth.
Our data demonstrate that collective performance on CNC word testing in the CI-only condition was associated with greater AIDs, regardless of low-frequency residual hearing. When patients with non-functional residual hearing were analyzed separately, the correlation between greater insertion depth and superior speech perception was strengthened. Therefore, the authors assert that for patients destined for electric-only stimulation, insertion of longer electrodes that maximize AIDs should be considered.
It should be emphasized that this study is retrospective and was not primarily designed to examine hearing preservation outcomes; however, we included such data due to its clinical relevance in discussions regarding electrode insertion depth. Our findings demonstrate that for patients with functional residual hearing pre-operatively, shallower AIDs are associated with less shift in low-frequency PTA at activation. Other studies have similarly shown that shallower insertion and use of shorter arrays leads to better low-frequency hearing preservation.9,24,25 This study alone cannot specifically address the clinical dilemma of whether to implant a shorter electrode and increase the likelihood of hearing preservation or choose a longer electrode and maximize cochlear coverage. To resolve this dilemma, studies comparing quality of life outcomes in patients using EAS with shallower insertions to those functioning in the CI-only condition with deeper insertions are warranted.
There are limitations that warrant discussion. The cohort was relatively small (48 ears), particularly for Flex 24 electrodes (5 ears), and patients were not randomized to electrode type, thus subjecting the study to possible selection bias. Other covariates not accounted for in this study may also confound outcomes. These include, but are not limited to, cognitive status, duration of deafness, duration of hearing aid use, and programming strategies. As all patients were post-lingual adults who failed an appropriate hearing aid trial, we feel that variability introduced by inability to control for duration of deafness is minimized. It should also be mentioned that CNC word testing in the CI-only ipsilateral condition is not reflective of some listener’s best-aided condition. Given that our data were accrued before commercial availability of the EAS processor, combined with the fact that most patients in our practice with residual hearing chose not to wear a hearing aid in the ipsilateral ear for acoustic stimulation, inclusion of speech scores in the EAS condition was not possible. Further study comparing the best aided speech score is warranted. Lastly, while we demonstrate a strong relationship between audiologic word recognition testing and AID, further investigations into sound quality, speech in noise, music perception, and quality of life measures are also needed.
CONCLUSION
This study examined the relationship between AID and audiologic outcomes in patients implanted with lateral wall electrode arrays positioned entirely within the scala tympani. When tested in the CI-only condition, a significant positive correlation (r=0.48) between greater AID and CNC scores was noted at 1 year following CI activation, regardless of post-operative hearing status. Deeper insertions were, however, associated with worse short-term hearing preservation. When patients without residual post-operative hearing (those destined for electric-only stimulation) were analyzed separately, the relationship between greater insertion depth and better performance was strengthened (r=0.57).
Acknowledgments
FINANCIAL MATERIAL AND SUPPORT: The project was supported by grants R01DC008408, R01DC014462, and R01DC014037 from the National Institute on Deafness and Other Communication Disorders and UL1TR000445 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not represent the official views of these institutes.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: Dr. Wanna is a consultant for MED-EL, Advanced Bionics, Oticon Medical, and Cochlear Americas. Dr. Haynes is a consultant for Cochlear, Advanced Bionics, Grace Medical, MedEl, Stryker, Synthes.
IRB APPROVAL: This study was approved by the Vanderbilt IRB #090155.
LEVEL OF EVIDENCE: Level 4
MEETING: This paper has been submitted for consideration for oral presentation at the Triological Society Combined Sections Meeting in New Orleans, LA, 2017.
References
- 1.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(Suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson ML, Driscoll CL, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32:962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connell BP, Cakir A, Hunter JB, et al. Electrode Location and Angular Insertion Depth Are Predictors of Audiologic Outcomes in Cochlear Implantation. Otol Neurotol. 2016 doi: 10.1097/MAO.0000000000001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(Suppl 6):S1–7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3:332–350. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 8.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope. 2016;126:962–973. doi: 10.1002/lary.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suhling MC, Majdani O, Salcher R, et al. The Impact of Electrode Array Length on Hearing Preservation in Cochlear Implantation. Otol Neurotol. 2016 doi: 10.1097/MAO.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 10.Yukawa K, Cohen L, Blamey P, Pyman B, Tungvachirakul V, O’Leary S. Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol Neurootol. 2004;9:163–172. doi: 10.1159/000077267. [DOI] [PubMed] [Google Scholar]
- 11.Hochmair I, Arnold W, Nopp P, Jolly C, Muller J, Roland P. Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryngol. 2003;123:612–617. [PubMed] [Google Scholar]
- 12.Buchman CA, Dillon MT, King ER, Adunka MC, Adunka OF, Pillsbury HC. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35:1773–1779. doi: 10.1097/MAO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 13.Verbist BM, Skinner MW, Cohen LT, et al. Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol Neurotol. 2010;31:722–730. doi: 10.1097/MAO.0b013e3181d279e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble JH, Labadie RF, Majdani O, Dawant BM. Automatic segmentation of intracochlear anatomy in conventional CT. IEEE Trans Biomed Eng. 2011;58:2625–2632. doi: 10.1109/TBME.2011.2160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Dawant BM, Labadie RF, Noble JH. Automatic localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv. 2014;17:331–338. doi: 10.1007/978-3-319-10404-1_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble JH, Dawant BM. Automatic graph-based localization of cochlear implant electrodes in CT. Lecture Notes in Computer Science - Proceedings of MICCAI. 2015;9350:152–159. doi: 10.1007/978-3-319-24571-3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope. 2010;120:2277–2283. doi: 10.1002/lary.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- 19.Dalbert A, Huber A, Baumann N, Veraguth D, Roosli C, Pfiffner F. Hearing Preservation After Cochlear Implantation May Improve Long-term Word Perception in the Electric-only Condition. Otol Neurotol. 2016;37:1314–1319. doi: 10.1097/MAO.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 20.Wanna GB, Noble JH, Gifford RH, et al. Impact of Intrascalar Electrode Location, Electrode Type, and Angular Insertion Depth on Residual Hearing in Cochlear Implant Patients: Preliminary Results. Otol Neurotol. 2015;36:1343–1348. doi: 10.1097/MAO.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer E, Karkas A, Attye A, Lefournier V, Escude B, Schmerber S. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol. 2015;36:422–429. doi: 10.1097/MAO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 22.van der Marel KS, Briaire JJ, Verbist BM, Muurling TJ, Frijns JH. The influence of cochlear implant electrode position on performance. Audiol Neurootol. 2015;20:202–211. doi: 10.1159/000377616. [DOI] [PubMed] [Google Scholar]
- 23.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34:342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurawitz MC, Buchner A, Harpel T, et al. Hearing preservation outcomes with different cochlear implant electrodes: Nucleus(R) Hybrid-L24 and Nucleus Freedom CI422. Audiol Neurootol. 2014;19:293–309. doi: 10.1159/000360601. [DOI] [PubMed] [Google Scholar]
- 25.Svrakic M, Roland JT, Jr, McMenomey SO, Svirsky MA. Initial Operative Experience and Short-term Hearing Preservation Results With a Mid-scala Cochlear Implant Electrode Array. Otol Neurotol. 2016 doi: 10.1097/MAO.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]


