Abstract
Adverse effects of advanced glycation end-products (AGEs) on the tissues are through nonreceptor- and receptor-mediated mechanisms. In the receptor-mediated mechanism, interaction of AGEs with its cell-bound receptor of AGE (RAGE) increases generation of oxygen radicals, activates nuclear factor-kappa B, and increases expression and release of pro-inflammatory cytokines resulting in the cellular damage. The deleterious effects of AGE and AGE–RAGE interaction are coined as “AGE-RAGE stress.” The body is equipped with defense mechanisms to counteract the adverse effects of AGE and RAGE through endogenous enzymatic (glyoxalase 1, glyoxalase 2) and AGE receptor-mediated (AGER1, AGER2) degradation of AGE, and through elevation of soluble receptor of AGE (sRAGE). Exogenous defense mechanisms include reduction in consumption of AGE, prevention of AGE formation, and downregulation of RAGE expression. We have coined AGE and RAGE as “stressors” and the defense mechanisms as “anti-stressors.” AGE–RAGE stress is defined as a shift in the balance between stressors and antistressors in the favor of stressors. Measurements of stressors or antistressors alone would not assess AGE–RAGE stress. For true assessment of AGE–RAGE stress, the equation should include all the stressors and antistressors. The equation for AGE–RAGE stress, therefore, would be the ratio of AGE + RAGE/sRAGE + glyoxalase1 + glyoxalase 2 + AGER1 +AGER2. This is, however, not practical in patients. AGE–RAGE stress may be assessed simply by the ratio of AGE/sRAGE. A high ratio of AGE/sRAGE indicates a relative shift in stressors from antistressors, suggesting the presence of AGE–RAGE stress, resulting in tissue damage, initiation, and progression of the diseases and their complications.
Keywords: advanced glycation end-products (AGE), receptor for AGE (RAGE), soluble receptor for AGE (sRAGE), AGE–RAGE stress, stressors, antistressors, glyoxalase 1, glyoxalase 2, AGER1, AGER2
Advanced glycation end-products (AGEs) and its cell receptor RAGE (receptor for AGEs) have been implicated in the pathogenesis of numerous diseases (atherosclerosis, 1 coronary artery disease, 2 3 hypertension, 4 5 cerebral vascular disease, 6 hyperthyroidism, 7 Alzheimer disease, 8 and end-stage renal disease, 9 and diabetes). 10 11 The adverse effects of AGEs are through nonreceptor- and receptor-mediated mechanisms. Nonreceptor-mediated mechanisms include enhanced synthesis of extracellular matrix, trapping of subendothelial low-density lipoprotein (LDL), and cross-binding with collagen. In receptor-mediated mechanism, there is an interaction of AGEs with RAGE resulting in the increased generation of oxygen radicals, activation of nuclear factor kappa B (NF-κB), and increased expressions of pro-inflammatory cytokines and cell adhesion molecules. 7 AGEs and AGE–RAGE interaction cause potential biological damage and hence we have coined AGE and RAGE as “stressors.” Stress is defined as a process of altered biochemical homeostasis produced by physiological or psychological or environmental stressors. 12 The body is equipped with anti-AGE–RAGE defense mechanisms such as degradation of AGE with enzymes and AGE receptor, and circulating soluble AGE-receptor called sRAGE which competes with RAGE for AGE, to counterbalance the effects of stressors (AGE and RAGE) and we have coined this as antistressors. Excessive levels of AGE and RAGE because of increased consumption of AGE, deficiency in the AGE degradative enzymes or receptors, reduced amount of sRAGE, and increased expression of RAGE would lead to AGE-RAGE stress. A shift in the balance between stressors (AGE and RAGE) and antistressors in favor of stressors, we have coined as AGE–RAGE stress. The ratio of AGEs/sRAGE has been reported as one of the important risk marker or biomarker for disease states. 13 AGE/sRAGE may be one of the most important determinants of AGE–RAGE stress.
This review focuses on the AGE–RAGE stress, stressors, and antistressors. It also discusses the consequences of AGE–RAGE interaction and defense mechanisms such as degradation of AGE, downregulation of RAGE, enhancement of levels of sRAGE, and lowering of AGEs levels.
Stressors (AGE and RAGE)
-
AGEs
AGEs comprise of chemical structures such as N-ε-carboxymethyl-lysine (CML), N-ε-carboxyethyl-lysine (CEL), pyrraline, pentosidine, and argpyrimidine. 14 CML modifications of proteins are predominant AGEs. 15 AGEs are heterogeneous groups of irreversible adducts formed by nonenzymatic glycation and glyoxidation of proteins, lipids, and nucleic acid with reducing sugars. 16 17 There are two sources of AGEs, in vivo, endogenous, and exogenous. Endogenous AGE formation in normal individual occurs slowly. Hyperglycemia accelerates formation of AGEs. 18 Endogenous sources comprise of glycation, polyol pathway, and glyoxidation. Glycation is nonenzymatic reaction of proteins, lipids, and nucleic acids with reducing sugars. 16 17 In the polyol pathway, aldolase reductase or sorbitol dehydrogenase acts on glucose to form intermediary products which bind to proteins to form AGEs. 19 20 Formation of AGE through glyoxidation pathway involves reactive oxygen species (ROS). Generation of superoxide anions in the mitochondria or redox-sensitive mechanism that generates hydroxyl radicals forms glyoxal and methylglyoxal (MGO). These agents react with different biomolecules to produce AGEs. 20 21 Exogenous sources of AGEs include foods high in AGE content (red meat, cheese, crispy brown crackers, fatty cookies sweetened with sugars, cream, and animal fat), 22 cooking at high temperature in dry heat (frying, broiling, grilling, roasting, and baking), 23 and cigarette smoking. 24
In humans, there is a significant increase in plasma levels of AGEs within 2 hours following oral intake of single AGE-rich diet. 25 There is a positive correlation between dietary AGE content and serum/tissue levels of AGEs. 26 About 10% to 30% of AGE (CML) is absorbed in the gastrointestinal tract and is delivered to the liver and other organs. 27 Thirty-three percent of the absorbed AGEs is excreted in the urine and the rest accumulates in the body. 28
-
Receptor for AGE
There are mainly three receptors for AGEs: (a) full-length RAGE, (b) cleaved RAGE (cRAGE), and (c) endogenous secretory RAGE (esRAGE). Full-length RAGE ( Fig. 1 ) is a multiligand receptor and a member of the immunoglobulin superfamily of cell surface molecule. 29 It has three extracellular domains including v-type that possesses ligand binding properties, and two c-type immunoglobulin domains C1 and C2, a transmembrane helix, and a short cytosolic tail. 30 The fourth transmembrane domain anchors RAGE in the membrane and is connected to the highly charged fifth intracellular domain that interacts with cytosolic transduction molecule. RAGE is expressed in a wide range of cells including monocytes, macrophages, endothelial cells, adipocytes, and podocytes. Besides AGEs, S100, calgranulin, 31 amphoterin 32 amyloid-B, and other fibrillar proteins 33 can bind with RAGE.
Fig. 1.
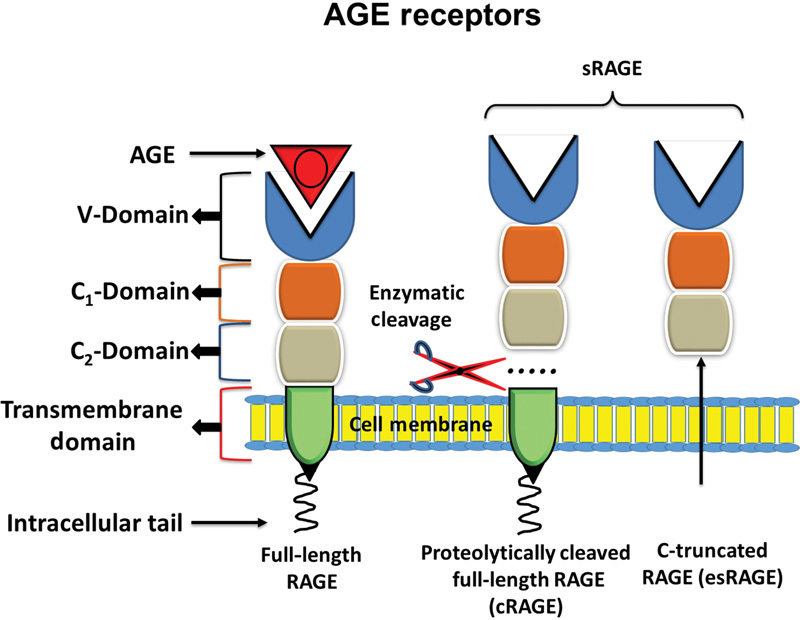
Diagrammatic representation of full-length RAGE and sRAGE. Full-length RAGE consists of intracellular tail, transmembrane domain, and extracellular domain, comprising of C 1 , C 2 , and V domain. V domain binds with AGE. sRAGE is comprised of cRAGE and esRAGE. Both cRAGE and esRAGE lack transmembrane domain and intracellular tail. AGE, advance glycation end-product; RAGE, cell bound receptor for AGE; sRAGE, soluble receptor for AGE; cRAGE, cleaved RAGE; esRAGE, endogenous secretory RAGE; C, constant; V, variable.
Adverse Effects of AGE and RAGE
AGE induces adverse effects in the body by two separate mechanisms: (a) nonreceptor and (b) receptor-mediated mechanisms.
-
Nonreceptor-mediated mechanism
Functional properties of extracellular matrix are affected by AGEs. Accumulation of AGEs on protein of extracellular matrix leads to the formation of cross-links, which traps other local macromolecules. 34 The properties of collagen are altered through AGE–RAGE intermolecular covalent bond or cross-linking. 35 Cross-linking of AGEs on collagen and elastin increases the extracellular matrix area which increases the stiffness of the artery. 36 Glycation increases the synthesis of collagen. 37 Cross-linking makes the collagen insoluble to the hydrolytic enzymes. 38 AGE-linked collagen is less susceptible to hydrolytic turnover and becomes stiff. Cross-linking of AGE with elastin reduces the elasticity of arterioles. Cross-linking increases the synthesis of collagen and reduces the quantity of elastin. AGE cross-linking with protein depends upon both sugar concentration and turnover rate of body proteins. LDL is sensitive to AGE cross-linking resulting in decreased uptake by LDL receptors. 39 AGE cross-linking of proteins of lens induces functional changes in lens 17 and AGE cross-linking of the proteins of renal tissue induces thickness of basement membrane of glomerulus, 40 and many other organs (retina, kidney nerves, hypertension, and atherosclerosis. 4 41
Glycation affects apoprotein B and phospholipid component of LDL resulting in functional alteration in LDL clearance and increased susceptibility to oxidative modification. 42 43 Glycation of LDL decreases its recognition by LDL receptors. 44 Glycated LDL has the capacity to stimulate the mitogen-activated protein kinase (MAPK) signaling pathways in vascular smooth muscle cells that increase the cell proliferation or differentiation. 40 45 AGEs interfere with the reverse cholesterol transport through suppression of scavenger receptor B1 (sR-B1)-mediated uptake of cholesterol ester from high-density lipoprotein (HDL) by liver and sR-B1-mediated cholesterol efflux from peripheral cells. 46 AGE induces accumulation of cholesterol and its ester in macrophages in vitro. 47
Glycated albumin alters the binding of drugs in plasma in diabetes. 48 It plays role in the platelet activation and aggregation. 49 Glycated fibrinogen impairs fibrinolysis 50 and increases fibrin gel permeability resulting in the formation of less thrombogenic fibrin network. 51 Glycation of immunoglobulin (IgG) is associated with inflammation and is target for auto-antibodies in rheumatoid arthritis. 52 Among all the AGEs, MGO is the major immune-suppressant in patients with diabetes. 52
-
Receptor-mediated mechanism
The effects of interaction of AGEs with RAGE are summarized in Fig. 2. Interaction of AGEs with RAGE results in the generation of ROS and activation of NF-κB. AGE–RAGE interaction directly increases the generation of ROS through activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, 53 which in turn activates NF-κB. Binding of AGEs with RAGE stimulates various signaling pathways including MAPKs, extracellular regulated kinases (Erk) 1 and 2, phosphatidyl-inositol 3 kinase/c-Jun-N-terminal kinase, p21 Ras, and the Janus kinases. 54 55 The net result of these signaling mechanisms is the activation of NF-κB and subsequent transcription of numerous proinflammatory genes shown in Table 1 . 31 35 56 Interaction of AGEs with RAGE in monocytes induces chemotaxis which accelerates the migration of monocytes into subendothelial space. 57 58 Binding of AGEs with RAGE in monocyte-macrophage increases the expression and generation of interleukin 1β (IL-1β), tumor necrosis factor-α (TNF-α), platelet-derived growth factor (PGDF), and insulin-like growth factor 1 (IGF-1) 59 60 61 and increases uptake of glycated LDL. 62 Interaction of AGEs with RAGE decreases endothelial barrier function and hence increases permeability of endothelial cell layer 63 64 and vascular smooth muscle cells proliferation and production of fibronectin. 65 66
Fig. 2.
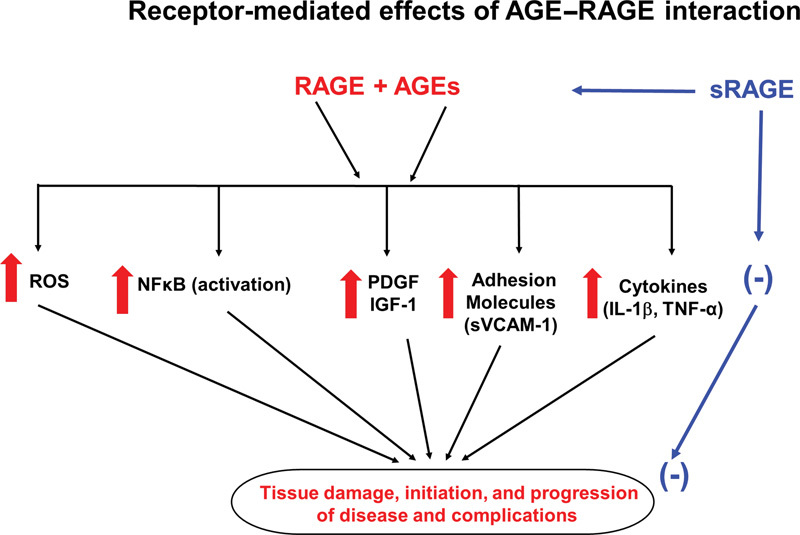
The effects of interaction AGE with RAGE or sRAGE. Interaction of AGE with RAGE increases ROS, NF-κB, VCAM-1, growth factor, and cytokines. Interaction of AGE with sRAGE counteracts the effect of AGE–RAGE interaction. ↑, increase; (-), decrease; AGE, advance glycation end-product; RAGE, cell bound receptor for AGE; sRAGE, soluble receptor for AGE; ROS, reactive oxygen species; NF-κB, nuclear factor kappa B; VCAM-1, vascular cell adhesion molecule 1; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; PDGF, platelet-derived growth factor; IGF-1, insulin-like growth factor-1.
Table 1. Increased gene expressions of some molecules by activated NF-κB 16 .
| Cell adhesion molecules | ELAM-1 |
| ICAM-1 | |
| VCAM-1 | |
| Cytokines and chemokines | IL-1, IL-1β, IL-2, IL-6, IL-8, TNF-α, G-CSF, M-CSF, MCP-1 |
| Acute phase proteins | Serum amyloid A precursor |
| Angiotensinogen | |
| Complement factor C4 | |
| Compliment factor B | |
| Others | Nitric oxide synthase |
| Hemeoxygenase-1 | |
| Growth factors |
Abbreviations: ELAM-1, endothelial leukocyte adhesion molecule 1; G-CSF, granulocyte colony stimulating factor; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; MCP-1, monocyte chemotactic protein; M-CSF, monocyte colony stimulating factor; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1.
Antistressors (AGE–RAGE Defense Mechanism)
The body is equipped with defense mechanisms to counteract the deleterious effects of AGE–RAGE stressors. These antistressors can be classified into two types: endogenous and exogenous.
-
Endogenous antistressors
Endogenous antistressors include enzymatic degradation of AGEs, AGE receptor-mediated degradation of AGE, and sRAGE.
-
Enzymatic degradation of AGEs
Glyoxalase-1 (GLO1) and glyoxalase-2 (GLO2) degrade reactive dicarbonyls prior to the formation of AGEs. Reactive dicarbonyls, MGO, react with reduced glutathione to form hemithioacetal. 67 The hemithioacetal is converted to s-2-hydroacetalglutathione by GLO1. GLO2 converts s-2-hydroacetalglutathione to α-hydroxyl and releases reduced glutathione. 68 Overexpression of GLO1 in endothelial cells in vitro under hyperglycemic conditions reduced the levels of dicarbonyls 69 and this effect was associated with correction of the defects in angiogenesis 70 and vascular relaxation. 71 Similarly, overexpression of GLO1 in lens and retinal capillary pericytes respectively protected against hyperglycemia-induced protein modifications 72 and apoptosis. 73 GLO1 is the key enzyme in antiglycation defense system because this is a rate-limiting step in the glyoxalase pathway and it prevents the storage of reactive dicarbonyls. 74
-
AGE-receptor-mediated degradation of AGE
Besides RAGE, AGE can bind to other cell surface receptors such as advanced glycation end-products receptors (AGER1, AGER2, AGER3). The first cell receptor discovered in connection with AGE endocytosis was AGER1. 75 This protein has significant AGE-specific binding capacity and hence was named AGER1.Although AGER2 does not directly binds with AGE, it is effectively phosphorylated by AGE and has been suggested to play a role in early stages of AGE signaling. 75 AGER3 has a high affinity to bind with AGE. 76 Its exact role is not known. However, it has been suggested that AGER3 may regulate the turnover of AGE and maintain the integrity of tissue. 77 It is upregulated in hyperglycemia and after exposure to AGEs. 78 AGER1 has been studied in detail. AGER1 protein is present in most of the cells and tissues including macrophages, 79 mesangial cells, 80 and mononuclear cells 81 mediate the uptake and degradation of AGE by kidney. 82 AGER1 accelerates the uptake and removal of AGE and blocks cellular AGE–RAGE-mediated generation of ROS and proinflammatory cytokines. 80 83 AGER1 also counteracts AGE-induced oxidative stress through inhibition of RAGE signaling. 80 83 Degradation of AGE by AGER1 produces AGE peptides which normally filter through glomerular membrane. The filtrate undergoes variable degree of tubular reabsorption or further catabolism in the proximal tubules and is excreted in the urine. 84 There is an inverse correlation between serum levels of AGE and renal function. 85 Renal disease is associated with reduced excretion of AGEs. 86 Since AGER1 and RAGE compete for AGE, low levels of AGER1 would increase the binding of AGE with RAGE and hence increase in the oxidative stress and inflammation.
There is a downregulation of AGER1 by AGE-rich diet 83 and diabetes. 87 There is a reduction in both RAGE and AGER1 with consumption of low AGE-rich diet. Also, there is an inverse correlation between AGER1 and intracellular levels of AGEs and positive correlation between AGER1 and urinary AGE levels in nondiabetic individuals. 88 However, the levels of RAGE are high while that of AGER1 reduced in diabetic patients in spite of full antidiabetic therapy. 89 With the consumption of low AGE-rich diet for 4 months, the levels of AGER1 were restored, while those of RAGE were suppressed. 89 These data suggest that the reduction in AGER1 gene contributes to the complications in diabetes due to elevation of AGE and consequences of AGE–RAGE interaction. It has been reported that interruption of AGER1-dependent uptake of AGEs and subsequent degradation accelerates glomerular renal pathologies in spontaneous nonobese diabetic mice. 87 It has also been shown that the patients with severe diabetic complications have reduced expression of AGER1 in circulating mononuclear cells and elevated serum levels of AGEs. 81 AGER1/RAGE ratio may serve as a biomarker for pathogenesis of AGE–RAGE-mediated disease conditions. High ratio of AGER1/RAGE would protect from the deleterious effects of interaction of AGE with RAGE.
Metabolic states including diabetes, hyperlipidemia, uremia, and aging are associated with upregulation of RAGE, AGER2, and AGER3, most probably because of elevation of AGE levels in these conditions. However, AGER1 expression is dependent on AGE levels. 81 Reduction in the expression of AGER1 in human monocytes is associated with elevated levels of sRAGE in patients with severe diabetic complications. 81 There was a positive correlation between expression of AGER1 and serum levels of sRAGE in complication-free diabetic patients suggesting that sRAGE modulates macrophage/monocyte AGER1. 81
-
Soluble receptor for AGE
There are three well-described receptors for AGE: full-length RAGE, cRAGE, and esRAGE. Full-length RAGE has been described in the detail in the receptor for AGE (RAGE) section of this review. sRAGE, cRAGE, and esRAGE are diagrammatically presented in Fig. 1 . cRAGE is proteolytically cleaved from full-length RAGE. 90 esRAGE is formed from alternative splicing of full-length RAGE mRNA. 91 Measurement of total soluble RAGE (sRAGE) includes cRAGE and esRAGE (sRAGE ELISA kit), while measurement of esRAGE measures only esRAGE (esRAGE ELISA kit). Since total sRAGE includes both cRAGE and esRAGE, cRAGE is determined by subtracting esRAGE from sRAGE. Serum sRAGE is approximately five times higher than esRAGE in healthy subjects. 9 92 93
cRAGE and esRAGE lack cytosolic tail and transmembrane domain and circulate in the blood. They bind with AGE but does not activate intracellular singling. 93 Soluble receptors are competitive inhibitor of AGE–RAGE interaction and may also serve as scavenger receptor for circulating AGEs. 94 sRAGE competes with full-length RAGE for binding with AGEs and functions as decoy, and hence has a cytoprotective effects against adverse effects of AGE–RAGE interaction. AGEs interact with sRAGE before they interact with full-length RAGE. 95 Low levels of serum sRAGE will allow high levels of AGEs to interact with RAGE and hence deleterious effect on the cells. Low serum levels of sRAGE have been implicated in the pathophysiology of numerous diseases. 1 2 3 4 5 6 7 8 9 10 11 However, diabetes and chronic renal disease and their complications are associated with high levels of serum sRAGE. 9 13 One would have expected that high levels of sRAGE would have protected the development of diabetes, chronic kidney disease. The reason for this discrepancy may be due to the elevation of levels of AGEs greater than the elevation of serum levels of sRAGE. Prasad et al 9 13 have reported that in the end-stage renal disease there is an increase in levels of both AGEs and sRAGE more so in AGEs than sRAGE. 9
It has been reported by Zhou et al 96 that the levels of AGEs and RAGE in carotid arterial wall are elevated in Zucker diabetic rats. They also showed that the balloon injury in carotid artery of these rats further increased the levels of AGE and RAGE and produced neointimal hyperplasia. Therapy with sRAGE reduced neointimal growth significantly. Treatment of diabetic apoE-deficient mice with sRAGE completely suppressed atherosclerosis. 97 McNair et al 98 have reported that low serum levels of sRAGE are a predictor of restenosis following percutaneous coronary intervention (PCI). These data suggest that sRAGE exerts an antagonist effects by binding RAGE ligands and preventing their signaling through membrane bound RAGE.
-
-
Exogenous antistressors
Exogenous antistressors can be categorized as follows: (I) reduction in AGE consumption, (II) cessation of smoking, (III) prevention of AGE formation, (IV) AGE breakers, (V) downregulation of RAGE expression, (VI) elevation of sRAGE, (VII) administration of recombinant sRAGE.
-
Reduction in AGE consumption
Consumption of AGE-rich diet such as read meat, cheese, cream, butter, animal fat, and sugars should be reduced. They increase the levels of AGEs in the body. 23
-
Cessation of smoking
Cigarette smoking should be stopped because it increases the serum levels of AGEs. 24
-
Prevention of AGE formation
The measures for the prevention of formation of AGE formation are summarized in Table 2 .
-
Cooking
Avoid cooking at high temperature with dry heat (frying, broiling, grilling, roasting, and baking). Cook at low temperature in moist heat. 23
Agents that reduce formation of AGEs
Acidic ingredients (lemon juice, vinegar). 99
Phytochemicals from pomegranates, 100 berries and grapes, 101 inhibit the formation of AGEs.
-
Drugs:
Aminoguanidine, a hydralazine compound, inhibits formation of AGEs. 102 It has been reported that aminoguanidine prevents the diabetic vascular complications in diabetic animals. 103 In a placebo controlled clinical trial, aminoguanidine reduced glomerular filtration rate and proteinuria and prevented deterioration of retinopathy in diabetic patients. 104 Action II trial reported that aminoguanidine produces side effects such as flu-like symptoms, hepatic abnormalities, gastrointestinal disorders, and anemia. 105 Further clinical trials were terminated because of concern over the side effects. 105
Angiotensin-converting enzyme (ACE) inhibitor ramipril reduces fluorescent AGE in diabetic patients. 106 Angiotensin II receptor blockers (telmisartan and losartan) reduced the formation of AGEs in cell culture. 107 Valsartan 108 and candesartan 109 lowered the serum levels of AGE in hypertensive patients with diabetes. Atorvastatin 110 and cerivastatin 111 reduced the serum levels of AGE in diabetic or prediabetic or diabetic kidney disease.
Biguanide derivative (metformin), an antidiabetic drug, reduces the serum levels of AGE in women with polycystic ovary syndrome. 112 Thiazolidine derivative (pioglitazone), an antidiabetic drug, inhibits AGE formation by trapping dicarbonyl compounds. 113 There are other drugs including α-lipoic acid, 114 aspirin, 115 taurine, 116 pentoxifylline, 113 resveratrol, 117 and curcumin 118 that are potential inhibitors of AGE formation.
-
Vitamins
Certain vitamins reduce the formation of AGEs. Benfotiamine (vitamin B1) 119 and pyridoxamine, a natural form of vitamin B6, 120 vitamin C, 121 vitamin D, 122 and vitamin E, 123 reduce the formation of AGEs.
-
AGE cross-link breaker
AGE cross-link breaker fragments α-carbonyl compounds by cleaving the carbon–carbon bond between carbonyls. Alagebrium (ALT-711) nonenzymatically breaks the established cross-linking AGE with adjacent long-lived collagen and elastin 124 and reduces the levels of AGE. It has also been shown to reduce arterial stiffness. 125
-
-
Downregulation of RAGE expression
Downregulation of RAGE expression would reduce the availability of RAGE to interact with AGE resulting in reduction of adverse effects of AGE–RAGE interaction. Table 3 shows the agents that reduce the expression of RAGE. In statin groups, simvastatin inhibits the expression of RAGE via decreases in the myeloperoxidase-dependent formation of AGEs. 126 RAGE expression is downregulated in vitro by atorvastatin. 127 Angiotensin II receptor blockers, telmisartan 128 and candesartan, 129 downregulate the expression of RAGE. Metformin, a biguanide derivative used in the treatment of diabetes, downregulates the expression of RAGE in the vascular endothelium. 130 Thiazolidinediones (pioglitazone, rosiglitazone) downregulate the expression of RAGE in human endothelial cells. 131 Nifedipine, a calcium channel blocker, reduces the RAGE expression in vascular endothelium exposed to AGE. 132 Curcumin, a condiment used in cooking, downregulates the RAGE expression in cultured hepatic cells. 133 Resveratrol downregulates the expression of RAGE in the vascular smooth muscle cells. 134 135
-
Elevation of soluble receptors (sRAGE, esRAGE)
The drugs that affect the expression and levels of soluble receptors (sRAGE, esRAGE) are shown in Table 4 . ACE inhibitors, ramipril, upregulated the expression of sRAGE in the aorta of streptozotocin-induced diabetic rats and increased the serum levels of sRAGE. 136 Perindopril increased the serum levels of sRAGE in patients with type 1 diabetes. 110
Among the statins, pitavastatin and pravastatin elevated the serum levels of sRAGE in angina patients with coronary atherosclerosis. 110 Atorvastatin increased the serum levels of sRAGE and esRAGE in hypercholesterolemic patients with type 2 diabetes and upregulated the expression of sRAGE and esRAGE in THP1 cells in vitro. 137 Lovastatin increases the sRAGE levels by inducing RAGE shedding. 138 Fluvastatin stimulates the production of sRAGE and esRAGE in vitro. 138 Antidiabetic drug, metformin, increased the serum levels of sRAGE in patients with metabolic syndrome. 139 Insulin increased the serum levels of sRAGE and esRAGE in Chinese patients with type 1 diabetes. 140 Insulin also stimulated the shedding of sRAGE from membrane-bound receptor for AGE in cell culture. 140 Rosiglitazone, a thiazolidine derivative, increased the serum levels of sRAGE and esRAGE. 141
-
Exogenous administration of sRAGE
Exogenous administration of sRAGE suppressed development of atherosclerosis and restenosis, prevented destabilization of vulnerable plaques, and reduced ischemia reperfusion-induced myocardial injuries. 16 Administration of recombinant sRAGE protected ischemic stroke in animal model, 142 reduced carotid artery stenosis in mice, 66 and completely suppressed atherosclerosis in apoE-deficient mice. 97 Possibility exists that administration of exogenous sRAGE would raise the serum levels of sRAGE which will combine with AGE, resulting in the reduced interaction of AGE with RAGE and hence reduction in pathophysiology of the disease.
Table 2. Agents that reduce the formation of AGEs.
| Cooking | Cooking food in moist heat 23 | |
| Acidic ingredients | Lemon juice and vinegar 99 | |
| Phytochemicals | Berries, grapes, 101 pomegranate 100 | |
| Drugs | Aminoguanidine | Pimagedine 102 |
| ACE-inhibitor | Ramipril 106 | |
| Ang II-receptor blockers | Telmisartan, 107 losartan, 107 valsartan, 108 candesartan 109 | |
| Statins | Atorvastatin, 110 cerivastatin 111 | |
| Antidiabetic drugs | Metformin, 112 pioglitazone 113 | |
| Other drugs | Aspirin, 115 pentoxifylline 113 | |
| Vitamins | Benfotiamine (B1), 119 pyridoxamine (B6), 120 vitamin C, 121 D, 122 E 123 | |
| Other | α-lipoic acid, 114 resveratrol, 117 curcumin 118 | |
Abbreviations: AGE, advance glycation end-product; ACE, angiotensin-converting enzyme; Ang II, angiotensin II.
Table 3. Agents that downregulate the expression of receptor for advanced glycation end-products.
Table 4. Agents that elevate sRAGE expression.
| Angiotensin-converting enzyme inhibitors | Ramipril, 136 Perindopril 136 |
| Statins | Atorvastatin, 137 pitavastatin, 110 pravastatin, 110 fluvastatin, 138 lovastatin 138 |
| Antidiabetic drugs | Rosiglitazone, 141 metformin, 139 insulin 140 |
Abbreviation: sRAGE, soluble receptor for advance glycation end-product.
Assessment of AGE–RAGE Stress
As mentioned earlier, AGE–RAGE stress is defined as a shift in balance between stressors and antistressors in favor of stressors ( Fig. 3 ). Measurements of stressors (AGE, RAGE) or antistressors (degradation of AGE by GLO1, GLO2, AGER1, and AGER 2), and sRAGE would not measure AGE–RAGE stress. A formula using all stressors and antistressors would provide a true AGE–RAGE stress. A ratio of AGE + RAGE/GLO1 + GLO2 + AGER1 + AGER2 + sRAGE would provide a true index of AGE–RAGE stress. This ratio of AGE–RAGE stress can be determined in animal studies. It would be cumbersome to use this ratio in human beings. AGE and sRAGE can be measured in the blood samples from human; however, human tissues are required to measure the receptors such as AGER1, AGER2, and RAGE and that is not easy. We have, therefore, suggested that the ratio of AGE/sRAGE would be a simple and feasible measure of AGE–RAGE stress. An increase in the ratio of AGE/sRAGE would indicate a relative shift in stressors from antistressors, suggesting the presence of AGE–RAGE stress at cellular and organ levels. AGE/RAGE ratio has been suggested as a risk marker of disease process. 13 Increased ratio of AGE/sRAGE has been implicated in pathogenesis of restenosis following PCI 98 hyperthyroidism, 7 and end-stage renal disease. 9 The sensitivity, specificity, positive and negative predictive value, and accuracy of AGE/sRAGE should be determined with large number of control and a particular group of patients. Using receiver operating characteristics (ROC) curve analysis, we have reported that the sensitivity and specificity of AGE/sRAGE ratio were 73% and 70%, respectively, in identifying patients with hyperthyroidism. 7 The sensitivity, specificity, positive predictive value, and negative predictive value of AGE/sRAGE ratio with optimal cut value of 2.75 were 84.88%, 80.95%, 94.81%, and 56.67%, respectively, in identifying patients with end-stage renal disease. 9 The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of AGE/sRAGE ratio were 100%,83%, 85%, 100%, and 91%, respectively, in predicting restenosis following PCI. 98 These values were obtained using methods described by Glas et.al. 143 The data suggest that the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the ratio of AGE/sRAGE in identifying the risk factor or predicting the disease condition appear to be excellent. The AGE/sRAGE ratio is, therefore, an appropriate measure of AGE–sRAGE stress.
Fig. 3.
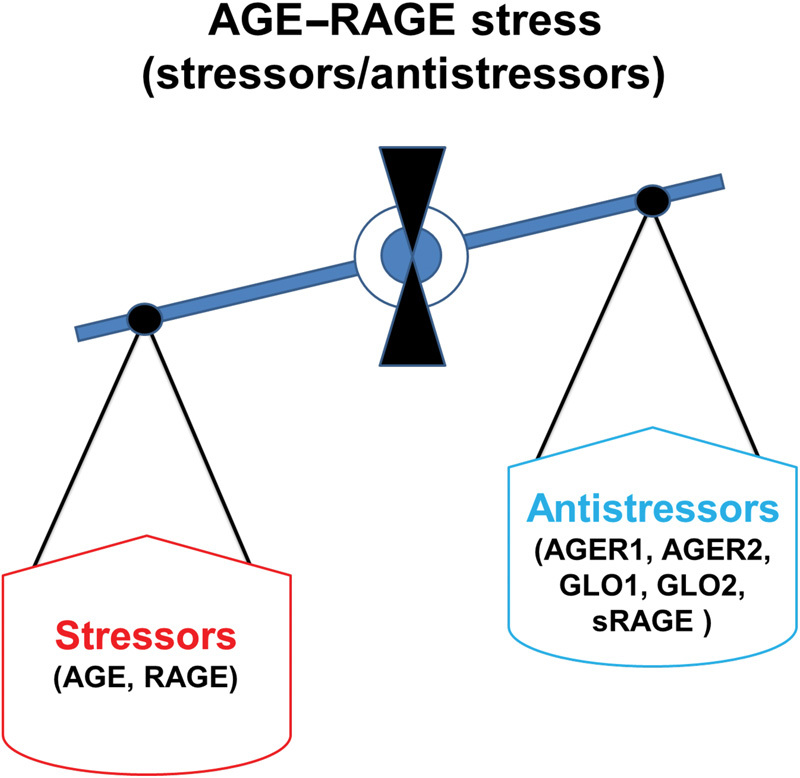
Schematic representation of AGE–RAGE stress, stressors, and antistressors. AGE, advance glycation end-product; RAGE, cell bound receptor for AGE; sRAGE, soluble receptor for AGE; AGER1, advance glycation end-product receptor 1; AGER2, advance glycation end-product receptor 2; GLO1, glyoxalase 1; GLO2, glyoxalase 2.
Conclusions
Stress is defined as a process of altered biochemical homeostasis produced by physiological, psychological or environmental stressors. In the present context, AGE and RAGE are stressors, and GLO1, GLO2, AGER1, and AGER 2 which degrade AGE and sRAGE are antistressors. Low levels of AGEs are due to reduction in the formation and consumption of AGE and degradation of AGE enzymatically (GLO1, GLO2) and through receptors (AGER1, AGER2). AGE–RAGE stress occurs when excess AGE and RAG are produced that could overwhelm the normal antistressors. In other word, AGE–RAGE stress is defined as a shift in the balance between stressors and antistressors in favor of stressors. Measurements of only stressors or antistressors would not provide an index of AGE–RAGE stress. The ratio of AGE + RAGE/sRAGE + GLO1 + GLO2 + AGER1 + AGER2 would be a true measure of AGE–RAGE stress. The measurement of this ratio is very feasible in animal studies. However, the measurement of this ratio of AGE–RAGE stress is not feasible in humans because one has to take tissue from human to measure RAGE and AGER1 and AGER2. We have, therefore, suggested that AGE/sRAGE ratio would be simple and feasible index of AGE–RAGE stress in clinical practice and for experimental studies. It is concluded that AGE/sRAGE ratio is one of the important determinants of AGE–RAGE stress. A high ratio would indicate a relative shift in stressors from antistressors suggesting the presence of AGE–RAGE stress that may partly be involved in the pathogenesis of numerous diseases and their complications.
Disclosure
None.
Footnotes
Conflict of Interest None.
References
- 1.Del Turco S, Basta G. An update on advanced glycation endproducts and atherosclerosis. Biofactors. 2012;38(04):266–274. doi: 10.1002/biof.1018. [DOI] [PubMed] [Google Scholar]
- 2.Falcone C, Emanuele E, D'Angelo A et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25(05):1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 3.McNair E D, Wells C R, Qureshi A M et al. Low levels of soluble receptor for advanced glycation end products in non-ST elevation myocardial infarction patients. Int J Angiol. 2009;18(04):187–192. doi: 10.1055/s-0031-1278352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad K, Mishra M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int J Angiol. 2017;26(01):1–11. doi: 10.1055/s-0037-1598183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20(03):242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Hudson B I, Moon Y P, Kalea A Z et al. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS) Atherosclerosis. 2011;216(01):192–198. doi: 10.1016/j.atherosclerosis.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspar-Bell G, Dhar I, Prasad K.Advanced glycation end products (AGEs) and its receptors in the pathogenesis of hyperthyroidism Mol Cell Biochem 2016414(1-2):171–178. [DOI] [PubMed] [Google Scholar]
- 8.Emanuele E, D'Angelo A, Tomaino C et al. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch Neurol. 2005;62(11):1734–1736. doi: 10.1001/archneur.62.11.1734. [DOI] [PubMed] [Google Scholar]
- 9.Prasad K, Dhar I, Zhou Q, Elmoselhi H, Shoker M, Shoker A.AGEs/sRAGE, a novel risk factor in the pathogenesis of end-stage renal disease Mol Cell Biochem 2016423(1-2):105–114. [DOI] [PubMed] [Google Scholar]
- 10.Yan S F, Ramasamy R, Schmidt A M. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl) 2009;87(03):235–247. doi: 10.1007/s00109-009-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilhovd B K, Berg T J, Birkeland K I, Thorsby P, Hanssen K F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22(09):1543–1548. doi: 10.2337/diacare.22.9.1543. [DOI] [PubMed] [Google Scholar]
- 12.Schneiderman N, Ironson G, Siegel S D. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol. 2014;23(01):11–16. doi: 10.1055/s-0033-1363423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70(02):138–151. [PubMed] [Google Scholar]
- 15.Reddy S, Bichler J, Wells-Knecht K J, Thorpe S R, Baynes J W. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34(34):10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 16.Prasad K. Soluble receptor for advanced glycation end products (sRAGE) and cardiovascular disease. Int J Angiol. 2006;15:57–68. [Google Scholar]
- 17.Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N. Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(01):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko M, Bucciarelli L, Hwang Y C et al. Aldose reductase and AGE-RAGE pathways: key players in myocardial ischemic injury. Ann N Y Acad Sci. 2005;1043:702–709. doi: 10.1196/annals.1333.081. [DOI] [PubMed] [Google Scholar]
- 20.Weiss M F, Erhard P, Kader-Attia F A et al. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57(06):2571–2585. doi: 10.1046/j.1523-1755.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed M U, Thorpe S R, Baynes J W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261(11):4889–4894. [PubMed] [Google Scholar]
- 22.Uribarri J, Woodruff S, Goodman S et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(06):911–1.6E13. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uribarri J, del Castillo M D, de la Maza M P et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6(04):461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad K, Dhar I, Caspar-Bell G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol. 2015;24(02):75–80. doi: 10.1055/s-0034-1396413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koschinsky T, He C J, Mitsuhashi T et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94(12):6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54(08):2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 27.Liardon R D-GD, Philippossian G, Finot P A. Identification of N. epsilon.-carboxymethyllysine: a new Maillard reaction product in rat urine. J Agric Food Chem. 1987;35(03):427–431. [Google Scholar]
- 28.Semba R D, Ang A, Talegawkar S et al. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: the Energetics Study. Eur J Clin Nutr. 2012;66(01):3–9. doi: 10.1038/ejcn.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neeper M, Schmidt A M, Brett J et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–15004. [PubMed] [Google Scholar]
- 30.Stern D M, Yan S D, Yan S F, Schmidt A M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1(01):1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann M A, Drury S, Fu C et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(07):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 32.Taguchi A, Blood D C, del Toro Get al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases Nature 2000405(6784):354–360. [DOI] [PubMed] [Google Scholar]
- 33.Yan S D, Zhu H, Zhu A et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med. 2000;6(06):643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt A M, Hasu M, Popov D et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci U S A. 1994;91(19):8807–8811. doi: 10.1073/pnas.91.19.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt A M, Hori O, Chen J X et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96(03):1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka S, Avigad G, Brodsky B, Eikenberry E F. Glycation induces expansion of the molecular packing of collagen. J Mol Biol. 1988;203(02):495–505. doi: 10.1016/0022-2836(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 37.Striker L J, Striker G E. Administration of AGEs in vivo induces extracellular matrix gene expression. Nephrol Dial Transplant. 1996;11 05:62–65. doi: 10.1093/ndt/11.supp5.62. [DOI] [PubMed] [Google Scholar]
- 38.Reiser K, McCormick R J, Rucker R B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992;6(07):2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- 39.Zieman S, Kass D.Advanced glycation end product cross-linking: pathophysiologic role and therapeutic target in cardiovascular disease Congest Heart Fail 20041003144–149., quiz 150–151 [DOI] [PubMed] [Google Scholar]
- 40.Ahmed N, Lüthen R, Häussinger D et al. Increased protein glycation in cirrhosis and therapeutic strategies to prevent it. Ann N Y Acad Sci. 2005;1043:718–724. doi: 10.1196/annals.1333.083. [DOI] [PubMed] [Google Scholar]
- 41.Poulsen M W, Hedegaard R V, Andersen J M et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 42.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993;90(14):6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinbrecher U P, Witztum J L. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 1984;33(02):130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- 44.Haberland M E, Fless G M, Scanu A M, Fogelman A M. Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophages. J Biol Chem. 1992;267(06):4143–4151. [PubMed] [Google Scholar]
- 45.Makita T, Tanaka A, Numano F. Effect of glycated low density lipoprotein on smooth muscle cell proliferation. Int Angiol. 1999;18(04):331–334. [PubMed] [Google Scholar]
- 46.Horiuchi S, Sakamoto Y, Sakai M.Scavenger receptors for oxidized and glycated proteins Amino Acids 200325(3-4):283–292. [DOI] [PubMed] [Google Scholar]
- 47.Brown B E, Dean R T, Davies M J. Glycation of low-density lipoproteins by methylglyoxal and glycolaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia. 2005;48(02):361–369. doi: 10.1007/s00125-004-1648-4. [DOI] [PubMed] [Google Scholar]
- 48.Barnaby O S, Cerny R L, Clarke W, Hage D S.Comparison of modification sites formed on human serum albumin at various stages of glycation Clin Chim Acta 2011412(3-4):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubenstein D A, Yin W. Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets. 2009;20(03):206–215. doi: 10.1080/09537100902795492. [DOI] [PubMed] [Google Scholar]
- 50.Dunn E J, Philippou H, Ariëns R A, Grant P J. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia. 2006;49(05):1071–1080. doi: 10.1007/s00125-006-0197-4. [DOI] [PubMed] [Google Scholar]
- 51.Jörneskog G, Hansson L O, Wallen N H, Yngen M, Blombäck M. Increased plasma fibrin gel porosity in patients with type I diabetes during continuous subcutaneous insulin infusion. J Thromb Haemost. 2003;1(06):1195–1201. doi: 10.1046/j.1538-7836.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad S, Moinuddin Khan R H, Ali A. Physicochemical studies on glycation-induced structural changes in human IgG. IUBMB Life. 2012;64(02):151–156. doi: 10.1002/iub.582. [DOI] [PubMed] [Google Scholar]
- 53.Wautier M P, Chappey O, Corda S, Stern D M, Schmidt A M, Wautier J L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(05):E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 54.Fleming T H, Humpert P M, Nawroth P P, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology. 2011;57(05):435–443. doi: 10.1159/000322087. [DOI] [PubMed] [Google Scholar]
- 55.Bierhaus A, Humpert P M, Morcos M et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 56.Reznikov L L, Waksman J, Azam T et al. Effect of advanced glycation end products on endotoxin-induced TNF-alpha, IL-1beta and IL-8 in human peripheral blood mononuclear cells. Clin Nephrol. 2004;61(05):324–336. doi: 10.5414/cnp61324. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt A M, Yan S D, Brett J, Mora R, Nowygrod R, Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993;91(05):2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992;89(24):12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vlassara H, Brownlee M, Manogue K R, Dinarello C A, Pasagian A.Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling Science 1988240(4858):1546–1548. [DOI] [PubMed] [Google Scholar]
- 60.Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirstein M, Aston C, Hintz R, Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992;90(02):439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein R L, Laimins M, Lopes-Virella M F. Isolation, characterization, and metabolism of the glycated and nonglycated subfractions of low-density lipoproteins isolated from type I diabetic patients and nondiabetic subjects. Diabetes. 1995;44(09):1093–1098. doi: 10.2337/diab.44.9.1093. [DOI] [PubMed] [Google Scholar]
- 63.Wautier J L, Zoukourian C, Chappey O et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97(01):238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989;170(04):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakata N, Meng J, Takebayashi S. Effects of advanced glycation end products on the proliferation and fibronectin production of smooth muscle cells. J Atheroscler Thromb. 2000;7(03):169–176. doi: 10.5551/jat1994.7.169. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi T, Yan S F, Yan S D et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111(07):959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mannervik B.Molecular enzymology of the glyoxalase system Drug Metabol Drug Interact 200823(1-2):13–27. [DOI] [PubMed] [Google Scholar]
- 68.Thornalley P J.Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation Biochem Soc Trans 200331(Pt 6):1343–1348. [DOI] [PubMed] [Google Scholar]
- 69.Shinohara M, Thornalley P J, Giardino I et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101(05):1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed U, Dobler D, Larkin S J, Rabbani N, Thornalley P J. Reversal of hyperglycemia-induced angiogenesis deficit of human endothelial cells by overexpression of glyoxalase 1 in vitro. Ann N Y Acad Sci. 2008;1126:262–264. doi: 10.1196/annals.1433.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brouwers O, Niessen P M, Haenen G et al. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53(05):989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gangadhariah M H, Mailankot M, Reneker L, Nagaraj R H. Inhibition of methylglyoxal-mediated protein modification in glyoxalase I overexpressing mouse lenses. J Ophthalmol. 2010;2010(10):274317. doi: 10.1155/2010/274317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller A G, Smith D G, Bhat M, Nagaraj R H. Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem. 2006;281(17):11864–11871. doi: 10.1074/jbc.M513813200. [DOI] [PubMed] [Google Scholar]
- 74.Kuhla B, Boeck K, Lüth H J et al. Age-dependent changes of glyoxalase I expression in human brain. Neurobiol Aging. 2006;27(06):815–822. doi: 10.1016/j.neurobiolaging.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z, Makita Z, Horii Y et al. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991;174(03):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vlassara H, Li Y M, Imani F et al. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1(06):634–646. [PMC free article] [PubMed] [Google Scholar]
- 77.Pugliese G, Pricci F, Leto G et al. The diabetic milieu modulates the advanced glycation end product-receptor complex in the mesangium by inducing or upregulating galectin-3 expression. Diabetes. 2000;49(07):1249–1257. doi: 10.2337/diabetes.49.7.1249. [DOI] [PubMed] [Google Scholar]
- 78.Goh S Y, Cooper M E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(04):1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 79.Vlassara H, Brownlee M, Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986;164(04):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu C, He J C, Cai W, Liu H, Zhu L, Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci U S A. 2004;101(32):11767–11772. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He C J, Koschinsky T, Buenting C, Vlassara H. Presence of diabetic complications in type 1 diabetic patients correlates with low expression of mononuclear cell AGE-receptor-1 and elevated serum AGE. Mol Med. 2001;7(03):159–168. [PMC free article] [PubMed] [Google Scholar]
- 82.Coughlan M T, Thallas-Bonke V, Pete J et al. Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: synergy or redundancy? Endocrinology. 2007;148(02):886–895. doi: 10.1210/en.2006-1300. [DOI] [PubMed] [Google Scholar]
- 83.Cai W, He J C, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci U S A. 2006;103(37):13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito A, Nagai R, Tanuma A et al. Role of megalin in endocytosis of advanced glycation end products: implications for a novel protein binding to both megalin and advanced glycation end products. J Am Soc Nephrol. 2003;14(05):1123–1131. doi: 10.1097/01.asn.0000062962.51879.f8. [DOI] [PubMed] [Google Scholar]
- 85.Vlassara H, Torreggiani M, Post J B, Zheng F, Uribarri J, Striker G E. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009;(114):S3–S11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- 86.Makita Z, Radoff S, Rayfield E J et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 87.He C J, Zheng F, Stitt A, Striker L, Hattori M, Vlassara H. Differential expression of renal AGE-receptor genes in NOD mice: possible role in nonobese diabetic renal disease. Kidney Int. 2000;58(05):1931–1940. doi: 10.1111/j.1523-1755.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- 88.Vlassara H, Striker G E. The role of advanced glycation end-products in the etiology of insulin resistance and diabetes. US Endocrinol. 2010;6(01):14–19. [Google Scholar]
- 89.Vlassara H, Cai W, Goodman S et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94(11):4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tam X H, Shiu S W, Leng L, Bucala R, Betteridge D J, Tan K C. Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in Type 2 diabetes. Clin Sci (Lond) 2011;120(02):81–89. doi: 10.1042/CS20100256. [DOI] [PubMed] [Google Scholar]
- 91.Yonekura H, Yamamoto Y, Sakurai Set al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury Biochem J 2003370(Pt 3):1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koyama H, Shoji T, Yokoyama H et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(12):2587–2593. doi: 10.1161/01.ATV.0000190660.32863.cd. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt A M, Yan S D, Yan S F, Stern D M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(07):949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malherbe P, Richards J G, Gaillard H et al. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res. 1999;71(02):159–170. doi: 10.1016/s0169-328x(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 95.Koyama H, Yamamoto H, Nishizawa Y.RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases Mol Med 200713(11-12):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Z, Wang K, Penn M S et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107(17):2238–2243. doi: 10.1161/01.CIR.0000063577.32819.23. [DOI] [PubMed] [Google Scholar]
- 97.Park L, Raman K G, Lee K J et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4(09):1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 98.McNair E D, Wells C R, Mabood Qureshi A et al. Soluble receptors for advanced glycation end products (sRAGE) as a predictor of restenosis following percutaneous coronary intervention. Clin Cardiol. 2010;33(11):678–685. doi: 10.1002/clc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramful D, Tarnus E, Rondeau P, Da Silva C R, Bahorun T, Bourdon E. Citrus fruit extracts reduce advanced glycation end products (AGEs)- and H 2 O 2 -induced oxidative stress in human adipocytes . J Agric Food Chem. 2010;58(20):11119–11129. doi: 10.1021/jf102762s. [DOI] [PubMed] [Google Scholar]
- 100.Liu W, Ma H, Frost L, Yuan T, Dain J A, Seeram N P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014;5(11):2996–3004. doi: 10.1039/c4fo00538d. [DOI] [PubMed] [Google Scholar]
- 101.Wang W, Yagiz Y, Buran T J, Nunes M CN, Gu L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res Int. 2011;44(09):2666–2673. [Google Scholar]
- 102.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A.Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking Science 1986232(4758):1629–1632. [DOI] [PubMed] [Google Scholar]
- 103.Hammes H P, Brownlee M, Edelstein D, Saleck M, Martin S, Federlin K. Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia. 1994;37(01):32–35. doi: 10.1007/BF00428774. [DOI] [PubMed] [Google Scholar]
- 104.Bolton W K, Cattran D C, Williams M E et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(01):32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 105.Freedman B I, Wuerth J P, Cartwright K et al. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II) Control Clin Trials. 1999;20(05):493–510. doi: 10.1016/s0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- 106.Sebeková K, Gazdíková K, Syrová D et al. Effects of ramipril in nondiabetic nephropathy: improved parameters of oxidatives stress and potential modulation of advanced glycation end products. J Hum Hypertens. 2003;17(04):265–270. doi: 10.1038/sj.jhh.1001541. [DOI] [PubMed] [Google Scholar]
- 107.Yoshida T, Yamagishi S, Matsui T et al. Telmisartan, an angiotensin II type 1 receptor blocker, inhibits advanced glycation end-product (AGE)-elicited hepatic insulin resistance via peroxisome proliferator-activated receptor-gamma activation. J Int Med Res. 2008;36(02):237–243. doi: 10.1177/147323000803600204. [DOI] [PubMed] [Google Scholar]
- 108.Komiya N, Hirose H, Saisho Y, Saito I, Itoh H. Effects of 12-month valsartan therapy on glycation and oxidative stress markers in type 2 diabetic subjects with hypertension. Int Heart J. 2008;49(06):681–689. doi: 10.1536/ihj.49.681. [DOI] [PubMed] [Google Scholar]
- 109.Saha S A, LaSalle B K, Clifton G D, Short R A, Tuttle K R. Modulation of advanced glycation end products by candesartan in patients with diabetic kidney disease--a dose-response relationship study. Am J Ther. 2010;17(06):553–558. doi: 10.1097/MJT.0b013e3181b96c27. [DOI] [PubMed] [Google Scholar]
- 110.Nozue T, Yamagishi S, Takeuchic M et al. Effect of statins on the serum soluble form of receptor for advanced glycation end-products and its association with coronary atherosclerosis in patients with angina pectoris. IJC Metab Endocr. 2014;4:47–52. [Google Scholar]
- 111.Scharnagl H, Stojakovic T, Winkler K, Rosinger S, März W, Boehm B O. The HMG-CoA reductase inhibitor cerivastatin lowers advanced glycation end products in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2007;115(06):372–375. doi: 10.1055/s-2007-973830. [DOI] [PubMed] [Google Scholar]
- 112.Diamanti-Kandarakis E, Alexandraki K, Piperi C et al. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism. 2007;56(01):129–134. doi: 10.1016/j.metabol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Rahbar S, Natarajan R, Yerneni K, Scott S, Gonzales N, Nadler J L.Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation Clin Chim Acta 2000301(1-2):65–77. [DOI] [PubMed] [Google Scholar]
- 114.Abdul H M, Butterfield D A. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and alpha-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease. Free Radic Biol Med. 2007;42(03):371–384. doi: 10.1016/j.freeradbiomed.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Urios P, Grigorova-Borsos A M, Sternberg M. Aspirin inhibits the formation of pentosidine, a cross-linking advanced glycation end product, in collagen. Diabetes Res Clin Pract. 2007;77(02):337–340. doi: 10.1016/j.diabres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 116.Nandhini A T, Thirunavukkarasu V, Anuradha C V. Taurine prevents collagen abnormalities in high fructose-fed rats. Indian J Med Res. 2005;122(02):171–177. [PubMed] [Google Scholar]
- 117.Mizutani K, Ikeda K, Yamori Y. Resveratrol inhibits AGEs-induced proliferation and collagen synthesis activity in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Biochem Biophys Res Commun. 2000;274(01):61–67. doi: 10.1006/bbrc.2000.3097. [DOI] [PubMed] [Google Scholar]
- 118.Tang Y, Chen A. Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Invest. 2014;94(05):503–516. doi: 10.1038/labinvest.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hammes H P, Du X, Edelstein D et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9(03):294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 120.Metz T O, Alderson N L, Thorpe S R, Baynes J W. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch Biochem Biophys. 2003;419(01):41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 121.Subratty A H, Aukburally N, Jowaheer V, Joonus N. Vitamin C and urea inhibit the formation of advanced glycation end products in vitro. Nutr Food Sci. 2010;40(05):456–465. [Google Scholar]
- 122.Salum E, Kals J, Kampus P et al. Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res Clin Pract. 2013;100(02):243–249. doi: 10.1016/j.diabres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 123.Baragetti I, Furiani S, Vettoretti S et al. Role of vitamin E-coated membrane in reducing advanced glycation end products in hemodialysis patients: a pilot study. Blood Purif. 2006;24(04):369–376. doi: 10.1159/000093678. [DOI] [PubMed] [Google Scholar]
- 124.Vasan S, Zhang X, Zhang Xet al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo Nature 1996382(6588):275–278. [DOI] [PubMed] [Google Scholar]
- 125.Kass D A, Shapiro E P, Kawaguchi M et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104(13):1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 126.Cuccurullo C, Iezzi A, Fazia M L et al. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26(12):2716–2723. doi: 10.1161/01.ATV.0000249630.02085.12. [DOI] [PubMed] [Google Scholar]
- 127.Xu L, Zang P, Feng B, Qian Q. Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy Sprague-Dawley rats. Diabetol Metab Syndr. 2014;6(01):102. doi: 10.1186/1758-5996-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamura K, Yamagishi S, Nakamura Y et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res. 2005;70(03):137–141. doi: 10.1016/j.mvr.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Fan Q, Liao J, Kobayashi M et al. Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant. 2004;19(12):3012–3020. doi: 10.1093/ndt/gfh499. [DOI] [PubMed] [Google Scholar]
- 130.Ouslimani N, Mahrouf M, Peynet J et al. Metformin reduces endothelial cell expression of both the receptor for advanced glycation end products and lectin-like oxidized receptor 1. Metabolism. 2007;56(03):308–313. doi: 10.1016/j.metabol.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 131.Marx N, Walcher D, Ivanova N et al. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004;53(10):2662–2668. doi: 10.2337/diabetes.53.10.2662. [DOI] [PubMed] [Google Scholar]
- 132.Yamagishi S, Takeuchi M. Nifedipine inhibits gene expression of receptor for advanced glycation end products (RAGE) in endothelial cells by suppressing reactive oxygen species generation. Drugs Exp Clin Res. 2004;30(04):169–175. [PubMed] [Google Scholar]
- 133.Lin J, Tang Y, Kang Q, Feng Y, Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br J Pharmacol. 2012;166(08):2212–2227. doi: 10.1111/j.1476-5381.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jing Y H, Chen K H, Yang S H, Kuo P C, Chen J K. Resveratrol ameliorates vasculopathy in STZ-induced diabetic rats: role of AGE-RAGE signalling. Diabetes Metab Res Rev. 2010;26(03):212–222. doi: 10.1002/dmrr.1076. [DOI] [PubMed] [Google Scholar]
- 135.Prasad K. Resveratrol, wine, and atherosclerosis. Int J Angiol. 2012;21(01):7–18. doi: 10.1055/s-0032-1306417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Forbes J M, Thorpe S R, Thallas-Bonke V et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16(08):2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 137.Tam H L, Shiu S W, Wong Y, Chow W S, Betteridge D J, Tan K C. Effects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetes. Atherosclerosis. 2010;209(01):173–177. doi: 10.1016/j.atherosclerosis.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 138.Quade-Lyssy P, Kanarek A M, Baiersdörfer M, Postina R, Kojro E. Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J Lipid Res. 2013;54(11):3052–3061. doi: 10.1194/jlr.M038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haddad M, Knani I, Bouzidi H, Berriche O, Hammami M, Kerkeni M. Plasma levels of pentosidine, carboxymethyl-lysine, soluble receptor for advanced glycation end products, and metabolic syndrome: the metformin effect. Dis Markers. 2016;2016(16):6.248264E6. doi: 10.1155/2016/6248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lam J K, Wang Y, Shiu S W, Wong Y, Betteridge D J, Tan K C. Effect of insulin on the soluble receptor for advanced glycation end products (RAGE) Diabet Med. 2013;30(06):702–709. doi: 10.1111/dme.12166. [DOI] [PubMed] [Google Scholar]
- 141.Tan K C, Chow W S, Tso A W et al. Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia. 2007;50(09):1819–1825. doi: 10.1007/s00125-007-0759-0. [DOI] [PubMed] [Google Scholar]
- 142.Tang S C, Wang Y C, Li Y I et al. Functional role of soluble receptor for advanced glycation end products in stroke. Arterioscler Thromb Vasc Biol. 2013;33(03):585–594. doi: 10.1161/ATVBAHA.112.300523. [DOI] [PubMed] [Google Scholar]
- 143.Glas A S, Lijmer J G, Prins M H, Bonsel G J, Bossuyt P M. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]


