Abstract
Postacute myocardial infarction (AMI) readmissions are common among Medicare beneficiaries (≥65 years) and are associated with significant resource utilization. However, patterns of AMI readmissions for younger age groups in the United States are not known. In the Nationwide Readmissions Database, a nationally representative all-payer database of in-patient hospitalizations, we identified 212,171 index AMI hospitalizations in January to November 2013, weighted to represent 478,247 hospitalizations nationally (mean age 66.9 years, 38% women, 29% low income). This included 26,516 cases in the 18 to 44 age group, 183,703 in the 45 to 64 age group, and 268,027 in the ≥65 age group. The overall 30-day readmission rate was 14.5% and varied across age groups (9.7% [18 to 44], 11.2% [45 to 64], and 17.3% [≥65]). The cumulative cost of 30-day readmissions was $1.1 billion, of which $365 million was spent on those <65 years of age. In multivariable hierarchical models, the risk of readmission was higher in women and in low-income patients, but the effect varied by age (p value for age-gender and age-income interactions <0.05) and was more prominent in the younger age groups. Further, patients in all age groups continue to have a high hospitalization burden beyond the typical 30-day readmission period, with an overall 24% post-AMI 90-day readmission rate. In conclusion, readmissions in young and middle-aged AMI survivors pose a substantial burden on patients and on U.S. health-care resources. Women and low-income patients with AMI, particularly those in younger age groups, are more frequently readmitted, and readmissions continue to burden the health-care system beyond the typical 30-day window. Future investigations would need to be targeted toward a better understanding and improvement of the rehospitalization burden for vulnerable patient groups.
Readmissions after acute myocardial infarction (AMI) are frequent in the Medicare population and pose a substantial burden on health-care resources.1,2 Although AMI readmissions are well studied in the fee-for-service Medicare population,3 patterns of readmission in younger patients (<65 years of age) at the national level, which constitute more than 40% of the AMI population in the United States, remain poorly understood.4 It is possible that in-hospital and postdischarge care differs in younger patients compared with older patients, which may influence readmission risk, particularly among certain vulnerable subgroups.5,6 For example, young women appear to be at a high relative risk of poor in-hospital outcomes possibly related to delays in AMI diagnosis,5,6 and regionally limited studies suggest that they may have worse posthospitalization outcomes.7 Likewise, patients with a lower socioeconomic status may face elevated postdischarge obstacles to successful recuperation after AMI and may be at an elevated readmission risk.8,9 Finally, the extent to which differences in revascularization during index admission impacts the future risk of readmission across age groups remains unclear. Previous studies, however, either are limited by the lack of sufficient data in patients <65 years of age and the availability of posthospitalization outcomes, or represent data from a limited set of hospitals. To address this gap in knowledge, we used the 2013 Nationwide Readmissions Database (NRD), a nationally representative all-payer database of hospital readmissions in the United States, to examine patterns of readmissions after AMI across different age groups. Furthermore, we compared whether the risk of 30-day readmission differed based on gender, income, and revascularization status in younger patients (<65 years of age) compared with older patients (≥65 years of age). Finally, we assessed the readmission burden after AMI beyond the typical 30-day readmission period.
Methods
The NRD is a nationally representative, all-payer database recently developed by the Agency of Healthcare Research and Quality (AHRQ) to specifically study readmission patterns in hospitalized patients.10 The NRD is constructed using discharge-level data for 100% of hospitalizations from the State Inpatient Databases of 21 geographically dispersed participating states capturing 49.3% of the total U.S. resident population, and representing 49.1% of all U.S. hospitalizations (see Supplementary Table S1 for the list of states).11 Each patient is assigned a unique deidentified patient linkage number allowing tracking of all patients across hospitals within a state throughout a calendar year. To allow national-level estimates, discharge weights are provided. Further details on the design of the NRD have been previously reported.10
Each observation in the dataset represents a unique hospitalization. Patient characteristics included demographics (age and gender) and visit information (calendar month and number of days between hospitalizations). Information on primary and secondary discharge diagnoses and procedures is also included as International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification (ICD-9 codes). For certain diagnoses and procedures, information was available using the clinical classification of diseases (CCS) codes, a validated combination of ICD-9 that represents broad categories. We used the CCS-coded discharge diagnoses for co-morbid conditions. When CCS codes were not available, we used ICD-9 codes. Other study variables included income status (household income based on the zip code of residence, available as quartiles of income),6 insurance status (Medicare, Medicaid, private, or others), admission status (elective or nonelective), and length of stay. The cost of hospitalization was obtained by multiplying hospital charges with AHRQ’s all-payer cost-to-charge ratios for each hospital.
We identified 212,171 patients aged ≥18 years discharged alive with a primary diagnosis of AMI (see Supplementary Table S2 for the list of codes). To ensure that 30-day follow-up was available for all patients, we restricted our cohort to patients discharged from January 1, 2013, to November 30, 2013. We also excluded patients who died during the primary hospitalization (n = 12,463, 5%) or were discharged against medical advice (n = 2,269, 1%). Patients who underwent interhospital transfer were included in our study. Data on such patients were combined to generate a single record to ensure that interhospital transfers were not counted as readmissions. Readmission in transferred patients was assigned to institutions where the final disposition occurred.
The primary outcome of the study, 30-day readmission, was defined as rehospitalization due to any cause within 30 days of discharge from the index AMI event identified using unique patient identifiers. First, we compared the characteristics of patients within the a priori defined age groups of 18 to 44, 45 to 64, and ≥65 years. Unadjusted estimates of 30-day readmission were obtained, both overall and for each of these age groups, and were expressed as percentage. The discharge weights provided in the NRD were applied to obtain national-level estimates of AMI readmissions.
Next, we obtained estimates of the hospitalization cost for both primary AMI admissions and all-cause rehospitalization. For these, we multiplied the claim charge reported in the NRD for each hospitalization with AHRQ’s all-payer cost-to-charge ratio for the respective hospitals. Next, we used multivariable hierarchical logistic regression models to identify associations between factors included in the current readmission models and the observed 30-day readmission rates in our study. Our models explicitly account for clustering of patients at hospitals and stratification in the design of NRD. Variables for inclusion in our risk-adjustment models were informed by the 30-day AMI readmission model developed by the Centers for Medicare and Medicaid Services (CMS),12 which has been validated for this purpose. The variables are listed in Supplementary Table S2. We included income quartile as an additional covariate in the model. We assessed for (1) model discrimination using the concordance (c)-statistic and (2) individual predictors of readmission in this model, both overall and in the 3 age groups.
Based on previous literature suggesting poorer outcomes in women and in low-income patients below 65 years of age,7 we additionally assessed for effect modification by including interaction terms for age categories with gender and income status as covariates in the model. Because these interaction tests were significant, we further evaluated readmission rates in subgroups defined by age-gender and age-income. We examined the effect of insurance status (insured vs uninsured) on these associations by including it as an additional covariate in the risk-adjustment model. Finally, to assess the effect of revascularization during the primary AMI hospitalization on readmission differences among age groups, we constructed another model that included revascularization as an additional covariate. All patient-level analyses were performed in accordance with the survey methods recommended by the Healthcare Cost and Utilization Project (HCUP).13–15
In a subgroup analysis, for index AMI admissions in January to September, that is, those with at least 90 days of follow-up, we also describe rates of 90-day readmissions, both overall and across the 3 age groups. In sensitivity analyses, we sought to further examine the nature of readmissions. We also enumerated the top 10 readmission diagnoses grouped into clinically meaningful categories using CCS codes. Using a previously suggested algorithm, we obtained rates for “unplanned” 30-day readmissions after excluding hospitalization for elective procedures or those not expected as part of usual care.5 Similarly, we also examined the proportion of readmissions that were related to a cardiac origin using a previously suggested approach based on the primary readmission diagnosis.16
All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). The level of significance was set at a p value of 0.05. The HCUP data are deidentified and were deemed exempt from the purview of the UT Southwestern Medical Center Institutional Review Board.
Results
A total of 212,171 adults (≥18 years of age) were discharged alive during January to November 2013 after a hospitalization for AMI (“index event”), with a weighted estimate of 478,247 discharges nationally. Of these, 210,222 (44%) were under 65 years of age. Overall, the mean age was 66.9 years and 37.9% were women, with women representing a higher proportion of AMI in the older age groups (28% in 18 to 44 age group, 29% in 45 to 64 age group, and 45% in ≥65 age group). Cardiovascular co-morbidities (diabetes and hypertension) were common and were more frequent in the ≥65 age group (Table 1). Overall, 61.5% of the patients underwent revascularization during their index hospitalization (percutaneous coronary intervention 52.2%, coronary artery bypass grafting 9.3%), which was more common in the younger age groups. Medicare was the primary payer in 57.9% of the patients overall and in 90.5% of those over 65 years. All patient characteristics are reported in Table 1. Differences between women and men, as well as between different income groups are presented in the Appendix S1 (Supplementary Tables S3 and S4). Overall, 71% were discharged to home or self-care, followed by 12% to a skilled nursing facility (Table 2). Younger patients were predominantly discharged to home or self-care compared with those ≥65 years of age. Overall, the average cost for index AMI hospitalization was U.S.$21,387, and was similar across age groups (Table 2).
Table 1.
Characteristics of patients with discharged alive after an acute myocardial infarction in the National Readmission Sample, overall- and by age-groups (index event)
| Characteristics | Overall | Ages (years) | p value (trend) | ||
|---|---|---|---|---|---|
|
| |||||
| 18–44 | 45–64 | ≥65 | |||
| Number of events (weighted numbers ± SD) | 478247 (11707) | 26517 (750) | 183703 (4782) | 268027 (6632) | |
| Patient characteristics | |||||
| Mean Age (SEM), years | 66.9 (0.1) | 39.2 (0.05) | 56.0 (0.02) | 77.1 (0.05) | <.0001 |
| Women | 39.9 (0.2) | 27.8 (0.5) | 28.7 (0.2) | 45.2 (0.2) | <.0001 |
| Income quartiles* | <.0001 | ||||
| Q1 (Lowest) | 28.9 (0.9) | 33.3 (1.1) | 30.2 (1.0) | 28.6 (0.9) | |
| Q2 | 28.4 (0.6) | 28.4 (0.8) | 28.5 (0.7) | 28.2 (0.6) | |
| Q3 | 24.0 (0.6) | 22.8 (0.7) | 23.7 (0.6) | 24.3 (0.6) | |
| Q4 (Highest) | 18.8 (0.8) | 15.6 (0.8) | 17.6 (0.8) | 19.9 (0.9) | |
| Secondary diagnoses/comorbidities | |||||
| Heart failure | 30.1 (0.3) | 13.2 (0.5) | 19.3 (0.3) | 39.2 (0.3) | <.0001 |
| Diabetes Mellitus | 40.2 (0.2) | 29.8 (0.5) | 39.1 (0.3) | 41.9 (0.3) | <.0001 |
| Hypertension | 76.4 (0.2) | 59.5 (0.6) | 71.6 (0.3) | 81.3 (0.3) | <.0001 |
| Valvular heart disease | 13.9 (0.3) | 4.6 (0.2) | 7.4 (0.2) | 19.3 (0.3) | <.0001 |
| Peripheral artery disease | 24.1 (0.3) | 10.5 (0.4) | 18.1 (0.3) | 29.5 (0.3) | <.0001 |
| Cerebrovascular disease | 5.1 (0.1) | 0.9 (0.1) | 3.0 (0.1) | 6.9 (0.1) | <.0001 |
| Acute stroke/transient ischemic attack | 1.4 (0.03) | 0.7 (0.1) | 1.0 (0.04) | 1.8 (0.04) | <.0001 |
| Cardiac arrhythmia | 30.4 (0.3) | 16.9 (0.5) | 21.9 (0.3) | 37.5 (0.3) | <.0001 |
| Liver disease | 3.6 (0.1) | 3.9 (0.2) | 3.7 (0.1) | 3.4 (0.1) | <.0001 |
| Asthma | 4.9 (0.1) | 5.6 (0.3) | 5.1 (0.1) | 4.8 (0.1) | <.0001† |
| Chronic obstructive pulmonary disease | 16.0 (0.2) | 3.9 (0.2) | 12.9 (0.3) | 19.4 (0.2) | <.0001 |
| Pneumonia | 6.5 (0.1) | 2.3 (0.2) | 3.9 (0.1) | 8.7 (0.1) | <.0001 |
| Sepsis | 1.6 (0.04) | 0.8 (0.1) | 1.2 (0.1) | 2.0 (0.1) | <.0001 |
| Solid malignancy | 10.3 (0.2) | 1.9 (0.2) | 5.1 (0.1) | 14.8 (0.2) | <.0001 |
| Leukemia/metastatic malignancy | 1.2 (0.03) | 0.3 (0.05) | 0.7 (0.03) | 1.7 (0.04) | <.0001 |
| Chronic skin ulcer | 1.9 (0.05) | 0.7 (0.1) | 1.2 (0.05) | 2.5 (0.1) | <.0001 |
| Delirium & dementia | 6.6 (0.1) | 0.2 (0.1) | 0.7 (0.03) | 11.4 (0.2) | <.0001 |
| Anemia | 21.9 (0.3) | 10.8 (0.4) | 15.1 (0.3) | 27.5 (0.4) | <.0001 |
| Malnutrition | 4.2 (0.1) | 1.5 (0.1) | 2.4 (0.1) | 5.7 (0.2) | <.0001 |
| Paralysis | 0.6 (0.02) | 0.5 (0.1) | 0.5 (0.03) | 0.6 (0.03) | <.0001 |
| Acute kidney injury | 14.4 (0.2) | 6.4 (0.3) | 9.0 (0.2) | 18.9 (0.3) | <.0001 |
| End stage renal disease/hemodialysis | 3.8 (0.1) | 2.5 (0.2) | 3.6 (0.1) | 4.0 (0.1) | <.0001 |
| Chronic kidney disease | 17.2 (0.2) | 4.6 (0.3) | 8.1 (0.2) | 24.6 (0.3) | <.0001 |
| Fluid/electrolyte disorder | 21.2 (0.3) | 15.0 (0.5) | 17.4 (0.3) | 24.5 (0.3) | <.0001 |
| Prior myocardial infarction | 12.7 (0.2) | 9.2 (0.4) | 11.7 (0.2) | 13.8 (0.2) | <.0001 |
| Prior percutaneous coronary intervention | 15.9 (0.2) | 10.9 (0.4) | 15.7 (0.3) | 16.5 (0.3) | <.0001 |
| Prior coronary artery bypass grafting | 8.5 (0.1) | 2.0 (0.2) | 5.2 (0.1) | 11.4 (0.2) | <.0001 |
| AMI characteristics/complications | |||||
| Anterior ST-elevation myocardial infarction | 10.0 (0.1) | 16.1 (0.4) | 12.8 (0.2) | 7.6 (0.1) | <.0001† |
| Cardiogenic shock | 3.8 (0.1) | 2.7 (0.2) | 3.9 (0.1) | 3.9 (0.1) | <.0001 |
| Cardiac arrest | 3.3 (0.1) | 4.4 (0.2) | 4.2 (0.1) | 2.6 (0.1) | <.0001† |
| Procedures | |||||
| Percutaneous coronary intervention | 52.2 (0.4) | 66.4 (0.7) | 64.3 (0.4) | 42.6 (0.5) | <.0001† |
| Coronary artery bypass grafting | 9.3 (0.2) | 6.1 (0.3) | 10.5 (0.3) | 8.8 (0.2) | <.0001† |
| Mechanical ventilation | 6.2 (0.1) | 3.5 (0.2) | 5.5 (0.1) | 6.9 (0.2) | <.0001 |
| Administrative/financial details | |||||
| Payment source | <.0001‡ | ||||
| Medicare | 57.9 (0.3) | 8.7 (0.3) | 17.4 (0.3) | 90.5 (0.3) | |
| Medicaid | 6.3 (0.2) | 18.0 (0.6) | 12.2 (0.4) | 1.1 (0.1) | |
| Private insurance | 25.1 (0.3) | 44.5 (0.7) | 49.2 (0.6) | 6.7 (0.2) | |
| Others | 10.7 (0.2) | 28.8 (0.6) | 21.2 (0.4) | 1.7 (0.1) | |
Abbreviations: SD = standard deviation; SEM = standard error of mean; C.I. = confidence interval.
All numbers in table represent percentages (standard errors), unless otherwise specified.
Median household income quartiles based on patient zip code. Trend for ‘lowest quartile (0–25th percentile)’ vs ‘others’.
p value for negative trend,
for Medicare vs others.
Table 2.
Outcomes of patients discharged alive after an acute myocardial infarction in the National Readmission Sample, overall- and by age-groups (index event)
| Characteristics | Overall | Ages (years) | p value (trend) | ||
|---|---|---|---|---|---|
|
| |||||
| 18–44 years | 45–64 years | ≥65 years | |||
| Disposition | <.0001 | ||||
| Home or self-care | 71.1 (0.4) | 92.7 (0.4) | 86.0 (0.3) | 58.8 (0.4) | |
| Short term hospital | 2.9 (0.1) | 2.4 (0.2) | 2.9 (0.1) | 3.0 (0.1) | |
| Skilled care facility | 12.2 (0.2) | 1.0 (0.1) | 3.1 (0.1) | 19.5 (0.3) | |
| Home health care | 13.8 (0.3) | 3.9 (0.3) | 8.0 (0.3) | 18.7 (0.3) | |
| Index hospitalization cost (US$) | 21,387 (246) | 20,035 (301) | 22,468 (261) | 20,778 (259) | <0.0001 |
| 30-day Readmission | |||||
| Unadjusted all-cause rate, % | 14.5 (0.1) | 9.7 (0.3) | 11.2 (0.2) | 17.3 (0.2) | <.0001 |
| Unplanned rate, % | 11.5 (0.1) | 7.3 (0.3) | 8.4 (0.2) | 14.1 (0.1) | <.0001 |
| Cardiac rate, % | 8.3 (0.1) | 6.5 (0.3) | 6.8 (0.1) | 9.5 (0.1) | <.0001 |
| All- cause risk-adjusted OR (95% C.I.)* | - | 0.69 (0.56, 0.86) | 0.69 (0.64, 0.74) | Reference | |
| Readmission cost (US$) | 14,885 (187) | 13,321 (561) | 15,436 (304) | 14,727 (205) | 0.49 |
Model details in Table 3.
The unadjusted rate of 30-day readmission was 14.5% in the overall population. There were prominent differences in readmission rates among demographic subgroups by age, gender, income, and revascularization status. Readmission rates were higher in the older age groups (p-trend <0.0001). Compared with men, women had higher rates of all-cause (13.1% vs 16.8%), unplanned (10.0% vs 14.1%), and cardiac (7.7% vs 9.3%, p <0.0001 for all) readmissions (Supplementary Table S3). All-cause readmission rates were higher among patients in the lowest-income subgroup and also trended down from the lowest to the highest-income quartile (Q1 [lowest quartile] 15.6%, Q2 14.6%, Q3 14.0%, and Q4 [highest quartile] 13.7%; ptrend < 0.0001), which was also observed in the cardiac and unplanned 30-day AMI readmission rates (Supplementary Table S4). Patients with AMI who underwent revascularization during primary hospitalization had fewer readmissions, both overall and across the 3 age groups (Supplementary Table S5).
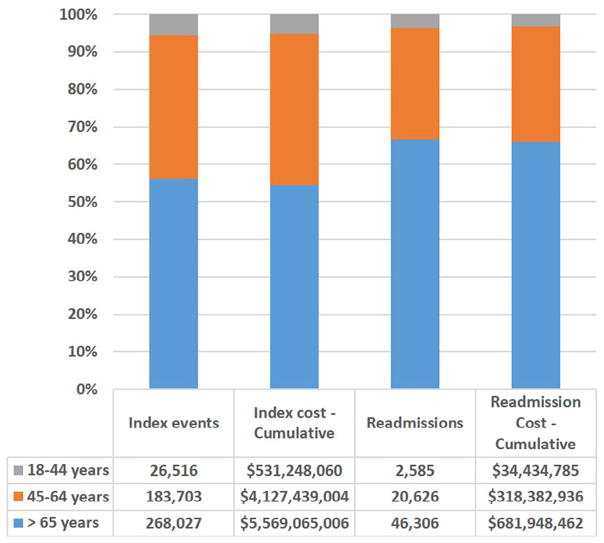
The mean cost of readmissions during 30 days after discharge after an AMI was U.S.$14,885, and was similar across age groups (Table 2). The cumulative national costs of index AMI events and 30-day readmissions for each age group are presented in Figure 1. Of the $1 billion estimated cost of AMI readmissions, over a third ($365 million, 34%) was spent in those <65 years of age.
Figure 1.
Cumulative cost of index AMI hospitalizations and 30-day readmission, overall and by age groups in the United States.
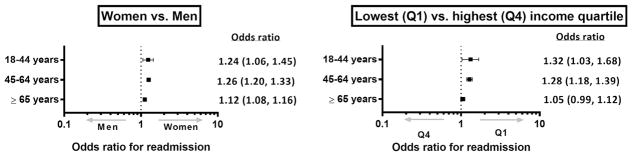
In risk-adjusted analyses using hierarchical models similar to those developed by CMS, the overall associations between risk factors and all-cause 30-day readmission AMI were consistent in the overall population (Table 3) and in individual age groups (Supplementary Table S6). Within our a priori defined age groups, compared with patients ≥65 years, patients <65 years of age had lower risk-adjusted odds for readmission. Compared with men, women had a higher risk of 30-day readmission. However, the above associations were more prominent in younger patients (p value for interaction <0.05; Figure 2, Supplementary Table S7 of Appendix S1). Likewise, patients in the lowest-income quartile had higher odds of readmission compared with those in the highest-income quartile. The previously mentioned association, however, was limited to patients <65 years of age where the odds for readmission were higher in the lower-income groups compared with the highest-income group (Figure 2, Supplementary Table S6). Notably, revascularization for AMI was associated with 23% lower risk-adjusted odds of subsequent hospitalizations (risk-adjusted odds ratio 0.77, 95% confidence interval 0.74 to 0.80). However, the addition of insurance status or revascularization as an additional covariate in the risk-adjusted model did not attenuate the differences by age, gender, or income (Supplementary Tables S8 and S9).
Table 3.
Acute myocardial infarction 30-day Readmission Logistic Regression Model (c-statistic 0.67)
| Covariates | Odds Ratio | 95% Confidence Limits | p-value | |
|---|---|---|---|---|
|
| ||||
| Lower Limit | Upper Limit | |||
| Age (in years) | 1.01 | 1.01 | 1.01 | <.0001 |
| Women vs. Men | 1.17 | 1.13 | 1.20 | <.0001 |
| Income quartiles*, Q1 vs. Q4 | 1.17 | 1.13 | 1.20 | <.0001 |
| Income quartiles*, Q2 vs. Q4 | 1.13 | 1.07 | 1.19 | 0.0059 |
| Income quartiles*, Q3 vs. Q4 | 1.07 | 1.02 | 1.13 | 0.34 |
| Heart failure | 1.47 | 1.42 | 1.51 | <.0001 |
| Chronic atherosclerosis | 0.99 | 0.95 | 1.03 | 0.6129 |
| Cardiac arrhythmia | 1.11 | 1.08 | 1.15 | <.0001 |
| Valvular disease | 1.10 | 1.06 | 1.14 | <.0001 |
| Stroke/transient ischemic attack | 1.13 | 1.02 | 1.26 | 0.0237 |
| Cerebrovascular disease | 1.11 | 1.05 | 1.17 | 0.0004 |
| Paralysis | 1.20 | 1.00 | 1.44 | 0.0452 |
| Peripheral vascular disease | 1.18 | 1.14 | 1.22 | <.0001 |
| Diabetes mellitus | 1.24 | 1.20 | 1.27 | <.0001 |
| Acute kidney injury | 1.13 | 1.09 | 1.18 | <.0001 |
| End state renal disease/hemodialysis | 2.11 | 1.99 | 2.23 | <.0001 |
| chronic kidney disease | 1.23 | 1.19 | 1.28 | <.0001 |
| Chronic obstructive pulmonary disease | 1.31 | 1.27 | 1.36 | <.0001 |
| Pneumonia | 1.08 | 1.03 | 1.14 | 0.0024 |
| Asthma | 1.18 | 1.11 | 1.26 | <.0001 |
| Fluid/electrolyte disorder | 1.13 | 1.09 | 1.17 | <.0001 |
| sepsis | 1.19 | 1.08 | 1.30 | 0.0004 |
| Solid malignancy | 1.10 | 1.05 | 1.15 | <.0001 |
| Leukemia/metastatic malignancy | 1.56 | 1.39 | 1.74 | <.0001 |
| Anemia | 1.21 | 1.18 | 1.25 | <.0001 |
| Chronic skin ulcer | 1.47 | 1.36 | 1.60 | <.0001 |
| Delirium/dementia | 1.00 | 0.95 | 1.05 | 0.93 |
| Malnutrition | 1.06 | 0.99 | 1.13 | 0.0825 |
| Anterior ST-elevation myocardial infarction | 1.09 | 1.04 | 1.14 | 0.0002 |
| Other ST-elevation myocardial infarction | 0.90 | 0.85 | 0.94 | <.0001 |
| Prior myocardial infarction | 1.04 | 1.00 | 1.09 | 0.082 |
| Prior percutaneous coronary intervention | 1.07 | 1.03 | 1.11 | 0.0004 |
| Prior coronary artery bypass grafting | 1.11 | 1.06 | 1.17 | <.0001 |
Median household income quartiles based on patient zip code.
Figure 2.
Risk-adjusted odds ratio for readmission in age groups by (1) gender (significant age-gender interaction, p = 0.01), and (2) income (significant age-income interaction, p = 0.007), lowest-income quartile versus highest quartile presented (other groups included in Supplementary Table S6 of Appendix S1).
In the subgroup analyses, 24.0% of the estimated 392,006 (unweighted n = 173,943) index AMI hospitalizations from January to September 2013 were followed by a readmission within 90 days with rates of 16.2% in the 18 to 44 age group, 18.6% in the 45 to 64 age group, and 28.4% in the ≥65 age group.
Finally, in the sensitivity analyses examining the nature of 30-day readmissions, the leading causes of readmission across age groups were cardiovascular (Table 4). However, whereas readmission events were related to manifestations of coronary artery disease, those in patients >65 years of age were associated with heart failure. Further, 11.5% of AMI hospitalizations were followed by “unplanned” 30-day readmissions, and 8.3% had readmissions related to a cardiac origin (Table 2). The unplanned and “cardiac” readmission rates closely followed the overall readmission rates in subgroups defined by age (Table 2), gender (Supplementary Table S3), and income quartiles (Supplementary Table S4).
Table 4.
The 10 most frequent primary diagnoses for 30-day readmissions, for the 3 study age-groups. The diagnoses are presented in the descending order of frequency
| Rank (most frequent first) | Ages | |||
|---|---|---|---|---|
|
| ||||
| Overall | 18–44 | 45–64 | >65 | |
| 1 | Congestive heart failure | Coronary atherosclerosis | Coronary atherosclerosis | Congestive heart failure |
| 2 | Acute myocardial infarction | Acute myocardial infarction | Acute myocardial infarction | Acute myocardial infarction |
| 3 | Coronary atherosclerosis | Nonspecific chest pain | Congestive heart failure | Coronary atherosclerosis |
| 4 | Nonspecific chest pain | Congestive heart failure | Nonspecific chest pain | Septicemia |
| 5 | Cardiac dysrhythmias | Complication of device; implant or graft | Complications of surgical procedures or medical care | Cardiac dysrhythmias |
| 6 | Septicemia | Complications of surgical procedures or medical care | Cardiac dysrhythmias | Acute and unspecified renal failure |
| 7 | Complications of surgical procedures or medical care | Hypertension (with complications or secondary) | Complication of device; implant or graft | Pneumonia |
| 8 | Complication of device; implant or graft | Cardiac dysrhythmias | Septicemia | Nonspecific chest pain |
| 9 | Acute and unspecified renal failure | Peri-; endo-; and myocarditis; cardiomyopathy | Acute cerebrovascular disease | Gastrointestinal hemorrhage |
| 10 | Pneumonia | Diabetes mellitus with complications | Pneumonia | Complications of surgical procedures or medical care |
Discussion
In our study, from a nationally representative all-payer sample of hospital discharges in the United States, we made the following key observations. First, although the risk of post-AMI 30-day readmission increases with advancing age, readmissions are not uncommon in younger patients. Over 1 in 10 patients with AMI below 65 years of age are readmitted within 30 days. Second, readmissions pose a substantial economic burden in both the young and the elderly, costing over $1 billion in 2013, and more than 1/3 of that cost ($350 million) is for those <65 years. Third, although women and lower-income patients had higher odds of readmission, these associations were more prominent in patients <65 years of age. Finally, patients in all age groups continue to have a high hospitalization burden beyond the currently measured 30-day readmission period.
Preventing posthospitalization readmissions has been identified as a potential avenue to mitigate extensive resource utilization in AMI care.2,17,18 However, contemporary data regarding burden of rehospitalization after AMI are largely derived from Medicare patients. Although associated with advanced age, the burden of readmission among younger patients is still substantial. Similar to elderly patients with AMI within Medicare,16 we found that readmissions were most frequently related to a cardiovascular diagnosis across all age groups. Further, consistent with previous studies, we also found that elderly patients with AMI were most frequently readmitted for heart failure16; however, readmissions in younger AMI survivors represented direct manifestations of coronary disease, particularly angina and recurrent myocardial infarction. Further, these differences did not merely represent a consequence of differences in the utilization of revascularization during the index hospitalization, because the higher revascularization rates in younger patients did not explain the observed age-, gender-, and income-based differences in our study. The factors that drive readmissions in the young would require dedicated future studies.
We found that, compared with men, the risk of readmission was higher in women, particularly in patients <65 years of age. We noted a disproportionately higher rate of risk-adjusted AMI readmissions in nonelderly women. A previous study from California hospitals focusing on patients with AMI ≤65 years of age during 2007 to 2009 suggested a 21% higher rate of readmission in women compared with men (risk-adjusted hazard ratio 1.21, 95% confidence interval 1.14 to 1.29).7 Our study confirms that the vulnerability of young women to elevated readmission risk are not regionally limited and are observed across the United States. Women frequently have worse post-AMI outcomes, including inhospital mortality and length of stay.5 Factors underlying the observed gender-age interaction for readmission remain poorly understood. Studied have shown that women are vulnerable to underdiagnosis of AMI and suboptimal care, driven in part by atypical presentation and lower suspicion for AMI.19 As a result, diagnosis and reperfusion treatment are often delayed, which may lead to higher rates of adverse postdischarge sequelae and possibly early rehospitalizations.19 Women also experience complications, such as bleeding, more frequently.5,14 Younger women may be at a greater risk of delayed higher-risk care due to a lower perceived risk of AMI compared with younger men,5,20–22 which might explain the gender-age interaction observed in our study.
Similarly, patients in the lower-income quartile had a higher risk of readmission after AMI. Income-related inequalities in AMI outcomes have also been previously suggested, particularly among elderly Medicare beneficiaries.23,24 However, in the present study, the association between income and outcomes was more prominent in younger patients. Further, despite lower rates of health insurance in the young, observed differences in the income-readmission relation were not explained by differences in insurance status alone. Although diminished access, poorer quality care,24,25 as well as social challenges after AMI hospitalization may portend higher readmission risk in low-income patients, our study does not capture these elements of care quality. Therefore, future studies are needed to better understand the reason for these differences.
Our study has several limitations. First, data on readmissions are available only for the year 2013. It is, therefore, unknown if these findings are consistent over time. Second, the NRD is constructed from State Inpatient Databases of the included states, and therefore, readmissions occurring in another state are not captured. However, through extensive data assessments, the AHRQ has reported that <5% of readmission estimates are affected by readmissions across state borders across all conditions.11 Third, NRD does not include observation-only hospital stays. However, this is consistent with CMS readmission metrics. Fourth, the NRD does not provide information on race and ethnicity, and therefore other aspects of socioeconomic class, and would need to be addressed in a future dedicated study. Finally, the diagnosis of AMI and co-morbidities used in the risk-adjustment models are all derived from administrative claims codes, and important clinical information on disease severity and therapeutic strategies that may affect readmission risk is not available. However, the performance of the CMS model for risk adjustment that we used in our study (c-statistic 0.67) was comparable with that in the original fee-for-service Medicare population (c-statistic 0.63).12
In conclusion, 30-day readmissions in young and middle-aged AMI survivors pose a substantial burden on U.S. health-care resources. AMI readmissions vary by both age and sociodemographic characteristics, with women and low-income AMI survivors representing the highest-risk groups. Finally, AMI survivors continue to have a high risk of rehospitalization beyond the usual 30-day follow-up period.
Supplementary Material
Acknowledgments
Dr. Girotra was supported by grant K08HL122527 and Dr. Khera was supported by grants 5T32HL125247-02 and UL1TR001105) from the National Institutes of Health (Bethesda, Maryland).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary data related with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.amjcard.2017.07.081.
References
- 1.Medicare Payment Advisory Commission. A path to bundled payment around a rehospitalization. Report to the Congress: reforming the delivery system; Washington, DC. June. 2005; pp. 83–103. [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 4.Bongiovanni D. [Accessed August 15, 2016];Readmissions penalties: are commercial payors following Medicare’s lead? 2015 Available at: http://www.besler.com/readmissions-penalties-commercial-payors/
- 5.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai NR, Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PLoS ONE. 2016;11:e0160492. doi: 10.1371/journal.pone.0160492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyer RP, Ranasinghe I, Wang Y, Dharmarajan K, Murugiah K, Nuti SV, Hsieh AF, Spertus JA, Krumholz HM. Sex differences in the rate, timing, and principal diagnoses of 30-day readmissions in younger patients with acute myocardial infarction. Circulation. 2015;132:158–166. doi: 10.1161/CIRCULATIONAHA.114.014776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood) 2014;33:778–785. doi: 10.1377/hlthaff.2013.0816. [DOI] [PubMed] [Google Scholar]
- 9.Bernheim SM, Spertus JA, Reid KJ, Bradley EH, Desai RA, Peterson ED, Rathore SS, Normand SL, Jones PG, Rahimi A, Krumholz HM. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153:313–319. doi: 10.1016/j.ahj.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Healthcare Cost and Utilization Project (HCUP) NRD overview. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2015. [Accessed August 15, 2016]. Available at: www.hcup-us.ahrq.gov/nrdoverview.jsp. [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project (HCUP) 2013 introduction to the NRD. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2015. [Accessed on August 15, 2016]. Available at: www.hcup-us.ahrq.gov/db/nation/nrd/NRD_Introduction_2013.jsp. [Google Scholar]
- 12.Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Mattera JA, Normand SL. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243–252. doi: 10.1161/CIRCOUTCOMES.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 14.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 15.Khera R, Krumholz HM. With great power comes great responsibility: big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. doi: 10.1161/CIRCOUTCOMES.117.003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff (Millwood) 2013;32:864–872. doi: 10.1377/hlthaff.2012.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368:1465–1468. doi: 10.1056/NEJMp1302981. [DOI] [PubMed] [Google Scholar]
- 19.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK American Heart Association Cardiovascular Disease in W, Special Populations Committee of the Council on Clinical Cardiology CoE, Prevention CoC, Stroke N, Council on Quality of C, Outcomes R. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 20.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosner S, Haasenritter J, Hani MA, Keller H, Sonnichsen AC, Karatolios K, Schaefer JR, Baum E, Donner-Banzhoff N. Gender differences in presentation and diagnosis of chest pain in primary care. BMC Fam Pract. 2009;10:79. doi: 10.1186/1471-2296-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastorius Benziger C, Bernabe-Ortiz A, Miranda JJ, Bukhman G. Sex differences in health care-seeking behavior for acute coronary syndrome in a low income country, Peru. Crit Pathw Cardiol. 2011;10:99–103. doi: 10.1097/HPC.0b013e318223e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindenauer PK, Lagu T, Rothberg MB, Avrunin J, Pekow PS, Wang Y, Krumholz HM. Income inequality and 30 day outcomes after acute myocardial infarction, heart failure, and pneumonia: retrospective cohort study. BMJ. 2013;346:f521. doi: 10.1136/bmj.f521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spatz ES, Beckman AL, Wang Y, Desai NR, Krumholz HM. Geographic variation in trends and disparities in acute myocardial infarction hospitalization and mortality by income levels, 1999–2013. JAMA Cardiol. 2016;1:255–265. doi: 10.1001/jamacardio.2016.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.