Abstract
Objective
To determine sucralose and acesulfame-K pharmacokinetics in breast milk following maternal ingestion of a diet soda.
Methods
Thirty-four exclusively breastfeeding women (14 normal weight, 20 obese) consumed twelve ounces of Diet Rite Cola™, sweetened with 68 mg sucralose and 41 mg acesulfame-potassium, prior to a standardized breakfast meal. Habitual LCS intake was assessed via a diet questionnaire. Breast milk was collected from the same breast prior to beverage ingestion and hourly for six hours.
Results
Due to one mother having extremely high concentrations, peak sucralose and acesulfame-potassium concentrations following ingestion of diet soda ranged from 4.0–7,387.9 ng/mL (median peak 8.1 ng/mL) and 299.0 – 4764.2 ng/mL (median peak 945.3 ng/mL), respectively.
Conclusions
Ace-K and sucralose transfer into breast milk following ingestion of a diet soda. Future research should measure concentrations after repeated exposure and determine whether chronic ingestion of sucralose and acesulfame-potassium via the breast milk has clinically relevant health consequences.
Clinical Trial Registration
NCT #02940795.
Keywords: low-calorie sweeteners, infant nutrition, taste, appetite, lactation
Introduction
In utero and early life exposures influence infant taste preferences and eating behavior (1), and may impact long-term weight trajectory and health (2). We have previously reported that non-nutritive sweeteners (NNS) such as sucralose, acesulfame-potassium (ace-K), and saccharin were present in breast milk of 20 lactating women measured randomly at a single time-point(3). Aspartame was not detected which is expected since it is immediately metabolized in the gut. With the exception of saccharin, which is not recommended for consumption during pregnancy, NNS are regarded as safe during pregnancy and lactation. Thus, it can be assumed that a large number of infants are exposed in utero and while nursing (3, 4). Given the innate preference for sweetness (1) and the known modulation of infant taste preferences by flavors to which they are repeatedly exposed (5), ingestion of NNS in breast milk may promote greater liking of sweet tasting foods and beverages. Since the predominant sugar in breast milk is lactose, which has a relatively low sweet taste intensity, addition of NNS to breast milk may possibly enhance its sweetness and thus encourage overconsumption. In fact, prior studies demonstrate that infants consume larger volumes of sweetened substances (6).
Beyond potentially influencing infant taste preferences, future food choice, and appetite regulation, early life NNS exposure may also influence the child’s metabolism and future health (4). NNS use in children and adults has been associated with obesity and metabolic abnormalities in epidemiologic studies (7), and a causal link between NNS, weight gain, and glucose intolerance has been demonstrated in rodents (8). If similar findings were demonstrated in humans, this would have critical implications for the developing infant, as exposure to other dietary constituents early in life, such as phthalates and bisphenols (9), exert a lasting influence on child cardiometabolic outcomes. Furthermore, NNS have been shown to alter the gut microbiome in rodents (8) and in a small human study (8). This is important, as the gut microbiome has emerged as an important mediator in immunity, inflammation, and metabolic disease (10).
Despite the ongoing debate about NNS effects, their consumption has continued to increase and is particularly prevalent among females and individuals with obesity (11). However, no data on the pharmacokinetics of NNS in breast milk are available. Therefore, this study aimed to evaluate the pharmacokinetics of sucralose and ace-K following maternal ingestion of a diet soda and to determine whether breast milk NNS concentrations differed in normal weight compared to obese mothers.
Methods
Thirty-four exclusively breastfeeding women (14 normal weight, 20 obese) and their infants who were approximately six weeks post-partum were enrolled. The protocol was approved by the Institutional Review Board at the University of Oklahoma Health Sciences Center and written informed consent was obtained in all mothers prior to testing (NCT #02940795).
NNS intake was assessed using a short food frequency questionnaire. Dietary intake and physical activity during the 24 hours prior to the study visit were recalled and recorded by the participant. Maternal weight was measured using a digital scale and standing height was measured using a standard stadiometer. Body mass index (BMI) was calculated (kg/m2) and weight status was determined using standard BMI cut-offs.
Participants were provided with a breast pump and collection containers for use during the study and detailed instructions for sample collection, storage, and transport. The day prior to testing, participants were asked to refrain from all soda, fruit juice and candy. Participants fasted overnight and provided a baseline breast milk sample at 6:00 am. Thereafter, women consumed 12 ounces of Diet Rite Cola™ (caffeine free, commercially-available diet soda containing 68 mg sucralose and 41 mg ace-K) prior to a standard breakfast meal (Jimmy Dean Breakfast Bowl Turkey Sausage breakfast). Participants then provided breast milk from the same breast hourly for six hours. Samples were immediately frozen after collection and were either delivered to the research team in an ice cooler or stored frozen in the home until pick-up by a study team member.
Sucralose and ace-K concentrations in the breast milk were measured in triplicate using liquid chromatography-mass spectrometry (LC-MS). Analyses were performed with an Acquity I-Class UPLC (Waters Corp., Milford, MA, USA) and an Acquity UPLC BEH C-18 column (2.1 mm × 50 mm, 1.7 μm) coupled with a Q-Exactive MS (Thermo Scientific, Waltham, MA, USA) with a HESI-II electrospray source. All analyses were performed using isotopically labelled sucralose as the internal standard for sucralose measurements and isotopically labelled acesulfame K for acesulfame K measurements. The %CV for the samples within day was 4.5% for sucralose and 0.8% for ace-K, respectively, and for samples between days was 5.8% for sucralose and 1.0% for ace-K, respectively.
Descriptive statistics were conducted for concentrations of both sweeteners, as well as demographic and anthropometric characteristics of the mothers and infants. The total area under the curve (AUC) was calculated using the trapezoidal method. The peak for both sucralose and ace-K was calculated as the maximum value over the 360-minute time course. Given that blood volume is greater in obese vs. lean individuals (12), adjustments for blood volume were performed. We multiplied each subject’s breast milk sucralose or acesulfame-potassium concentrations by their blood volume, which we estimated using a validated equation (12). Differences based on weight status and NNS consumption were assessed using paired t-tests and one-way ANOVA, respectively. P-values <0.05 were considered statistically significant.
Results
Demographic data are shown in Table 1. The majority of participants were non-Hispanic white. The distribution of age and race/ethnicity was similar across the normal weight (n=14) and obese (n=20) mothers. Among 19 infants with demographic and/or anthropometric information available (infant data were only available if the infants participated in other studies, as the current study was a milk pharmacokinetics study), 89% (17 of 19) were non-Hispanic white and 81% (13 of 16) were male. Mean infant birth weight was 3.5 ± 0.4 kg and mean gestational age was 39.4 ± 1.0 weeks.
Table 1.
Participant characteristics and plasma sweetener concentrations following ingestion of sucralose containing beverages
| All (n=34) | Normal Weight Mothers (n=14) | Obese Mothers (n=20) | |
|---|---|---|---|
| Age (years, mean ± SD) | 29.5 ± 2.6 | 29.4 ± 1.9 | 29.5 ± 3.0 |
| Weight (kg) | 75.1 ± 20.1 | 62.2 ± 7.2 | 91.8 ± 16.8* |
| BMI (kg/m2) | 27.9 ± 6.4 | 23.3 ± 1.6 | 34 ± 4.5* |
| NNS Consumption1 | |||
| Any NNS (n, %) | |||
| Never or < 1/week | 13, 39% | 5, 36% | 8, 42% |
| ≥ 1/week | 12, 36% | 6, 43% | 6, 32% |
| ≥ 1/day | 8, 24% | 3, 21% | 5, 26% |
| NNS Food (n, %)2 | |||
| Never or < 1/week | 25, 76% | 9, 64% | 16, 84% |
| ≥ 1/week | 4, 12 % | 3, 21% | 1, 5% |
| ≥ 1/day | 4, 12% | 2, 14% | 2, 11% |
| Splenda™ Packets (n, %)3 | |||
| Never or < 1/week | 31, 94% | 13, 93% | 18, 95% |
| ≥ 1/month | 0, 0% | 0, 0% | 0, 0% |
| ≥ 1/day | 2, 6% | 1, 7% | 1, 5% |
| Sucralose (median (25th,75th percentile)) ng/ml | |||
| Baseline | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| 60 min | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.0) |
| 120 min | 1.3 (1.0, 2.8) | 1.6 (1.0, 2.8) | 1.1 (1.0, 1.9) |
| 180 min | 3.5 (2.5, 5.1) | 4.1 (2.6, 5.7) | 3.1 (2.4, 4.6) |
| 240 min | 5.3 (3.9, 8.4) | 5.6 (4.9, 9.8) | 4.8 (3.6, 6.6) |
| 300 min | 7.4 (5.7, 10.1) | 9.0 (6.8, 12.4) | 7.0 (4.8, 8.1) |
| 360 min | 7.9 (5.9, 11.5) | 8.4 (7.9, 14.5) | 7.2 (5.7, 9.6) |
| AUC (ng/mL/360 min) | 1244.2 (997.2, 2038.8) | 1508.1 (1184.4, 2186.8) | 1141.2 (932.8, 1447.6) |
| Peak (ng/mL) | 8.1 (7.1, 11.5) | 9.8 (8.2, 14.5) | 7.6 (6.0, 9.7) |
| Ace-K (median (25th,75th percentile)) ng/ml | |||
| Baseline | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| 60 min | 163.7 (85.2, 246.5) | 155.3 (91.3, 238.4) | 163.7 (81.5, 324.1) |
| 120 min | 457.8 (314.6, 980.5) | 660.9 (364.2, 1068.0) | 400.8 (291.6, 722.0) |
| 180 min | 880.3 (596.8, 1460.2) | 1055.0 (647.3, 1524.4) | 774.1 (569.7, 1175.8) |
| 240 min | 864.5 (634.3, 1417.9) | 1141.7 (744.4, 1590.9) | 776.1 (529.9, 1216.8) |
| 300 min | 907.4 (651.6, 1290.4) | 1132.0 (714.3, 1407.3) | 816.5 (482.0, 1205.0) |
| 360 min | 777.4 (540.1, 1169.6) | 931.9 (646.4, 1394.5) | 678.9 (439.7) |
| AUC (ng/mL/360 min) | 218,885.1 (166,281.7, 361774.8) | 290,041.0 (188,830.2, 368,196.8) | 196,270.8 (139,907.5, 289488.4) |
| Peak (ng/mL) | 945.3 (714.5, 1529.8) | 1243.5 (764.5, 1604.4) | 876.6 (614.2, 1227.6) |
Indicates statistically significant difference between normal weight and obese mothers based on a p-value < 0.05. When excluding the one outlier, ID #206, there were no statistical differences in breast milk concentrations between normal weight and obese mothers.
Not all percentages for NNS consumption add up to 100% due to rounding.
Thirty-nine percent of mothers reported never or rarely consuming NNS, while 36% and 24% reported weekly and daily consumption, respectively. Only two mothers reported consumption of Splenda™ packets, in which sucralose is the active ingredient. Seventy-six percent of mothers reported never or rarely consuming foods containing NNS (which typically are sweetened with sucralose and/or ace-K), while 12% reported weekly and 12% reported daily consumption of NNS containing foods. Reported NNS use was similar among normal weight and obese mothers.
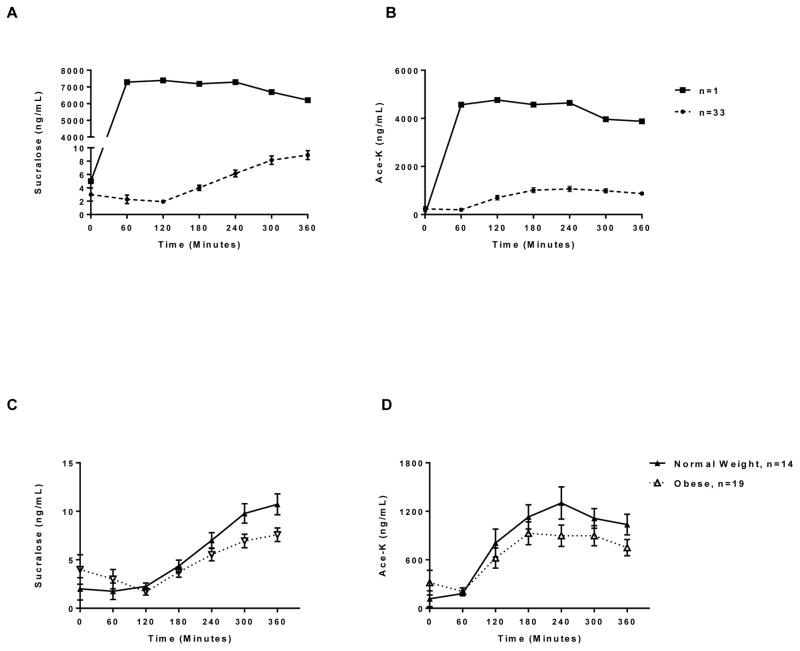
At baseline, prior to ingestion of the diet soda, sucralose and ace-K were detected in the breast milk of 21% (0.4–5.2 ng/mL sucralose) and 21% (11.9–733.2 ng/mL ace-K) of mothers, respectively. Due to one mother having extremely high concentrations, peak sucralose concentrations following ingestion of diet soda ranged from 4.0–7,387.9 ng/mL (median peak 8.1 ng/mL) and the AUC ranged from 526.1 – 2,337,351 ng/mL (median AUC 1244.2 ng/mL). Peak ace-K concentrations ranged from 299.0 – 4764.2 ng/mL (median peak 945.3 ng/mL) and the AUC ranged from 46,396.7–1,466,799 ng/mL (median AUC 218,885.1 ng/mL). Median values and interquartile ranges for sucralose and ace-K at each time point, along with peaks and AUCs, are shown in Table 1.
In Figure 1A, the time course of the mean NNS concentrations over the 360 minute time course are shown (n=33), with one mother with strikingly high sucralose concentrations shown separately (n=1). Concentrations of sucralose were higher in normal weight compared to obese mothers (median sucralose peak 9.8 vs. 7.6 ng/mL, p=0.01 in normal vs. obese, respectively) and a similar trend was observed for ace-K (1243.5 vs. 876.6 ng/mL, p=0.14) (Figure 1B). However, this trend disappeared after adjustment for blood volume. Concentrations of either sweetener also did not differ based on reported overall NNS consumption, Splenda™ use, nor NNS containing food intake.
Figure 1. Low-calorie sweetener concentrations in breast milk following ingestion of diet soda.
Average breast milk sucralose (A, top left) and acesulfame-potassium (B, top right) concentrations (ng/mL) prior to and for 360 minutes following ingestion of diet soda containing 68 mg sucralose and 41 mg ace-K. N=1 reflects concentrations for one particular mother with strikingly high sucralose concentrations and who was therefore an extreme outlier. Average breast milk sucralose (C, bottom left) and acesulfame-potassium (D, bottom right) concentrations (ng/mL) prior to and for 360 minutes following ingestion of diet soda containing 68 mg sucralose and 41 mg ace-K among normal weight (n=14) compared to obese (n=19) mothers, excluding the one extreme outlier. Values are expressed as mean ± se.
Discussion
Our findings demonstrate that ace-K and sucralose transfer into breast milk following ingestion of a diet soda. Ace-k was immediately detectable, whereas sucralose appeared in breast milk two hours following ingestion, with concentrations varying markedly between individuals. Given that ‘real-life’ NNS consumption often occurs on multiple occasions over the course of several days, concentrations reported in the current analysis may underestimate true infant exposure via the breast milk. Ace-K concentrations peaked approximately four hours after maternal ingestion of the diet soda, whereas sucralose concentrations continued to rise over the six hour time course, suggesting that peak levels had not yet been reached. Compared to plasma concentrations previously determined in adults, ace-K concentrations (13) in breast milk were very similar, but sucralose concentrations were approximately 10-fold lower in breast milk at the 2 hour time point (14).
There are several potential implications of early life exposure to NNS during lactation (3, 4). These include alteration of the gut microbiome (8), which is highly dynamic throughout the first three years of life and is known to be influenced by diet and nutrition during infancy, among many other factors (15). In addition to altering gut microbiota, the presence of NNS in breast milk may also alter the composition of human milk oligosaccharides, known to be associated with infant growth and body composition (16). NNS ingestion has also been proposed to increase the preference for sweet taste (17), and to promote energy intake (18, 19), weight gain (18, 19), and glucose intolerance (20). Due to immature drug transporters (e.g. P-glycoprotein) and drug-metabolizing enzymes (e.g. CYP3A) in neonates(21), ingestion of even small quantities of sucralose (which induces P-gp and CYP3A in rodents (18)), may lead to higher NNS concentrations in the infants’ bloodstream than in adults. Given that the threshold of NNS required to exert metabolic and health effects is unknown, whether rapid sucralose and ace-K appearance in breast milk following maternal ingestion of diet soda plays a clinically relevant role in infant diet and health requires further study.
Limitations of this study include the small sample size, inclusion of NNS consumers and non-consumers, and inability to quantify intake of specific NNS among NNS-consumers. Furthermore, no maternal plasma was obtained and thus, plasma sucralose and ace-K concentrations were not measured. Comparisons between breast milk and blood concentrations could only be based on historic data. In addition, six hours of measurement for was not sufficient to achieve peak sucralose concentrations and no information on infant NNS concentrations or infant health outcomes was collected. Study strengths include serial NNS measurements and the inclusion and comparison of normal weight and obese mothers.
While the current analysis assessed NNS concentrations following a single diet soda ingestion, future research should determine NNS concentrations after repeated exposure. Most importantly, these results further emphasize the need to determine whether chronic ingestion of NNS via the breast milk has clinically relevant consequences such as alteration of taste preferences, gut microbiota, metabolism, and weight trajectory.
What is Known
In utero and early life exposures influence infant taste preferences and eating behavior and may impact long-term weight trajectory and health.
Non-nutritive sweeteners (NNS) such as sucralose, acesulfame-potassium (ace-K), and saccharin are present in human breast milk.
What is New
Acesulfame-potassium rapidly transferred into breast milk following ingestion of a diet soda, whereas sucralose appeared two hours following ingestion, with concentrations varying markedly between individuals.
Concentrations of sucralose were higher in normal weight compared to obese mothers, but was no longer the case after adjustment for blood volume.
Breast milk sweetener concentrations did not differ based on reported overall NNS consumption, Splenda™ use, nor NNS containing food intake.
Acknowledgments
This work was supported in part by an award from Harold Hamm Diabetes Center at the University of Oklahoma and by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also in part supported by the National Institute of Child Health and Human Development (NICHD R01HD080444).
List of Abbreviations
- Ace-K
Acesulfame-potassium
- AUC
Area under the curve
- BMI
Body mass index
- LC-MS
Liquid chromatography-mass spectrometry
- NNS
Non-nutritive sweeteners
- RSD
Relative standard deviation
- US
United States of America
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest.
Clinical Trial Information: NCT #02940795, https://clinicaltrials.gov/ct2/show/NCT02940795?term=David+Fields&draw=3&rank=11
Author Contributions: KIR, ACS, and DAF designed the research. KIR and ACS wrote the first draft of the manuscript. PJW and HMG analyzed the data. All authors read and approved the final version of the manuscript.
References
- 1.Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am J Clin Nutr. 2014;99(3):704S–11S. doi: 10.3945/ajcn.113.067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 3.Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive Sweeteners in Breast Milk. J Toxicol Environ Health A. 2015;78(16):1029–32. doi: 10.1080/15287394.2015.1053646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rother KI, Sylvetsky AC, Schiffman SS. Non-nutritive sweeteners in breast milk: perspective on potential implications of recent findings. Arch Toxicol. 2015;89(11):2169–71. doi: 10.1007/s00204-015-1611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics. 2004;113(4):840–5. doi: 10.1542/peds.113.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventura AK, Mennella JA. Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care. 2011;14(4):379–84. doi: 10.1097/MCO.0b013e328346df65. [DOI] [PubMed] [Google Scholar]
- 7.Fowler SP. Low-calorie sweetener use and energy balance: Results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 9.Philips EM, Jaddoe VW, Trasande L. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58(Suppl 2):44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- 11.Sylvetsky AC, Jin Y, Clark EJ, et al. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J Acad Nutr Diet. 2017 doi: 10.1016/j.jand.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 13.International Programme on Chemical Safety. Acesulfame Potassium. http://www.inchem.org/documents/jecfa/jecmono/v28je13.htm.
- 14.Sylvetsky AC, Bauman V, Blau JE, et al. Plasma concentrations of sucralose in children and adults. Toxicological and Environmental Chemistry. 2016:1–8. doi: 10.1080/02772248.2016.1234754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cong X, Henderson WA, Graf J, et al. Early Life Experience and Gut Microbiome: The Brain-Gut-Microbiota Signaling System. Adv Neonatal Care. 2015;15(5):314–23. doi: 10.1097/ANC.0000000000000191. quiz E1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderete TL, Autran C, Brekke BE, et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102(6):1381–8. doi: 10.3945/ajcn.115.115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang GH, Chen ML, Liu SS, et al. Effects of mother’s dietary exposure to acesulfame-K in Pregnancy or lactation on the adult offspring’s sweet preference. Chem Senses. 2011;36(9):763–70. doi: 10.1093/chemse/bjr050. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A. 2008;71(21):1415–29. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- 19.Swithers SE, Laboy AF, Clark K, et al. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res. 2012;233(1):1–14. doi: 10.1016/j.bbr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suez J, Korem T, Zilberman-Schapira G, et al. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6(2):149–55. doi: 10.1080/19490976.2015.1017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet. 2002;41(12):959–98. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]