Abstract
Background
Isoniazid preventive therapy (IPT) reduces mortality among people living with HIV (PLHIV), and is recommended for those without active tuberculosis (TB) symptoms. Heavy alcohol use, however, is contraindicated for liver toxicity concerns. We evaluated the risks and benefits of IPT at antiretroviral therapy (ART) initiation to ART alone for PLHIV who are heavy drinkers in three high TB/HIV burden countries.
Methods
We developed a Markov simulation model to compare ART alone to ART with either 6 or 36 months of IPT for heavy drinking PLHIV enrolling in care in Brazil, India, and Uganda. Outcomes included non-fatal toxicity, fatal toxicity, life expectancy, TB cases and TB death.
Results
In this simulation, 6 months of IPT+ART (IPT6) extended life expectancy over both ART alone and 36 months of IPT+ ART (IPT36) in India and Uganda, but ART alone dominated in Brazil in 51.5% of simulations. Toxicity occurred in 160/1000 persons on IPT6, and 415/1000 persons on IPT36, with fatal toxicity in 8/1000 on IPT6 and 21/1000 on IPT36. Sensitivity analyses favored IPT6 in India and Uganda with high toxicity thresholds.
Conclusions
The benefits of IPT for heavy drinkers outweighed its risks in India and Uganda when given for a 6-month course. The toxicity/efficacy trade-off was less in Brazil where TB incidence is lower. IPT6 resulted in fatal toxicity in 8/1000 people, whereas even higher toxicities of IPT36 negated its benefits in all countries. Data to better characterize IPT toxicity among HIV-infected drinkers are needed to improve guidance.
Keywords: Tuberculosis, isoniazid, prevention, HIV, alcohol, isoniazid preventive therapy
INTRODUCTION
Tuberculosis (TB) is the leading cause of mortality for people living with HIV (PLHIV) worldwide accounting for nearly one-third of all HIV deaths.1 Although anti-retroviral therapy (ART) significantly reduces TB incidence in PLHIV, there is an increased risk of TB disease during the first months after ART initiation.2 Isoniazid Preventive Therapy (IPT) reduces all-cause mortality and TB disease among PLHIV by 32-62%,4,5 extending beyond the benefit of ART alone.6 The World Health Organization (WHO) thus recommends 36 months of empiric IPT, without diagnostic testing for latent TB infection, for all PLHIV in resource-limited countries without symptoms of active TB disease.7,8 The 2011 guidelines, however, state that “regular and heavy alcohol use” is a contraindication to IPT, presumably for concern of increased hepatotoxicity. The WHO also acknowledges that implementation of 36 months of IPT is extremely low; where IPT is implemented, six month courses remain predominant8.
Grade 3 or 4 drug toxicity is reported in 0.1-4.0% of individuals taking isoniazid.9 Alcohol users are considered higher risk as isoniazid is metabolized by the liver. One observational US study in 1978 reported daily drinkers had more than four times the risk of toxicity compared to non-drinkers,10 but those high toxicity rates are not consistently observed.11 There is also theoretical concern about isoniazid and ART interactions, but higher rates of toxicity with concomitant ART initiation were not observed in trials.6,12
Heavy alcohol consumption, defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria of ≥ 4 drinks per day or 14 drinks per week for men and ≥ 3 drinks per day or 7 drinks per week for women, is common among PLHIV. In studies of PLHIV in sub-Saharan Africa, Brazil, and India, as many as 25% of participants self-reported heavy alcohol consumption.13–16 Current guidance may thus exclude 25% of PLHIV from IPT because of their heavy drinking. Additionally, heavy drinking is associated with a three-fold increase in the risk of TB disease, slower TB treatment response, and higher mortality on therapy compared to non-drinkers.17–19
We hypothesized that the benefit of a six-month course of IPT for heavy alcohol drinking PLHIV in high TB/HIV burden countries is greater than the elevated risk for Grade 3/4 drug toxicity. To investigate, we developed a decision analytic model to compare the risks and benefits of providing IPT for either six months or 36 months at initiation of ART to ART alone for PLHIV who heavily consume alcohol. We validated the model in three high TB/HIV burden countries—Brazil, India, and Uganda—to further compare the impact of TB prevalence and mortality on the benefit of IPT.
METHODS
Analytic Overview
We developed a Markov model to compare ART alone to ART with either six months or 36 months of empiric IPT for heavy drinking PLHIV enrolling in care in three high TB/HIV burden countries: Brazil, India, and Uganda (supplement Figure 1). We constructed the model using TreeAge Pro 2016 (TreeAge Software Inc., Williamstown, MA). All analyses simulated a closed cohort of PLHIV classified as heavy drinkers by NIAAA criteria initiating ART. The model utilized a lifetime horizon. Outcomes included life expectancy in years, cumulative TB cases, TB deaths, and fatal and non-fatal toxicity events. We developed inputs to replicate epidemiology, TB disease incidence, and outcomes specific to each country. We performed one-and two-way deterministic sensitivity analyses to evaluate the impact of parameters on model outcomes. To characterize uncertainty around the base case findings, we also performed probabilistic sensitivity analyses. We defined a probability density function around each parameter value and used second-order Monte Carlo simulation to replicate the simulation 1,000 times. We reported all results with an associated 95% confidence range.20
Model Structure
The model employed a Markov framework with a monthly time cycle. The simulation cohort entered the model and initiated ART either alone or with six or 36 months of IPT. During months on IPT, a portion of the cohort experienced symptomatic drug toxicity at which point IPT was discontinued. A portion of IPT toxicity events were fatal. For the six-month IPT course, we assumed the IPT protective benefit extended for six-months beyond therapy after which the incidence of TB returned to that expected without IPT. For the 36-month IPT course, the benefit also extended six months beyond therapy, and the risk of IPT toxicity declined over time. The lifetime simulation included five health states: 1) alive without TB 2) alive with TB disease 3) alive after treatment for TB disease, 4) dead, TB-attributable, 5) dead, other causes. In the base case, we assumed no TB relapse after treatment and then relaxed that assumption in sensitivity analyses. The cohort also experienced mortality from causes other than TB, including HIV-related and age/sex adjusted non-HIV competing risks of death.
Base case parameters
Table 1 summarizes model parameters for cohort characteristics, tuberculosis infection, IPT toxicity and effectiveness, and mortality with ranges used for deterministic sensitivity analyses.
Table 1.
Model input parameters for a comparative analysis of the risks and benefits from isoniazid preventive therapy (IPT) for either six or 36 months plus antiretroviral therapy (ART) versus ART alone among people living with HIV who heavily consume alcohol. Ranges in parenthesis were used for deterministic sensitivity analyses.
| Model Parameter | Brazil (Range) | India (Range) | Uganda (Range) | Source(s) |
|---|---|---|---|---|
| Proportion female | 0.35 (0.20-0.55) | 0.40 (0.27-0.60) | 0.66 (0.45-0.70) | 21–23 |
|
| ||||
| Baseline Age (years) | 33.0 (25-42) | 33.0 (27-40) | 33.3 (20-40) | 24–26 |
|
| ||||
| Monthly incidence of TB per | ||||
| 1000 persons stratified by duration of ART | ||||
| 0-3 months of ART | 9.94 | 59.7 | 20.4 | 2,26–28 |
| 3-6 months of ART | 5.00 | 30.0 | 10.3 | |
| 6-12 months of ART | 2.31 | 13.9 | 4.75 | |
| 12-24 months of ART | 0.86 | 5.13 | 1.76 | |
| 24-36 months of ART | 0.65 | 3.90 | 1.33 | |
| > 36 months of ART | 0.44 | 2.66 | 0.91 | |
|
| ||||
| TB Case Fatality Ratio | 13% (4-25%) | 13% (4-25%) | 13% (4-25%) | 38,39 |
|
| ||||
| Monthly Probability of Grade | 6,12,32 | |||
| 3 or 4 IPT toxicity | ||||
| • 0–12 months IPT | 0.029 | 0.029 | 0.029 | |
| • 13–24 months IPT | 0.011 | 0.011 | 0.011 | |
| • 25–36 months IPT | 0.002 | 0.002 | 0.002 | |
|
| ||||
| Probability of Grade 3 or 4 IPT toxicity in Months 0-12 – ATS Estimate | 0.05 | 0.05 | 0.05 | 9,10 |
|
| ||||
| Probability of fatal IPT toxicity among those with Grade 3 or 4 toxicity | 0.05 (0 – 0.23) | 0.05 (0 – 0.23) | 0.05 (0 – 0.23) | 6,12,33 |
|
| ||||
| Duration IPT effect (months) | (0-120) | (0-120) | (0-120) | 35–37 |
| • 6 month course IPT | 12 | 12 | 12 | |
| • 36 month course IPT | 42 | 42 | 42 | |
|
| ||||
| IPT Effectiveness | 0.9 (0 -1) | 0.9 (0 -1) | 0.9 (0 -1) | 34 |
TB = Tuberculosis; ATS = American Thoracic Society
Cohort Characteristics
We derived the proportion female for each country from the United Nations Programme on HIV/AIDS country progress reports.21–23 Baseline age was taken as the median age reported in cohort studies of PLHIV initiating ART in each country.24–26
Tuberculosis Disease Incidence
We modeled the relative risk of TB disease by time on ART, such that the probability of developing TB disease was the highest during the first three months of ART.2 We derived cumulative incidence of TB disease from country-specific observational data among cohorts initiating ART.2,26–28 The base case assumed no TB disease relapse after cure, but sensitivity analyses explored TB disease relapse with rates informed by the American Thoracic Society (ATS) guidelines from 0-6% per year.29
IPT Toxicity and Effectiveness
We estimated IPT toxicity among drinkers initiating ART in two steps. First, we abstracted the rate of Grade 3/4 adverse events in the early ART+IPT arm of the TEMPRANO ANRS trial.6 Second, to estimate the effect of alcohol use on risk of IPT toxicity, we identified a cohort study in Botswana that reported IPT hepatotoxicity rates among participants on ART stratified by alcohol dependence characterized by the CAGE questionnaire.12,30 We applied the risk ratio of 2.37 found in this study to the rate of toxicity observed in TEMPRANO. Because data about the effect of alcohol on IPT toxicity are limited, we developed an additional estimate and report findings for both. For the second estimate, we applied the risk ratio for isoniazid toxicity among daily drinkers in the general population (not PLHIV specific) referenced in the ATS documents (RR = 4.14).9,10 For the 36-month course of IPT, we reduced the probability of developing IPT toxicity after 12 months and again after 24 months. We calculated the risk ratios for relative reductions over time from the adverse event rates reported from two clinical sites in Swaziland,31,32 and applied these ratios to our base case toxicity estimates. Fatal drug toxicity depended on the development IPT toxicity. We derived the base case estimate from the Botswana cohort and extrapolated the range for sensitivity analyses from the 95% confidence interval reported by the National Institutes of Health isoniazid drug record.33 We assume that underlying ART toxicity is equivalent in all strategies. To estimate the toxicity attributable to alcohol, we performed sensitivity analyses applying the rate of toxicity events seen in the TEMPRANO ANRS trial, and compared the proportion of toxicity and toxicity deaths to our base case.
We derived the effectiveness of IPT while on therapy from the BOTUSA trial34. In the six-month IPT arm, the protective effectiveness of IPT extended for one-year, six months of active therapy plus six months of extended benefit, after which participants resumed monthly risk for developing TB disease corresponding to the cycle month of the model at that point in time.35–37 Similarly, we extended the benefit of the 36-month IPT course an additional six months.
Mortality
We derived the case fatality ratio (CFR) for TB disease by combining a weighted average of the pooled CFR reported in a meta-analysis of HIV infected patients on ART38 with the CFR reported for HIV infected patients who default on TB treatment, assuming a 20% defaulter rate in the base case.39
We estimated non-TB mortality, stratified by age, using country specific life tables.43–45 Because TB-related morality likely impacts national-level life expectancy in endemic zones, we adjusted life-tables to remove TB mortality. To do so, we estimated the TB attributable mortality rate in each country as the product of prevalence of TB disease * country mortality rate of those with TB disease. The country mortality rates were extracted from WHO TB reports for each country. We then subtracted TB attributable mortality rates from the all-cause mortality rates (Supplement Table 1).
Model Validation
We validated the model in each country against median life expectancy and cumulative TB incidence.
Sensitivity Analyses
We performed deterministic one-way sensitivity analyses for all model parameters. The ranges for deterministic sensitivity analyses were informed by the 95% confidence intervals from observational studies, and based on expert opinion when no quantitative measure of uncertainty was available. In the sensitivity analyses for IPT toxicity, we explored a range of toxicity in order to capture the additional effect of viral hepatitis (B and C) co-infection. For the sensitivity analysis of TB incidence stratified by duration on ART, we used a multiplier to proportionally scale up and down the base case incidence rates while maintaining the trend for decreasing incidence by longer duration of ART. These analyses not only account for uncertainty in the parameter estimates, but also model the increased risk for TB disease among the heaviest drinkers. We explored a wide range for the TB CFR to simulate excellent treatment retention and effectiveness on one extreme, and high default rates or poor treatment effectiveness as may be seen with MDR TB cases on the other extreme. For non-TB mortality, we applied a multiplier to proportionally scale-up base case background mortality rates to simulate higher mortality at lower CD4 counts. We also assessed parameters to allow for TB relapse after TB disease treatment completion, and varied IPT effectiveness to simulate both poor medication compliance and decreased effectiveness for the prevention of isoniazid resistant TB.
We constructed tornado diagrams from the one-way sensitivity analyses for each country to evaluate the impact of model parameters on the life expectancy outcome. For parameters that demonstrated high impact, we performed several two-way sensitivity analyses. We utilized threshold sensitivity analyses around the IPT toxicity assumptions to determine the maximum toxicity levels allowable that would favor IPT in each setting.
Lastly, we conducted probabilistic sensitivity analyses to characterize uncertainty in the simulation results (see supplement Table 2). We employed the beta distribution to generate probability density functions around each probability parameter, based on the counts of events from observational studies. For baseline age and the duration of IPT effect, we assumed a normal distribution of all values specified in the range tested for deterministic sensitivity analyses. For the multiplier variables used to vary TB incidence, IPT toxicity over time, and background non-TB mortality outlined above, we applied a uniform distribution.
RESULTS
Base Case
The strategy of six months of IPT+ART (IPT6) extended life expectancy over both ART alone and 36 months of IPT+ ART (IPT36) in India and Uganda, but neither IPT strategy improved life expectancy in Brazil over ART alone (Table 2). In India, IPT6 extended life expectancy by 0.5 years, IPT36 by 0.3 years. IPT6 reduced TB incidence from 801 TB cases per 1000 persons to 706 cases per 1000 persons, and deaths by 12 per 1000 persons, whereas IPT36 further reduced TB incidence to 665 cases per 1000 persons, and deaths by 17 per 1000 persons. In Uganda, IPT6 extended life expectancy 0.1 years beyond ART alone, whereas IPT36 reduced life expectancy 0.2 years compared to ART alone. IPT6 reduced TB cases by 82 per 1000 persons, and TB deaths by 10 per 1000 persons. ART alone extended life expectancy in Brazil by 0.1 years compared to IPT6, and 0.5 years compared to IPT36. The cumulative cases of TB decreased from 259 cases per 1000 persons on ART to 209 on IPT6, and 193 on IPT36, with 6-8 additional TB fatalities per 1000 persons on ART alone. In all countries, IPT6 resulted in Grade 3/4 toxicity in 158-160 per 1000 persons treated with 8 toxicity deaths per 1000 persons. Between 406-415 persons per 1000 treated developed Grade 3/4 toxicity in the IPT36 arm with 20-21 deaths per 1000 treated.
Table 2.
Base case projected outcomes by country—Brazil, India, Uganda— for an analysis of the risks and benefits from isoniazid preventive therapy (IPT) for either six (IPT 6) or 36 months (IPT 36) plus antiretroviral therapy (ART) versus ART alone among people living with HIV who heavily consume alcohol. The 95% confidence ranges (95% CR) are presented from probabilistic sensitivity analyses.
| Life Expectancy (Years)* (95% CR) | Toxicity Per 1000 Persons (95% CR) | Alcohol Attributable Tox. per 1000 Persons | Toxicity Deaths per 1000 persons (95% CR) | Alcohol Attributable Tox. Deaths per 1000 | TB Cases per 1000 persons (95% CR) | TB Deaths per 1000 persons (95% CR) | |
|---|---|---|---|---|---|---|---|
| Brazil | |||||||
| IPT 6+ART | 42.8 (35.1-51.6) | 160 (90-290) | 89 | 8 (0-30) | 4 | 209 (110-312) | 26 (8-58) |
| IPT 36+ART | 42.4 (34.6-51.1) | 415 (250-650) | 213 | 21 (0-69) | 11 | 193 (99-293) | 24 (11-61) |
| ART | 42.9 (35.2-51.5) | 0 | 0 | 0 | 0 | 259 (142-374) | 32 (21-78) |
| India | |||||||
| IPT 6+ART | 38.1 (31.7-46.85) | 158 (89-291) | 88 | 8 (0-28) | 4 | 706 (487-841) | 86 (12-210 |
| IPT 36+ART | 37.9 (31.6-46.4) | 406 (253-638) | 209 | 20 (0-68) | 10 | 665 (453-809) | 81 (10-198) |
| ART | 37.6 (30.9-46.9) | 0 | 0 | 0 | 0 | 801 (594-908) | 98 (17-243) |
| Uganda | |||||||
| IPT 6+ART | 36.5 (27.4-47.7) | 160 (93-288) | 89 | 8 (0-32) | 4 | 336 (194-503) | 41 (7-97) |
| IPT 36+ART | 36.2 (26.9-47.3) | 412 (437-652) | 212 | 21 (1-58) | 11 | 308 (172-468) | 38 (10-93) |
| ART | 36.4 (27.5-47.6) | 0 | 0 | 0 | 418 (248-577) | 51 (10-122) | |
Years of life expectancy after entry into the simulation; Tox. = Toxicity; TB = Tuberculosis; ART = anti-retroviral therapy; IPT = isoniazid preventive therapy
Deterministic Sensitivity Analyses
One-way sensitivity analyses of input parameters are shown in Figure 1 and in the supplement (supplement Figures 2 – 8). Parameters with the greatest impact on the risk-benefit ratio of IPT varied by country, with monthly incidence of TB and monthly probability of TB death consistently in the top four (supplement Figures 4, 5 and 9). Fatal and non-fatal IPT toxicity were impactful parameters in Brazil and Uganda, while duration of IPT effect superseded fatal IPT toxicity in India. Toxicity and fatal toxicity attributable to alcohol was between 50-55.6% (Table 2). The risk-benefit favored IPT6 in Brazil when Grade 3/4 IPT toxicity was below 0.023, (base case estimate 0.029, ATS comparator 0.05), and favored IPT36 when toxicity decreased to 0.01 (Figure 1). The threshold probability of IPT toxicity in India that shifted IPT6 to ART alone was 0.087 (three times the base case estimate), and 0.044 for IPT36 to ART alone (1.5 times the base case). In Uganda, the strategy shifted against IPT6 to ART alone at a toxicity threshold of 0.044, and against IPT36 to ART alone when the toxicity exceeded 0.02.
Figure 1.
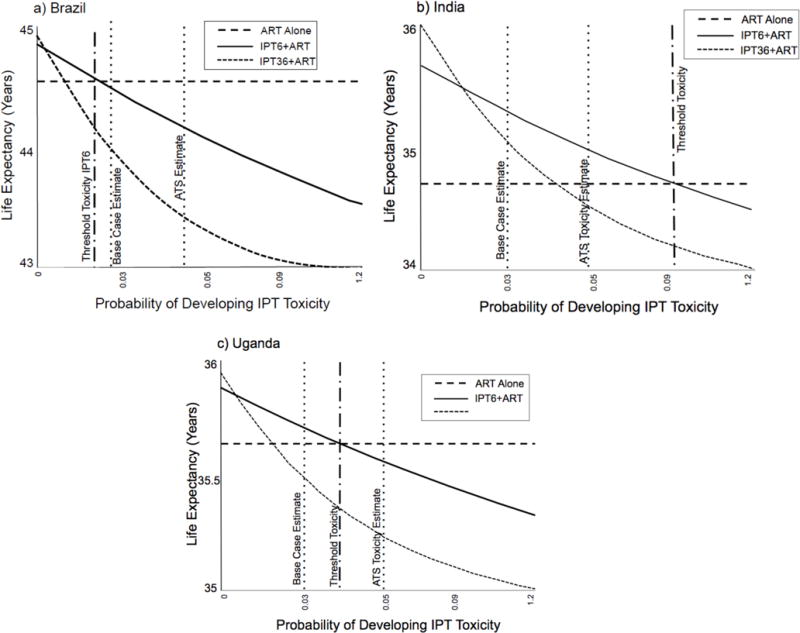
One-way sensitivity analyses of the threshold probability of isoniazid preventive therapy (IPT) toxicity at the initiation of antiretroviral therapy (ART) compared to the base case estimate and American Thoracic Society (ATS) estimate among people with HIV infection who heavily consume alcohol in a) Brazil b) India and c) Uganda.
The benefit of IPT6 exceeded the risk when the probability of fatal toxicity was less than 0.04 in Brazil, 0.13 in India, and 0.07 in Uganda (base case 0.05 in all countries) (supplement Figure 6). The probabilities of fatal toxicity required to shift the results against IPT36 were 0.02 in Brazil, 0.07 in India, and 0.04 in Uganda. Varying the effectiveness of IPT up to 100% did not change the preferred strategy of ART alone in Brazil. When the effectiveness of IPT was below 37% in India, the preferred strategy shifted from IPT36 to no IPT, but the minimal level of effectiveness of IPT needed to shift from favoring IPT6 to no IPT was 4%. In Uganda, the preferred strategy was ART alone if IPT effectiveness was below 49.4% (supplement Figure 7).
Two-way sensitivity analyses of the monthly probability of TB death and the incidence of TB during the first three months of ART found the intersection of base case values favored no IPT in Brazil with a narrow margin—small increases in TB mortality or TB incidence shifted the strategy to IPT6. IPT6 was clearly favored in India, and while still favored in Uganda, the margin was narrower (Figure 2). These trends persisted in two-way sensitivity analyses of the probability of developing IPT toxicity and the incidence of TB disease during the first 3 months of ART (Supplement Figure 10). The intersection point of base case values for each parameter favored IPT6 in India and Uganda, whereas ART alone dominated the strategies in Brazil. However, for the heaviest drinkers, with up to three times greater risk of developing TB disease, the results favored IPT6 when IPT toxicity was up to twice that of the base case estimate. IPT36 was preferred only with increased risk for TB disease along with reduced IPT toxicity, scenarios less likely among the heaviest drinkers.
Figure 2.
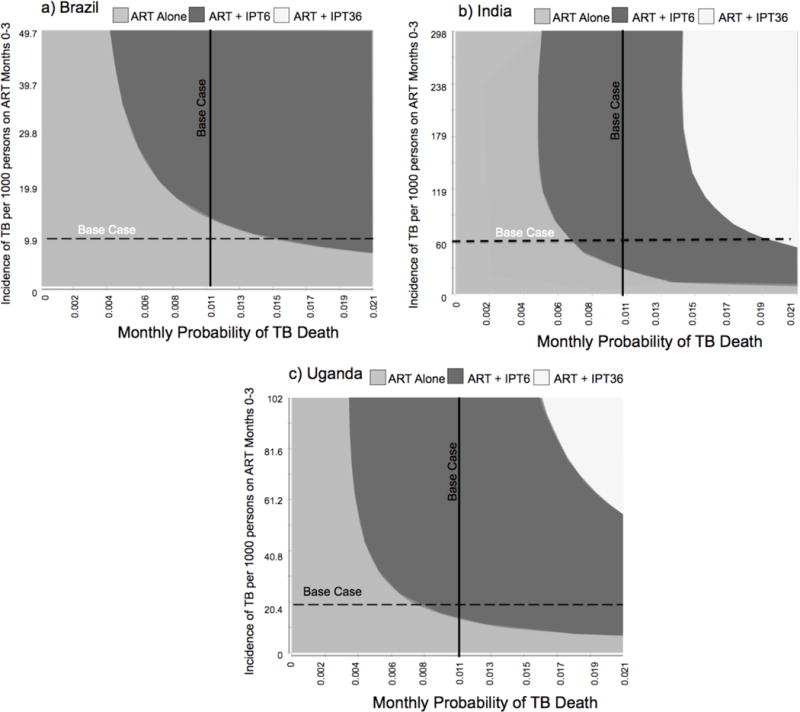
Two-way sensitivity analyses of the monthly probability of death from tuberculosis (TB) disease and monthly TB disease incidence per 1000 people during the first 3 months of ART among people with HIV infection who heaviliy consume alcohol in a) Brazil b) India and c) Uganda.
Probabilistic Sensitivity Analyses
In Brazil, ART alone remained dominant in 51.5% of simulations, while IPT6 was selected 41.1% of the time and IPT36 7.4% (Figure 3, and supplement Table 3). Strategy selection was less robust to uncertainly in India with IPT6 selected 47.5%, IPT36 27.9%, and ART alone 24.6% of the time. In Uganda, ART alone dominated 44.4% of simulations, while the strategy favored IPT6 in 43.2% and IPT36 in 12.4% of simulations.
Figure 3.
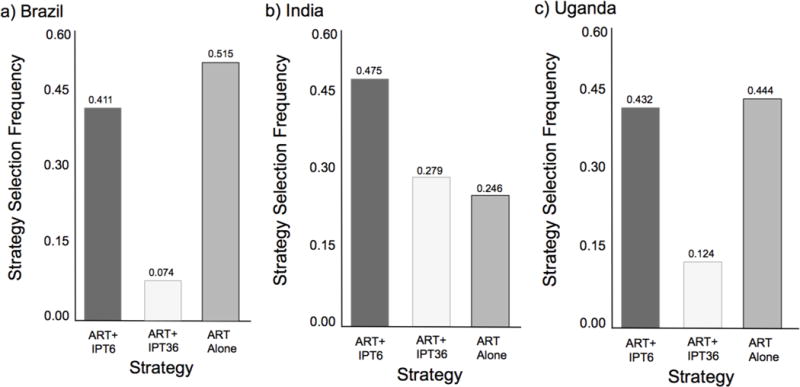
Histograms depicting strategy selection frequency of antiretroviral therapy (ART) plus six-months of isoniazid preventive therapy (IPT), ART plus 36-months of IPT, or ART alone from probabilistic sensitivity analyses of a model simulating a cohort of people living with HIV who heavily consume alcohol enrolling in care in a) Brazil b) India, and c) Uganda.
DISCUSSION
In this simulation model, the benefits of a six-month course of IPT at initiation of ART among heavy drinking PLHIV compared to ART alone outweighed the risks in high TB/HIV prevalence settings, as seen in India and Uganda. The risk-benefit of IPT was less in Brazil where TB incidence is lower. Overall, the 36-month course of IPT reduced the cumulative incidence of TB disease and death compared to the six-month course of IPT and ART alone in all simulated countries; however, the increased cases of IPT toxicity and deaths that accumulated over the 36-month course negated its benefits beyond the six-month comparison. The uncertainty in strategy selection seen in probabilistic sensitivity analyses, particularly in Uganda, highlight the need to better characterize IPT toxicity in heavy drinkers.
Global TB and HIV guidelines currently do not reflect the differential impact of IPT in varying country settings that we report. Under conditions of high TB incidence, such as in India and Uganda, IPT toxicity thresholds favoring IPT6 were much higher than our most conservative estimates. These sensitivity analyses also support that IPT benefits are likely to outweigh the increased toxicity risk among heavy drinkers with concomitant viral hepatitis co-infection in high prevalence TB settings. In contrast, data from Brazil showed that in a country with lower TB incidence the risk of IPT exceeded the benefit unless the true toxicity rate of IPT is 21% less than our base case estimate. The heterogeneity seen between countries suggests that having a single global guideline for IPT among HIV-infected drinkers in resource-limited countries is not optimal.
Though we found that empiric IPT extended life expectancy in many settings, it is clear that strategies to minimize IPT-related morbidity and mortality among drinkers are important for real-world implementation. Instructing patients to stop IPT should symptoms of appetite loss, malaise, or jaundice develop can help prevent hepatic failure and death. Further risk mitigation with liver enzyme monitoring may also prompt discontinuation of therapy prior to symptom development and irreversible hepatotoxicity. Unfortunately, in settings where IPT is most beneficial, liver enzyme monitoring is often not feasible. However, close to patient diagnostics for liver enzyme monitoring are in development, and may make monitoring possible in some settings.46 Another strategy is to better understand the heterogeneity of toxicity risk among drinkers, which clinical or alcohol use characteristics are associated with complications, and identify those who could be safely treated.
We note limitations to this analysis. Few studies to date report IPT toxicity stratified by alcohol consumption, and only one among PLHIV.12,47–49 Thus, this parameter has the most uncertainty, as it was derived from a combination of trial data and prospective cohort data. The base case estimates may in fact double count for some alcohol use as the TEMPRANO trial did not explicitly exclude those who consume alcohol, and the general population toxicity risk may also overestimate as it is not HIV specific and does not exclude drinkers. We employed sensitivity analyses to account for uncertainty in the estimates, and we present toxicity thresholds for whether IPT is favored in each setting.
Second, we included all HIV-infected drinkers, and did not investigate providing IPT only for those who have a positive tuberculin skin test (TST), where the benefits of IPT appear to be the greatest.5 However, TST is not currently part of the WHO guidance,7 and often not available in resource-limited settings with high TB burden. Where TB incidence and mortality are lower, screening strategies for latent TB infection like TST are likely effective to target and treat only those who are infected and decrease unnecessary exposure to IPT toxicity. We also did not simulate TB reinfection or transmission, meaning our model is conservative in that it does not incorporate the indirect benefit of averted TB transmission. Furthermore, this model strictly evaluated life years gained, cases of TB reduced, and deaths avoided. It did not include utility measures such as health-related quality of life. The comparisons thus did not capture the benefits of improved quality of life among those who avoid TB disease, or the potential decrease in quality of life associated with taking daily medications (i.e. for six or 36 months). Lastly, we did not investigate newer regimens, such as 12 weekly doses of rifapentine plus IPT, which have shown promising safety, efficacy, and adherence results,51 but are not yet approved for use in developing countries.
Our findings suggest IPT benefits for PLHIV who heavily consume alcohol outweigh the potential risks of increased drug toxicity where TB incidence and mortality are high, and among those with increased TB disease risk. For countries with lower TB incidence, like Brazil, IPT toxicity must be lower than our estimates for the benefits to exceed the risks. These results highlight the need for more nuanced recommendations for IPT stratified by TB incidence and/or TB mortality such that countries can implement the policy most applicable to the epidemiology of TB within their borders as opposed to a global one-size-fits-all guideline. Furthermore, there is a clear need for prospective studies of IPT toxicity among PLHIV who consume alcohol. Such data could inform strategies to increase the safety profile for those at the highest toxicity risk, instead of current recommendations to withhold beneficial therapy from a substantial proportion of those in greatest need.
Supplementary Material
Acknowledgments
FUNDING
This project was supported by the National Center for Advancing Translational Sciences (grant number 1KL2TR001411), the National Institute of Allergy and Infectious Diseases (grant numbers 5T32AI052074-10, 5P30AI042853-18, and R01 AI119037-02), the National Institute on Alcohol Abuse and Alcoholism (grant numbers K24 AA022586, U01 AA20776), and the National Institute on Drug Abuse (grant number P30DA040500). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data from this project were presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, February 2017. Dr. Freiman received a Young Investigators Scholarship to attend the conference.
Conflicts of interest: none to report
References
- 1.World Health Organization. Global Tuberculosis Report 2015. Geneva: [Google Scholar]
- 2.Liu E, Makubi A, Drain P, et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. Aids. 2015;29(11):1391–1399. doi: 10.1097/QAD.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr. 2011;56(4):349–355. doi: 10.1097/QAI.0b013e3181f9fb39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayele HT, Mourik MS, Debray TP, Bonten MJ. Isoniazid Prophylactic Therapy for the Prevention of Tuberculosis in HIV Infected Adults: A Systematic Review and Meta-Analysis of Randomized Trials. PLoS One. 2015;10(11):e0142290. doi: 10.1371/journal.pone.0142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database of Systematic Reviews. 2010;(Issue 1) doi: 10.1002/14651858.CD000171.pub3. Art. No.: CD000171. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danel C, Moh R, Gabillard D, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for intensified tuberculosis case finding and Isoniazid preventive therapy for people living with HIV in resource constrained settings. Geneva: Switzerland; 2011. [Google Scholar]
- 8.WHO Guidelines Approved by the Guidelines Review Committee. Recommendation on 36 Months Isoniazid Preventive Therapy to Adults and Adolescents Living with HIV in Resource-Constrained and High TB- and HIV-Prevalence Settings: 2015 Update. Geneva: World Health Organization Copyright (c) World Health Organization 2015; 2015. [PubMed] [Google Scholar]
- 9.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 10.Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117(6):991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 11.Comstock GW. Prevention of tuberculosis among tuberculin reactors: maximizing benefits, minimizing risks. Jama. 1986;256(19):2729–2730. [PubMed] [Google Scholar]
- 12.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in hiv-infected adults in Botswana. Am J Respir Crit Care Med. 2010;182(2):278–285. doi: 10.1164/rccm.200911-1783OC. [DOI] [PubMed] [Google Scholar]
- 13.Thakarar K, Asiimwe SB, Cheng DM, et al. Alcohol Consumption in Ugandan HIV-Infected Household-Brewers Versus Non-Brewers. AIDS Behav. 2016 doi: 10.1007/s10461-016-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wandera B, Tumwesigye NM, Nankabirwa JI, et al. Alcohol Consumption among HIV-Infected Persons in a Large Urban HIV Clinic in Kampala Uganda: A Constellation of Harmful Behaviors. PLoS One. 2015;10(5):e0126236. doi: 10.1371/journal.pone.0126236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Sachdeva RK, Kumar M, Nehra R, Nakra M, Jones D. Effects of Lifetime History of Use of Problematic Alcohol on HIV Medication Adherence. J Int Assoc Provid AIDS Care. 2014;13(5):450–453. doi: 10.1177/2325957413491430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva CM, Mendoza-Sassi RA, da Mota LD, Nader MM, de Martinez AM. Alcohol use disorders among people living with HIV/AIDS in Southern Brazil: prevalence, risk factors and biological markers outcomes. BMC Infect Dis. 2017;17(1):263. doi: 10.1186/s12879-017-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkmann T, Moonan PK, Miramontes R, Oeltmann JE. Tuberculosis and excess alcohol use in the United States, 1997–2012. Int J Tuberc Lung Dis. 2015;19(1):111–119. doi: 10.5588/ijtld.14.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amoakwa K, Martinson NA, Moulton LH, Barnes GL, Msandiwa R, Chaisson RE. Risk Factors for Developing Active Tuberculosis After the Treatment of Latent Tuberculosis in Adults Infected With Human Immunodeficiency Virus. Open Forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. Second. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 21.UNAIDS. The HIV and AIDS Uganda Country Progress Report 2014. 2015 [Google Scholar]
- 22.UNAIDS. The HIV and AIDS Brazil Country Progress Report. 2015 [Google Scholar]
- 23.UNAIDS. The HIV and AIDS India Country Progress Report. 2015 [Google Scholar]
- 24.Hahn JA, Emenyonu NI, Fatch R, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction. 2016;111(2):272–279. doi: 10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta SH, McFall AM, Srikrishnan AK, et al. Morbidity and Mortality Among Community-Based People Who Inject Drugs With a High Hepatitis C and Human Immunodeficiency Virus Burden in Chennai, India. Open Forum Infect Dis. 2016;3(3):ofw121. doi: 10.1093/ofid/ofw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH. The impact of anti-retroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21 doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Uria G, Pakam R, Midde M, Naik PK. Incidence and mortality of tuberculosis before and after initiation of antiretroviral therapy: an HIV cohort study in India. J Int AIDS Soc. 2014;17:19251. doi: 10.7448/IAS.17.1.19251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. Aids. 2007;21(6):713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. United States. [DOI] [PubMed] [Google Scholar]
- 30.Ewing JA. Detecting alcoholism. The CAGE questionnaire. Jama. 1984;252(14):1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 31.Muller Y. Implementation of 36 months of isoniazid preventive therapy for patients living with HIV/AIDS in two clinics of Shilselwini region, Kingdom of Swaziland. Medecins sans frontieres; 2016. [Google Scholar]
- 32.Mueller Y, Mpala Q, Kerschberger B, et al. Adherence, tolerability, and outcome after 36 months of isoniazid-preventive therapy in 2 rural clinics of Swaziland: A prospective observational feasibility study. Medicine (Baltimore) 2017;96(35):e7740. doi: 10.1097/MD.0000000000007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institutes of Health. LiverTox: Isoniazid. Clinical and Research Information on Drug-Induced Liver Toxicity. https://livertox.nih.gov/Isoniazid.htm. Accessed June 21, 2016.
- 34.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JL, Okwera A, Hom DL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. Aids. 2001;15(16):2137–2147. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 36.Sumner T, Houben RM, Rangaka MX, et al. Post-treatment effect of isoniazid preventive therapy on tuberculosis incidence in HIV-infected individuals on antiretroviral therapy. Aids. 2016;30(8):1279–1286. doi: 10.1097/QAD.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 37.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384(9944):682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odone A, Amadasi S, White RG, Cohen T, Grant AD, Houben R. The Impact of Antiretroviral Therapy on Mortality in HIV Positive People during Tuberculosis Treatment: A Systematic Review and Meta-Analysis. In: Kranzer K, editor. PLoS One. Vol. 9. San Francisco, USA: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis. 2009;13(3):283–303. [PubMed] [Google Scholar]
- 40.World Health Organization. Tuberculosis Profile. Uganda: 2015. [Google Scholar]
- 41.World Health Organization. Tuberculosis Profile. India: 2015. [Google Scholar]
- 42.World Health Organization. Tuberculosis Profile. Brazil: 2015. [Google Scholar]
- 43.World Health Organization. Life Table. Uganda: 2015. [Google Scholar]
- 44.World Health Organization. Life Table. India: 2015. [Google Scholar]
- 45.World Health Organization. Life Table. Brazil: 2015. [Google Scholar]
- 46.Jain S, Rajasingham R, Noubary F, et al. Performance of an Optimized Paper-Based Test for Rapid Visual Measurement of Alanine Aminotransferase (ALT) in Fingerstick and Venipuncture Samples. PLoS One. 2015;10(5):e0128118. doi: 10.1371/journal.pone.0128118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bliven-Sizemore EE, Sterling TR, Shang N, et al. Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc Lung Dis. 2015;19(9):1039–1044. i–v. doi: 10.5588/ijtld.14.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- 49.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168(4):443–447. doi: 10.1164/rccm.200303-390OC. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JL, Nyole S, Okwera A, et al. Instability of tuberculin and Candida skin test reactivity in HIV-infected Ugandans. The Uganda-Case Western Reserve University Research Collaboration. Am J Respir Crit Care Med. 1998;158(6):1790–1796. doi: 10.1164/ajrccm.158.6.9803104. [DOI] [PubMed] [Google Scholar]
- 51.Pease C, Hutton B, Yazdi F, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC Infect Dis. 2017;17(1):265. doi: 10.1186/s12879-017-2377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


